Abstract
Cytokine and activation of lymphocytes are critical for tumor growth. We investigated whether interleukin (IL)-32β overexpression changes other cytokine levels and activates cytotoxic lymphocyte, and thus modify tumor growth. Herein, IL-32β inhibited B16 melanoma growth in IL-32β-overexpressing transgenic mice (IL-32β mice), and downregulated the expressions of anti-apoptotic proteins (bcl-2, IAP, and XIAP) and cell growth regulatory proteins (Ki-67 antigen (Ki-67) and proliferating cell nuclear antigen (PCNA)), but upregulated the expressions of pro-apoptotic proteins (bax, cleaved caspase-3, and cleaved caspase-9). IL-32β also inhibited colon and prostate tumor growth in athymic nude mice inoculated with IL-32β-transfected SW620 colon or PC3 prostate cancer cells. The forced expression of IL-32β also inhibited cell growth in cultured colon and prostate cancer cells, and these inhibitory effects were abolished by IL-32 small interfering RNA (siRNA). IL-10 levels were elevated, but IL-1β, IL-6, and tumor necrosis factor-alpha (TNF-α) levels were reduced in the tumor tissues and spleens of IL-32β mice, and athymic nude mice. The number of cytotoxic T (CD8+) and natural killer (NK) cells in tumor tissues, spleen, and blood was significantly elevated in IL-32β mice and athymic nude mice inoculated with IL-32β-transfected cancer cells. Constituted activated NF-κB and STAT3 levels were reduced in the tumor tissues of IL-32β mice and athymic nude mice, as well as in IL-32β-transfected cultured cancer cells. These findings suggest that IL-32β inhibits tumor growth by increasing cytotoxic lymphocyte numbers, and by inactivating the NF-κB and STAT3 pathways through changing of cytokine levels in tumor tissues.
Keywords: IL-32β, lymphocytes, NF-κB, STAT3, tumor growth
Emerging evidence indicates that tumor microenvironments have critical roles in tumor development and progression.1 A vast diversity of cytokines, growth factors, and inflammatory mediators released from tumor-associated stromal cells may directly influence the behavior of tumor cells or indirectly influence them by inducing changes in growth signals or by activating cytotoxic lymphocytes.2 Cytokines are known to be significantly involved in tumor development and progression.3 Interleukin (IL)-6, IL-8, and IL-22 increase tumorigenic activity,4, 5, 6 whereas IL-1α, IL-10, IL-21, and IL-27 inhibit tumor growth.7, 8, 9, 10
NF-κB and STAT family proteins have been proposed to be important regulators of tumor development.3, 11 The constitutive activation of NF-κB has been described in a number of tumors.12, 13, 14 The NF-κB signal could be modified by cytokines in tumors and tumor environments.1 IL-6 and IL-8 are strongly associated with NF-κB activation during tumor growth and metastasis.5, 15 However, IL-10 and IL-1α inhibit tumor growth by inhibiting NF-κB activity.16, 17 STAT3 is constitutively activated in diverse tumors and also involved in tumor growth.18, 19, 20 Many cytokines including IL-6, IL-8, IL-15, and IL-17 are crucial for the activation of STAT3 in tumors, and thus stimulate tumor growth.21, 22 However, IL-10, IL-21, and IL-23 inhibit tumor growth by inhibiting STAT3 in tumor.7, 8, 23 Several studies have demonstrated crosstalk between STAT3 and NF-κB in tumors. Persistently, activated STAT3 was found to maintain constitutive NF-κB activity in tumors, which resulted in the stimulation of tumor growth.24, 25
Cytokines can also directly influence the retardation or promotion of tumor growth through the activation of lymphocytes.26 Furthermore, the activation of NF-κB and/or STAT3 in tumor-associated stromal inflammatory and immune cells can indirectly influence tumor growth by affecting the tumor-inhibiting activity of lymphocytes, or by increasing the number of tumor-inhibiting lymphocytes.27, 28 Taken together, interaction between tumors and immune cells either enhance or inhibit cancer development and/or progression, and changes of cytokines could affect the activation of lymphocytes, and the activation of NF-κB and STAT3 signals, which thus control tumor growth.
Apoptosis is a biological process that is essential to all living organisms and is a key feature of cancer development.29, 30 Apoptosis is mediated by the two major pathways: the extrinsic (extracellular receptor and ligand) and the intrinsic (mitochondria-associated) pathways.31, 32 The extrinsic pathway is triggered by death receptor, which initiates a signaling cascade mediated by caspase-8 activation. Caspase-8 activates caspase-3 and stimulates the release of cytochrome c by the mitochondria. Caspase-3 activation leads to the degradation of cellular proteins to maintain cell survival and death. The intrinsic pathway is initiated by the release of cytochrome c from the mitochondria. Cytochrome c interacts with Apaf-1 and caspase-9 to promote the activation of caspase-3, and regulated by the pro-apoptotic B cell lymphoma protein-2 (Bcl-2) family of proteins, such as Bax, Bid, and Bak, and by the anti-apoptotic Bcl-2 family of proteins, such as Bcl-2, IAP, and XIAP. The Ki-67 antigen (Ki-67) and proliferating cell nuclear antigen (PCNA) are classic markers of cellular proliferation that have been widely applied in the diagnostic procedures.33 Many investigations for proliferative activity of tumor cell have used PCNA and Ki-67 to evaluate cell proliferation in tumors.34, 35, 36
IL-32 is a recently discovered inflammatory cytokine produced by T lymphocytes, natural killer (NK) cells, epithelial cells, and blood monocytes.28 IL-32 induces tumor necrosis factor-alpha (TNF-α), IL-1β, IL-6, and IL-10,37 and thereby may have an important role in tumor development. In the present study, considering the significance of cytokines with respect to lymphocyte activation and the modification of NF-κB and STAT3 pathways, we investigated whether IL-32β regulates tumor growth via the activation of cytotoxic lymphocytes, and the inactivation of NF-κB and STAT3 pathways by the changes of cytokine levels.
Results
Generation of IL-32β transgenic mice and IL-32β expression in mouse tissues
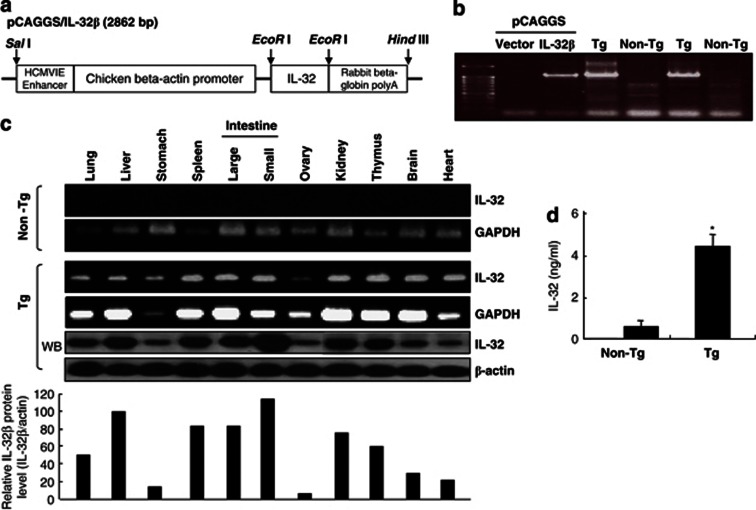
As described previously,38 we generated human IL-32β-overexpressing transgenic mice (IL-32β mice) by subcloning IL-32β cDNA into the mammalian expression vector pCAGGS (Figure 1a). The success of procedure was confirmed by PCR of mouse tail genomic DNA using allele-specific primers (Figure 1b). The transgene was successfully transmitted to 50% of pups from each littermate, as evaluated by genotyping and western blotting. These founder mice were each back-crossed into the C57BL6/J background for eight generations. The male/female ratio was 50% for IL-32β transgenic and nontransgenic littermates. IL-32β transgenic mice are viable, fertile, and have no tissue or organ abnormalities. RT-PCR and western blotting analysis revealed that human IL-32β was ubiquitously expressed in various tissues such as liver, kidney, and intestine, spleen and thymus, whereas human IL-32β was not expressed in the tissues of nontransgenic mice (Figure 1c). Furthermore, IL-32 levels in the sera of IL-32β transgenic mice (∼4.4 ng/ml) were found to be >10 times that in wild-type mice (∼0.3 ng/ml, Figure 1d).
Figure 1.
Generation of IL-32β transgenic mice. (a) Scheme for IL-32β transgenic generation. (b) PCR analysis (genotyping) was performed to analyze the existence of IL-32β gene in transgenic mice, as described in Materials and Methods. (c) RT-PCR and western blott analyses for IL-32β in the tissues of IL-32β transgenic and nontransgenic mice. (d) Detection of IL-32 in the sera of transgenic or nontransgenic mice. The results are expressed as mean±SD of three mice. * Significant difference from nontransgenic mice (*P<0.05)
IL-32β inhibited tumor growth in IL-32β transgenic mice and in BALB/c athymic nude mice inoculated with IL-32β-transfected colon and prostate cancer cells, and IL-32β also inhibited cultured cancer cell growth
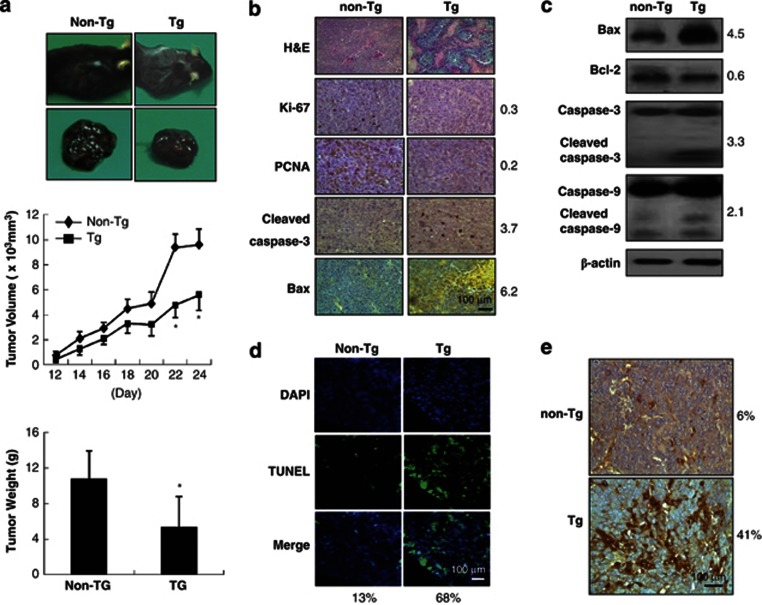
To elucidate the effect of IL-32β on tumor growth in vivo, B16 melanoma cells were inoculated subcutaneously into IL-32β mice and nontransgenic mice (n=10), tumor growth was monitored for 24 days. Whereas B16 melanoma inoculated into nontransgenic mice grew vigorously, it did not grow well in IL-32β mice, in which tumor volumes and weights were significantly reduced (Figure 2a). H&E staining showed the presence of large necrotic areas in tumor sections of IL-32β mice (Figure 2b). Consistent with tumor growth inhibition, numbers of cells immunoreactive for Ki-67 (30% lower than nontransgenic mice) and PCNA (20% lower than nontransgenic mice) was significantly decreased, whereas numbers of cells immunoreactive for cleaved caspase-3 (3.7-fold) and Bax (6.2-fold) were increased in the tumor tissues of IL-32β mice (Figure 2b). Western blot analysis also showed that the expression of cleaved caspase-3 (3.3-fold), cleaved caspase-9 (2.1-fold), and Bax (4.5-fold) was increased, but bcl-2 expression (60%) was decreased in the tumor tissues of IL-32β mice (Figure 2c). Numbers of TUNEL-positive cells (apoptotic cell death, 5-fold) were greater in the tumor tissues of IL-32β mice than in those of nontransgenic mice (Figure 2d). Furthermore, immunohistochemical analysis showed that numbers of IL-32β immunoreactive cells were higher (about 7-fold) in the tumor tissues of IL-32β mice (Figure 2e).
Figure 2.
Effect of IL-32β on tumor growth in IL-32β transgenic mice. (a) Tumor images (upper panel), volumes (middle panel), and weights (lower panel) were measured at study termination (on day 24 in transgenic mice (a) as described in Materials and Methods section. The results are expressed as mean±SD from 10 mice; *P<0.05 compared with the nontransgenic mice. (b) tumor sections were analyzed by immunohistochemistry. (c) Tumor extracts were analyzed by western blotting as described in Materials and Methods section. Each images and band are representative of three independent mice. (d) Apoptotic cells were examined by TUNEL staining. (e) Tumor sections were analyzed by immunohistochemistry for detection of IL-32β expression in tumor tissues. Each image is representative of three independent mice. The values on the right or bottom of panels are average fold difference from three independent nontransgenic mice *Significant difference from nontransgenic mice (*P < 0.05)
We further examined the inhibitory effect of IL-32β on colon and prostate tumor growth. As cancer cells were rejected when they were inoculated into IL-32β mice, we used BALB/c athymic nude mice. Colon (SW620) and prostate cancer cells (PC3) were transfected with an empty vector or IL-32β, and then inoculated subcutaneously into BALB/c athymic nude mice (n=10). Relative tumor growth was then monitored for 38 days. Tumor growth was delayed in mice that were inoculated with IL-32β-transfected colon or prostate cancer cells (named as IL-32βC and IL-32βp nude mice) compared with control mice that were inoculated with vector-transfected cancer cells (IL-32βv mice) (Supplementary Figure 1A). Similar to IL-32β transgenic mice, H&E staining showed the presence of large necrotic areas in tumor sections of IL-32βC and IL-32βp nude mice (Supplementary Figure 1B). Consistent with the observed tumor growth inhibition, the numbers of immunoreactive cells for Ki-67 (30% in both IL-32βC and IL-32βp mice) and PCNA (32% in IL-32βC and 40% in IL-32βp) were significantly decreased, whereas the numbers of immunoreactive cells for cleaved caspase-3 (3-fold in IL-32βC and 3.3-fold in IL-32βp nude mice) and Bax (5-fold in IL-32βC and 3-fold in IL-32βp nude mice) in tumor tissues of IL-32βC and IL-32βp nude mice were significantly increased (Supplementary Figure 1B). Western blot analysis also showed that IL-32β increased the expression of cleaved caspase-3 (1.4-fold in IL-32βC and 2.5-fold in IL-32βp nude mice), cleaved caspase-9 (2.7-fold in IL-32βC mice and 3.2-fold in IL-32βp nude mice), and Bax (4.5-fold in IL-32βC and 3.2-fold in IL-32βp nude mice), but decreased bcl-2 expression (10% in IL-32βC and 30% in IL-32βp nude mice) in the tumor tissues of IL-32βC and IL-32βp nude mice, and in all cases, these changes were associated with a large increase of IL-32β (Supplementary Figure 1C). The number of TUNEL-positive cells (indicating apoptotic cell death, 5-fold in IL-32βC and 4.5-fold in IL-32βp mice) were increased in the tumor tissues of IL-32βC and IL-32βp nude mice, as compared with those of IL-32βV nude mice (Supplementary Figure 1D).
We also found that the forced expression of IL-32β in melanoma, human colon cancer cell lines (SW620), and human prostate cancer cell lines (PC3) resulted in the inhibition of cell growth in enforced IL-32β in a dose-dependent manner (Supplementary Figure 2a), as compared with cancer cells transfected with empty vector. DAPI and TUNEL assay showed that the introduction of IL-32β increased the number of TUNEL-positive cells by five-fold (melanoma cells), four-fold (SW620), or five-fold (PC3) (Supplementary Figure 2B). Moreover, IL-32 small interfering RNA (siRNA) treatment abrogated these IL-32β-induced inhibitory effects on cancer cell growth and apoptosis (Supplementary Figures 2A and 2B). These cell growth inhibition and apoptotic cell death induction were paralleled by increases in the enforced expression of IL-32β (Supplementary Figure 2C).
IL-32β-modulated cytokine levels in tumor and spleen tissues
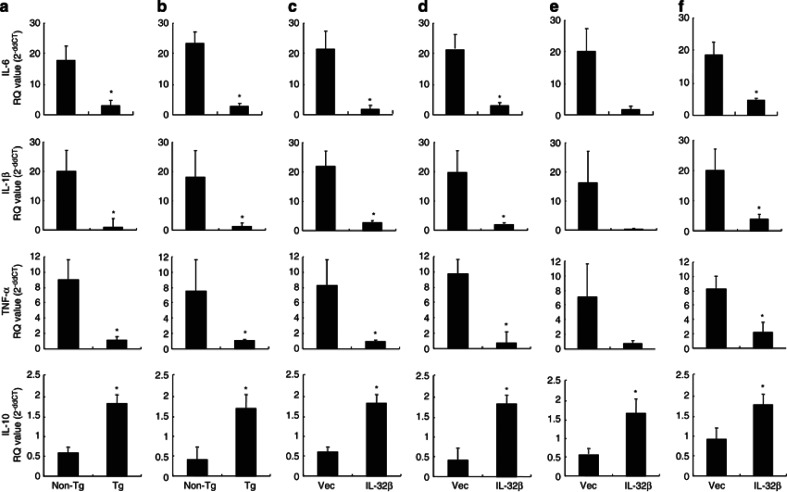
Cytokines are critically required for tumor growth because they directly influence cancer growth or they indirectly contribute to antitumor activities of lymphocytes.39 Therefore, we investigated whether IL-32β also changes other cytokine levels in these tissues. We analyzed the mRNA levels of IL-6, IL-10, TNF-α, and IL-1β, which are known to regulate tumor growth. The mRNA levels of IL-6, TNF-α, and IL-1β (tumor-promoting cytokines) were almost undetectable in the tumor tissues of IL-32β mice, whereas the level IL-10 was concomitantly elevated (Figure 3a). In tumor microenvironments, cytokines released from lymphocytes could influence tumor growth by conveying tumor growth signals from the microenvironment to tumor. Thus, we examined cytokine levels in spleen tissues of IL-32β mice. As was observed for cytokine levels in tumor tissues, IL-6, TNF-α, and IL-1β levels were depressed, but IL-10 levels were elevated in the spleen of IL-32β mice (Figure 3b). In addition, in the tumor (Figures 3c and e) and spleen tissues (Figures 3d and f) of IL-32βC (Figures 3c and d) and IL-32βp (Figures 3e and f) nude mice, IL-6, TNF-α, and IL-1β levels were decreased, but IL-10 levels were elevated as compared with those in IL-32βV mice.
Figure 3.
Effect of IL-32β on cytokine levels in tumor and spleen tissues. The level of IL-6, IL-1β, TNF-α, and IL-10 was determined by qRT-PCR in the tumor (a) and spleen (b) of tumor-bearing IL-32β-transgenic mice. The cytokine levels of tumor (c and e) or spleen (d and f) of athymic nude mice inoculated with IL-32β-transfected colon cancer cells (c and d) or prostate cancer cells (e and f). The results are expressed as mean±SD of five mice. *Significant difference from nontransgenic mice or athymic nude mice inoculated with vector-transfected cancer cells (*P<0.05)
IL-32β increased the number of CD8+ cytotoxic T cells and NK cells in blood, spleen, and tumor tissues
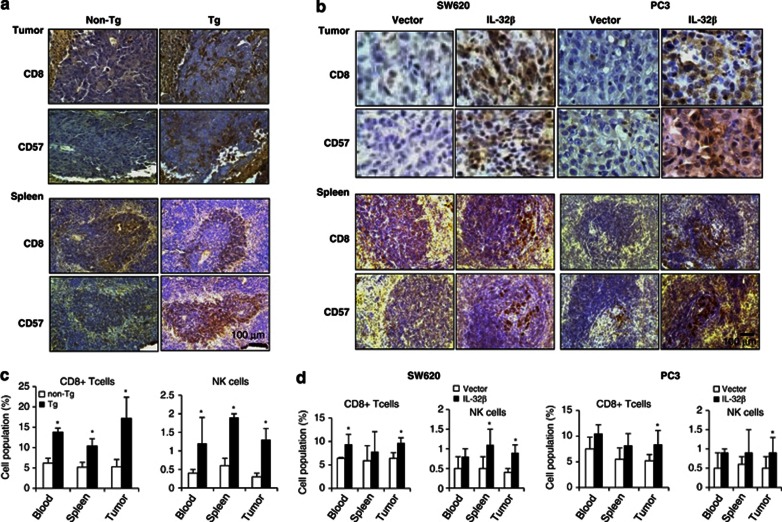
As changes of cytokine levels modify the activation or infiltration of lymphocytes into tumor, leading to their antitumor immunity, we counted numbers of infiltrating T and NK cells in tumor tissues. Immunohistochemical staining showed much higher number of CD8+ cytotoxic T cells and CD57+ NK cells spreading diffusely throughout entire tumor and spleen sections of IL-32β mice, and of IL-32βC and IL-32βp nude mice (Figures 4a and b). Fluorescence-activated cell sorting (FACS) analysis also showed that CD8+ T and NK cell numbers and total B cell numbers in blood, spleen, and tumor tissues of IL-32β mice and IL-32βC, and IL-32βp nude mice were significantly higher than in nontransgenic mice and IL-32βv nude mice (Figures 4c and d). These findings suggest that the antitumor immunity of IL-32β is associated with promoting the infiltration of CD8+ T cells and NK cells into tumor tissues, and increasing the numbers of these cells in immune tissues and blood.
Figure 4.
Effect of IL-32β on CD8+ cytotoxic T cell and CD57+ NK cell number in tumor and spleen tissues, and blood of athymic nude mice inoculated with IL-32β-trasfected colon and prostate cancer cells. (a) Effect of IL-32β on the number of CD8+ cytotoxic T cells and CD57+ NK cells in tumor and spleen tissue section of IL-32β-transgenic mice, as determined in immunohistochemistry. (b) Effect of IL-32β on the number of CD8+ cytotoxic T cells and CD57+ NK cells in tumor and spleen tissue sections of athymic nude mice inoculated with IL-32β-trasfected colon and prostate cancer cells, as determined in immunohistochemistry. The images shown in (a) and (b) are representative of three sections from each mouse (n=3). (c and d) Subpopulation of immune cells determined after FACS analysis, as described in Materials and Methods section. The values in each area are the average subpopulation of immune cells (NK and CD8+ cells) *Significant difference from nontransgenic mice (*P < 0.05).
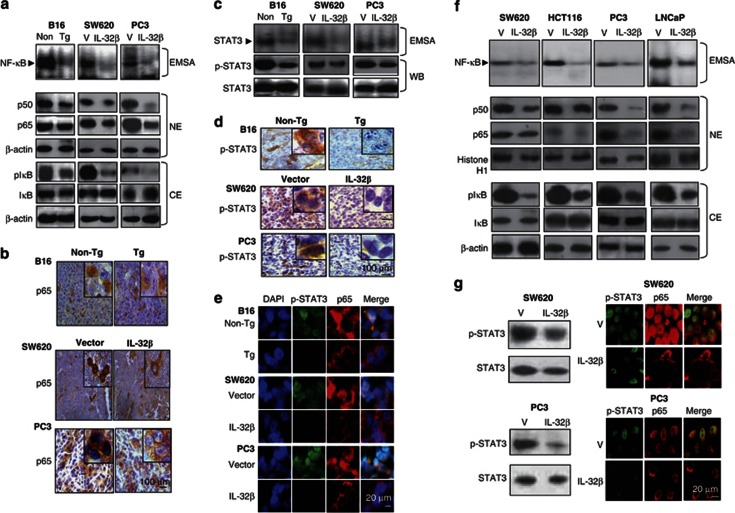
IL-32β inhibited NF-κB and STAT3 signals in tumor
The changes of cytokines could influence the tumor growth signals such as NF-κB and STAT3, which are critical signals to control tumor growth. To determine whether IL-32β inhibits the activation of NF-κB in tumors, and cultured colon and prostate cancer cells, we measured the DNA-binding activity of NF-κB by electromobility shift assay (EMSA), and translocations of p50 and p65 into the nucleus and IκBα protein degradation by western blotting and immunohistochemical analysis. The activity of NF-κB, the nuclear translocations of p50 and p65, and the phosphorylation of IκB in the tumor tissues of IL-32β mice, and in IL-32βC and IL-32βp nude mice were decreased as compared with those of nontransgenic and IL-32βV mice (Figure 5a). Immunohistochemical analysis also verified that the numbers of immunoreactive cells for NF-κB (p65) were much less in the tumors of IL-32β mice, and IL-32βC and IL-32βp nude mice (Figure 5b). Similar to NF-κB, the DNA-binding activity of STAT3 was lower in the tumor tissues of IL-32β mice, and IL-32βC and IL-32βp nude mice than that of nontransgenic and IL-32βV mice (Figure 5c), and phosphorylated STAT3 levels were also lower in the tumor tissues of these mice (Figure 5c). Immunohistochemistry confirmed that the numbers of immunoreactive cells for STAT3 in the tumor tissues of IL-32β mice, and IL-32βC and IL-32βp nude mice were lower than in those of nontransgenic mice and IL-32βV mice (Figure 5d). Confocal microscopy confirmed that the nuclear translocations of p65 and p-STAT3, and the colocalization of p-STAT3 and p65 were reduced by IL-32β in the tumor tissues of IL-32β mice, and IL-32βC and IL-32βp nude mice as compared with those in the tumor tissues of nontransgenic mice, and in IL-32βV mice and colon cancer cells (Figure 5e). As NF-κB is highly activated in colon and prostate cancer cells, the DNA-binding activity of NF-κB was measured after the introduction of vector or IL-32β into colon and prostate cancer cells. In agreement with in vivo data and a previous report,39 the introduction of IL-32β inhibited the constitutive DNA-binding activity in colon and prostate cancer cells (Figure 5f). IL-32β also prevented the nuclear translocations of p50 and p65 by inhibiting the phosphorylation of IκB (Figure 5f). The phosphorylation of STAT3 (STAT3 activity) was also reduced in colon and prostate cancer cells transfected with IL-32β (Figure 5g left panel). Confocal microscopy confirmed that the nuclear translocations of p65 and p-STAT3 were reduced by IL-32β in both colon and prostate cancer cells (Figure 5g right panel). These results suggest that IL-32β inactivated NF-κB and STAT3 in tumor tissues, which are associated with the antitumor activity of IL-32β.
Figure 5.
Effect of IL-32β on the activation of NF-κB and STAT3 in tumor tissues, and cultured colon and prostate cancer cells. (a) The DNA-binding activity of NF-κB was determined by EMSA in the nuclear extracts of tumor tissues of IL-32β-overexpressed transgenic mice or the athymic nude mice (a, upper panel). Expression of p50 and p65 in nuclear extracts (NE, middle panel of a), IκB phosphorylation in the cytosol extracts (CE, lower panel of a), and DNA-binding activity and STAT3 phosphorylation (c) in total lysates of tumors were determined by EMSA (upper panel) or by western blotting (lower panel). (b and d) Expression of p65 (b), and phosphorylated STAT3 (d) in murine tumors were determined by immunohistochemistry. (e) Cellular localization p-STAT3 (green) and p65 (red) in tumor tissues. IL-32β transgenic and athymic nude mice were observed by fluorescence microscopy after Immunofluorescence staining, as described in Materials and Methods section. Each image and band are representative of three independent mice. (f) Colon and prostate cancer cells were transfected with the vector or the IL-32β and cultured for 24 h, and then NF-κB was determined by EMSA (f, upper panel). Expression of p50 and p65 in nuclear extracts (NE, middle panel) and IκB phosphorylation in the cytosol extracts (CE, lower panel) were determined by western blotting. (g) Phosphorylation of STAT3 in total cell extracts was analyzed by western blotting (g, left panel). Cellular localization of p-STAT3 (green) and p65 (red) in IL-32β-transfected colon and prostate cancer cells was determined with confocal microscopy, as described in Materials and Methods (f, right panel). Each band is representative of three independent experiments
Discussion
In the present study, it was originally found that IL-32β inhibits melanoma, colon, and prostate tumor growth via the activation of cytotoxic T cells and NK cells, and the inactivation of NF-κB and STAT3.
Interaction between tumor and stromal cells, such as inflammatory cells and lymphocytes, is important when considering tumor progression (or regression) because stromal cells spread and induce antitumor immune responses through the release of several mediators such as cytokine and growth factors.39 Cytokines can cause activation of T cells (cytotoxic CD8+ T cells) and NK cells in tumor tissues, which can induce the apoptosis of tumor cells.40 IL-10 is known to inhibit tumor growth and metastasis via NK cell-dependent mechanism.27 Furthermore, it has been reported that after active T lymphocyte levels had been reconstituted, hepatic carcinoma cell apoptosis was increased, depending on serum IL-10 and VEGF levels.41 In contrast, IL-6 that is released from stromal cells stimulates cancer cell growth.42 Furthermore, lowering the level of IL-6 was found to be critical for antitumor activity against prostate cancer by combined purine nucleoside phosphorylase and docetaxel by increasing lymphocyte infiltration, and modulating cytokine levels.43 We found that tumor-promoting proinflammatory cytokines (IL-1β, IL-6, and TNF-α) were decreased, but levels of tumor growth-inhibitory cytokine IL-10 were elevated in spleen and tumor tissues. Concomitantly, we also found that IL-32β mice, and IL-32βC and IL-32βp nude mice had higher levels of CD8+ T cells and CD57+ NK cells in tumor tissues, blood, spleen, and thymus than nontransgenic mice and IL-32βv mice. These observations indicate that changes of cytokines by IL-32β could influence the activation of cytotoxic lymphocytes, leading to the inhibition of tumor growth. It is not clear how the T cells migrated in nude mice, as these mice do not have functional thymus and therefore cannot produce mature T cells. However, extrathymic maturation (spleen, lymph node, and bone marrow) of T cells (NK cells) could be possible when these mice have been burdened with virus or tumor, as reported by several other investigators.44, 45 Cheng et al.46 also report that selenium increased peripheral and spleen CD4+ and CD8+ cells in the nude mice bearing prostate cancer cells. Moreover, it is noteworthy that postnatal thymic-independent T cell differentiation (maturation) in the extrathymic origin (such as in skin) could be possible.47
Owing to the abilities of NF-κB and STAT3 signals to induce the expression of a large number of cytokines and act as core transcription factors during diverse immune responses in tumor microenvironments, they are considered to be the major pathways responsible for cytokine-associated cancer development and tumor immunity.48, 49 In the present study, we found that in agreement with the activation of cytotoxic lymphocytes, IL-32β inactivated NF-κB in tumor and cultured cancer cells. These findings suggest that the inactivation of NF-κB could induce IL-32β-induced tumor growth inhibition through the activation of lymphocytes directly or indirectly by changes of other cytokine level. In addition, in the present study, STAT3 activity was also found to be lower in the tumors of IL-32β mice, IL-32βC and IL-32βp nude mice, and IL-32β-transfected cancer cells than in nontransgenic mice, and IL-32βv mice as well as nontransfected cancer cells. In agreement with these observations, in a previous study, ablating STAT3 was found to activate macrophages, NK cells, and neutrophils, and have an antitumor effect.50 It is well known that inactivation of STAT3 is required for IL-10-mediated immunosuppressive signaling.51 Tumor-killing cytokines were found to induce an NK cell-mediated antitumor immune response to human cancer cells via the inactivation of STAT3.52
Considering the roles of NF-κB and STAT3 in the regulation of genes responsible for cancer cell growth, apoptosis, and tumor development and/or progression, it appears that the direct inhibitory effects of IL-32β on NF-κB and STAT3 in tumor tissues might be also the fundamental mechanisms underlying tumor growth inhibition by IL-32β. It remains to be determined how IL-32β modulates STAT3 and NF-κB signals, but it is noteworthy that several cytokines can modulate the activities of STAT3 and NF-κB, and increase gene transcription. For example, IL-6 activates STAT3, but IL-10 inhibits the activation of STAT3.53 Furthermore, the acetylation and/or recruitment of p300 protein are critical for the cytokine-induced activations of STAT3 and NF-κB; IL-6 prolongs the nuclear retentions of STAT3 and NF-κB.54
Thus, in the present study, we conclusively demonstrate the inhibitory effect of IL-32β on tumor growth and these findings provide novel evidence that IL-32β has a suppressive effect on tumor growth through the activation of cytotoxic lymphocytes, and inactivation of NF-κB and STAT3 signals by alterations in cytokine levels.
Materials and Methods
Generation of IL-32-transgenic mice
To generate transgenic mice that express hIL-32β, concentrated hIL-32β cDNA was prepared. To generate IL-32β-transgenic mice, a 705-bp fragment of the human IL-32β gene was subcloned into the EcoRI sites of the pCAGGs expression vector. The sequence of the IL-32β insert was confirmed by automated sequencing. The IL-32β gene-containing genomic fragment was released from the vector using SalI/HindIII digestion, and then separated from the pCAGGs vector using a low melt-point agarose and transverse alternating field-gel electrophoresis. The fragment was purified and microinjected at a concentration of 4 ng/μl into the embryos of BDF1 mice. The experimental treatments were carried out according to the guidelines for animal experiments of the Faculty of Disease Animal Model Research Center, Korea Research Institute of Bioscience and Biotechnology (Daejeon, Korea), as well as the Guidelines for the welfare and use of animals in cancer research.55
IL-32β insertion was confirmed by the amplification of genomic DNA isolated from the transgenic mice tails using Super Taq PLUS Pre-mix (RexGene BioTech, Ochang, Chungbuk, Korea) and the following specific primer set: sense, 5′-GAAGGTCCTCTCTGATGACA-3′ and antisense, 5′-GAAGAGGGACAGCTATGACTG-3′ (nt 2245–2225). pCAGGS/IL-32β was used as a positive control, and pCAGGS (4.8 kb) was used as a negative control. GAPDH was used as an internal control. Genomic DNA samples were extracted from transgenic mice tails, and PCR analysis was performed for detection of the insertion of IL-32β in the genome. The following conditions were used for the TaKaRa PCR Thermal Cycler: 94 °C for 10 min, followed by 35 cycles of 94 °C for 1 min, 63 °C for 1 min, and 72 °C for 1.5 min, with a final step of 72 °C for 10 min. The IL-32β transgenic mice were crossback mated with C57Bl6J mice for eight generations in order to establish IL-32β transgenic line. In order to analyze IL-32 gene expression in various tissues of transgenic mice, total RNAs were extracted and RT-PCR analysis was performed using the following primer sets: sense: 5′-GAAGGT CCTCTCTGATGACA-3′, antisense: 5′-GGGGTTCAGAGCACTTCT-3′ (371 bp). GAPDH was used as an internal control. Sense: 5′-ACCACAGTCCATGCCATCAC-3′, antisense: 5′-TCCACCACCCTGTTGCTGTA-3′ (450 bp).
Reagents and cell culture
The HCT116 and SW620 colon cancer cell lines and B16 melanoma cells were obtained from the American Type Culture Collection (Manassas, VA, USA). Colon cancer cells were grown at 37 °C in 5% CO2-humidified air in RPMI 1640 medium that contained 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin. B16 melanoma cells were grown at the same conditions in DMEM medium. RPMI 1640, DMEM, penicillin, streptomycin, and FBS were purchased from Gibco Life Technologies (Grand Island, NY, USA). siRNA species for IL-32, p50, and p65, and a nontargeting control siRNA were purchased from Bioneer (Daejeon, Korea); siRNA for STAT3 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Transfection
Colon and prostate cancer cells (5 × 104 cells per well) were plated in 24-well plates, and stable transfected with 0.4 μg of the empty vector or the constitutively activated full-length IL-32β plasmid per well by using a mixture of plasmid. To establish constitutive expression systems of IL-32, cancer cells were transfected with the pcDNA3.1+-6 × Myc or pcDNA3.1+-6 × Myc-IL-32α vector using the Neon transfection system (Invitrogen, Carlsbad, CA, USA). G418 (900 μg/ml)-resistant cells were screened for 3 weeks, and single cell-expanded clones were obtained by serial dilutions.
Cell viability
To determine viable cell numbers, the colon and prostate cancer cells were seeded onto 24-well plates (5 × 104 cells per well). The cells were trypsinized, pelleted by centrifugation for 5 min at 1500 r.p.m., resuspended in 10 ml of phosphate-buffered saline (PBS), and 0.1 ml of 0.2% trypan blue was added to the tumor cell suspension in each solution (0.9 ml each). Subsequently, a drop of suspension was placed in a Neubauer chamber, and the living cancer cells were counted. Cells that showed signs of trypan blue uptake were considered to be dead, whereas those that excluded trypan blue were considered to be viable. Each assay was carried out in triplicate.
Detection of apoptosis
TUNUL assays were performed using the In situ Cell Death Detection Kit (Roche Diagnostics GmbH, Mannheim, Germany). The total cell number in a given area was determined based on DAPI nuclear staining. The apoptotic index was calculated as the number of DAPI-stained, TUNUL-positive cells divided by the total number of cells counted s × 100.
Anti-tumor activity of IL-32β in IL-32β-transgenic mice
Male, 6–8 week-old IL-32β transgenic and nontransgenic mice were maintained in accordance with the guidelines proscribed by the Chungbuk National University Animal Care Committee. B16 melanoma cells were injected subcutaneously (5 × 105 tumor cells in 0.1 ml PBS per animal) into transgenic and nontransgenic mice. The weights and tumor volumes of the animals were monitored twice weekly. The tumor volumes were measured with Vernier calipers and calculated using the following formula: (A × B2)/2, where A is the larger and B is the smaller of the two dimensions. At the end of the experiment, the animals were killed and the tumors were separated from the surrounding muscles.
In vivo antitumor activity of IL-32β in a xenograft animal model
Six-week-old male BALB/c athymic nude mice were purchased from Japan SLC (Hamamatsu, Japan). All experiments were approved and carried out according to the Guide for the Care and Use of Animals (Chungbuk National University Animal Care Committee, Korea). Human colon cancer SW620 cells and prostate cancer PC3 cells that had been transfected with the vector or the IL-32β plasmid (6 μg/1 × 106 cells) were injected subcutaneously (1 × 107 tumor cells in 0.1 ml PBS per animal) into the right-lower flanks of the carrier mice. The body weights and tumor volumes of the animals were monitored twice weekly. The formula described above was used to calculate tumor volume. At the end of the experiment, the animals were killed by cervical dislocation. The tumors were separated from the surrounding muscles and dermis, excised, and weighed.
Western blotting and electromobility shift assay
The membranes were immunoblotted with the following primary antibodies: mouse monoclonal antibodies directed against p65, p50, STAT3, and p-STAT3 (Santa Cruz Biotechnology), rabbit polyclonal antibodies directed against bax, and PARP (Santa Cruz Biotechnology) and against caspase-3, cleaved caspase-3, caspase-9, Bcl-2, XIAP and cIAP (Cell Signaling Technology, Beverly, MA, USA). The monoclonal anti-hIL-32 antibody KU32-52 was used. A gel EMSA was performed according to the manufacturer's recommendations (Promega, Madison, WI, USA).
Detection of IL-32 in the sera of transgenic mouse
The level of IL-32 in the sera was detected by ELISA methods using mouse Il-32 antibody, as describe in elsewhere.56
Fluorescence microscopy
The fixed cells and tissues were exposed to the following primary antibodies: p50, p65, and p-STAT3 (1 : 100 dilutions in blocking serum; Santa Cruz Biotechnology) at room temperature for 1 h. After incubation, the cells were washed twice with ice-cold PBS and incubated with an anti-rabbit or mouse secondary antibody conjugated to Alexa Fluor 488 or 568 (Invitrogen-Molecular Probes, Carlsbad, CA, USA) at room temperature for 1 h. Immunofluorescence images were acquired using an inverted fluorescent microscope Zeiss Axiovert 200 M (Carl Zeiss, Thornwood, NY, USA).
Quantitative real-time PCR
For mRNA quantification, total RNA was extracted using the RNAqueous kit and the cDNA was synthesized by 1 μg of total RNA using High Capacity RNA-to-cDNA kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's protocol. Quantitative real-time PCR was performed using specific primers for GAPDH (Mm99999915_g1), IL-6 (Mm00446190_m1), TNF-α (Mm00443258_m1), IL-10 (Mm00439616_ m1), IL-1β (Mm00434228_m1) in a 7500 Real-Time PCR System (Applied Biosystems). Thermocycling conditions consisted of an initial denaturation of 20 s at 95 °C, followed by 60 cycles of 95 °C for 30 s and 60 °C for 30 s). The values obtained for the target gene expression were normalized to GAPDH and quantified relative to the expression in control samples. For the calculation of relative quantification, the 2–▵▵Ct formula was used, where –▵▵Ct=(Ct, target−Ct, GAPDH) experimental sample – (Ct, target–Ct, GAPDH) control sample.
Immunohistochemistry
All specimens were fixed in formalin and embedded in paraffin for examination. Sections (4-μm thickness) were stained with H&E and analyzed by immunohistochemistry using primary anti-mouse PCNA, Ki-67, bax (1 : 200 dilution), CD3 (1 : 10), and CD57 (1 : 50) monoclonal antibodies or primary rabbit anti-human cleaved caspase-3 polyclonal antibody (1 : 100), and secondary biotinylated anti-mouse and anti-rabbit antibodies.
Fluorescence-activated cell sorting analysis for immune cell populations
Immune cell populations in the whole blood, spleen, and tumor were analyzed by FACS analysis. A total of 100 l of whole blood was collected using a hematocrit capillary tube and blocked with Fc-block (eBioscience, San diego, CA, USA) to reduce nonspecific antibody binding for 3 min at room temperature. Cells were then incubated in the dark with 10 μl of the appropriate fluorochrome-conjugated antibodies from eBioscience – T cells (anti-CD3-FITC, 1 : 25), B cells (anti-CD19-PE, 1 : 25), and NK cells (anti-CD49-APC, 1 : 50) for 20 min at 4 °C. Cells were washed with 500 μl of FACS (fluorescence-activated cell sorter) buffer containing 0.02% sodium azide and 2% FBS in PBS. The red blood cells were lysed for 5 min with FACS lysis buffer (BD Bioscience, Franklin Lakes, NJ, USA) at room temperature, then re-washed with FACS buffer. Finally, each sample was fixed with 1% paraformaldehyde until further analysis. Flow cytometry analysis was performed on the FACSCalibur system (BD Biosciences). Control samples were matched for each fluorochrome. Data were analyzed using CellQuest software (Becton Dickinson, Franklin Lakes, NJ, USA). For immune cell-population analysis in the spleens and tumor, tissues were disrupted by forcing them through a 70-μm cell strainer into 10 ml of cold PBS using a rubber-tipped syringe plunger. The cell suspensions were then centrifuged at 1500 r.p.m. for 10 min and the supernatants were discarded. The cells were resuspended in 3-ml ACK lysing buffer (LONZA, Walkersville, MD, USA) for 3 min and the debris was sedimented by centrifuging at 1500 r.p.m. for 10 min. Cell concentrations were determined by haemocytometer counting using trypan blue dye exclusion, and were adjusted to 1 × 106 cells/ml. Single-cell suspensions were stained with the following fluorochrome-conjugated antibodies from BD Biosciences: B cells (anti-B220-APC, 1 : 100), T cells (anti-CD3-FITC, 1 : 400), NK cells (anti-CD49-APC, 1 : 50), and cytotoxic T cells (anti-CD8-FITC, 1 : 100). Flow cytometry and data analysis were done as mentioned above in blood.
Statistical analysis
The data were analyzed using the GraphPad Prism 4 software (GraphPad Software, La Jolla, CA, USA). Data (tumor volumes and weights) are presented as mean±SD. The homogeneity of variances was assessed using the Bartlett test. When the variances were homogeneous, differences were assessed by one-way analysis of variance (ANOVA). Other data were assessed by ANOVA. When the P-value in the ANOVA test indicated statistical significance, the differences were assessed by the Dunnett's test. A value of P<0.05 was considered to be statistically significant.
Acknowledgments
We would like to acknowledge Dr. Howard P. Glauert (University of Kentucky) who provided invaluable assistance for preparation of manuscripts. This work was supported by the Korea Research Foundation of Korea (NRF) grant funded by the Korea Government (MEST) (2012R1A2A2A 02008751 and MRC 2011-0028213), and by the National R&D Program for Cancer Control, Ministry for Health, Welfare and Family affairs, Republic of Korea. DY Yoon was partially from the basic research program (2012R1A2A2A 02008751, 2012-0006686) and D Yu from KRIBB Research Initiative Program Grant.
Glossary
- Bax
Bcl-2-associated X protein
- Bcl-2
B cell lymphoma protein-2
- CD8
cluster of differentiation 8
- IL-32
interleukin-32
- Ki-67
Ki-67 antigen
- NK cells
natural killer cells
- NF-κB
nuclear factor-kappaB
- PCNA
proliferating cell nuclear antigen
- STAT3
signal transducer and activator of transcription 3
- TNF-α
tumor necrosis factor-alpha
- VEGF
vascular endothelial growth factor
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by Stephanou
Supplementary Material
References
- Danese S, Mantovani A. Inflammatory bowel disease and intestinal cancer: a paradigm of the Yin-Yang interplay between inflammation and cancer. Oncogene. 2010;29:3313–3323. doi: 10.1038/onc.2010.109. [DOI] [PubMed] [Google Scholar]
- Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jee SH, Shen SC, Chiu HC, Tsai WL, Kuo ML. Overexpression of interleukin-6 in human basal cell carcinoma cell lines increases anti-apoptotic activity and tumorigenic potency. Oncogene. 2001;20:198–208. doi: 10.1038/sj.onc.1204076. [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Akimoto K, Nagashima Y, Kojima Y, Sasaki T, Ishiguro-Imagawa Y, et al. aPKClambda/iota promotes growth of prostate cancer cells in an autocrine manner through transcriptional activation of interleukin-6. Proc Natl Acad Sci USA. 2009;106:16369–16374. doi: 10.1073/pnas.0907044106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber NL, Bailey SR, Schuster R, Ogle CK, Lentsch AB, Pritts TA. Prior thermal injury accelerates endotoxin-induced inflammatory cytokine production and intestinal nuclear factor-kappaB activation in mice. J Burn Care Res. 2012;33:279–285. doi: 10.1097/BCR.0b013e3182331d75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng LM, Ojcius DM, Garaud F, Roth C, Maxwell E, Li Z, et al. Interleukin-10 inhibits tumor metastasis through an NK cell-dependent mechanism. J Exp Med. 1996;184:579–584. doi: 10.1084/jem.184.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondergaard H, Frederiksen KS, Thygesen P, Galsgaard ED, Skak K, Kristjansen PE, et al. Interleukin 21 therapy increases the density of tumor infiltrating CD8+ T cells and inhibits the growth of syngeneic tumors. Cancer Immunol Immunother. 2007;56:1417–1428. doi: 10.1007/s00262-007-0285-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JK, Park MH, Choi DY, Yoo HS, Han SB, Yoon do Y, et al. Deficiency of C-C chemokine receptor 5 suppresses tumor development via inactivation of NF-kappaB and upregulation of IL-1Ra in melanoma model. PLoS One. 2012;7:e33747. doi: 10.1371/journal.pone.0033747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisada M, Kamiya S, Fujita K, Belladonna ML, Aoki T, Koyanagi Y, et al. Potent antitumor activity of interleukin-27. Cancer Res. 2004;64:1152–1156. doi: 10.1158/0008-5472.can-03-2084. [DOI] [PubMed] [Google Scholar]
- Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- Muerkoster S, Arlt A, Sipos B, Witt M, Grossmann M, Kloppel G, et al. Increased expression of the E3-ubiquitin ligase receptor subunit betaTRCP1 relates to constitutive nuclear factor-kappaB activation and chemoresistance in pancreatic carcinoma cells. Cancer Res. 2005;65:1316–1324. doi: 10.1158/0008-5472.CAN-04-1626. [DOI] [PubMed] [Google Scholar]
- Shukla S, MacLennan GT, Fu P, Patel J, Marengo SR, Resnick MI, et al. Nuclear factor-kappaB/p65 (Rel A) is constitutively activated in human prostate adenocarcinoma and correlates with disease progression. Neoplasia. 2004;6:390–400. doi: 10.1593/neo.04112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlt A, Schafer H. NFkappaB-dependent chemoresistance in solid tumors. Int J Clin Pharmacol Ther. 2002;40:336–347. doi: 10.5414/cpp40336. [DOI] [PubMed] [Google Scholar]
- Xu Y, Josson S, Fang F, Oberley TD, St, Clair DK, Wan XS, et al. RelB enhances prostate cancer growth: implications for the role of the nuclear factor-kappaB alternative pathway in tumorigenicity. Cancer Res. 2009;69:3267–3271. doi: 10.1158/0008-5472.CAN-08-4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schottelius AJ, Mayo MW, Sartor RB, Baldwin AS., Jr Interleukin-10 signaling blocks inhibitor of kappaB kinase activity and nuclear factor kappaB DNA binding. J Biol Chem. 1999;274:31868–31874. doi: 10.1074/jbc.274.45.31868. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Hikiba Y, Nakagawa H, Hayakawa Y, Yanai A, Akanuma M, et al. Inhibitor of kappaB kinase beta regulates gastric carcinogenesis via interleukin-1alpha expression. Gastroenterology. 2010;139:226–238 e226. doi: 10.1053/j.gastro.2010.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, et al. Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- Kim G, Khanal P, Lim SC, Yun HJ, Ahn SG, Ki SH, et al. Interleukin-17 induces AP-1 activity and cellular transformation via upregulation of tumor progression locus 2 activity. Carcinogenesis. 2012;34:341–350. doi: 10.1093/carcin/bgs342. [DOI] [PubMed] [Google Scholar]
- Hyun YS, Han DS, Lee AR, Eun CS, Youn J, Kim HY. Role of IL-17A in the development of colitis-associated cancer. Carcinogenesis. 2012;33:931–936. doi: 10.1093/carcin/bgs106. [DOI] [PubMed] [Google Scholar]
- Bollrath J, Phesse TJ, von Burstin VA, Putoczki T, Bennecke M, Bateman T, et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15:91–102. doi: 10.1016/j.ccr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Malamut G, El Machhour R, Montcuquet N, Martin-Lanneree S, Dusanter-Fourt I, Verkarre V, et al. IL-15 triggers an antiapoptotic pathway in human intraepithelial lymphocytes that is a potential new target in celiac disease-associated inflammation and lymphomagenesis. J Clin Invest. 2010;120:2131–2143. doi: 10.1172/JCI41344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamut G, El Machhour R, Montcuquet N, Martin-Lanneree S, Dusanter-Fourt I, Verkarre V, et al. IL-15 triggers an antiapoptotic pathway in human intraepithelial lymphocytes that is a potential new target in celiac disease-associated inflammation and lymphomagenesis. J Clin Invest. 120:2131–2143. doi: 10.1172/JCI41344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Du H, Qin Y, Roberts J, Cummings OW, Yan C. Activation of the signal transducers and activators of the transcription 3 pathway in alveolar epithelial cells induces inflammation and adenocarcinomas in mouse lung. Cancer Res. 2007;67:8494–8503. doi: 10.1158/0008-5472.CAN-07-0647. [DOI] [PubMed] [Google Scholar]
- Cheon S, Lee JH, Park S, Bang SI, Lee WJ, Yoon DY, et al. Overexpression of IL-32alpha increases natural killer cell-mediated killing through up-regulation of Fas and UL16-binding protein 2 (ULBP2) expression in human chronic myeloid leukemia cells. J Biol Chem. 2011;286:12049–12055. doi: 10.1074/jbc.M110.159756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H, Thompson RG, et al. ‘Re-educating' tumor-associated macrophages by targeting NF-kappaB. J Exp Med. 2008;205:1261–1268. doi: 10.1084/jem.20080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerondakis S, Strasser A, Metcalf D, Grigoriadis G, Scheerlinck JY, Grumont RJ. Rel-deficient T cells exhibit defects in production of interleukin 3 and granulocyte-macrophage colony-stimulating factor. Proc Natl Acad Sci USA. 1996;93:3405–3409. doi: 10.1073/pnas.93.8.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JW, Choi SC, Cho MC, Kim HJ, Kim JH, Lim JS, et al. A proinflammatory cytokine interleukin-32beta promotes the production of an anti-inflammatory cytokine interleukin-10. Immunology. 2009;128 (1 Suppl:e532–e540. doi: 10.1111/j.1365-2567.2008.03025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- van Diest PJ, Brugal G, Baak JP. Proliferation markers in tumours: interpretation and clinical value. J Clin Pathol. 1998;51:716–724. doi: 10.1136/jcp.51.10.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews GM, Newbold A, Johnstone RW. Intrinsic and extrinsic apoptotic pathway signaling as determinants of histone deacetylase inhibitor antitumor activity. Adv Cancer Res. 2012;116:165–197. doi: 10.1016/B978-0-12-394387-3.00005-7. [DOI] [PubMed] [Google Scholar]
- Keating J, Tsoli M, Hallahan AR, Ingram WJ, Haber M, Ziegler DS. Targeting the inhibitor of apoptosis proteins as a novel therapeutic strategy in medulloblastoma. Mol Cancer Ther. 2012;11:2654–2663. doi: 10.1158/1535-7163.MCT-12-0352. [DOI] [PubMed] [Google Scholar]
- Myoung H, Kim MJ, Lee JH, Ok YJ, Paeng JY, Yun PY. Correlation of proliferative markers (Ki-67 and PCNA) with survival and lymph node metastasis in oral squamous cell carcinoma: a clinical and histopathological analysis of 113 patients. Int J Oral Maxillofac Surg. 2006;35:1005–1010. doi: 10.1016/j.ijom.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Figueroa A, Correnti M, Avila M, Andea A, DeVilliers P, Rivera H. Keratocystic odontogenic tumor associated with nevoid basal cell carcinoma syndrome: similar behavior to sporadic type. Otolaryngol Head Neck Surg. 2010;142:179–183. doi: 10.1016/j.otohns.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Pontes HA, Pontes FS, Silva BS, Cury SE, Fonseca FP, Salim RA, et al. Immunoexpression of Ki67, proliferative cell nuclear antigen, and Bcl-2 proteins in a case of ameloblastic fibrosarcoma. Ann Diagn Pathol. 2010;14:447–452. doi: 10.1016/j.anndiagpath.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Bologna-Molina R, Mosqueda-Taylor A, Molina-Frechero N, Mori-Estevez AD, Sanchez-Acuna G. Comparison of the value of PCNA and Ki-67 as markers of cell proliferation in ameloblastic tumors. Med Oral Patol Oral Cir Bucal. 18:e174–e179. doi: 10.4317/medoral.18573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Han SY, Azam T, Yoon DY, Dinarello CA. Interleukin-32: a cytokine and inducer of TNFalpha. Immunity. 2005;22:131–142. doi: 10.1016/j.immuni.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Oh JH, Cho MC, Kim JH, Lee SY, Kim HJ, Park ES, et al. IL-32gamma inhibits cancer cell growth through inactivation of NF-kappaB and STAT3 signals. Oncogene. 2011;30:3345–3359. doi: 10.1038/onc.2011.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurin MR, Shurin GV, Lokshin A, Yurkovetsky ZR, Gutkin DW, Chatta G, et al. Intratumoral cytokines/chemokines/growth factors and tumor infiltrating dendritic cells: friends or enemies. Cancer Metastasis Rev. 2006;25:333–356. doi: 10.1007/s10555-006-9010-6. [DOI] [PubMed] [Google Scholar]
- Moretta L, Biassoni R, Bottino C, Mingari MC, Moretta A. Human NK-cell receptors. Immunol Today. 2000;21:420–422. doi: 10.1016/s0167-5699(00)01673-x. [DOI] [PubMed] [Google Scholar]
- Wang KF, Ye SL, Song LJ, Weng YQ. The metastasis of hepatocarcinoma can be inhibited by T lymphocytes reconstitution in nude mice model. Hepatogastroenterology. 2010;57:1220–1226. [PubMed] [Google Scholar]
- Bromberg J, Wang TC. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell. 2009;15:79–80. doi: 10.1016/j.ccr.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PP, Joshi S, Russell PJ, Verma ND, Wang X, Khatri A. Molecular chemotherapy and chemotherapy: a new front against late stage hormone refractory prostate cancer. Clin Cancer Res. 2011;17:4006–4018. doi: 10.1158/1078-0432.CCR-11-0248. [DOI] [PubMed] [Google Scholar]
- Holland AM, Zakrzewski JL, Tsai JJ, Hanash AM, Dudakov JA, Smith OM, et al. Extrathymic development of murine T cells after bone marrow transplantation. J Clin Invest. 2012;122:4716–4726. doi: 10.1172/JCI60630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy RL, Darne J, Davies R, Price A. Thyrotoxicosis and hyperemesis gravidarum associated with a serum activity which stimulates human thyroid cells in vitro. Clin Endocrinol (Oxf) 1992;36:83–89. doi: 10.1111/j.1365-2265.1992.tb02906.x. [DOI] [PubMed] [Google Scholar]
- Cheng WH, Holmstrom A, Li X, Wu RT, Zeng H, Xiao Z. Effect of dietary selenium and cancer cell xenograft on peripheral T and B lymphocytes in adult nude mice. Biol Trace Elem Res. 2012;146:230–235. doi: 10.1007/s12011-011-9235-2. [DOI] [PubMed] [Google Scholar]
- Romano R, Palamaro L, Fusco A, Iannace L, Maio S, Vigliano I, et al. From murine to human nude/SCID: the thymus, T-cell development and the missing link. Clin Dev Immunol. 2012;2012:467101. doi: 10.1155/2012/467101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov. 2009;8:33–40. doi: 10.1038/nrd2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortylewski M, Kujawski M, Herrmann A, Yang C, Wang L, Liu Y, et al. Toll-like receptor 9 activation of signal transducer and activator of transcription 3 constrains its agonist-based immunotherapy. Cancer Res. 2009;69:2497–2505. doi: 10.1158/0008-5472.CAN-08-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kasmi KC, Holst J, Coffre M, Mielke L, de Pauw A, Lhocine N, et al. General nature of the STAT3-activated anti-inflammatory response. J Immunol. 2006;177:7880–7888. doi: 10.4049/jimmunol.177.11.7880. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Nakazato H, Matsui H, Hasumi M, Shibata Y, Ito K, et al. NK cell-mediated anti-tumor immune response to human prostate cancer cell, PC-3: immunogene therapy using a highly secretable form of interleukin-15 gene transfer. J Leukoc Biol. 2001;69:531–537. [PubMed] [Google Scholar]
- Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou T, Ray S, Lee C, Brasier AR. The STAT3 NH2-terminal domain stabilizes enhanceosome assembly by interacting with the p300 bromodomain. J Biol Chem. 2008;283:30725–30734. doi: 10.1074/jbc.M805941200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman P, Aboagye EO, Balkwill F, Balmain A, Bruder G, Chaplin DJ, et al. Guidelines for the welfare and use of animals in cancer research. Br J Cancer. 2010;102:1555–1577. doi: 10.1038/sj.bjc.6605642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kim S, Bae S, Choi J, Hong J, Ryoo S, et al. Interleukin-32 gamma specific monoclonal antibody and developing IL-32 specific ELISA. Hybridoma (Larchmt) 2010;29:501–509. doi: 10.1089/hyb.2010.0059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.