Abstract
Background
Studies of resource utilization by patients with new-onset atrial fibrillation after coronary artery bypass grafting have addressed only length of stay and bed charges.
Objective
To compare resource utilization between patients with new-onset atrial fibrillation and patients without atrial fibrillation after isolated coronary artery bypass grafting.
Methods
Retrospective review of clinical and administrative electronic databases for 720 subjects who underwent isolated coronary artery bypass grafting with cardiopulmonary bypass in 25 months at one medical center. The prevalence of atrial fibrillation was determined, and resource utilization in various hospital cost centers was compared between subjects with and without atrial fibrillation.
Results
The prevalence of new-onset atrial fibrillation was 33.1%. Compared with subjects without atrial fibrillation, subjects with atrial fibrillation had a longer stay (5.8 ± 2.4 vs 4.4 ± 1.2 days, P< .001), more days receiving mechanical ventilation (P=.002) and oxygen therapy (P< .001), and higher rates of readmission to the intensive care unit (4.6% vs 0.2%, P< .001). Subjects with atrial fibrillation also had more laboratory tests (P< .001) and more days receiving cardiac drugs, heparin, diuretics, and electrolytes. Subjects with atrial fibrillation had higher total postoperative charges ($57261 ± $17 101 vs $50905 ± $10062, P= .001), a mean difference of $6356. The mean differences were greatest for bed charges ($1642), laboratory charges ($1215), pharmacy ($989), and respiratory care ($582).
Conclusion
The economic impact of atrial fibrillation after coronary artery bypass grafting has been underestimated.
Atrial fibrillation is the most common complication after coronary artery bypass grafting (CABG), a procedure undergone by about 400 000 adults each year.1 The reported prevalence of this complication ranges from 5% to 40%.2–6 Many researchers have attempted to determine the risk factors for atrial fibrillation after CABG and to develop prediction models or prophylactic strategies.2,5,7–20 Although the development of atrial fibrillation after CABG affects patients’ mortality21 and morbidity to some degree,1,13,22–24 this complication most commonly results in lengthened hospitalization.8,24,25 Estimated charges for these additional hospital days range from $1500 per patient5 to $10 000 per patient2 for bed and room charges alone. In only one other study4 has resource utilization been critically examined (in addition to length of stay) for patients with atrial fibrillation after CABG. As a result, the economic impact of atrial fibrillation after CABG has most likely been underestimated. Further study in this area is warranted to determine the economic severity of the problem and to provide a baseline whereby the effectiveness of cost-containment measures can be assessed. Developing practice patterns that minimize resource utilization may reduce the costs of caring for patients with atrial fibrillation after CABG, even if the prevalence is not reduced.
The purposes of this study were to determine the prevalence of new-onset atrial fibrillation in a large sample of patients undergoing isolated standard CABG with cardiopulmonary bypass in a single medical center and to compare resource utilization between patients with and patients without atrial fibrillation.
Materials and Methods
After receiving approval from the institutional review board (March 13, 1998), we retrospectively obtained data for a 25-month interval (May 1, 1996 through May 31, 1998) from the Medical Archival System (MARS) at the University of Pittsburgh Medical Center-Health System. The MARS is a repository for information forwarded from the health system’s electronic clinical, administrative, and financial databases. MARS is indexed on every word and can be used to determine all encounters with a given patient between specified dates.26 All subjects were more than 18 years old and underwent isolated CABG (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] procedure codes 36.10–36.16 from the medical records discharge abstracts). Exclusion criteria were (1) history of atrial fibrillation (both active and inactive), (2) prior or current heart valve replacement or repair, (3) prior or current ventricular assist device, (4) CABG procedures that did not use cardiopulmonary bypass (minimally invasive direct-vision CABG or off-pump CABG), (5) perioperative or postoperative myocardial infarction, (6) prior or current heart, lung, or heart-lung transplantation, (7) any other surgical procedure during current admission, and (8) death in the operating room or within 12 hours of surgery. Prior CABG was not an exclusion criterion. All subjects participated in the routine prophylaxis strategy for atrial fibrillation that was in effect during the study period, which included magnesium supplementation in the operating room, upon admission to the intensive care unit (ICU), and on the first postoperative day and β-blocker administration beginning the first postoperative day, if heart rate, blood pressure, and cardiac index were acceptable.
Patients were initially selected by using procedure codes and then reviewing operative reports to determine whether the patient had undergone isolated CABG with cardiopulmonary bypass and to determine the number and location of vessels bypassed. To determine which patients had new-onset atrial fibrillation, we first used the MARS to select patients who had an ICD-9-CM code for atrial fibrillation (427.31) from the administrative database. Because ICD-9-CM code assignment does not differentiate between new-onset and preexisting atrial fibrillation, discharge summaries for all patients with code 427.31 were reviewed to verify that atrial fibrillation had occurred and to determine if the atrial fibrillation was a newly acquired or a prior problem (full chart review if necessary). Second, the clinical database for all remaining patients (no ICD-9-CM code 427.31) who underwent CABG or minimally invasive direct-vision CABG was subjected to a MARS word search (for atrial fibrillation, atrial fib, atrial dysrhythmia, and word variations), and we determined whether the atrial fibrillation was of new onset or preexisting. Third, the pharmacy database was queried for procainamide administration for all patients who did not have new-onset atrial fibrillation according to the results of the preceding 2 steps. The charts of patients receiving procainamide were reviewed to determine the rationale for drug administration and then assigned to the appropriate research group (new-onset atrial fibrillation, no atrial fibrillation, excluded).
If a patient’s atrial fibrillation status was in question (new onset vs preexisting, yes vs no), the patient was eliminated from the study. A total of 997 patients had undergone isolated CABG with or without cardiopulmonary bypass within the 25-month time frame. Of these, 63 were excluded because of preexisting atrial fibrillation (active or inactive), 94 because of off-pump surgery, and 120 because they either had more than 1 surgical procedure during the admission or they died during or within 12 hours of surgery. A total of 238 subjects had new-onset atrial fibrillation: (1) 140 according to ICD-9-CM code, (2) 86 according to the word search, and (3) 12 according to the procainamide query. The remaining 482 subjects who met entry criteria did not experience atrial fibrillation. Therefore, the final sample included 720 subjects.
Demographic (age, sex, race), clinical, and fiscal data were extracted from the MARS database. Preoperative condition was assessed by using Medi-Qual Systems’ Atlas (MedisGroups, Westborough, Mass) scores. Atlas scores are an estimate of risk for in-hospital mortality determined on the basis of key clinical findings upon admission by using the scale 0 = no risk for clinical instability, 1 = minimal risk of clinical instability, 2 = moderate risk, 3 = severe risk, and 4 = maximal risk. The admission characteristics upon which Atlas scores were based are as follows: admission source, admission type (emergent vs elective), age, cardiac dysrhythmias, cardiogenic shock, cardiomyopathy, conduction disorders, diabetes, dialysis, sex, heart failure, hypertension with complications, hypertension without complications, infarct site, malignant neoplasm, payer, prior CABG surgery, and renal failure. Atlas scores compare favorably with Acute Physiology and Chronic Health Evaluation II scores for predicting in-hospital death.27 In an effort to assess past medical problems, ICD-9-CM codes within similar disease categories were clustered, and only diseases with cumulative frequencies greater than 75 were selected for analysis. Hypertension was excluded from this process because it was individually coded in 74% of the sample. The number of vessels bypassed was obtained by review of the operative report (obtained from MARS). Administrative and finance database components of MARS were used to determine the operating room time.
Resource utilization was evaluated with regard to (1) operating room time (charge hours), (2) length of stay (LOS), namely, total postoperative LOS, LOS in the postoperative ward, postoperative LOS in the ICU, and readmission to the ICU, (3) laboratory tests (type and number performed), (4) respiratory care (number of days of mechanical ventilation and oxygen therapy), (5) number of electrocardiograms, and (6) pharmacy utilization. Pharmacy usage was evaluated by identifying the total postoperative days of drug therapy within classifications for both oral and intravenous cardiac drugs (β-blockers, calcium channel blockers, angiotensin-converting enzyme inhibitors, digoxin, adenosine, procainamide, lidocaine infusion, quinidine, amiodarone), anticoagulants (heparin, warfarin), diuretics (furosemide, bumetanide), and electrolytes (potassium, calcium, magnesium). These data are reported by drug days: the number of days for which the subject received a drug from within a specific drug classification. This method was chosen to provide an approximate basis for comparison between the groups, because comparing doses or dosages for various drug formulations would be difficult. Drug days were measured from and including the operative day through the day of discharge. Unless otherwise noted, or if the drug was available for only a single route of administration, the drug days represent either an oral or an intravenous preparation. If subjects received both an oral and intravenous preparation from a drug class on the same day, it was counted as only 1 drug day.
Disposition at discharge was extracted from the administrative database by using MARS. Charge comparisons between subjects with and subjects without atrial fibrillation were made for the resources just listed. Charges were extracted from MARS, which stores each charge transaction. Charges were used (rather than costs) because they are considered less proprietary, more readily shared in the literature, and could reflect between-group differences in the same manner as costs. In order to account for inflationary increases, charges for subjects admitted during months 13 to 25 of the study were decreased by 3%.
Statistical Analysis
Data were assembled by the MARS staff, prepared with subject identifiers to preserve confidentiality, and presented to the research team for transfer and into the Microsoft Excel (Microsoft Office 97, Microsoft Corporation, Redmond, Wash) research database. The χ2 test for proportions in the Statistical Package for the Social Sciences (SPSS, Inc, Chicago, Ill) was used to examine differences in the prevalence of atrial fibrillation. Between-group comparisons were made by using a Student t test for continuous variables and a χ2 test for categorical variables. A P value less than .05 was considered significant. When several variables in a category were analyzed, a Bonferroni correction was made and the alpha was divided evenly across multiple variables. The number of subjects for the analyses varied depending on the data available for the variable tested.
Results
Demographics
From the total sample of 720 subjects who underwent isolated CABG with cardiopulmonary bypass, 238 had new-onset atrial fibrillation postoperatively, yielding a prevalence of 33.1% for this complication. The demographic characteristics of subjects with and without atrial fibrillation after CABG are compared in Table 1. The subjects in the 2 groups did not differ on the basis of sex, race, body surface area, or past medical problems. Subjects in whom atrial fibrillation developed were significantly older than were subjects without atrial fibrillation. The groups also varied with respect to Atlas scores (P = .001); only 24% of the patients without atrial fibrillation were moderate to severe risk for complications while hospitalized, compared with 36% of the patients with atrial fibrillation. Subjects in whom atrial fibrillation developed also had significantly more vessels bypassed (P= .014); 59% of subjects with, but only 52% of subjects without, atrial fibrillation had 4 or more vessels bypassed.
Table 1.
Characteristics of subjects with and without atrial fibrillation after coronary artery bypass graft surgery
| Characteristic | No fibrillation (n= 482) |
Fibrillation (n =238) |
P |
|---|---|---|---|
| Age, mean (SD), years | 63.9 (10.1) | 69.5 (7.5) | <.001 |
| Male sex | 67.2 | 69.6 | .05 |
| White race | 92.5 | 92.0 | .68 |
| Body surface area, mean (SD), m2 |
1.95 (0.23)* | 1.96 (0.21)† | .62 |
| Atlas score | .001 | ||
| 0 = no risk | 1.0 | 0.4 | |
| 1 = minimal risk | 75.0 | 63.9 | |
| 2 = moderate risk | 22.4 | 32.8 | |
| 3 = severe risk | 1.7 | 3.0 | |
| 4 = maximal risk | 0 | 0 | |
| Past medical problems defined by ICD-9-CM code |
|||
| Diabetes | 27 | 31.9 | .17 |
| Old myocardial infarction | 29.5 | 29.0 | .90 |
| Heart failure | 9.5 | 9.2 | .90 |
| Chronic obstructive pulmonary disease |
10.2 | 10.9 | .75 |
| No. of vessels bypassed | .014 | ||
| 1 | 3.7 | 0 | |
| 2 | 11.6 | 10.1 | |
| 3 | 33.0 | 31.5 | |
| 4 | 38.6 | 39.9 | |
| 5 or more | 13.1 | 18.5 |
Other than P values, numbers in table are percentages of patients with that characteristic in each group, unless otherwise indicated. Because of rounding, percentages for Atlas scores do not total 100. ICD-9-CM indicates International Classification of Diseases, Ninth Revision, Clinical Modification.
n=235.
n=479.
Resource Utilization
The utilization of resources is compared between subjects with atrial fibrillation and subjects without atrial fibrillation in Table 2. The groups did not differ with respect to the number of operating room charge hours (P=.54), suggesting that the length of the operative procedure was similar for both groups. The total postoperative LOS was significantly longer for subjects with atrial fibrillation than for subjects without atrial fibrillation (P<.001). The subjects with atrial fibrillation also had a significantly longer postoperative stay in the ICU (P=.001) and were more likely to require readmission to the ICU (P<.001). Subjects with atrial fibrillation also had a longer postoperative stay in the hospital ward than did subjects without atrial fibrillation (P<.001).
Table 2.
Resource utilization for subjects with and without atrial fibrillation after coronary artery bypass graft surgery
| Resource | No fibrillation (n = 482) |
Fibrillation (n= 238) |
P |
|---|---|---|---|
| Time in operating room, mean (SD), charge hours |
3.9 (0.9) | 3.8 (0.8) | .54 |
| Length of stay | |||
| Total postoperative, mean (SD), days |
4.4 (1.2) | 5.8 (2.4) | <.001 |
| Intensive care unit postoperative, mean (SD), days |
1.2 (0.5)* | 1.5 (1.5)† | .001 |
| Readmission to intensive care unit, No. (%) of patients |
1 (0.2) | 11 (4.6) | <.001 |
| Ward postoperative, mean (SD), days |
3.3 (1.1) | 4.3 (1.7) | <.001 |
| No. of laboratory tests per subject, mean (SD) |
38.6 (16.6) | 53.7 (29.5) | <.001 |
| Respiratory care | |||
| Mechanical ventilation, mean (SD), days |
1.4 (0.5) | 1.7 (1.3) | .002 |
| Oxygen therapy, mean (SD), days |
4.1 (1.3) | 5.0 (2.3) | <.001 |
| No. of electrocardiograms per subject, mean (SD) |
1.9 (0.8) | 2.5 (1.3) | <.001 |
| Discharge disposition, No. (%) of patients |
.017 | ||
| Home | 167 (34.6) | 70 (29.4) | |
| Home health agency | 252 (52.3) | 119 (50.0) | |
| Posthospital care facility | 63 (13.1) | 47 (19.7) | |
| Mortality | 0 (0) | 2 (0.8) |
n = 477
n = 235
Subjects in the atrial fibrillation group tended to have more laboratory tests postoperatively than did subjects without atrial fibrillation (P<.001), although variance within the atrial fibrillation group was greater. Subjects who experienced atrial fibrillation also had more postoperative charge days for mechanical ventilation (P=.002) and days of oxygen therapy (P<.001), with more variance within the atrial fibrillation group. Subjects in the atrial fibrillation group also had more electrocardiograms obtained (P<.001). Additionally, subjects varied with respect to disposition at discharge (P= .017); 19.7% of subjects with atrial fibrillation and 13.1% of subjects without atrial fibrillation were discharged to posthospital care facilities.
Laboratory Test Utilization
Further examination was done to determine if a specific category of laboratory tests contributed to the difference noted between the groups (Table 3). Subjects in the atrial fibrillation group had almost twice as many blood chemistry assays done as the subjects without atrial fibrillation had done (P<.001). The atrial fibrillation group also had more hematologic tests done (P<.001), including more prothrombin and partial thromboplastin times (P=.001). More arterial blood gas analyses were performed for subjects with atrial fibrillation than for subjects without atrial fibrillation (P= .009). As expected, a much larger percentage of the patients with atrial fibrillation had procainamide/N-acetylprocainamide levels measured, although the numbers of tests per subject were similar in the 2 groups (P = .28). Only a few subjects with atrial fibrillation and none of the subjects without atrial fibrillation had quinidine or digoxin levels measured. The most striking finding is the difference between the groups in the number of blood chemistry assays performed.
Table 3.
Laboratory utilization for subjects with and without atrial fibrillation after coronary artery bypass graft surgery
| No fibrillation (n = 482) |
Fibrillation (n = 238) |
||||
|---|---|---|---|---|---|
| Laboratory test | No. (%) of subjects who had tests |
No. of tests per subject, mean (SD) |
No. (%) of subjects who had tests |
No. of tests per subject, mean (SD) |
P* |
| Total no. of tests per patient |
482 (100) | 38.4 (16.6) | 238 (100) | 53.7 (29.5) | <.001 |
| Blood chemistry assays | 481 (99.8) | 11.8 (10.1) | 238 (100) | 23.0 (17.7) | <.001 |
| Hematologic tests | 481 (99.8) | 10.7 (4.6) | 238 (100) | 13.5 (8.3) | <.001 |
| Prothrombin and partial thromboplastin times |
180 (37.3) | 1.5 (0.9) | 109 (45.8) | 2.3 (2.2) | .001 |
| Arterial blood gas analyses |
478 (99.2) | 4.0 (1.2) | 238 (100) | 4.2 (1.6) | .009 |
| Procainamide/ N-acetylprocainamide levels |
3 (0.6) | 2 (0) | 133 (55.9) | 3.6 (2.6) | .28 |
| Quinidine level | 0 (0) | 0 (0) | 3 (1.3) | 1.7 (0.6) | ND |
| Digoxin level | 0 (0) | 0 (0) | 4 (1.7) | 1.3 (1.3) | ND |
ND indicates that statistical analysis was not done because of the small number of subjects for whom this test was ordered.
P values are based on comparison of data for no. of tests per subject.
Pharmacy Product Utilization
The comparison of pharmacy product utilization between subjects with and without atrial fibrillation is presented in Table 4. Although similar percentages of both groups of subjects received β-blockers, the subjects in the atrial fibrillation group had more drug days than did the subjects without atrial fibrillation (P<.001). Although a higher percentage of subjects with atrial fibrillation received calcium channel blockers, the groups did not differ in drug days (P = .04) when a Bonferroni correction for 5 variables was applied. Patients in the atrial fibrillation group had a greater number of drug days for angiotensin-converting enzyme inhibitors (P= .002). The 2 groups did not differ in the number of drug days for digoxin (P= .09). As expected, more patients with atrial fibrillation received procainamide, but the number of drug days were comparable between the groups (P = .12). The numbers of subjects who received adenosine, quinidine, and amiodarone were too low to analyze.
Table 4.
Pharmacy utilization for subjects with and without atrial fibrillation after coronary artery bypass graft surgery
| No fibrillation (n = 482) |
Fibrillation (n= 238) |
||||
|---|---|---|---|---|---|
| Drug | No. (%) of subjects receiving drug |
No. of drug days per subject mean (SD) |
No. (%) of subjects receiving drug |
No. of drug days per subject mean (SD) |
P* |
| Oral and intravenous cardiac drugs† |
|||||
| β-Blockers | 446 (92.5) | 4.2 (1.4) | 221 (92.9) | 5.1 (2.5) | <.001 |
| Calcium channel blockers |
156 (32.4) | 2.2 (1.8) | 137 (57.6) | 2.7 (2.2) | .04 |
| Angiotensin- converting enzyme inhibitors |
138 (28.6) | 3.6 (2.0) | 79 (33.2) | 4.7 (2.8) | .002 |
| Digoxin | 52 (10.8) | 3.1 (1.9) | 142 (59.7) | 3.8 (2.4) | .09 |
| Adenosine | 0 (0) | 0 (0) | 6 (2.5) | 1 (1) | ND |
| Procainamide | 4 (0.8) | 1.8 (0.5) | 186 (78.2) | 3.8 (2.6) | .12 |
| Lidocaine infusion | 10 (2.1) | 1 (0) | 7 (2.9) | 1 (0) | ND |
| Quinidine | 0 (0) | 0 (0) | 0 (0) | 0 | ND |
| Amiodarone | 2 (0.4) | 3 (2.8) | 4 (1.7) | 3.3 (0.9) | ND |
| Anticoagulants | |||||
| Heparin | 461 (95.6) | 1.2 (0.9) | 234 (98.3) | 2.1 (2.1) | <.001 |
| Warfarin | 64 (13.3) | 2.8 (1.5) | 49 (20.6) | 3.1 (2.0) | .49 |
| Diuretics | |||||
| Furosemide | 477 (99.0) | 4.0 (1.4) | 236 (99.2) | 5.3 (2.1) | <.001 |
| Bumetanide | 9 (1.9) | 2.0 (2.0) | 3 (1.3) | 2.0 (1) | ND |
| Electrolytes | |||||
| Potassium | 481 (99.8) | 4.2 (1.3) | 237 (99.6) | 5.3 (2.0) | <.001 |
| Calcium | 317 (65.8) | 1.0 (0.1) | 164 (68.9) | 1.1 (0.3) | .42 |
| Magnesium | 481 (99.8) | 1.7 (0.9) | 238 (100) | 2.7 (1.1) | <.001 |
ND indicates that statistical analysis was not done because of the small number of subjects who received this medication.
P values are based on comparison of data for no. of drug days per subject.
Bonferroni correction for 5 variables significant at P= .01.
The subjects with atrial fibrillation had more drug days of heparin therapy than did subjects without atrial fibrillation (P<.001). Although a greater percentage of subjects with atrial fibrillation than without received warfarin therapy, the 2 groups did not differ with respect to drug days (P=.49). Subjects with atrial fibrillation also had significantly more drug days of furosemide therapy (P<.001). In terms of electrolyte replacement, the subjects with atrial fibrillation had significantly more drug days for oral and intravenous potassium (P<.001), intravenous magnesium (P<.001), and intravenous calcium (P<.001) supplementation.
Charges
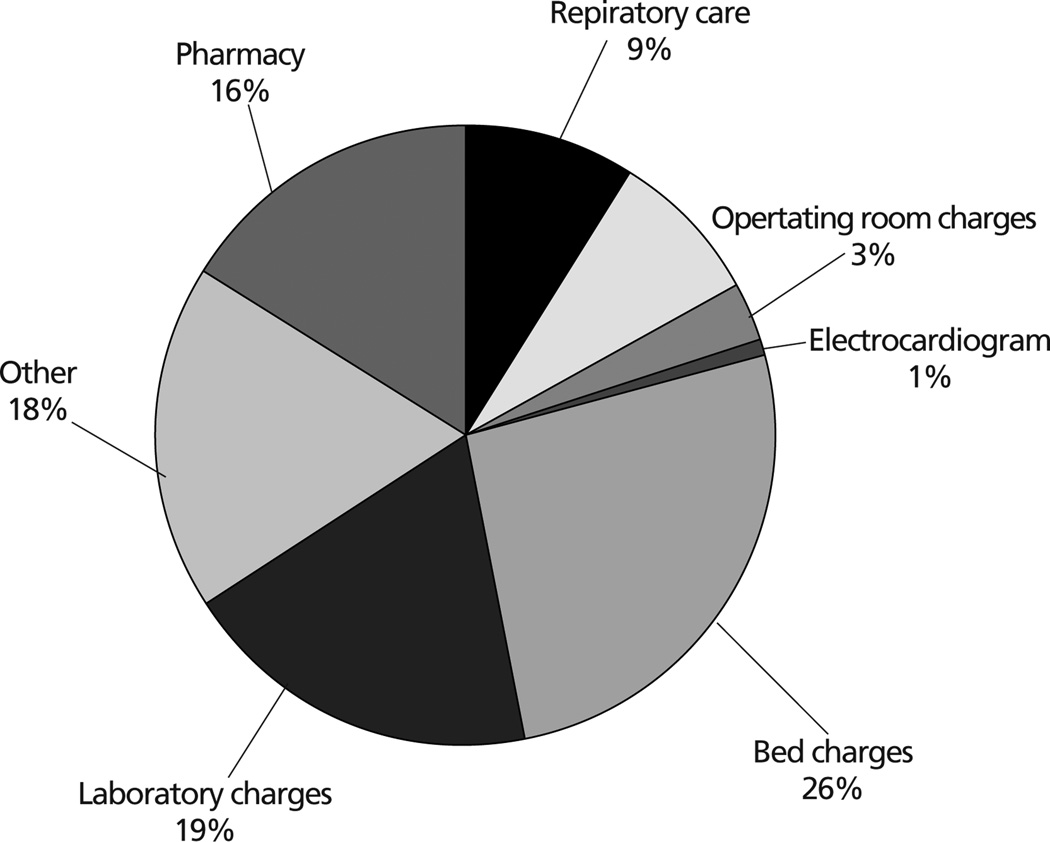
The charges for CABG patients with and without atrial fibrillation are listed in Table 5. Patients with atrial fibrillation had significantly higher charges for room, operating room, laboratory, pharmacy, and respiratory care cost centers. The difference in total postoperative charges was significant (P = .001), with a mean difference in total charges between groups of $6356 (see Figure). The largest mean difference was for bed and room charges ($1642); other mean differences were laboratory charges ($1215), pharmacy ($989), respiratory care ($582), room supplies ($518), operating room ($203), and electrocardiography ($67). Charges accrued for services or products not examined in the study (eg, radiology, microbiology, physical therapy) account for a mean difference of $1140.
Table 5.
Charges accrued by subjects with and without atrial fibrillation after coronary artery bypass graft surgery
| Variable | No fibrillation (n = 482) |
Fibrillation (n= 238) |
P |
|---|---|---|---|
| Total postoperative charges | 50.90 (10.06) | 57.26 (17.10) | .001 |
| Room* | |||
| Intensive care unit bed | 3.01 (1.35) | 3.91 (4.02) | .001 |
| Intensive care unit supplies | 0.41 (0.34) | 0.62 (1.29) | .02 |
| Ward bed | 2.22 (0.71) | 2.96 (1.12) | <.001 |
| Ward supplies | 0.65 (0.33) | 0.96 (0.61) | <.001 |
| Operating room related | |||
| Operating room total | 19.91 (2.02) | 20.11 (1.88) | .19 |
| Operating room time | 6.69 (1.59) | 6.72 (1.44) | .71 |
| Perfusion charges | 6.61 (1.07) | 6.70 (0.93) | .22 |
| Laboratory total | 3.20 (1.11) | 4.42 (2.14) | .001 |
| Pharmacy total | 2.34 (1.11) | 3.33 (2.21) | <.001 |
| Respiratory care | |||
| Mechanical ventilation | 0.97 (0.36) | 1.16 (0.89) | .001 |
| Oxygen therapy | 1.70 (0.81) | 2.09 (1.54) | .001 |
Other than P values, all numbers in table are in thousands of US dollars, presented as mean (SD)
Bonferroni correction for 4 variables, P= .01.
Distribution of the difference in total mean charges ($6356) between subjects with and subjects without atrial fibrillation after coronary artery bypass graft surgery.
Discussion
Resource Utilization
In this study, the mean postoperative stay in the hospital was 1.4 days longer for subjects with new-onset atrial fibrillation than for subjects without atrial fibrillation (P<.001). However, most of their lengthened stay was on the ward (mean difference in ward stay was 1 day vs mean difference in ICU stay of 0.3 days). Others1,6,19,28–31 have noted that the usual onset of atrial fibrillation is 2 to 4 days after surgery, so it would be expected that any atrial fibrillation occurred after transfer from the ICU and treatment most likely was given on the ward. Eleven (4.6%) of the subjects who developed atrial fibrillation were readmitted to the ICU, compared with only 1 (0.2%) of the patients without atrial fibrillation.
Mathew et al5 noted that subjects with atrial fibrillation remained a mean of 0.5 days in the ICU and 2.0 days on the ward. Aranki et al8 noted an overall increase in length of stay of 4.9 days for subjects with atrial fibrillation and an ICU readmission rate of 9% for patients with atrial fibrillation compared with 2% for patients without atrial fibrillation. Almassi et al1 reported a longer stay in the hospital of 3 days (10 days for patients with atrial fibrillation vs 7 days for patients without), longer time in the ICU (3.6 days for patients with atrial f ibrillation vs 2 days for patients without), and more frequent ICU readmission (13% for patients with atrial fibrillation vs 3% for patients without).
Thus, the reported LOSs vary widely between centers. This variance may be due to geographic considerations (Europe vs United States), time (more recent trend to fast track), and variations in ICU admission criteria. However, all reports agree that patients with atrial fibrillation stay in the ICU and on the ward longer, and our results concur with those of others. Determining the reason for the lengthened stay is difficult. To some degree, the difference might be explained by the greater number of vessels bypassed and the higher risk according to Atlas scores for subjects with atrial fibrillation. Another hypothesis is that more hospitalization time is required to implement interventions to convert to sinus rhythm, verify stabilization of the therapy (eg, therapeutic procainamide levels), or institute and monitor use of anticoagulants for subjects who do not convert. Still another hypothesis is that the older age of subjects with atrial fibrillation indicates that they are more fragile subjects. In a study of 436 subjects, 23% of whom had atrial fibrillation develop, Borzak et al32 noted that subjects with atrial fibrillation had a longer stay in the ICU (2.7 days for subjects with atrial fibrillation vs 1.7 days for subjects without) and on the ward (9.4 days for subjects with atrial fibrillation vs 6.3 days for subjects without). However, according to multivariate analysis with adjustments for age, sex, and race, the postoperative hospital stay was still longer (P<.001) for subjects with atrial fibrillation (9.2 ± 5.3 days) than for subjects without atrial fibrillation (6.4± 5.3 days). This results yields the conclusion that although subjects in whom atrial fibrillation developed were older, the increased length of stay was attributable to the atrial fibrillation and not to the patient’s age itself. Paone et al16 reported similar findings. It therefore appears that the former hypotheses for increased LOS after atrial fibrillation (more baseline coronary disease, more time needed for application and adjustment of therapies) is the more likely explanation for the difference, rather than the age of the patient.
Use of mechanical ventilation was significantly higher among subjects with atrial fibrillation, although less so from a clinical standpoint (mean difference between groups was only 0.3 days), whereas the difference between groups for oxygen therapy was 1 day. (Note that the total number of days of mechanical ventilation plus days of oxygen therapy exceeds the number for the postoperative LOS. This anomaly is most likely due to patients’ being charged for both mechanical ventilation and oxygen therapy on the day of extubation. Additionally, LOS data were based on admission/discharge/transfer data, which were based on the location of the patient at midnight. Therefore, patients could be charged for items on the day of discharge but not have the discharge day calculated in the LOS data so long as the patients were discharged before midnight.) These data closely correspond to the data for mean difference in LOS. It is unknown whether or not the subjects with atrial fibrillation required oxygen therapy longer because of a clinical indication such as decreased oxygen saturation or if the therapy was routinely applied during the longer hospitalization.
Laboratory
In most other studies, examination of resource consumption was limited to LOS data. In this study, we examined the utilization of resources within several large categories. In addition to increased LOS, we noted that standard CABG patients with atrial fibrillation used significantly more respiratory care services and had more laboratory tests performed. The most striking finding is that subjects with atrial fibrillation had almost twice the number of blood chemistry assays performed as did subjects without atrial fibrillation. Although this outcome could, in part, be attributed to the longer LOS for patients with atrial fibrillation, the twofold increase is not explained by possible “daily” electrolyte monitoring alone. More likely, patients in whom atrial fibrillation develops have electrolyte analyses ordered more often, or before and after additional supplementation. In the future, this might be an area for study in this population of patients, because the number of such analyses may vary regionally.
Pharmacy
Although many researchers have examined drug therapies for prophylaxis of atrial fibrillation, no other published report discusses potential differences in the utilization of a wide variety of drugs when patients have atrial fibrillation from the standpoint of resource utilization. β-Blockers were received by nearly equal percentages of patients in both groups (92.9% of patients with atrial fibrillation vs 92.5% of patients without) and for numbers of drug days (5.1 ± 2.5 days for patients with atrial fibrillation and 4.2 ± 1.4 days for patients without) that were nearly compatible with their respective lengths of stay although significantly different between groups. This finding is in keeping with current practice in the medical center for prophylaxis of atrial fibrillation (unless contraindicated by hemodynamics data or cardiac rhythm). Apparently, 78.2% of subjects who developed atrial fibrillation received procainamide therapy. A larger percentage of patients with atrial fibrillation (59.7%) than patients without atrial fibrillation (10.8%) also received digoxin, although the numbers of drug days within each group were comparable (3.8 ± 2.4 days for patients with atrial fibrillation vs 3.1 ± 1.9 days for patients without).
Although patients from both groups may have received digoxin preoperatively, the larger number of patients receiving the drug in the atrial fibrillation group is presumably explained by use of this drug as a treatment for atrial fibrillation in conjunction with procainamide. A higher percentage of patients with atrial fibrillation (57.6%) than without (32.4%) also received calcium channel blockers, although the numbers of drug days were comparable in the 2 groups. Although patients from both groups may have been receiving this class of drugs for blood pressure control in both the preoperative and postoperative phases, the greater use among patients with atrial fibrillation suggests that this drug class may be being used as a treatment for atrial fibrillation. This topic is another worthy of exploration during the development of a treatment protocol for atrial fibrillation. Because the charges for calcium channel blockers are nearly 7 times as high as charges for procainamide, procainamide may be the drug of choice for the protocol unless the patients should not receive procainamide according to defined guidelines within the protocol. Patients with atrial fibrillation also had more drug days for diuretics and for electrolytes. Presumably this information indicates that patients with atrial fibrillation require more diuresis and experience more electrolyte abnormalities, conditions that may, in turn, potentiate the genesis of atrial fibrillation. Another target area for protocol development might be institution of a more comprehensive program for routine electrolyte replacement therapy and monitoring of fluid balance.
Charges
Other researchers have noted an increase in cost related to care of patients with atrial fibrillation, from as little as $1616 per patient for bed and room costs alone5 to $10 000 per patient.2 Only 1 other investigator provided a more comprehensive analysis of cost breakdown per cost center. Kowey et al4 noted an overall increase of $20 000 per patient, with a mean difference of $7000 for room charges, $4000 for pharmacy charges, $3000 for laboratory charges, and $1599 for respiratory care. These figures are much larger than those we found in our data analysis. However, the results are similar to ours in that the increased costs incurred by subjects with atrial fibrillation are not limited to bed and room charges alone, but are accrued across most hospital cost centers in proportions that were similar for the most part in both studies. Therefore, the economic impact of atrial fibrillation after CABG is underestimated in most previous reports.
Limitations
Our study had several limitations. The study was based on data obtained from an electronic medical record and therefore has risks inherent to use of retrospective data. ICD-9-CM codes do not distinguish between new-onset and existing problems and therefore may lead to underestimates of the true occurrence of the diseases, although measures were undertaken to increase the likelihood of detecting all subjects with atrial fibrillation. Finally, economic impact was estimated by using charges, rather than costs or the ratio of costs to charges. Nevertheless, use of charges is consistent with reports from other researchers.
Implications
The development of new-onset atrial fibrillation after CABG remains an important clinical problem. Our findings indicate that the economic impact of the development of this complication was underestimated in the past. The development of predictive models has had only limited success,19,33 and the application of prophylactic strategies has resulted in some decrease in the prevalence but not in the eradication of atrial fibrillation. We can continue to await more effective prophylactic strategies, but in the meantime the costliness of this complication compels us to find more cost-effective ways to manage the problem and the associated care once atrial fibrillation has occurred.
Our research suggests that much of the increased resource utilization parallels the longer stay (mean difference values for oxygen therapy days and drug days closely correspond to the mean difference for LOS) and can be influenced by testing protocols that indicate treatment leading to more rapid conversion to normal sinus rhythm or that require little adjustment and monitoring after implementation, thereby decreasing LOS. Second, pharmacological strategies for conversion could be compared (use of diltiazem vs procainamide) to determine if less costly drugs are acceptably efficacious, or conversely, if more expensive drugs minimize the LOS and the attendant associated costs we described and are therefore ultimately more cost-effective. Third, costs can be affected by examining whether some therapies or tests done every hospital day are applied by routine or could be safely eliminated (eg, more liberal potassium and magnesium replacement with lesser routine monitoring of levels for patients with normal renal function). We recommend that treatment protocols that address these areas be developed and tested in the future.
ACKNOWLEDGMENTS
We thank Julie F. Cuneo, RN, MSN, CRNP, and Stacey Dapos for assistance with data collection and screening and John M. Clochesy, PhD, and Bartley Griffith, MD, for input on study design.
REFERENCES
- 1.Olshansky B. Management of atrial fibrillation after coronary artery bypass graft. Am J Cardiol. 1996;78:27–34. doi: 10.1016/s0002-9149(96)00563-2. [DOI] [PubMed] [Google Scholar]
- 2.Almassi GH, Schowalter T, Nicolasi AC, et al. Atrial fibrillation after cardiac surgery: a major morbid event? Ann Surg. 1997;226:501–513. doi: 10.1097/00000658-199710000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bietz DS. Rapid recovery in the elderly. Ann Thorac Surg. 1997;64:1222–1223. doi: 10.1016/s0003-4975(97)00642-5. [DOI] [PubMed] [Google Scholar]
- 4.Kowey PR, Dallessandro DA, Herbertson R, et al. Effectiveness of digitalis with or without acebutolol in preventing atrial arrhythmias after coronary artery surgery. Am J Cardiol. 1997;79:1114–1117. doi: 10.1016/s0002-9149(97)00059-3. [DOI] [PubMed] [Google Scholar]
- 5.Mathew JP, Parks R, Savino JS, et al. Atrial fibrillation following coronary artery bypass graft surgery: predictors, outcomes and resource utilization. JAMA. 1996;276:300–306. [PubMed] [Google Scholar]
- 6.Kalman JM, Munawar M, Howes LG, et al. Atrial fibrillation after coronary artery bypass grafting is associated with sympathetic activation. Ann Thorac Surg. 1995;60:1709–1715. doi: 10.1016/0003-4975(95)00718-0. [DOI] [PubMed] [Google Scholar]
- 7.Ali IM, Abulkasim AA, Clark V. Beta-blocker effects on postoperative atrial fibrillation. Eur J Cardiothorac Surg. 1997;11:1154–1157. doi: 10.1016/s1010-7940(97)01215-3. [DOI] [PubMed] [Google Scholar]
- 8.Aranki SF, Shaw DP, Adams DH, et al. Predictors of atrial fibrillation after coronary artery surgery: current trends and impact on hospital resources. Circulation. 1996;94:390–397. doi: 10.1161/01.cir.94.3.390. [DOI] [PubMed] [Google Scholar]
- 9.Asimakopoulos G, Santa RD, Taggart DP. Effects of posterior pericar-diotomy on the incidence of atrial fibrillation and chest drainage after coronary revascularization: a prospective randomized trial. J Cardiovasc Surg. 1997;113:797–799. doi: 10.1016/s0022-5223(97)70242-3. [DOI] [PubMed] [Google Scholar]
- 10.Crosby LH, Pifalo B, Woll KR, Burkholder JA. Risk factors for atrial fibrillation after coronary artery bypass grafting. Am J Cardiol. 1990;66:1520–1522. doi: 10.1016/0002-9149(90)90550-k. [DOI] [PubMed] [Google Scholar]
- 11.Frost L, Lund B, Pilegaard H, Christiansen EH. Re-evaluation of the role of P-wave duration and morphology as predictors of atrial fibrillation and flutter after coronary artery bypass surgery. Eur Heart J. 1996;1:1065–1071. doi: 10.1093/oxfordjournals.eurheartj.a015003. [DOI] [PubMed] [Google Scholar]
- 12.Frost L, Jacobsen CJ, Christiansen EH, Molgaard H, Pilegaars H, Hjortholm K. Hemodynamic predictors of atrial fibrillation or atrial flutter after coronary artery bypass grafting. Acta Anaesthesiol Scand. 1995;39:690–697. doi: 10.1111/j.1399-6576.1995.tb04149.x. [DOI] [PubMed] [Google Scholar]
- 13.Lauer MS, Eagle KA, Buckley MJ, DeSanctis DS. Atrial fibrillation following coronary artery bypass surgery. Prog Cardiovasc Dis. 1989;31:367–378. doi: 10.1016/0033-0620(89)90031-5. [DOI] [PubMed] [Google Scholar]
- 14.Leitch JW, Thomson D, Baird DK, Harris PJ. The importance of age as a predictor of atrial fibrillation and flutter after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 1990;3:338–342. [PubMed] [Google Scholar]
- 15.Mendes LA, Connelly GP, McKenney PA, et al. Right coronary artery stenosis: an independent predictor of atrial fibrillation after CABG. J Am Coll Cardiol. 1995;25:198–202. doi: 10.1016/0735-1097(94)00329-o. [DOI] [PubMed] [Google Scholar]
- 16.Paone G, Higgins RSD, Havstad SL, Silverman NA. Does age limit the effectiveness of clinical pathways after coronary artery bypass graft surgery? Circulation. 1998;98(19 suppl):II41–II45. [PubMed] [Google Scholar]
- 17.Willems S, Weiss C, Meinertz T. Tachyarrhythmias following coronary artery bypass surgery: epidemiology, mechanisms, and current therapeutic strategies. Thorac Cardiovasc Surg. 1997;45:232–237. doi: 10.1055/s-2007-1013733. [DOI] [PubMed] [Google Scholar]
- 18.DeJong MJ, Morton PG. Patients at risk for atrial dysrhythmias after coronary bypass grafting. Dimens Crit Care Nurs. 1998;17:114–126. doi: 10.1097/00003465-199805000-00001. [DOI] [PubMed] [Google Scholar]
- 19.DeJong MJ, Gonce Morton P. Predictors of atrial dysrhythmias for patients undergoing coronary artery bypass grafting. Am J Crit Care. 2000;9:388–396. [PubMed] [Google Scholar]
- 20.Hravnak M, Hoffman LA, Saul MI, et al. Atrial fibrillation: prevalence after minimally invasive direct and standard coronary artery bypass. Ann Thorac Surg. 2001;71:1491–1495. doi: 10.1016/s0003-4975(01)02477-8. [DOI] [PubMed] [Google Scholar]
- 21.Rady MY, Ryan T, Starr N. Perioperative determinants of morbidity and mortality in elderly patients undergoing cardiac surgery. Crit Care Med. 1998;26:225–235. doi: 10.1097/00003246-199802000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Fuller JA, Adams G, Buxton B. Atrial fibrillation after coronary artery bypass grafting: is it a disorder of the elderly? J Thorac Cardiovasc Surg. 1989;97:821–825. [PubMed] [Google Scholar]
- 23.Taylor GJ, Malik SA, Colliver JA, Dove JT. Usefulness of atrial fibrillation as a predictor of stroke after isolated coronary artery bypass grafting. Am J Cardiol. 1987;60:905–907. doi: 10.1016/0002-9149(87)91045-9. [DOI] [PubMed] [Google Scholar]
- 24.Creswell LL, Schuessler RB, Rosenbloom M, Cox JL. Hazards of postoperative atrial arrhythmias. Ann Thorac Surg. 1993;56:539–549. doi: 10.1016/0003-4975(93)90894-n. [DOI] [PubMed] [Google Scholar]
- 25.Kolkevar S, D’Souza AD, Akhatar P, Reek C, Garratt C, Spyt T. Role of atrial ischaemia in development of atrial fibrillation following coronary artery bypass surgery. Eur J Cardiothorac Surg. 1997;11:70–75. doi: 10.1016/s1010-7940(96)01095-0. [DOI] [PubMed] [Google Scholar]
- 26.Yount RJ, Vries JK, Councill CD. The Medical Archival Retrieval system: an information retrieval system based on distributed parallel processing. Inform Process Manag. 1991;27:1–11. [Google Scholar]
- 27.Landon B, Iezzoni LI, Ash AS, et al. Judging hospitals by severity-adjusted mortality rates: the case of CABG surgery. Inquiry. 1996;33:155–166. [PubMed] [Google Scholar]
- 28.Klemperer JD, Klein IL, Ojamaa K, et al. Triiodothyronine therapy lowers the incidence of atrial fibrillation after cardiac operations. Ann Thorac Surg. 1996;61:1323–1329. doi: 10.1016/0003-4975(96)00102-6. [DOI] [PubMed] [Google Scholar]
- 29.Pehkonen EJ, Makynen PJ, Kataja MJ, Tarkka MR. Atrial fibrillation after blood and crystalloid cardioplegia in CABG patients. Thorac Cardiovasc Surg. 1995;43:200–203. doi: 10.1055/s-2007-1013209. [DOI] [PubMed] [Google Scholar]
- 30.Stafford PJ, Kolkevar S, Cooper J, et al. Signal averaged P wave compared with standard electrocardiography or echocardiography for prediction of atrial fibrillation after coronary artery bypass grafting. Heart. 1997;77:417–422. doi: 10.1136/hrt.77.5.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaman AG, Alamgir F, Richens T, Williams R, Rothman MT, Mills PG. The role of signal averaged P-wave and serum magnesium as a combined predictor of atrial fibrillation after elective coronary artery bypass surgery. Heart. 1997;77:527–531. doi: 10.1136/hrt.77.6.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borzak S, Tisdale JE, Amin NB, et al. Atrial fibrillation after bypass surgery: does the arrhythmia or the characteristics of the patient prolong hospital stay? Chest. 1998;113:1489–1491. doi: 10.1378/chest.113.6.1489. [DOI] [PubMed] [Google Scholar]
- 33.Hravnak M, Hoffman LA, Zullo T, Whitman G, Clochesy J, Saul M. Atrial fibrillation following coronary artery bypass grafting: prevalence, predictors and impact [abstract] Circulation. 2000;102:II512. [Google Scholar]