Abstract
Methyl viologen and phenazine methosulfate (photosystem I electron acceptors), 3-(3,4-dichlorophenyl)-1, 1-dimethylurea (DCMU, electron-transport inhibitor), and methylamine (photophosphorylation uncoupler) were used to study the dependence of nitrite reduction on electron transport in chloroplasts.
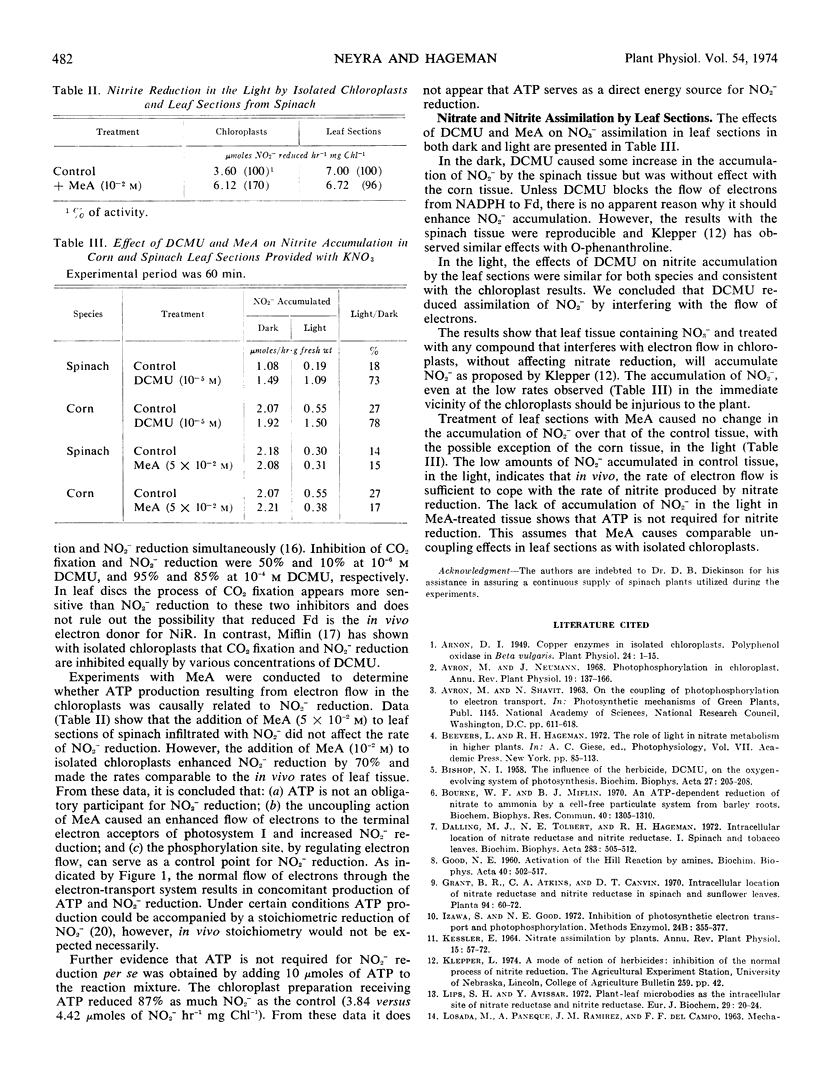
DCMU, methyl viologen, and phenazine methosulfate markedly inhibited, whereas methylamine stimulated NO2− reduction in isolated, intact spinach (Spinacia oleracea L.) chloroplasts. The addition of DCMU to leaf sections of spinach and corn, (Zea mays L. var. XL81), incubated with No3−, caused no inhibition of nitrate reduction but inhibited nitrite reduction leading to the accumulation of NO2− in the light. The addition of methylamine to comparable leaf sections did not affect either nitrate or nitrite reduction.
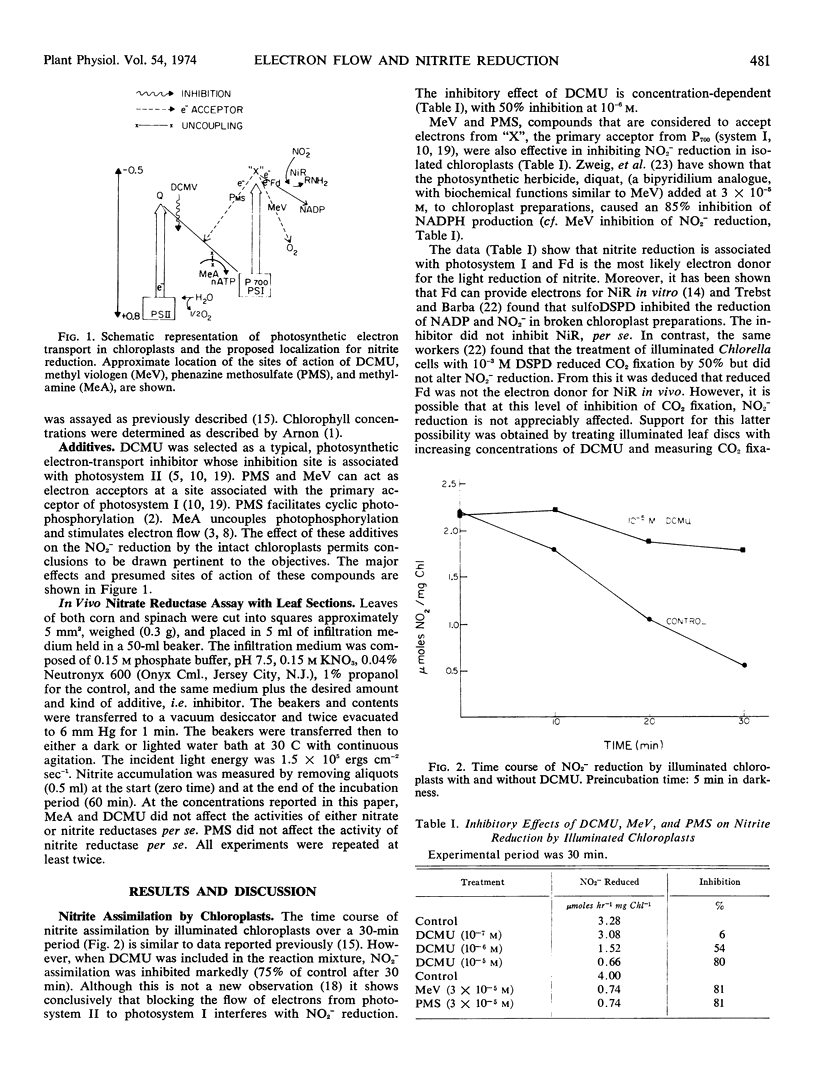
We concluded that: (a) nitrite reduction is functionally associated with the electron transport arising from the light reactions of the chloroplast and this provides additional support for the localization of nitrite reductase in the chloroplast; (b) nitrite reduction is associated with photosystem I and ferredoxin is the most likely donor in leaf tissue; and (c) ATP is not involved directly in nitrite reduction. However, ATP synthesis, by regulating electron flow to photosystem I, can affect nitrite reduction in the light.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP N. I. The influence of the herbicide, DCMU, on the oxygen-evolving system of photosynthesis. Biochim Biophys Acta. 1958 Jan;27(1):205–206. doi: 10.1016/0006-3002(58)90313-5. [DOI] [PubMed] [Google Scholar]
- Beevers L., Hageman R. H. The role of light in nitrate metabolism in higher plants. Photophysiology. 1972;(7):85–113. [PubMed] [Google Scholar]
- Bourne W. F., Miflin B. J. An ATP dependent reduction of nitrate to ammonia by a cell free particulate system from barley roots. Biochem Biophys Res Commun. 1970 Sep 30;40(6):1305–1310. doi: 10.1016/0006-291x(70)90008-2. [DOI] [PubMed] [Google Scholar]
- Dalling M. J., Tolbert N. E., Hageman R. H. Intracellular location of nitrate reductase and nitrite reductase. I. Spinach and tobacco leaves. Biochim Biophys Acta. 1972 Dec 14;283(3):505–512. doi: 10.1016/0005-2728(72)90266-6. [DOI] [PubMed] [Google Scholar]
- GOOD N. E. Activation of the Hill reaction by amines. Biochim Biophys Acta. 1960 Jun 3;40:502–517. doi: 10.1016/0006-3002(60)91391-3. [DOI] [PubMed] [Google Scholar]
- Lips S. H., Avissar Y. Plant-leaf microbodies as the intracellular site of nitrate reductase and nitrite reductase. Eur J Biochem. 1972 Aug 18;29(1):20–24. doi: 10.1111/j.1432-1033.1972.tb01952.x. [DOI] [PubMed] [Google Scholar]
- Magalhaes A. C., Neyra C. A., Hageman R. H. Nitrite assimilation and amino nitrogen synthesis in isolated spinach chloroplasts. Plant Physiol. 1974 Mar;53(3):411–415. doi: 10.1104/pp.53.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann J., Arntzen C. J., Dilley R. A. Two sites for adenosine triphosphate formation in photosynthetic electron transport mediated by photosystem I. Evidence from digitonin subchloroplast particles. Biochemistry. 1971 Mar 2;10(5):866–873. doi: 10.1021/bi00781a021. [DOI] [PubMed] [Google Scholar]
- PANEQUE A., RAMIREZ J. M., DELCAMPO F. F., LOSADA M. LIGHT AND DARK REDUCTION OF NITRITE IN A RECONSTITUTED ENZYMIC SYSTEM. J Biol Chem. 1964 Jun;239:1737–1741. [PubMed] [Google Scholar]
- Ritenour G. L., Joy K. W., Bunning J., Hageman R. H. Intracellular localization of nitrate reductase, nitrite reductase, and glutamic Acid dehydrogenase in green leaf tissue. Plant Physiol. 1967 Feb;42(2):233–237. doi: 10.1104/pp.42.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweig G., Shavit N., Avron M. Diquat (I,I'-ethylene-2,2'-dipyridylium dibromide) in photo-reactions of isolated chloroplasts. Biochim Biophys Acta. 1965 Nov 29;109(2):332–346. [PubMed] [Google Scholar]



