Abstract
Normally, the glomerular filtration barrier almost completely excludes circulating albumin from entering the urine. Genetic variation and both pre- and postnatal environmental factors may affect albuminuria in humans. Here we determine whether glomerular gene expression in mouse strains with naturally occurring variations in albuminuria would allow identification of proteins deregulated in relatively ‘leaky' glomeruli. Albuminuria increased in female B6 to male B6 to female FVB/N to male FVB/N mice, whereas the number of glomeruli/kidney was the exact opposite. Testosterone administration led to increased albuminuria in female B6 but not female FVB/N mice. A common set of 39 genes, many expressed in podocytes, were significantly differentially expressed in each of the four comparisons: male versus female B6 mice, male versus female FVB/N mice, male FVB/N versus male B6 mice, and female FVB/N versus female B6 mice. The transcripts encoded proteins involved in oxidation/reduction reactions, ion transport, and enzymes involved in detoxification. These proteins may represent novel biomarkers and even therapeutic targets for early kidney and cardiovascular disease.
Keywords: albuminuria, gender difference, glomerulus, nephron number, podocyte
Normally, macromolecules such as albumin are almost completely excluded from entering the filtrate by the glomerular filtration barrier consisting of endothelia, podocytes, and glomerular basement membrane.1 Major barrier disruptions, as occur in individuals with mutations of slit diaphragm genes, cause massive protein leakage.2, 3 More moderate albumin excretion above the normal range, or ‘microalbuminuria' (30–300 mg per 24 h), may also be clinically important, as may variations within the so-called normal range. In individuals with diabetes mellitus, microalbuminuria generally precedes, and may predict progression to nephropathy.4, 5 Indeed, filtered proteins, or bound molecules, may be tubulotoxic.6, 7 Microalbuminuria is an independent risk factor for cardiovascular mortality and morbidity not only in individuals with diabetes mellitus or systemic hypertension but also in the general population.8 This association may be explained by increased albuminuria being just one manifestation of a generalized microvascular disturbance.8
In normotensive US adolescents, albumin excretion rate is higher in blacks than in whites.9 In US adults, the prevalence of microalbuminuria is greater in non-Hispanic blacks and Mexican Americans as compared with non-Hispanic whites.10 The importance of genetic background in determining albuminuria is supported by observations of inbred ‘normal' mice.11 Tsaih et al.11 examined mice at advanced ages of 12–24 months, reporting up to a 100-fold difference of albuminuria between strains. Within certain strains (for example, A/J, C57BL/10J, and FVB/NJ), males excreted more albumin than females, whereas the opposite held in other strains (for example, BUB/BnJ and SJL/J). In healthy adults in the Netherlands, men had a higher average urinary albumin excretion rate than women (10 vs. 8 mg per 24 h), and the prevalence of microalbuminuria was twofold higher in males, even after controlling for smoking, hypercholesterolemia, and obesity.12 In nondiabetic white UK adults, men had a higher albumin excretion rate than women; microalbuminuria in men was associated with short stature, whereas hypertension positively correlated with albumin excretion rate in women.13
The antenatal environment to which an individual is exposed may influence urinary albuminuria. Adults gestated during the Dutch famine in World War II had an increased risk of microalbuminuria.14 Possibly, maternal undernutrition led to birth of individuals whose kidneys contained fewer nephrons than normal and subsequent compensatory changes would have resulted in loss of filtration barrier functionality.15, 16 Indeed, rodent embryos exposed to maternal low-protein diet form kidneys with fewer glomeruli than normal.17 Interestingly, within the general human population there exists considerable variation in the numbers of glomeruli per kidney,18 with normotensives having about 1–2 × 106 glomeruli per kidney and adults with essential hypertension having approximately 0.5–1 × 106 glomeruli per kidney.
The above evidence is consistent with the contentions that genetic background and sex modify albumin excretion rate. We speculated such variations in albuminuria would relate to alterations in glomerular biology and/or numbers. We hypothesized that studying mice with naturally occurring variations in albuminuria would allow us to identify genes deregulated in ‘leaky' glomeruli.
RESULTS
Urinary albumin excretion in B6 and FVB/N mice
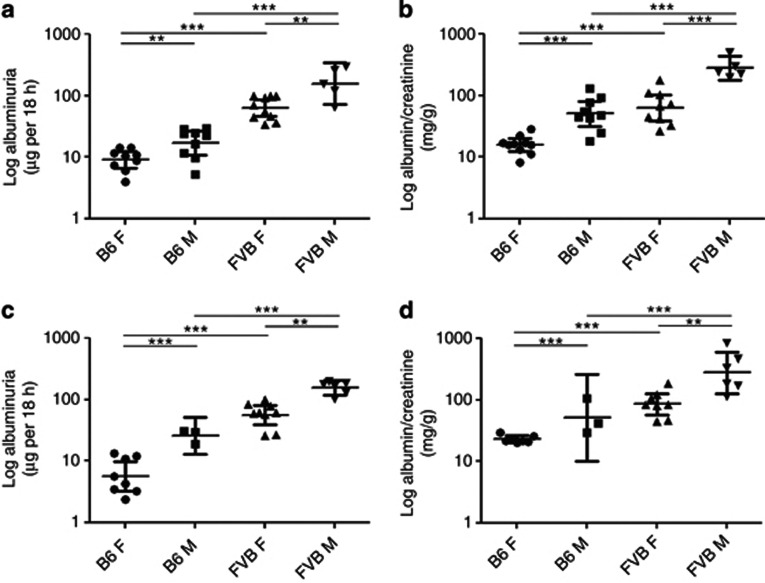
The urinary albumin excretion rate in adult (18 weeks old) female and male FVB/NHanHsd (FVB/N) mice was, on average, eightfold more than age- and sex-matched C57BL/6JOlaHsd (B6) mice (Figure 1a). Within each strain, males had increased albumin excretion rates than females (twofold elevations in both B6 and FVB/N strains; Figure 1a). The same patterns were apparent when albumin/creatinine concentration was measured (Figure 1b). Albumin excretion rates (Figure 1c) and albumin/creatinine (Figure 1d) were quantified in separate sets of mice aged 13 weeks, with the same patterns noted between the strains and sexes. There were no significant differences in albuminuria between mice of the same sex and strain at 13 versus 18 weeks.
Figure 1.
Albuminuria in B6 and FVB/N mice. Overnight (a, c) albumin excretion and (b, d) albumin-to-creatinine ratios were evaluated in (a, b) 18-week-old and (c, d) 13-week-old adult mice. Data were log transformed before analysis and are presented as geometric means and confidence interval. There was a significant increase in urinary albumin in male (M) and female (F) FVB/N mice compared with sex-matched B6 animals. Within each strain, males had elevated albuminuria versus females (**P<0.01, ***P<0.001 between groups).
Glomerular gene expression in vivo
Eighteen-week-old mice were perfused with magnetic beads that accumulate in glomerular capillaries (Figure 2a). Magnetically isolated glomeruli usually consisted of the tuft alone, although others also contained a capsule (Figure 2b). mRNA integrity was preserved in isolated glomeruli (Figure 2c). For each group, we undertook three sets of RNA microarrays, each from a separate mouse. We identified a common set of 39 genes significantly differentially expressed in each of the comparisons: female FVB/N versus female B6 mice; male FVB/N versus male B6 mice; male versus female B6 mice; and male versus female FVB/N mice. Expression levels of 34 transcripts positively correlated with albuminuria (Table 1), whereas levels of five others negatively correlated with albuminuria (Table 2). Several upregulated transcripts coded for proteins involved in oxidation/reduction reactions (Aass, Aldh1l1, Cyp4a12a, Hsd3b2, and Ldhd) or ion transport (Slc22a2, Slc22a6, Slc5a8, and Slco1a1). Other upregulated transcripts included: Acy3 and Tst, coding for enzymes involved in detoxification; Treh, an enzyme hydrolyzing trehalose; Aqp1, coding for aquaporin-1; and Hpn, coding for hepsin, a serine protease. Downregulated transcripts included: Trf, encoding transferrin; Cldn11, coding for the tight junction protein claudin 11; and Pln, encoding phospholamban, a membrane protein that regulates the calcium ion pump in muscle. Real-time quantitative reverse transcriptase–PCR (qPCR) was performed for selected upregulated (Acy3, Cyp4a12a, Hsd3b2, and Treh) and downregulated (Pln and Trf) transcripts using independent samples. These results (Figure 2d–i) concurred with the directions indicated by the microarray. In the case of Hsd3b2, changes were seen between strains but not sex; this may be because of the microarray probes also detecting Hsd3b3 and Hsd3b6 that may contribute to the differences seen between male and female mice. Note that the relative fold change in the levels of any particular transcript as assessed by the array and qPCR analyses did not necessarily exactly correspond to the numerical fold change in albuminuria documented between sexes and also between strains.
Figure 2.
Isolation of glomeruli and real-time PCR for selected genes. (a) Glomeruli (g) were isolated by perfusion of magnetic beads (arrows) that accumulated in the glomerular vessels. (b) Isolated glomeruli retain intact morphology with the majority simply comprising the tuft (left panel) whereas on occasions the tuft was surrounded by Bowman's capsule (arrows, right panel); (c) RNA integrity was preserved. FU, fluorescence units. Real-time quantitative reverse transcriptase–PCRs (qPCRs) for (d) Acy3, (e) Cyp4a12a, (f) Treh, and (g) Hsd3b2; transcripts positively correlating with albuminuria in array analyses. qPCRs for (h) Pln and (i) Trf, transcripts negatively correlating with albuminuria in array analyses. F, female; M, male. Hypoxanthine-guanine phosphoribosyltransferase (hprt) was used as a housekeeping gene. Fold changes in expression are expressed relative to B6 female mice where average expression was given an arbitrary value of 1 (a=P<0.05, b=P<0.01, and c=P<0.001 compared with B6 female mice; d=P<0.01 compared with B6 male mice; e=P<0.001 compared with B6 male mice; f=P<0.001 compared with FVB/N female mice, n=4 in each group). Bar in a is 50 μm and in b is 20 μm. Data were log transformed before analysis and are presented as geometric means and confidence interval.
Table 1. Genes significantly upregulated in glomeruli by both strain and sex.
|
Fold change compared with B6 female |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Probe set | Gene symbol | Gene title | Mouse chromosomal location (chromosome (Chr):Mb) | Human chromosomal location (Chr:Mb) | B6 male | FVB/N female | FVB/N male | P-value (sex) | P-value (strain) |
| 1450947_at | 2610528J11Rik | RIKEN cDNA 2610528J11 gene | 4:118.5 | 1:43.7 | 2.5 | 2.0 | 6.8 | 0.017 | 0.012 |
| 1431904_at | 4933427G17Rik | RIKEN cDNA 4933427G17 gene | 7:121.0 | NA | 4.8 | 2.8 | 8.0 | 0.024 | 0.045 |
| 1423523_at | Aass | Aminoadipate-semialdehyde synthase | 6:23.1 | 7:121.7 | 3.0 | 1.6 | 6.8 | 0.013 | 0.043 |
| 1448539_a_at | Acy3 | Aspartoacylase (aminoacylase) 3 | 19:4.0 | 11:67.4 | 4.8 | 1.7 | 12.8 | 0.001 | 0.009 |
| 1424400_a_at | Aldh1l1 | Aldehyde dehydrogenase 1 family, member L1 | 6:90.5 | 3:125.8 | 2.7 | 2.0 | 6.1 | 0.041 | 0.042 |
| 1416203_at | Aqp1 | Aquaporin 1 | 6:55.3 | 7:30.9 | 2.1 | 2.6 | 6.8 | 0.032 | 0.003 |
| 1418914_s_at | Bhmt2 | Betaine-homocysteine methyltransferase 2 | 13:93.7 | 5:78.4 | 2.9 | 2.0 | 9.3 | 0.045 | 0.037 |
| 1451625_a_at | C8g | Complement component 8, γ polypeptide | 2:25.5 | 9:139.8 | 2.7 | 2.5 | 9.5 | 0.020 | 0.006 |
| 1418976_s_at | Cideb | Cell death-inducing DNA fragmentation factor, α subunit-like effector B | 14:55.8 | 14:24.8 | 2.2 | 1.6 | 6.8 | 0.013 | 0.012 |
| 1424811_at | Cml5 | Camello-like 5 | 6:85.8 | 2:73.9 | 10.8 | 6.4 | 65.7 | 0.030 | 0.019 |
| 1423701_at | Coasy | Coenzyme A synthase | 11:101.1 | 17:40.7 | 2.1 | 1.7 | 6.5 | 0.037 | 0.029 |
| 1424352_at | Cyp4a12a | Cytochrome P450, family 4, subfamily a, polypeptide 12a | 4:115.3 | 1:47.4 | 28.6 | 2.2 | 359.8 | 0.003 | 0.022 |
| 1428738_a_at | D14Ertd449e/// ENSMUSG00000072676 | DNA segment, Chr 14, ERATO Doi 449, expressed/// predicted gene, ENSMUSG00000072676 | 14:26.1 | 10:81.8 | 1.6 | 1.6 | 6.8 | 0.035 | 0.013 |
| 1417393_a_at | Fam132a | Family with sequence similarity 132, member A (also known as C1qdc2) | 4:156.0 | 1:1.2 | 1.5 | 1.6 | 3.4 | 0.023 | 0.005 |
| 1417422_at | Gnmt | Glycine N-methyltransferase | 17:46.8 | 6:42.9 | 3.0 | 2.3 | 8.9 | 0.037 | 0.024 |
| 1420712_a_at | Hpn | Hepsin | 7:31.1 | 19:35.5 | 2.0 | 1.6 | 6.4 | 0.033 | 0.025 |
| 1460232_s_at | Hsd3b2/// Hsd3b3/// Hsd3b6 | Hydroxy-δ-5-steroid dehydrogenase, 3 β- and steroid δ-isomerase 2/// hydroxy-δ-5-steroid dehydrogenase, 3 β- and steroid δ-isomerase 3/// hydroxy-δ-5-steroid dehydrogenase, 3 beta- and steroid δ-isomerase 6 | 3:98.7 | 1:120.0 | 4.4 | 4.5 | 11.5 | 0.025 | 0.005 |
| 1428614_at | Ldhd | Lactate dehydrogenase D | 8:111.6 | 16:75.1 | 5.0 | 1.8 | 15.4 | 0.002 | 0.008 |
| 1419504_at | LOC100047046/// Mogat1 | Similar to monoacylglycerol O-acyltransferase 1/// monoacylglycerol O-acyltransferase 1 | 1:78.5 | 2:223.5 | 8.3 | 6.0 | 69.4 | 0.005 | 0.002 |
| 1419520_at | Nat8 | N-acetyltransferase 8 | 6:85.8 | 2:73.9 | 3.2 | 2.6 | 23.3 | 0.008 | 0.003 |
| 1455490_at | Pigr | Polymeric immunoglobin receptor, | 1:130.8 | 1:207.1 | 3.9 | 2.4 | 25.5 | 0.004 | 0.003 |
| 1419117_at | Slc22a2 | Solute carrier family 22 (organic cation transporter), member 2 | 17:12.6 | 6:160.6 | 2.5 | 1.8 | 9.0 | 0.029 | 0.023 |
| 1417072_at | Slc22a6 | Solute carrier family 22 (organic anion transporter), member 6 | 19:8.6 | 11:62.7 | 3.7 | 1.7 | 16.8 | 0.020 | 0.046 |
| 1425606_at | Slc5a8 | Solute carrier family 5 (iodide transporter), member 8 | 10:88.9 | 12:101.5 | 3.8 | 1.5 | 15.3 | 0.008 | 0.027 |
| 1436667_at | Slc6a20b | Solute carrier family 6, member 20b | 9:123.6 | 3:45.8 | 2.1 | 2.1 | 6.5 | 0.036 | 0.009 |
| 1449301_at | Slc7a13 | Solute carrier family 7, (cationic amino acid transporter, y+ system) member 13 | 4:19.8 | 8:87.2 | 224.0 | 3.9 | 1267.6 | <0.001 | 0.005 |
| 1420379_at | Slco1a1 | Solute carrier organic anion transporter family, member 1a1 | 6:141.9 | 12:21.4 | 36.0 | 3.4 | 567.4 | <0.001 | 0.002 |
| 1449409_at | Sult1c2 | Sulfotransferase family, cytosolic, 1C, member 2 | 17:53.8 | 2:108.9 | 1.5 | 2.5 | 5.9 | 0.036 | 0.001 |
| 1451528_at | Tmem82 | Transmembrane protein 82 | 4:141.6 | 1:16.1 | 1.7 | 1.7 | 6.7 | 0.036 | 0.009 |
| 1430889_a_at | Tpmt | Thiopurine methyltransferase | 13:47.0 | 6:18.1 | 2.1 | 1.8 | 13.7 | 0.020 | 0.007 |
| 1449430_a_at | Treh | Trehalase (brush-border membrane glycoprotein) | 9:44.7 | 11:118.5 | 3.4 | 3.6 | 41.9 | 0.008 | 0.001 |
| 1448609_at | Tst | Thiosulfate sulfurtransferase, mitochondrial | 15:78.4 | 22:37.4 | 2.2 | 2.1 | 7.3 | 0.015 | 0.004 |
| 1451373_at | Ugt3a1 | UDP glycosyltransferases 3 family, polypeptide A1 | 15:9.3 | 5:36.0 | 3.1 | 1.9 | 13.7 | 0.028 | 0.022 |
| 1460244_at | Upb1 | Ureidopropionase, β | 10:75.4 | 22:24.9 | 1.8 | 1.6 | 5.2 | 0.048 | 0.030 |
All genes were significantly altered by both strain and gender (P<0.05), followed the direction of albuminuria in the four groups assessed, and varied by >1.5-fold in individual comparisons between different sexes in both B6 and FVB/N mice and between mice of the same sex but from different strains. Fold changes are presented relative to B6 female mice (given a value of 1) that had the lowest albumin excretion rate. NA, not available.
Table 2. Genes significantly downregulated in glomeruli by both strain and sex.
|
Fold change compared with B6 female |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Probe set | Gene symbol | Gene title | Mouse chromosomal location | Human chromosomal location | B6 male | FVB/N female | FVB/N male | P-value (sex) | P-value (strain) |
| 1445938_at | 5930427L02Rik | RIKEN cDNA 5930427L02 gene | NA | NA | −1.9 | −1.5 | −29.4 | 0.049 | 0.017 |
| 1416003_at | Cldn11 | Claudin 11 | 3:31.1 | 3:170.1 | −2.6 | −2.2 | −15.5 | 0.036 | 0.009 |
| 1450952_at | Pln | Phospholamban | 10:53.3 | 6:118.9 | −1.5 | −1.6 | −4.7 | 0.010 | 0.002 |
| 1425546_a_at | Trf | Transferrin | 9:103.2 | 3:133.5 | −1.8 | −1.5 | −3.4 | 0.046 | 0.042 |
| 1446477_at | Zfp622 | Zinc-finger protein 622 | 15:26.0 | 5:16.5 | −2.0 | −2.1 | −3.4 | 0.023 | 0.004 |
All genes were significantly altered by both strain and gender (P<0.05), followed the direction of albuminuria in the four groups assessed, and varied by >1.5-fold in individual comparisons between different sexes in both B6 and FVB/N mice and between mice of the same sex but from different strains. Fold changes are presented relative to B6 female mice (given a value of 1) that had the lowest albumin excretion rate. NA, not available.
Relation of candidate genes to mouse albuminuria loci
We compared the location of these genes with published genetic analyses of albuminuria loci in mice and rats.19 Acy3, Cyp4a12a, and Hsd3b2 were found to be within the confidence intervals of mouse and rat quantitative tract loci (QTLs) for albuminuria. We also interrogated the array data with respect to candidate genes previously implicated in albuminuria using a genome-wide association approach, namely Aspa, Atic, Cyp24a1, Fdn4, Fn1, Mbp, Myo16, Negr1, Olfr381, Olfr389, Olfr392, Prdm5, Ripk2, Spata22, Trpv1, Trpv3, and Zfp236.11 There was no significant effect of sex on the expression levels of these genes. However, in FVB/N versus B6 glomeruli of either sex, we found decreased transcript levels of: Negr1 (19.3- and 4.0-fold in males and females, respectively, P<0.01), encoding a cell adhesion molecule; Prdm5 (2-fold in both sexes, P<0.01), encoding a transcription factor; and Trpv1 (10.7- and 2.3-fold in male and female mice, respectively, P<0.05), encoding the vanilloid receptor-1 ion channel.
Glomerular expression of candidate molecules
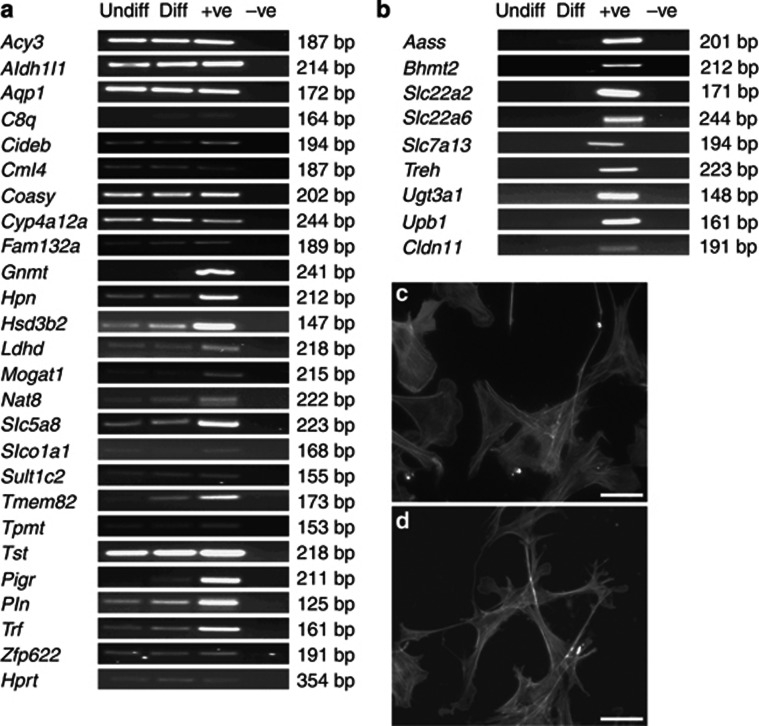
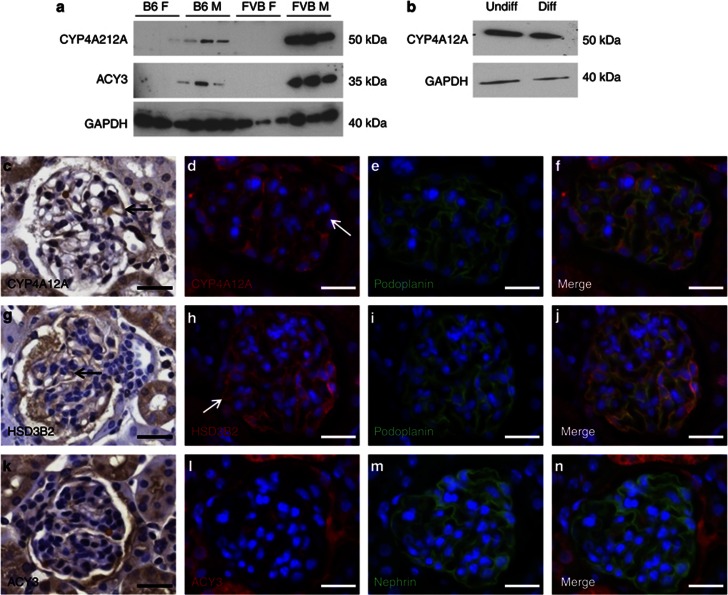
Assessed by reverse transcriptase–PCR (RT–PCR) (Figure 3a), over half of the set of transcripts identified by the array were expressed in a podocyte line,20 whereas others were not detected in the same cells (Figure 3b). Using protein lysates from isolated glomeruli, we undertook semiquantitative western blotting for CYP4A12A, a cytochrome P450, and aspartocyclase 3 (ACY3), respectively the proteins encoded by Cyp4a12a and Acy3. Reliable signals for HSD3B2 could not be obtained with available antibodies. Levels of CYP4A12A and ACY3 appeared markedly and reproducibly greatest in FVB/N males; lesser levels of these proteins were detected in B6 male glomeruli but they were barely detectable or undetectable in female glomeruli of either strain (Figure 4a). Immunohistochemistry of FVB/N male kidneys demonstrated CYP4A12A (Figure 4c–f) and HSD3B2 (Figure 4g–j) in the glomeruli; with some of the signal in podocytes as evidenced by double labeling with podoplanin. Furthermore, CYP4A12A was detected in western blots of both undifferentiated and differentiated cultured podocytes (Figure 4b). In tissue sections, ACY3 was predominantly immunolocalized in parietal glomerular epithelia, with a faint signal in glomerular tufts (Figure 4k–n); we did not detect ACY3 in western blots of cultured podocytes.
Figure 3.
Expression of candidate genes in cultured podocytes. (a, b) RNA was collected from undifferentiated (Undiff) and differentiated (Diff) podocytes and PCR performed for all annotated genes found to be differentially expressed in the microarray analysis. Positive controls (+ve) of reactions consisted of mouse whole kidney RNA. Negative controls (−ve) consisted of reactions without cDNA template. Panel a comprises genes that were found to be expressed in cultured podocytes and panel b comprises transcripts that were not detected using this methodology. Representative pictures of phalloidin-stained undifferentiated (c) and differentiated (d) podocytes showing the extensive process formation characteristic of the in vivo podocyte phenotype. (c, d) Bar=50 μm.
Figure 4.
Expression of candidate proteins in glomeruli. (a) Protein lysates from isolated glomeruli (n=3 kidneys from each group) were used to immunoblot for CYP4A12A and ACY3. Levels of CYP4A12A and ACY3 appeared markedly and reproducibly greatest in FVB/N males; lesser levels of these proteins were detected in B6 male (M) glomeruli but they were barely detectable or undetectable in female (F) glomeruli of either strain; glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a housekeeping gene to assess loading. (b) Protein isolates from both undifferentiated (Undiff) and differentiated (Diff) podocytes contained CYP4A12A protein. Using immunohistochemistry, CYP4A12A was immunodetected in the glomeruli of tissue sections obtained from FVB/N male kidneys; some of the signal was found in podocytes as evidenced by double labeling with podoplanin (c–f, arrows). HSDS3B2 was also detected in the podocytes of the glomeruli (g–j, arrows). ACY3 was predominately immunolocalized to parietal glomerular epithelia, with a faint signal observed in glomerular tufts (k–n). (c–n) Bars=20 μm.
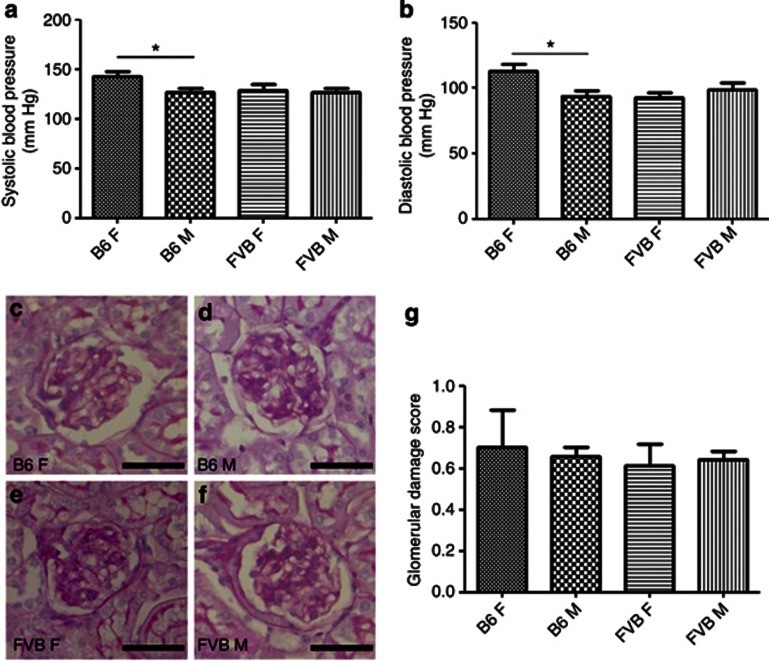
Blood pressure, glomerular histology, and expression levels of other glomerular genes
Systolic and diastolic blood pressures were similar in male B6, male FVB/N, and female FVB/N mice, with female B6 mice tending to have higher pressures (Figure 5a and b). Glomerular histology was similar in the groups and sclerotic glomeruli were rare (Figure 5c–h). We interrogated microarray data, comparing transcript levels of genes implicated in the biology of podocytes (Actn4, Fat1, Nck1, Nck2, Nphs1, Nphs2, Podxl, Synpo, Trpc6, and Wt1), endothelia (Ang1, Ang2, Ang3, Pecam1, Tie1, Tie2, Vegfa, and Vegfr2), and glomerular basement membrane (Agrn, Col4a1-5, Fn1, Hspg2, Lama1, Lama5, Lamb1, Lamb2, and Lamc1). There was no significant effect of sex on their expression levels. However, in FVB/N versus B6 glomeruli of either sex, levels of the following transcripts were altered: Col4a1 (decreased 1.4-fold; P<0.05), Nphs2 (decreased 2-fold; P<0.001), Podxl (decreased 2–3-fold; P<0.01), and Synpo (decreased 2-fold; P<0.01).
Figure 5.
Blood pressure and glomerular histology in 18-week-old B6 and FVB/N mice. (a) Systolic and (b) diastolic blood pressure was not positively associated with alterations in albuminuria, with the only differences found between B6 female (F) and male (M) mice, with the former having elevated pressures. Blinded analysis of glomerular histology revealed that there were no differences between (c) B6 female, (d) B6 male, (e) FVB/N female, and (f) FVB/N male mice; (g) quantification is shown. *P<0.05, n=5 for blood pressure analysis and n=3 for glomerular analysis in each group. (c–f) Bars=30 μm. All data are presented as means and s.e.m.
Testosterone administration in female mice
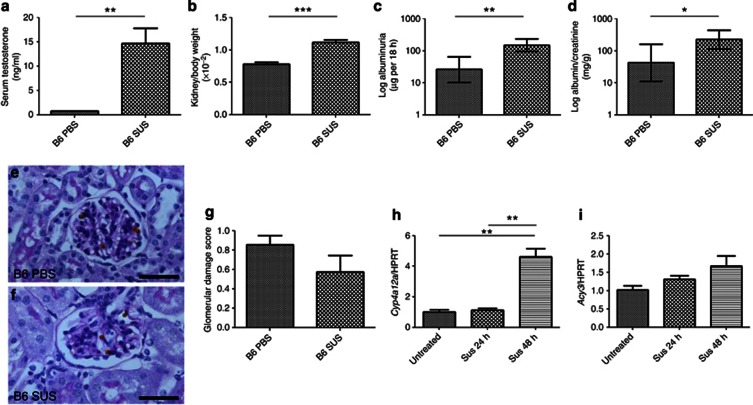
Sustanon 250, comprising testosterone esters, was administered to 5-week-old B6 and FVB/N females. At the start of the experiment, FVB/N tended to have higher excretion rates than B6 mice (18±5 vs. 8±2 μg per 18 h respectively, P=0.07, n=12 in each group). When evaluated at 9 weeks of age, Sustanon-exposed female B6 mice had increased serum testosterone than vehicle-administered controls (Figure 6a) and higher kidney/body weight ratios (Figure 6b). Compared with vehicle-treated mice, the Sustanon-exposed group had increases in albumin excretion rate (373±71%, P<0.01; Figure 6c) and albumin/creatinine ratios (312±87%, P<0.05; Figure 6d) but glomerular histology was similar (Figure 6e–g). Sustanon administration was associated with a 7-fold upregulation of Acy3 and a 1072-fold upregulation of Cyp4a12a (n=4 in each group, P<0.05 in both cases). In vitro, addition of Sustanon to differentiating podocytes for 48 h caused significant elevation of Cyp4a12a transcripts (Figure 6h), with a tendency (P=0.09) for Acy3 levels to increase (Figure 6i). Sustanon administration to female FVB/N mice led to elevated serum testosterone levels (19±3 vs. placebo 0.7±0.1 ng/ml, P<0.001, n=6 in each group) and increased kidney/body weight ratios (1.3±0.1 vs. vehicle alone 0.9±0.1 × 10−2, P<0.001), but no significant effect on albuminuria.
Figure 6.
Administration of testosterone to female B6 mice. Sustanon-250 injection for 4 weeks in 5-week-old B6 mice (n=6 in each group) led to a significant increase in (a) serum testosterone, (b) kidney/body weight, (c) albumin excretion rate, and (d) albumin/creatinine ratio. Despite these changes, blinded analysis of glomerular histology showed that there was no difference between B6 female mice injected with either (e) phosphate-buffered saline (PBS) or (f) Sustanon (SUS); (g) quantification is shown. (h) Stimulation of mouse podocytes in vitro (n=3 in each group) with Sustanon-250 led to a significant increase in Cyp4a12a mRNA levels; hypoxanthine-guanine phosphoribosyltransferase (hprt) was used as a housekeeping gene. (i) Acy3 mRNA tended to be elevated following Sustanon-250 exposure but this was not significant *P<0.05, **P<0.01; ***P<0.001 between groups. (e, f) Bars=30 μm. All data are presented as means and s.e.m. except c and d that were log transformed before analysis and are presented as geometric means and confidence interval.
We considered the fact that exogenous testosterone increased albuminuria in B6 but not FVB/N females might be explained if the latter had higher endogenous testosterone levels. However, in 18-week-old mice there were no differences in testosterone levels between sex-matched animals of either the FVB/N or the B6 strain (B6 females, 0.5±0.1; FVB/N females 0.4±0.1; B6 males 5.1±1.8; and FVB/N males 7.2±3.4 ng/ml, n=9 in each group).
Glomerular numbers in male and female FVB/N and B6 mice
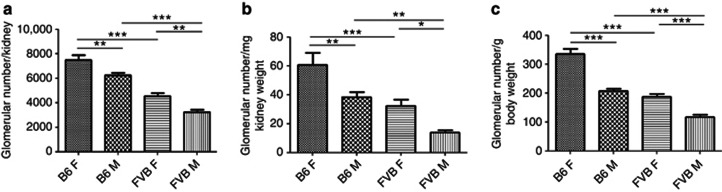
We measured glomerular numbers/kidney in male and female 18-week-old B6 and FVB/N mice. Here, it should be noted that mouse glomerulogenesis starts a week before birth and continues through the first postnatal week; no new glomeruli can form after this period because the nephrogenic zone disappears.21 Across the strains and sexes, the numbers of glomeruli (whether expressed as the total/kidney, or factored for either kidney or body weight) was inversely correlated with albuminuria. Therefore, glomerular numbers were: female B6>male B6>female>FVB/N>male FVB/N, whereas the degree of albuminuria was the exact opposite (Figure 7a–c).
Figure 7.
Glomerular counts in B6 and FVB/N 18-week-old mice. (a) A reduction in glomerular number was observed in male (M) mice compared with strain-matched females (F); in addition, FVB/N had significantly lower number of glomeruli compared with sex-matched B6 mice. Similar changes were calculated when either (b) kidney or (c) body weight of the mice was taken into account (*P<0.05, **P<0.01; ***P<0.001 between groups, n=6 in each group). All data are presented as means and s.e.m.
DISCUSSION
Albuminuria is often quantified as urinary albumin/creatinine concentrations but up to one half of urinary creatinine in mice is derived from tubular secretion, and this contribution may vary between sexes.22 Therefore, to compare albuminuria between sexes, a more robust measure might be to measure the absolute amount of albumin excreted per unit time. We found that, as assessed by either parameter, a characteristic and reproducible level of urinary albumin excretion had been established by early adulthood for each of the four groups of healthy mice. Specifically, we found increasing albuminuria in female B6<male B6<female FVB/N<male FVB/N mice. Considering that a human is ∼2000 times heavier than a mouse, the albumin excretion rate range between female B6 and male FVB/N mice (∼15–150 μg/day) is similar to the range for human microalbuminuria (30–300 mg/day). Hence, our models may have relevance to humans with modestly elevated albuminuria.
Proximal tubules endocytose filtered macromolecules.23 Although this will modify the amount of albumin in the final urine, we reasoned that because the glomerular filtration barrier is the main player in determining albuminuria, then the degree of albuminuria should reflect glomerular biology changes. Within whole glomeruli in vivo, across both strains and sexes, we identified reproducible changes in gene expression correlating with albuminuria. Most of these transcripts were also detected in cultured podocytes; perhaps those that were not would be expressed by other tuft cells such as endothelia and/or mesangial cells. Alternatively, some of them, such as genes encoding transporters, might have been expressed by Bowman's capsule epithelia observed, adhering to tufts in some isolated glomeruli.
Cyp4a12a encodes a cytochrome P450 involved in degradation of long-chain fatty acids.24 For arachidonic acid, this yields 20-hydroxyeicosatetraenoic acid, implicated in pathogenesis of systemic hypertension.25 Thus, a possible explanation for differences in albuminuria between mouse sexes and strains were variations of systemic blood pressure, assuming higher pressures would increase hydrostatic pressure within glomerular capillaries and/or trigger physical damage, manifested for example by glomerulosclerosis.15 We found no evidence for a positive relation between albuminuria and blood pressure or glomerular dysmorphology. High glucose elevates CYP4A protein and 20-hydroxyeicosatetraenoic acid in podocytes, the effects ameliorated by pharmacological CYP4A inhibition.26 In diabetic nephropathy mice, glomerular CYP4A is elevated, and blockade of this pathway attenuates albuminuria.26
Hsd3b2 encodes 3-β-hydroxysteroid dehydrogenase, essential for steroid synthesis.27 Hsd3b2 and related steroidogenic transcripts are expressed in fetal kidneys, although their specific localization was unreported.28 Pezzi et al.28 speculated that low levels of the encoded proteins might produce local, intrarenal effects. HSD3B2 modulates biosynthesis of glucocorticoids that themselves alter glomerular biology.29 Isolated glomeruli express the glucocorticoid receptor,30 and here glucocorticoid exposure has antiapoptotic and anti-inflammatory effects.31, 32 In contrast, high-dose glucocorticoids in normal adult mice cause proteinuria, mesangial expansion, and glomerulosclerosis,33 and exposure to developing murine kidneys retards organ growth34 and glomerulogenesis.35
Acy3 encodes aspartoacylase 3, implicated in detoxifying xenobiotics36 and metal ion binding.37 ACY3 is upregulated during mycophenolate mofetil treatment of a collagen-4α3-deficient renal fibrosis model.38 The enzyme binds cobalt and nickel37 and, notably, anionic glycosaminoglycans in the glomerular basement membrane also bind divalent cations.39 Pushkin et al.36 reported expression of the protein in proximal tubules; in this study, we found that ACY3 was present in parietal glomerular epithelia. In cultured podocytes, RT–PCR detected Acy3 transcripts but the encoded protein was not detectable on western blotting. As demonstrated in Figure 2, a subset of harvested glomeruli have a Bowman's capsule, and thus the most likely interpretation is that the association of levels of Acy3 transcripts with albuminuria may predominantly reflect a secondary effect of ultrafiltered protein on parietal epithelia.
Our study indicated that male sex was associated with elevated albuminuria, and hence we hypothesized that androgens would increase albumin excretion. Testosterone administered to B6 females did so, accompanied by upregulated glomerular Acy3 and Cyp4a12a transcripts; levels of the latter also increased in testosterone-exposed cultured podocytes. Indeed, Muller et al.24 demonstrated that, in whole kidneys, Cyp4a12a transcript and protein levels in males exceeded those in females, and administration of testosterone increased the levels. They showed differences in expression between males of different strains correlating with the capacity of renal microsomes to hydroxylate arachidonic acid.24 Supporting the contention that sex steroids affect expression of transcripts implicated in albuminuria, five genes identified in the array contain predicted androgen response elements (Aass, Aqp1, D14Ertd449e, Fam132a, and Slc22a6). The testosterone receptor has been shown to be expressed in isolated glomeruli40 and cultured podocytes,41 and hence these cells may be a direct target for sex steroids. Furthermore, incubation of cultured podocytes with testosterone increases apoptosis, which can be abolished by androgen receptor blockade.41 In vivo, testosterone supplementation to ovariectomized B6 wild-type mice increases albuminuria, glomerulosclerosis, and podocyte apoptosis.40, 41 Hsd3b2 is also essential for biosynthesis of testosterone29 and perhaps increased levels of Hsd3b2 within glomeruli increase local testosterone concentrations, with detrimental effects.
Sustanon increased albuminuria in B6 females but not in FVB/N females. From our microarray, we did not find changes in glomerular transcript levels of the androgen receptor between FVB/N and B6 mice of either sex. Another possible explanation for sex differences in albuminuria would be that estrogen is protective. Indeed, 14 of the genes implicated in our array analyses contain predicted estrogen response elements (Acy3, Aqp1, Cideb, D14Ertd449e, Gnmt, Pigr, Slc5a8, Slc6a20b, Slc7a13, Slco1a1, Sult1c2, Tmem82, Tpmt, and Zfp622). Estrogen receptors are expressed on podocytes41, 42 and estradiol attenuates podocyte apoptosis induced by puromycin or testosterone.41, 42 Furthermore, mice lacking estrogen receptor-α develop albuminuria and glomerulosclerosis.40, 41 Although circulating estrogen was not measured in this study, estrogen receptor 1 was increased in FVB/N versus B6 glomeruli (by 3.5- and 1.9-fold in male and females, P<0.01). If estrogen effects were to be higher in FVB/N than B6 glomeruli, this may explain why the filtration barrier of FVB/N female animals was resistant to Sustanon-250 administration.
Our experiments using Sustanon were only partially consistent with the contention that androgens enhance albuminuria. Furthermore, male FVB/N mice had elevated albuminuria versus male B6 mice, although both had similar circulating testosterone levels. Therefore, we sought a different explanation for the variation in albuminuria between sexes and strains. Strikingly, numbers of glomeruli/kidney for the four experimental groups showed exactly the inverse pattern to the degree of albuminuria. It should be noted that we used acid dissociation17 rather than a ‘gold standard' sterology method43 to assess glomerular numbers. However, the dissociation technique should allow valid relative comparisons between numbers of glomeruli/kidney between the groups studied, none of which had anatomically deranged or sclerotic kidneys. Recurrent observations have shown that low glomerular numbers per kidney correlate with systemic hypertension in white subjects,18, 44 but we did not observe an inverse relationship between blood pressure and glomerular counts in this study.
A glomerular deficiency could potentially lead to glomerular hyperfiltration that may cause microalbuminuria associated with activation of the renin–angiotensin system.15, 45 Although we found no positive correlation of albuminuria with systemic blood pressure in FVB/N compared with B6 mice, we noted increased glomerular transcript levels of Ace1 (4.8-fold in males and 1.8-fold in females, P=0.02) and Ace2 (2.6-fold in males and 1.6-fold in females, P=0.02) suggesting that renin–angiotensin system may be enhanced in the FVB/N strain of mice. There was no evidence of glomerular structural changes between sex- or strain-matched male and female B6 and FVB/N mice. However, there were some changes in the mRNA expression of several glomerular genes with loss of podocyte genes Nphs2, Podxl, Synpo, and the basement membrane gene Col4a1. These differences indicate that the molecular make-up of the filtration barrier may differ between FVB/N and B6 mice that potentially could lead to albuminuria.
We have demonstrated that low numbers of glomeruli and testosterone are potential mechanisms leading to albuminuria in mice. Several unsuspected genes have been identified that correlate with albuminuria, but further studies are needed to demonstrate whether these changes are simply reactions to albuminuria or may drive glomerular pathobiology. Some of these genes may also influence glomerulogenesis, although none have yet been functionally implicated in kidney development per se. Several of the genes coding for transcripts highlighted in this study are located within the mapped intervals for albuminuria QTL in mice and rats. For example, Cyp4a12a lies within the interval of an albuminuria QTL found in a cross between C57BL/6J and A/J46 and a cross between C57BL/6J and NZM.47 Acy3 is located within albuminuria QTL found in crosses between C57BL/6J and A/J mice46 and between MWF and SHR rats.48 Mogat1 maps within the albuminuria QTL found in a cross between MWF and SHR rats48 and both Hsd3b2 and Mogat1 map within albuminuria QTL found from a cross between SS and SHR rats.49 However, it is important to note that the QTL intervals are large and contain hundreds of genes. Expression analysis in the kidney for the above-mentioned genes in these mouse and rat strains would be necessary to establish whether these could be candidate genes for the QTL. Most interestingly, the human homolog of Nat8 has been associated with estimated glomerular filtration rate in several human genome-wide association studies.50, 51 Furthermore, Juhanson et al.52 resequenced the NAT8 promoter and suggested that polymorphisms therein determined systolic blood pressure and glomerular filtration rate. Proteins encoded by these transcripts could be novel biomarkers and therapeutic targets for early kidney and vascular disease.
MATERIALS AND METHODS
Assessment of B6 and FVB/N mice
Reagents were obtained from Sigma Chemical Company (Gillingham, Dorset, UK) unless stated. C57BL/6JOlaHsd and FVB/NHanHsd male and female mice (Harlan, Oxfordshire, UK) were provided with Teklad 18% protein rodent diet (Harlan) and water ad libitium and used in protocols approved by the UK Home Office. Urine was collected from 13- and 18-week-old mice by housing them individually in metabolic cages for 18 h. Albumin concentrations were measured by enzyme-linked immunosorbent assay (Bethyl Laboratories, Montgomery, TX). Urinary creatinine concentrations were assessed by a commercially available assay (Cusabio, Newark, DE). At 18 weeks, systolic blood pressure was assessed using a noninvasive blood pressure system (Kent Scientific, Torrington, CT53); measurements were taken in duplicate at the same time on subsequent days after animals had been trained every day for the prior two weeks. Mice were then killed and body and kidney weights measured. Blood was collected and testosterone levels assessed using a commercially available assay (Alpha Diagnostic International, San Antonio, TX54).
Microarray analysis of isolated glomeruli
Eighteen-week-old male and female B6 and FVB/N mice (n=3 in each group) were anesthetized and perfused with 1 × 108 Dynabeads (Invitrogen, Paisley, UK) through the left ventricle of the heart.55 Kidneys were removed, decapsulated, minced, and digested,55 and the glomeruli containing Dynabeads were gathered by a magnetic particle concentrator and RNA prepared using RNeasy kit (Qiagen, Crawley, UK). RNA quality was assessed on the Bioanalyser 2100 (Agilent Technologies, Palo Alto, CA); subsequent cDNA and cRNA synthesis was performed and hybridized to mouse MOE430 2.0 GeneChips. Functional categories for genes were assigned using the Database for Annotation, Visualization and Integrated Discovery software (http://david.abcc.ncifcrf.gov). For transcripts up- or downregulated with albuminuria, we used the ClustalW tool (http://www.ebi.ac.uk/Tools/clustalw/index.html) to determine whether the 1000 bp upstream of the transcription start site, in addition to either the complete 5′-untranslated region or the first exon of the 5′-untranslated region when this is split over several exons, contained sequences with at least 75% homology to the consensus sequence of the androgen response element (AGAACAnnnTGTTCT)56 or estrogen response element (AGGTCAnnnTGACCT).57 We compared our data with prior studies that have identified QTLs related to albumin excretion in the mouse19 and candidate genes implicated in albuminuria using a genome-wide association approach.11
Quantitative real-time PCR
Independent glomerular extracts were obtained from 18-week-old male and female B6 and FVB/N mice (n=4 in each group) for qPCR. cDNA was prepared and 1 μg used to examine Acy3, Cyp4a12a, Hsd3b2, Pln, Treh, and Trf using previously described methods,58 with Hprt as a housekeeping gene. All measurements were performed in duplicate; primer details are available on request.
Western blotting of isolated glomeruli
Protein was extracted from isolated glomeruli using radioimmunoprecipitation assay buffer, quantified using bicinchoninic acid protein assay kit (Thermo Scientific, Rockford, IL) and 10 μg separated on a 4–15% Mini-Protean gel and transferred to a nitrocellulose membrane (Bio-Rad, Hemel Hempstead, UK). Blots were probed with either rabbit anti-ACY3 (Abcam, Cambridge, UK) or rabbit anti-CYP4A12A.24 Appropriate secondary antibodies were used and blots visualized using chemiluminescence. To confirm loading, blots were stripped and reprobed with mouse anti-glyceraldehyde 3-phosphate dehydrogenase (Abcam).
Histological analysis and assessment of glomerular numbers
Kidneys were fixed in 4% paraformaldehyde, embedded, and 5 μm sections were then cut for staining with periodic acid–Schiff reagent. A total of 50 glomeruli were scored by a blinded observer using the following system: 0, normal glomerular structure; 1, glomeruli with mesangial expansion but some capillary loops still observed; and 2, sclerotic glomeruli with no capillary loops; an average score was obtained for each kidney. Immunohistochemistry was performed in FVB/N male mice as described59 for ACY3 and CYP4A12A using the primary antibodies above and rabbit anti-HSD3B2; double labeling for these proteins was performed with either guinea-pig anti-nephrin (Progen, Heidelberg, Germany) or hamster anti-podoplanin antibodies (Abcam). Negative controls consisted of omission of primary antibodies or substitution with preimmune serum. Glomerular number was assessed from one kidney of 18-week-old male and female B6 and FVB/N mice using the acid digestion method.17
Sustanon administration
Five-week-old female B6 and FVB/N mice were administered either 10 μl of phosphate-buffered saline or Sustanon-250 (Organon Laboratories, Hoddesdon, UK) intramuscularly every 2 weeks (n=6 in each group). After 4 weeks, overnight urine was collected and blood obtained to assess systemic testosterone levels. Animals were killed following Dynabead perfusion; half a kidney was embedded in 4% paraformaldehyde and the remaining material used to extract glomerular RNA for qPCR to examine Cyp4a12a and Acy3.
Cell culture
Podocytes isolated from 10-week-old H-2Kb-tsAJ58 mice20 were cultured as described60 and induced to differentiate for 7 days; at this time point, they formed extensive processes. Using TRI Reagent, 1 mg of RNA was isolated from proliferating and differentiating podocytes and cDNA prepared for RT–PCR of the genes identified in the microarray analysis. In addition, 30 μg of protein was extracted and western blotting performed for CYP4A12A and ACY3. Some cells were also plated onto chamber slides for phalloidin staining to visualize actin filaments. In other experiments, podocytes differentiated for 7 days were exposed to Sustanon-250 (250 μg/ml) for either 24 or 48 h and qPCR performed for Cyp4a12a and Acy3.
Statistical methods
Microarray
Signal values for transcripts on the Affymetrix array were calculated using the MAS 5.0 algorithm to generate .chp files that were exported to GeneSpring 9.0 (Agilent Technologies, Wokingham, Berkshire, UK) for further analysis. The MAS 5.0–generated values were log2 transformed, normalized to the median within each array (to control for array loading), and these values were then baseline transformed to the median value of each transcript. Transcripts were filtered to exclude genes whose expression did not reach a threshold value for reliable detection (based on the relaxed Affymetrix MAS 5.0 probability of detection; P⩽0.1) in at least 1 of the 12 chips assessed. To determine genes modified by strain and sex, a two-way analysis of variance analysis was performed. Transcripts that were shown to be significantly altered by both strain and sex (P<0.05 after applying the Benjamini and Hochberg false discovery multiple testing correction61) were used in subsequent analysis. We then examined expression levels in female FVB/N versus female B6 mice, male FVB/N versus male B6 mice, male versus female B6 mice, and male versus female FVB/N mice, and genes were excluded if mean normalized expression levels did not vary by >1.5-fold in all of these individual comparisons. Genes were also excluded if expression levels did not follow the degree of albuminuria observed in the four groups assessed. Expression differences are presented relative to B6 female mice (given a relative value of 1) that had the lowest albumin excretion rate.
Other statistical analyses
Blood pressure, glomerular histology, and number and qPCR were presented as mean±s.e.m. and assessed by one-way analysis of variance with least significant difference correction. In experiments where testosterone administration was provided to female mice, the vehicle versus treated animals were assessed using unpaired two-tailed t-tests. In cases where the data were not normally distributed (albuminuria and some of the qPCR analysis; Figure 2d–i), the results were log transformed and presented as geometric mean (±95% confidence intervals) and then analyzed by one-way analysis of variance with least significant difference correction. Significance was accepted at P<0.05.
Acknowledgments
We thank UCL Biological Services and Genomics for their assistance with animal and microarray experiments, respectively. We thank Professor Peter Scambler and Dr Paul Winyard (UCL Institute of Child Health) and Dr Fabiola Terzi (INSERM, Paris, France) for helpful discussions regarding this work. The podocyte line was a gift from Peter Mundel, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA. CYP4A12A antibody was a gift from Wolf-Hagen Schunck, Max Delbrueck Center for Molecular Medicine, Berlin, Germany. DAL is supported by a Kidney Research UK Senior Non-Clinical Fellowship and a Medical Research Council New Investigator Award. EP is supported by a Wellcome Trust Postdoctoral Training Fellowship for MB/PhD graduates. Other support for this work was provided from Diabetes UK, Kids Kidney Research, and the University of London Central Research Fund. ASW acknowledges grant support from the Manchester Biomedical Research Centre.
All the authors declared no competing interests.
References
- Patrakka J, Tryggvason K. Molecular make-up of the glomerular filtration barrier. Biochem Biophys Res Commun. 2010;396:164–169. doi: 10.1016/j.bbrc.2010.04.069. [DOI] [PubMed] [Google Scholar]
- Boute N, Gribouval O, Roselli S, et al. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet. 2000;24:349–354. doi: 10.1038/74166. [DOI] [PubMed] [Google Scholar]
- Kestilä M, Lenkkeri U, Männikkö M, et al. Positionally cloned gene for a novel glomerular protein–nephrin–is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1:575–582. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- Viberti GC, Hill RD, Jarrett RJ, et al. Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet. 1982;1:1430–1432. doi: 10.1016/s0140-6736(82)92450-3. [DOI] [PubMed] [Google Scholar]
- Mogensen CE, Christensen CK. Predicting diabetic nephropathy in insulin-dependent patients. N Engl J Med. 1984;311:89–93. doi: 10.1056/NEJM198407123110204. [DOI] [PubMed] [Google Scholar]
- Kamijo A, Kimura K, Sugaya T, et al. Urinary free fatty acids bound to albumin aggravate tubulointerstitial damage. Kidney Int. 2002;62:1628–1637. doi: 10.1046/j.1523-1755.2002.00618.x. [DOI] [PubMed] [Google Scholar]
- Guo JK, Marlier A, Shi H, et al. Increased tubular proliferation as an adaptive response to glomerular albuminuria. J Am Soc Nephrol. 2012;23:429–437. doi: 10.1681/ASN.2011040396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir MR. Microalbuminuria and cardiovascular disease. Clin J Am Soc Nephrol. 2007;2:581–590. doi: 10.2215/CJN.03190906. [DOI] [PubMed] [Google Scholar]
- Hanevold CD, Pollock JS, Harshfield GA. Racial differences in microalbumin excretion in healthy adolescents. Hypertension. 2008;51:334–338. doi: 10.1161/HYPERTENSIONAHA.107.098095. [DOI] [PubMed] [Google Scholar]
- Jones CA, Francis ME, Eberhardt MS, et al. Microalbuminuria in the US population: third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2002;39:445–459. doi: 10.1053/ajkd.2002.31388. [DOI] [PubMed] [Google Scholar]
- Tsaih SW, Pezzolesi MG, Yuan R, et al. Genetic analysis of albuminuria in aging mice and concordance with loci for human diabetic nephropathy found in a genome-wide association scan. Kidney Int. 2010;77:201–210. doi: 10.1038/ki.2009.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhave JC, Hillege HL, Burgerhof JG, et al. Cardiovascular risk factors are differently associated with urinary albumin excretion in men and women. J Am Soc Nephrol. 2003;14:1330–1335. doi: 10.1097/01.asn.0000060573.77611.73. [DOI] [PubMed] [Google Scholar]
- Gould MM, Mohamed-Ali V, Goubet SA, et al. Microalbuminuria: associations with height and sex in non-diabetic subjects. BMJ. 1993;306:240–242. doi: 10.1136/bmj.306.6872.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter RC, Roseboom TJ, van Montfrans GA, et al. Microalbuminuria in adults after prenatal exposure to the Dutch famine. J Am Soc Nephrol. 2005;16:189–194. doi: 10.1681/ASN.2004060474. [DOI] [PubMed] [Google Scholar]
- Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more the other. Am J Hypertens. 1988;1:335–347. doi: 10.1093/ajh/1.4.335. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Osmond C, Forsen TJ, et al. Trajectories of growth among children who have coronary events as adults. N Engl J Med. 2005;353:1802–1809. doi: 10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- Welham SJM, Riley PR, Wade A, et al. Maternal diet programs embryonic kidney gene expression. Physiol Genomics. 2005;22:48–56. doi: 10.1152/physiolgenomics.00167.2004. [DOI] [PubMed] [Google Scholar]
- Keller G, Zimmer G, Mall G, et al. Nephron number in patients with primary hypertension. N Engl J Med. 2003;348:101–108. doi: 10.1056/NEJMoa020549. [DOI] [PubMed] [Google Scholar]
- Garrett MR, Pezzolesi MG, Korstanje R. Integrating human and rodent data to identify the genetic factors involved in chronic kidney disease. J Am Soc Nephrol. 2010;21:398–405. doi: 10.1681/ASN.2009080881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundel P, Reiser J, Zuniga Mejia Borja A, et al. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res. 1997;236:248–258. doi: 10.1006/excr.1997.3739. [DOI] [PubMed] [Google Scholar]
- Hartman HA, Lai HL, Patterson LT. Cessation of renal morphogenesis in mice. Dev Biol. 2007;310:379–387. doi: 10.1016/j.ydbio.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner C, Faulhaber-Walter R, Wang Y, et al. Major contribution of tubular secretion to creatinine clearance in mice. Kidney Int. 2010;77:519–526. doi: 10.1038/ki.2009.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsellem S, Gburek J, Hamard G, et al. Cubilin is essential for albumin reabsorption in the renal proximal tubule. J Am Soc Nephrol. 2010;21:1859–1867. doi: 10.1681/ASN.2010050492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller DN, Schmidt C, Barbosa-Sicard E, et al. Mouse Cyp4a isoforms: enzymatic properties, gender- and strain-specific expression, and role in renal 20-hydroxyeicosatetraenoic acid formation. Biochem J. 2007;403:109–118. doi: 10.1042/BJ20061328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao CM, Breyer MD. Physiologic and pathophysiologic roles of lipid mediators in the kidney. Kidney Int. 2007;71:1105–1115. doi: 10.1038/sj.ki.5002192. [DOI] [PubMed] [Google Scholar]
- Eid AA, Gorin Y, Fagg BM, et al. Mechanisms of podocyte injury in diabetes: role of cytochrome P450 and NADPH oxidases. Diabetes. 2009;58:1201–1211. doi: 10.2337/db08-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Salavaggione E, Pelleymounter L, et al. Human 3beta-hydroxysteroid dehydrogenase types 1 and 2: gene sequence variation and functional genomics. J Steroid Biochem Mol Biol. 2007;107:88–99. doi: 10.1016/j.jsbmb.2007.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzi V, Mathis JM, Rainey WE, et al. Profiling transcript levels for steroidogenic enzymes in fetal tissues. J Steroid Biochem Mol Biol. 2003;87:181–189. doi: 10.1016/j.jsbmb.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Rainey WE, Nakamura Y. Regulation of the adrenal androgen biosynthesis. J Steroid Biochem Mol Biol. 2008;108:281–286. doi: 10.1016/j.jsbmb.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan K, Kudo A, Hirano H, et al. Subcellular localization of glucocorticoid receptor protein in the human kidney glomerulus. Kidney Int. 1999;56:65–73. doi: 10.1046/j.1523-1755.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- Wada T, Pippin JW, Marshall CB, et al. Dexamethasone prevents podocyte apoptosis induced by puromycin aminonuleoside: role of p53 and Bcl-2-related family proteins. J Am Soc Nephrol. 2005;16:2615–2625. doi: 10.1681/ASN.2005020142. [DOI] [PubMed] [Google Scholar]
- Xing CY, Saleem MA, Coward RJ, et al. Direct effects of dexamethasone on human podocytes. Kidney Int. 2006;70:1038–1045. doi: 10.1038/sj.ki.5001655. [DOI] [PubMed] [Google Scholar]
- Chen A, Sheu LF, Ho YS, et al. Administration of dexamethasone induces proteinuria of glomerular origin in mice. Am J Kidney Dis. 1998;31:443–452. doi: 10.1053/ajkd.1998.v31.pm9506681. [DOI] [PubMed] [Google Scholar]
- Chan SK, Riley PR, Price KL, et al. Corticosteroid-induced kidney dysmorphogenesis is associated with deregulated expression of known cystogenic molecules, as well as Indian hedgehog. Am J Physiol Renal Physiol. 2010;298:F346–F356. doi: 10.1152/ajprenal.00574.2009. [DOI] [PubMed] [Google Scholar]
- De Vries WB, van den Borne P, Goldschmeding R, et al. Neonatal dexamethasone treatment in the rat leads to kidney damage in adulthood. Pediatr Res. 2010;67:72–76. doi: 10.1203/PDR.0b013e3181bf570d. [DOI] [PubMed] [Google Scholar]
- Pushkin A, Carpenito G, Abuladze N, et al. Structural characterization, tissue distribution and functional expression of murine aminoacylase III. Am J Physiol Cell Physiol. 2004;286:C848–C856. doi: 10.1152/ajpcell.00192.2003. [DOI] [PubMed] [Google Scholar]
- Tsirulnikov K, Abuladze N, Newman D, et al. Mouse aminoacylase 3: a metalloenzyme activated by cobalt and nickel. Biochim Biophys Acta. 2009;1794:1049–1057. doi: 10.1016/j.bbapap.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova DT, Brehmer F, Schultze FC, et al. Differential kidney proteome profiling in a murine model of renal fibrosis under treatment with mycophenolate mofetil. Pathobiology. 2011;78:162–170. doi: 10.1159/000324597. [DOI] [PubMed] [Google Scholar]
- Templeton DM. Interaction of toxic cations with the glomerulus: binding of Ni to purified glomerular basement membrane. Toxicology. 1987;43:1–15. doi: 10.1016/0300-483x(87)90070-9. [DOI] [PubMed] [Google Scholar]
- Elliot SJ, Berho M, Korach K, et al. Gender-specific effects of endogenous testosterone: female alpha-estrogen receptor-deficient C57Bl/6J mice develop glomerulosclerosis. Kidney Int. 2007;72:464–472. doi: 10.1038/sj.ki.5002328. [DOI] [PubMed] [Google Scholar]
- Doublier S, Lupia E, Catanuto P, et al. Testosterone and 17β-estradiol have opposite effects on podocyte apoptosis that precedes glomerulosclerosis in female estrogen receptor knockout mice. Kidney Int. 2011;79:404–413. doi: 10.1038/ki.2010.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer S, Jeruschke S, Wegerich LV, et al. Estrogen receptor alpha expression in podocytes mediates protection against apoptosis in-vitro and in-vivo. PLoS One. 2011;6:e27457. doi: 10.1371/journal.pone.0027457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen-McEwen LA, Drago J, Bertram JF. Nephron endowment in glial cell line-derived neurotrophic factor (GDNF) heterozygous mice. Kidney Int. 2001;60:31–36. doi: 10.1046/j.1523-1755.2001.00767.x. [DOI] [PubMed] [Google Scholar]
- Hughson MD, Douglas-Denton R, Bertram JF, et al. Hypertension, glomerular number, and birth weight in African Americans and white subjects in the southeastern United States. Kidney Int. 2006;69:671–678. doi: 10.1038/sj.ki.5000041. [DOI] [PubMed] [Google Scholar]
- Helal I, Fick-Brosnahan GM, Reed-Gitomer B, et al. Glomerular hyperfiltration: definitions, mechanisms and clinical implications. Nat Rev Nephrol. 2012;8:293–300. doi: 10.1038/nrneph.2012.19. [DOI] [PubMed] [Google Scholar]
- Doorenbos C, Tsaih SW, Sheehan S, et al. Quantitative tract loci for urinary albumin in crosses between C57BL/6J and A/J inbred mice in the presence and absence of Apoe. Genetics. 2008;179:693–699. doi: 10.1534/genetics.107.085142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel L, Rudofsky UH, Longmate JA, et al. Polygenic control of susceptibility to murine systemic lupus erythematosus. Immunity. 1995;1:219–229. doi: 10.1016/1074-7613(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Schulz A, Standke D, Kovacevic L, et al. A major gene locus links early onset albuminuria with renal interstitial fibrosis in the MWF rat with polygenetic albuminuria. J Am Soc Nephrol. 2003;14:3081–3089. doi: 10.1097/01.asn.0000100126.62370.25. [DOI] [PubMed] [Google Scholar]
- Siegel AK, Kossmehl P, Planert M, et al. Genetic linkage of albuminuria and renal injury in Dahl salt-sensitive rats on a high-salt diet: comparison with spontaneously hypertensive rats. Physiol Genomics. 2004;18:218–225. doi: 10.1152/physiolgenomics.00068.2004. [DOI] [PubMed] [Google Scholar]
- Köttgen A, Pattaro C, Böger CA, et al. New loci associated with kidney function and chronic kidney disease. Nat Genet. 2010;42:376–384. doi: 10.1038/ng.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers JC, Zhang W, Lord GM, et al. Genetic loci influencing kidney function and chronic kidney disease. Nat Genet. 2010;42:373–375. doi: 10.1038/ng.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhanson P, Kepp K, Org E, et al. N-acetyltransferase 8, a positional candidate for blood pressure and renal regulation: resequencing, association and in silico study. BMC Med Genet. 2008;9:25. doi: 10.1186/1471-2350-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku CH, White KE, Dei Cas A, et al. Inducible overexpression of sFlt-1 in podocytes ameliorates glomerulopathy in diabetic mice. Diabetes. 2008;57:2824–2833. doi: 10.2337/db08-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat GK, Sea TL, Olatinwo MO, et al. Influence of a leptin deficiency on testicular morphology, germ cell apoptosis, and expression levels of apoptosis-related genes in the mouse. J Androl. 2006;27:302–310. doi: 10.2164/jandrol.05133. [DOI] [PubMed] [Google Scholar]
- Takemoto M, Asker N, Gerhardt H, et al. A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol. 2002;161:799–805. doi: 10.1016/S0002-9440(10)64239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PS, Clegg N, Arnold H, et al. The program of androgen-responsive genes in neoplastic prostate epithelium. Proc Natl Acad Sci USA. 2002;99:11890–11895. doi: 10.1073/pnas.182376299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber CJ, Gruber DM, Gruber IM, et al. Anatomy of the estrogen response element. Trends Endocrinol Metab. 2004;15:73–78. doi: 10.1016/j.tem.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Price KL, Long DA, Jina N, et al. Microarray interrogation of human metanephric mesenchymal cells highlights potentially important molecules in vivo. Physiol Genomics. 2007;28:193–202. doi: 10.1152/physiolgenomics.00147.2006. [DOI] [PubMed] [Google Scholar]
- Long DA, Woolf AS, Suda T, et al. Increased renal angiopoietin-1 expression in folic-acid induced nephropathy in mice. J Am Soc Nephrol. 2001;12:2721–2731. doi: 10.1681/ASN.V12122721. [DOI] [PubMed] [Google Scholar]
- Yates LL, Papakrivopoulou J, Long DA, et al. The planar cell polarity gene Vangl2 is required for mammalian kidney-branching morphogenesis and glomerular maturation. Hum Mol Genet. 2010;19:4663–4676. doi: 10.1093/hmg/ddq397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pounds SB. Estimation and control of multiple testing error rates for microarray studies. Brief Bioinform. 2006;7:25–36. doi: 10.1093/bib/bbk002. [DOI] [PubMed] [Google Scholar]