Abstract
Background
Febrile neutropenia (FN) is a common cause of mortality in cancer patients. We examined guideline and non-guideline-based care for patients hospitalized with FN and examined how initial treatment influenced outcomes.
Methods
The Perspectives database was used to examine the treatment of cancer patients with FN from 2000–2010. To capture initial decision-making, we examined treatment within 48 hours of admission. We determined use of guideline-based antibiotics and non-guideline-based treatments, vancomycin and granulocyte-colony stimulating factors (GCSF). Hierarchical models were developed to examine the factors associated with treatment. Patients were stratified into low and high-risk groups and the effect of initial treatment on outcome (non-routine discharge and death) examined.
Findings
Among 25,231 admitted with FN, guideline-based antibiotics were administered to 79%, vancomycin to 37%, and GCSF to 63%. Patients treated at high-FN volume hospitals, by high-FN volume physicians and patients managed by hospitalists were more likely to receive guideline-based antibiotics (p<0.05). Vancomycin use increased from 17% in 2000 to 55% in 2010 while GCSF use only decreased from 73% to 55%. Among low-risk patients, prompt initiation of guideline-based antibiotics decreased discharge to a nursing facility (OR=0.77; 95% CI, 0.65–0.92) and death (OR=0.63; 95% CI, 0.42–0.95).
Conclusion
While use of guideline-based antibiotics is high, use of the non-guideline-based treatments, vancomycin and GCSF, is also high. Physician and hospital factors are the strongest predictors of both guideline and non-guideline-based treatment.
Introduction
Febrile neutropenia (FN) from myelosuppressive chemotherapy results in substantial morbidity, typically requires hospitalization, results in high medical costs, and is associated with significant mortality.1–5 A review of data from 115 centers in the U.S. noted that the in-patient mortality rate for febrile neutropenia was 9.5%. The same study noted that the median cost per episode of FN was over $19,000 and the average hospital stay 11.5 days.2 In addition to the direct consequences of FN, neutropenia often results in reductions in the chemotherapy dose intensity that may impact oncologic outcomes.2
A better understanding of the etiology, natural history, and prevention of febrile neutropenia has led to reductions in morbidity for patients with FN over the last two decades.5–7 Much of the improved outcome for FN has been the result of the recognition of the importance of early administration of empiric, broad spectrum antibiotics.5–7 A large body of literature has now emerged evaluating new antibiotics, alternate treatment regimens, and strategies for the use of granulocyte colony stimulating factors (GCSF) to promote neutrophil production. These data have not only increased the number of treatment options available to clinicians, but also dramatically increased the complexity and costs of therapy.6–9
To guide management, a number of professional societies have developed practice guidelines for the treatment of FN.6–9 In addition to recommendations for empiric antibiotic therapy, these guidelines address more controversial and costly treatments such as the use of therapeutic GCSF, antifungal and antiviral agents, and empiric vancomycin.6–9 While therapeutic GCSF for patients with FN may minimally reduce the length of hospitalization, randomized trials have reported that its use does not impact mortality and these agents are not recommended.6–17 Likewise, there appears to be little benefit to the use of empiric vancomycin outside of specific clinical scenarios.6,7,18
Despite the fact that consensus guidelines for febrile neutropenia have been in place for over a decade, little is known about adherence to these recommendations by clinicians. Small institutional series and surveys have suggested that there are wide variations in practice patterns among oncologists.19–21 This is problematic in that underuse of beneficial treatments and overuse of ineffective treatments may not only result in adverse outcomes, but also has substantial impact on cost and resource utilization. The objective of our study was to examine compliance with guideline-based recommendations for FN treatment, explore the factors that influence adherence to consensus guidelines, and analyze how the use of guideline-based care impacts outcomes.
Methods
Data Source
Data from the Perspective database (Premier, Charlotte, North Carolina) was utilized. Perspectives is a voluntary, fee-supported database that captures data from more than 600 acute-care hospital from throughout the U.S. In addition to patient demographics, disease characteristics, and procedures, the database collects information on all billed services rendered during a patient’s hospital stay. Data in Perspectives undergoes a rigorous quality control process and this dataset has been utilized in a number of outcomes studies.22 –25 In 2006 nearly 5.5 million hospital discharges that represents approximately 15% of all hospitalizations, were captured in Perspectives.22
Patient Selection
We analyzed patients with neutropenia treated from 2000–2010. Only patients with an admitting or primary diagnosis of neutropenia (ICD-9 code 288.0) in combination with an ICD-9 code for a solid tumor were included. Prior studies have captured admissions for neutropenia using a variety of methods often classifying patients with a primary diagnosis of fever or infection as febrile neutropenia.1–3,26 To capture initial decision-making and treatment, we focused our analysis on only hospitalized patients with a primary or admitting diagnosis of neutropenia. Primary tumor sites were classified into the following groups: colorectal, other gastrointestinal, head and neck, lung, breast, skin, soft tissue, genitourinary, gynecologic, lymphoma, and brain.
While numerous risk stratification systems for FN have attempted to use clinical scenarios associated with “high-risk” neutropenia, no consensus exists and there is currently not an objective system to stratify risk using population-based, administrative data.6,7 We performed a series of sensitivity analyses to develop a risk stratification schema using administrative data. We first developed univariate regression models to examine the risk of in-hospital death associated with each of the clinical, demographic, and disease characteristics of our cohort (Table 1). Based on data from these analyses we then developed a series of models sequentially incorporating combinations of the variables associated with death. A final model incorporating the characteristics that remained associated with death was developed. In the model pneumonia, hypotension, sepsis, ICU admission, and mechanical ventilation remained independently associated with death. We classified patients as high-risk if they had any of these 5 clinical characteristics.
Table 1.
Risk of death based on selected complications in patients with febrile neutropenia.
| Risk of death | |
|---|---|
| Univariate models (models included individual parameters only) | |
| Pneumonia | 3.77 (3.19–4.45)* |
| Hypotension | 4.79 (2.66–8.64)* |
| Sepsis | 6.90 (5.99–7.96)* |
| ICU | 10.74 (9.23–12.49)* |
| Mechanical ventilation | 50.64 (40.82–62.84)* |
| Multivariate models (model including all the parameters) | |
| Pneumonia | 1.80 (1.47–2.21)* |
| Hypotension | 3.16 (1.57–6.35)* |
| Sepsis | 3.66 (3.09–4.33)* |
| ICU | 2.69 (2.19–3.31)* |
p<0.05
Clinical and Demographic Characteristics
Clinical data analyzed included age (< 60 and ≥ 60 years), date of admission (2000–2003, 2004–2006, 2007–2010), race (white, black, other including Hispanic, Asian and patients with undefined race), marital status, and insurance status (Medicare, Medicaid, commercial, self-pay, and unknown). Each patient’s admitting physician was noted and their specialty classified as: medical oncology (including hematology), internal medicine (other than medical oncology), family practice, hospitalist, other, and unknown. Hospitals in which patients were treated were characterized based on location (metropolitan, non-metropolitan), region of the country (northeast, midwest, west, south), size (<400 beds, 400–600 beds, and >600 beds) and teaching status (teaching, non-teaching).
Risk adjustment for comorbid conditions was performed using the Charlson comorbidity index.27,28 Each physician and hospital’s annual FN volume was estimated by dividing the number of subjects admitted with FN by the number of years an individual hospital or physician contributed at least one FN patient to the cohort. The distribution of annual FN volume was analyzed and cut-points selected to create three tertiles of physician (low <1.4 cases/year, intermediate 1.4–2.7 cases/year, high >2.7 cases/year) and hospital FN volumes (<8.375 cases/year, intermediate 8.375–14.59 cases/year, high ≥14.6 cases/year) as previously described.29,30
Outcomes
Three primary endpoints were analyzed: use of guideline-based antibiotics, use of vancomycin, and use of granulocyte colony stimulating factors. These outcomes were based on a review of published treatment guidelines for FN.6–12,31 We chose a permissive definition of guideline-based antibiotics that included all of the antibiotics that have been recommended by consensus groups in guidelines over the last decade.6,10,32 Administration of one dose of any of the following antibiotics within 48 hours of admission was considered guideline-based antibiotic therapy: ceftazidime, cefepime, imipenem, meropenem, piperacillin/tazobactam, and an aminoglycoside (any) in combination with any of the aforementioned agents or ciprofloxacin or ticarcillin/clavulanate.6,10,32 Use of vancomycin was defined as at least one dose of vancomycin during the first 48 hours of hospitalization.6,10,32 GCSF use was defined as utilization of one dose of either filgrastim or pegfilgrastim during the hospitalization.8,11,12,31–33 For patients who received filgrastim, we calculated the total number of days in which the drug was given.
We examined how use of guideline-based therapy influenced non-routine discharge (discharge to a nursing home, skilled nursing facility, or acute or subacute rehabilitation center), in hospital mortality, and cost. Among patients who received GCSF we examined the numbers of days in which filgrastim was administered. Cost estimates for the total number of doses administered to low and high-risk patients were then calculated using published 2010 Medicare reimbursement schedules (filgrastim 300 microgram daily dose at $233.43 per dose).
Statistical Analysis
Frequency distributions between categorical variables were compared using χ2 tests. We used hierarchical logistic regression analysis to determine the factors associated with use of guideline-based antibiotics, vancomycin, GCSF use, non-routine discharge, and death. These models included all patient, physician, and hospital characteristics as well as physician-specific and hospital-specific random effects. Separate models were developed for low-risk and high-risk patients. A priori with our sample size of approximately 25,000 patients we estimated that with an alpha of 0.05 and power of 80% that the minimum detectable odds ratio for the detection of an outcome of interest even for a relatively uncommon characteristic (20%) was 1.11. All analyses were performed with SAS version 9.2 (SAS Institute Inc, Cary, North Carolina).
Results
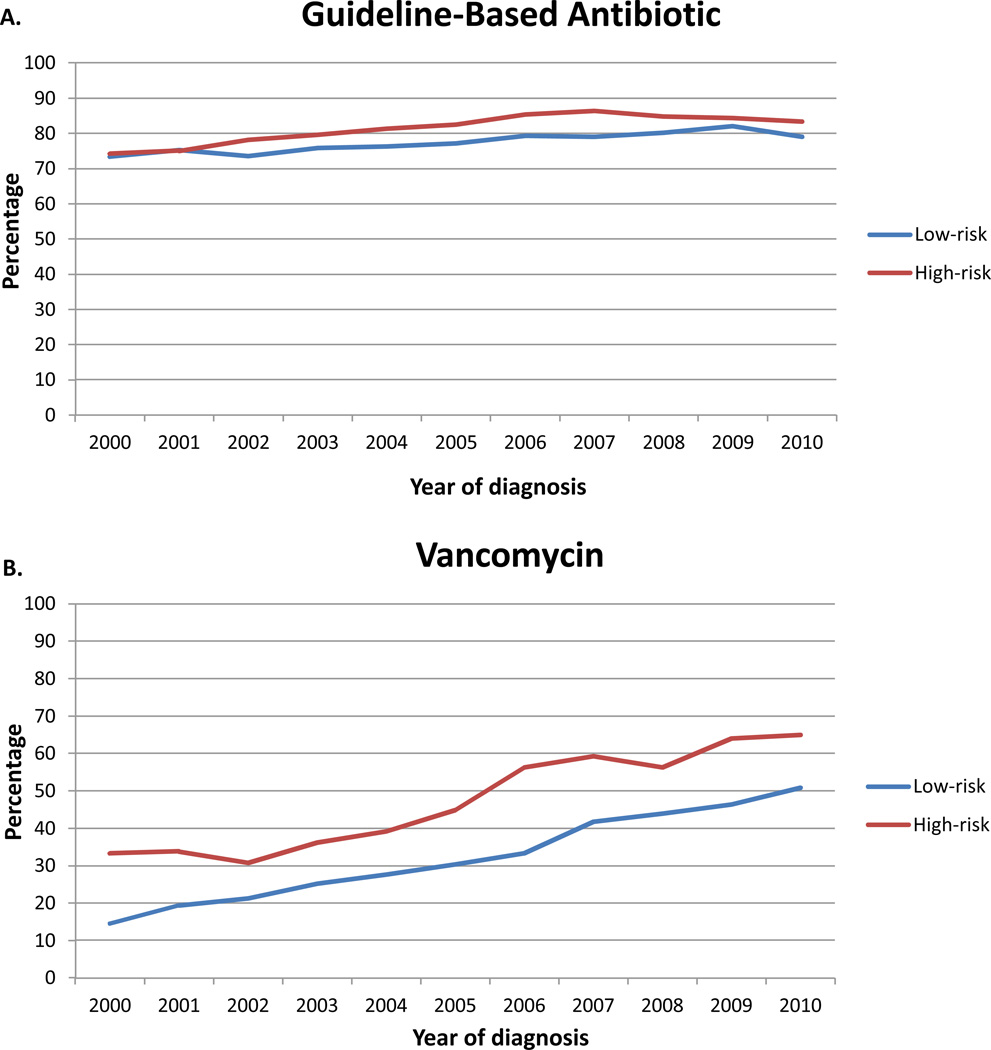
A total of 25,231 patients with FN were identified. Guideline-based antibiotics were administered to 19,897 (78.9%) subjects (Table 2). The use of guideline-based antibiotics increased minimally over time from 73.4% in 2000 to 80.3% in 2010 (p<0.0001) (Figure 1A). Guideline-based antibiotics were utilized in 77.8% of low-risk and 82.7% (p<0.0001) of high-risk patients.
Table 2.
Univariate and multivariate analysis of factors associated with use of guideline-based antibiotics.
| N | (%)1 | Univariate OR | Multivariate OR | ||
|---|---|---|---|---|---|
| 19,897 | (78.9) | ||||
| Age | |||||
| <60 years | 9111 | (81.3) | Referent | Referent | |
| ≥60 years | 10,786 | (77.0) | 0.77 (0.72–0.82)* | 0.89 (0.81–0.98)* | |
| Gender | |||||
| Male | 8057 | (80.8) | Referent | Referent | |
| Female | 11,840 | (77.6) | 0.82 (0.77–0.88)* | 0.82 (0.75–0.90)* | |
| Year of diagnosis | |||||
| 2000–2003 | 5563 | (75.1) | Referent | Referent | |
| 2004–2006 | 5807 | (78.9) | 1.24 (1.15–1.34)* | 1.17 (1.06–1.28)* | |
| 2007–2010 | 8527 | (81.5) | 1.46 (1.36–1.57)* | 1.40 (1.27–1.53)* | |
| Race | |||||
| White | 14,541 | (79.4) | Referent | Referent | |
| Black | 1934 | (81.3) | 1.13 (1.01–1.26)* | 1.12 (0.98–1.27) | |
| Other | 3422 | (75.5) | 0.80 (0.74–0.87)* | 0.96 (0.87–1.07) | |
| Marital status | |||||
| Married | 11,293 | (79.4) | Referent | Referent | |
| Single | 2974 | (81.5) | 1.14 (1.04–1.25)* | 0.98 (0.87–1.09) | |
| Unknown | 5630 | (76.5) | 0.84 (0.79–0.90)* | 0.91 (0.84–0.99)* | |
| Insurance status | |||||
| Medicare | 8251 | (76.4) | Referent | Referent | |
| Medicaid | 1746 | (81.9) | 1.39 (1.24–1.57)* | 1.25 (1.07–1.46)* | |
| Commercial | 8928 | (80.5) | 1.27 (1.19–1.36)* | 1.19 (1.08–1.30)* | |
| Uninsured | 484 | (83.0) | 1.51 (1.21–1.88)* | 1.39 (1.07–1.80)* | |
| Unknown | 488 | (78.0) | 1.09 (0.90–1.32) | 1.03 (0.82–1.30) | |
| Location | |||||
| Metropolitan | 17,312 | (79.0) | Referent | Referent | |
| Non-metropolitan | 2585 | (78.2) | 0.95 (0.87–1.04) | 1.05 (0.91–1.21) | |
| Teaching status | |||||
| Non-teaching | 10,697 | (76.0) | Referent | Referent | |
| Teaching | 9200 | (82.5) | 1.49 (1.40–1.59)* | 1.32 (1.18–1.48)* | |
| Hospital size | |||||
| <400 beds | 8804 | (76.7) | Referent | Referent | |
| 400–600 beds | 5799 | (78.7) | 1.12 (1.04–1.20)* | 0.94 (0.84–1.07) | |
| >600 beds | 5294 | (82.9) | 1.47 (1.36–1.59)* | 0.97 (0.82–1.13) | |
| Hospital location | |||||
| Eastern | 2706 | (82.9) | Referent | Referent | |
| Midwest | 4076 | (81.0) | 0.88 (0.79–0.99)* | 0.90 (0.76–1.06) | |
| Southern | 10,535 | (78.2) | 0.74 (0.67–0.81)* | 0.74 (0.64–0.86)* | |
| West | 2580 | (74.6) | 0.60 (0.54–0.68)* | 0.77 (0.64–0.92)* | |
| Comorbidity | |||||
| <2 | 2662 | (79.6) | Referent | Referent | |
| 2 | 3267 | (79.2) | 0.98 (0.87–1.09) | 0.94 (0.82–1.07) | |
| >2 | 13,968 | (78.6) | 0.94 (0.86–1.03) | 0.89 (0.79–0.99)* | |
| Physician specialty | |||||
| Medical oncology | 12,741 | (79.5) | Referent | Referent | |
| Internal medicine | 3916 | (79.1) | 0.98 (0.91–1.06) | 1.12 (0.99–1.26) | |
| Family practice | 1084 | (76.0) | 0.82 (0.72–0.93)* | 0.98 (0.83–1.17) | |
| Hospitalist | 715 | (82.8) | 1.24 (1.04–1.49)* | 1.49 (1.18–1.88)* | |
| Other | 863 | (72.1) | 0.67 (0.59–0.76)* | 0.76 (0.63–0.93)* | |
| Unknown | 578 | (76.4) | 0.84 (0.70–0.99)* | 1.02 (0.74–1.40) | |
| Physician volume | |||||
| Low | 6417 | (77.2) | Referent | Referent | |
| Intermediate | 6664 | (78.6) | 1.09 (1.01–1.17)* | 1.10 (0.98–1.23) | |
| High | 6816 | (80.8) | 1.24 (1.15–1.34)* | 1.19 (1.03–1.38)* | |
| Hospital volume | |||||
| Low | 6256 | (74.8) | Referent | Referent | |
| Intermediate | 6527 | (78.4) | 1.23 (1.14–1.32)* | 1.22 (1.08–1.37)* | |
| High | 7114 | (83.3) | 1.68 (1.55–1.81)* | 1.56 (1.34–1.81)* | |
| Primary tumor site | |||||
| Colorectal | 1647 | (76.9) | 0.88 (0.80–0.98)* | 0.87 (0.65–1.16) | |
| Other gastrointestinal | 1513 | (78.8) | 1.00 (0.89–1.12) | 0.87 (0.65–1.17) | |
| Head and neck | 922 | (82.2) | 1.25 (1.07–1.46)* | 1.06 (0.78–1.44) | |
| Lung | 4800 | (77.0) | 0.86 (0.81–0.93)* | 0.83 (0.63–1.10) | |
| Breast | 5257 | (78.5) | 0.97 (0.91–1.04) | 0.99 (0.75–1.30) | |
| Skin | 180 | (84.5) | 1.47 (1.01–2.13)* | 1.46 (0.91–2.34) | |
| Soft tissue | 894 | (88.3) | 2.08 (1.71–2.53)* | 1.74 (1.24–2.44)* | |
| Genitourinary | 1104 | (77.3) | 0.91 (0.80–1.03) | 0.77 (0.58–1.03) | |
| Gynecologic | 1056 | (72.4) | 0.69 (0.61–0.77) | 0.75 (0.55–1.01) | |
| Lymphoma | 2582 | (84.1) | 1.48 (1.34–1.64)* | 1.25 (0.94–1.66) | |
| Brain | 169 | (74.1) | 0.77 (0.57–1.03) | 0.60 (0.39–0.93)* | |
| Infections | |||||
| Sepsis | 2572 | (83.3) | 1.39 (1.25–1.53)* | 1.32 (1.17–1.48)* | |
| Pneumonia | 1698 | (83.2) | 1.36 (1.21–1.54)* | 1.40 (1.22–1.61)* | |
| Intensive care use | 227 | (14.0) | 1.70 (1.48–1.97)* | 1.62 (1.36–1.93)* | |
| Hypotension | 78 | (83.0) | 1.31 (0.76–2.24) | 1.28 (0.70–2.33) | |
| Mechanical ventilation | 336 | (86.4) | 1.71 (1.28–2.29)* | 1.17 (0.83–1.65) | |
Reflects the percentage of patients who received guideline-concordant care.
p<0.05
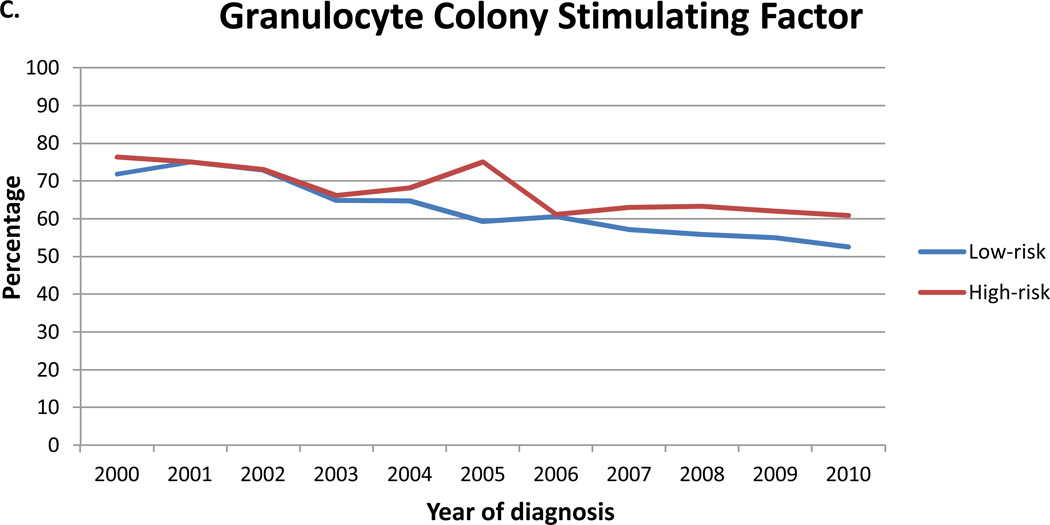
Figure 1.
Use of guideline and non-guideline based therapy for patients with febrile neutropenia stratified into low and high-risk groups. A. Guideline-base antibiotics. B. Vancomycin. C. Granulocyte colony stimulating factor. The black line represents the overall mean for each treatment.
Patients treated more recently (OR=1.40; 95% CI, 1.27–1.53), black patients (OR=1.13; 95% CI, 1.01–1.26), those at teaching hospitals (OR=1.32; 95% CI, 1.18–1.48), patients treated at high-volume hospitals (OR=1.56; 95% CI, 1.34–1.81) and by high-FN volume physicians (OR=1.19; 95% CI, 1.03–1.38), those cared for by hospitalists (OR=1.49; 95% CI, 1.18–1.88) and patients treated in the ICU or with sepsis or pneumonia were more likely to receive guideline-based antibiotics (Table 2). Likewise, compared to those with Medicare, Medicaid recipients and patients with commercial insurance were more likely to receive guideline-based antibiotics. In contrast, older patients (OR=0.89; 95% CI, 0.81–0.98) and women (OR=0.82; 95% CI, 0.75–0.90) were less likely to receive guideline-based antibiotics.
Upfront vancomycin was administered to 9311 (36.9%) patients and increased with time from 17.2% in 2000 to 54.9% in 2010 (p<0.0001) (Figure 1B). Vancomycin was utilized in 33.1% of low-risk and 50.8% of high-risk patients. Patients with more severe disease (i.e., sepsis, pneumonia, in the ICU) more often received vancomycin (Table 3). Black patients (OR=1.21; 95% CI, 1.08–1.35), subjects with >2 comorbidities (OR=1.12; 95% CI 1.02–1.24) and those treated at large hospitals (OR=1.30; 95% CI, 1.13–1.50) were more likely to receive vancomycin. Non-metropolitan residents (OR=0.71; 95% CI, 0.62–0.81) were less likely to receive vancomycin. Use of vancomycin was inversely associated with physician case volume. Compared to medical oncologists, other internists (OR=1.38; 95% CI, 1.23–1.54) and hospitalists (OR=1.61; 95% CI, 1.32–1.96) more often used vancomycin.
Table 3.
Univariate and multivariate analysis of factors associated with use of non-guideline based care, vancomycin and granulocyte colony stimulating factors.
| Vancomycin | Granulocyte colony stimulating factor | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | (%)1 | Univariate OR | Multivariate OR | N | (%)1 | Univariate OR | Multivariate OR | ||
| 9311 | (36.9) | 15,880 | (62.9) | ||||||
| Age | |||||||||
| <60 years | 4484 | (40.0) | Referent | Referent | 6775 | (60.5) | Referent | Referent | |
| ≥60 years | 4827 | (34.4) | 0.77 (0.72–0.84)* | 0.77 (0.71–0.84)* | 9105 | (64.9) | 1.22 (1.15–1.28)* | 1.10 (1.01–1.20)* | |
| Gender | |||||||||
| Male | 3824 | (38.3) | Referent | Referent | 6442 | (64.6) | Referent | Referent | |
| Female | 5487 | (36.0) | 0.90 (0.86–0.95)* | 1.00 (0.93–1.09) | 9438 | (61.9) | 0.89 (0.84–0.94)* | 1.03 (0.94–1.12) | |
| Year of diagnosis | |||||||||
| 2000–2003 | 1691 | (22.8) | Referent | Referent | 5261 | (71.0) | Referent | Referent | |
| 2004–2006 | 2526 | (34.3) | 1.56 (1.44–1.70)* | 1.73 (1.58–1.89)* | 4613 | (62.7) | 0.69 (0.64–0.74)* | 0.58 (0.53–0.63)* | |
| 2007–2010 | 5094 | (48.7) | 2.97 (2.73–3.22)* | 3.40 (3.11–3.71)* | 6006 | (57.4) | 0.55 (0.52–0.59)* | 0.42 (0.38–0.46)* | |
| Race | |||||||||
| White | 6705 | (36.6) | Referent | Referent | 11,257 | (61.5) | Referent | Referent | |
| Black | 1064 | (44.7) | 1.12 (1.01–1.25)* | 1.21 (1.08–1.35)* | 1524 | (64.1) | 1.12 (1.02–1.22)* | 1.17 (1.04–1.32)* | |
| Other | 1542 | (34.0) | 0.94 (0.85–1.03) | 0.92 (0.84–1.01) | 3099 | (68.4) | 1.36 (1.27–1.45)* | 1.22 (1.10–1.35)* | |
| Marital status | |||||||||
| Married | 5198 | (36.6) | Referent | Referent | 8983 | (63.2) | Referent | Referent | |
| Single | 1592 | (43.6) | 1.06 (0.97–1.16) | 1.10 (1.00–1.22)* | 2275 | (62.3) | 0.96 (0.89–1.04) | 1.03 (0.93–1.14) | |
| Unknown | 2521 | (34.2) | 0.98 (0.91–1.06) | 0.96 (0.89–1.04) | 4622 | (62.8) | 0.98 (0.93–1.04) | 0.96 (0.88–1.04) | |
| Insurance status | |||||||||
| Medicare | 3761 | (34.4) | Referent | Referent | 7048 | (65.3) | Referent | Referent | |
| Medicaid | 818 | (38.4) | 0.96 (0.85–1.09) | 0.94 (0.83–1.08) | 1341 | (62.9) | 0.90 (0.82–0.99)* | 1.05 (0.91–1.20) | |
| Commercial | 4275 | (38.5) | 1.04 (0.96–1.12) | 1.05 (0.97–1.15) | 6729 | (60.7) | 0.82 (0.78–0.87)* | 0.93 (0.85–1.01) | |
| Uninsured | 219 | (37.6) | 0.87 (0.70–1.06) | 0.84 (0.67–1.05) | 384 | (65.9) | 1.03 (0.86–1.22) | 1.04 (0.82–1.31) | |
| Unknown | 238 | (38.0) | 1.09 (0.90–1.33) | 1.07 (0.86–1.32) | 378 | (60.4) | 0.81 (0.69–0.96)* | 0.87 (0.70–1.09) | |
| Location | |||||||||
| Metropolitan | 8319 | (37.9) | Referent | Referent | 13,815 | (63.0) | Referent | Referent | |
| Non-metropolitan | 992 | (30.0) | 0.75 (0.61–0.92)* | 0.71 (0.62–0.81) | 2065 | (62.4) | 0.98 (0.90–1.05) | 0.90 (0.77–1.06) | |
| Teaching status | |||||||||
| Non-teaching | 4828 | (34.3) | Referent | Referent | 9212 | (65.4) | Referent | Referent | |
| Teaching | 4483 | (40.2) | 1.16 (0.96–1.39) | 1.10 (0.99–1.22) | 6668 | (59.8) | 0.79 (0.75–0.83)* | 0.71 (0.63–0.80)* | |
| Hospital size | |||||||||
| <400 beds | 3867 | (33.7) | Referent | Referent | 7308 | (63.7) | Referent | Referent | |
| 400–600 beds | 2560 | (34.7) | 1.06 (0.87–1.29) | 0.97 (0.87–1.09) | 4871 | (66.1) | 1.11 (1.05–1.18)* | 1.22 (1.07–1.40)* | |
| >600 beds | 2884 | (45.2) | 1.34 (1.01–1.78)* | 1.30 (1.13–1.50)* | 3701 | (58.0) | 0.79 (0.74–0.84)* | 0.80 (0.67–0.95)* | |
| Hospital location | |||||||||
| Eastern | 1344 | (41.2) | Referent | Referent | 2073 | (63.5) | Referent | Referent | |
| Midwest | 2020 | (40.2) | 1.09 (0.85–1.40) | 1.06 (0.92–1.22) | 3055 | (60.7) | 0.89 (0.81–0.97)* | 0.72 (0.61–0.86)* | |
| Southern | 4765 | (35.4) | 1.03 (0.81–1.31) | 0.97 (0.85–1.11) | 8396 | (62.3) | 0.95 (0.88–1.03) | 0.96 (0.82–1.12) | |
| West | 1182 | (34.2) | 0.89 (0.67–1.18) | 0.84 (0.71–0.99)* | 2356 | (68.1) | 1.23 (1.11–1.36)* | 1.10 (0.91–1.34) | |
| Comorbidity | |||||||||
| <2 | 1162 | (34.8) | Referent | Referent | 2058 | (61.5) | Referent | Referent | |
| 2 | 1468 | (35.6) | 0.99 (0.89–1.11) | 0.98 (0.88–1.10) | 2505 | (60.7) | 0.97 (0.88–1.06) | 0.95 (0.85–1.08) | |
| >2 | 6681 | (37.6) | 1.13 (1.03–1.23)* | 1.12 (1.02–1.24)* | 11,317 | (63.7) | 1.10 (1.02–1.18)* | 1.04 (0.94–1.16) | |
| Physician specialty | |||||||||
| Medical oncology | 5674 | (35.4) | Referent | Referent | 9899 | (61.7) | Referent | Referent | |
| Internal medicine | 2163 | (43.7) | 1.28 (1.16–1.41)* | 1.38 (1.23–1.54)* | 3306 | (66.8) | 1.25 (1.17–1.33)* | 1.23 (1.08–1.40)* | |
| Family practice | 498 | (34.9) | 0.94 (0.81–1.09) | 0.93 (0.79–1.10) | 870 | (61.0) | 0.97 (0.87–1.08) | 0.97 (0.80–1.16) | |
| Hospitalist | 438 | (50.7) | 1.62 (1.36–1.93)* | 1.61 (1.32–1.96)* | 533 | (61.7) | 1.00 (0.87–1.45) | 0.99 (0.79–1.25) | |
| Other | 381 | (31.8) | 0.78 (0.67–0.92)* | 0.75 (0.62–0.91)* | 757 | (63.2) | 1.07 (0.94–1.20) | 1.03 (0.83–1.28) | |
| Unknown | 157 | (20.7) | 1.03 (0.79–1.35) | 1.10 (0.80–1.52) | 515 | (68.0) | 1.32 (1.13–1.54)* | 0.68 (0.47–0.98)* | |
| Physician volume | |||||||||
| Low | 3343 | (40.2) | Referent | Referent | 5350 | (64.3) | Referent | Referent | |
| Intermediate | 3107 | (36.6) | 0.99 (0.91–1.07) | 0.94 (0.85–1.04) | 5407 | (63.8) | 0.98 (0.92–1.04) | 1.02 (0.90–1.15) | |
| High | 2861 | (33.9) | 0.88 (0.80–0.97)* | 0.77 (0.67–0.88)* | 5123 | (60.7) | 0.86 (0.80–0.91)* | 0.88 (0.74–1.04) | |
| Hospital volume | |||||||||
| Low | 2742 | (32.8) | Referent | Referent | 5492 | (65.7) | Referent | Referent | |
| Intermediate | 2979 | (35.8) | 1.03 (0.85–1.24) | 1.25 (1.12–1.39)* | 5276 | (63.4) | 0.91 (0.85–0.97)* | 0.95 (0.83–1.08) | |
| High | 3590 | (42.0) | 1.21 (0.92–1.57) | 1.45 (1.27–1.65)* | 5112 | (59.8) | 0.78 (0.73–0.83)* | 1.04 (0.89–1.23) | |
| Primary tumor site | |||||||||
| Colorectal | 793 | (37.0) | 1.17 (0.92–1.48) | 1.21 (0.94–1.56) | 1476 | (68.9) | 1.34 (1.22–1.47)* | 1.29 (0.98–1.70) | |
| Other gastrointestinal | 743 | (38.7) | 1.24 (0.98–1.58) | 1.31 (1.01–1.69)* | 1257 | (65.5) | 1.13 (1.02–1.24)* | 1.18 (0.89–1.56) | |
| Head and neck | 475 | (42.3) | 1.38 (1.08–1.77)* | 1.42 (1.09–1.86)* | 753 | (67.1) | 1.21 (1.07–1.38)* | 1.24 (0.92–1.65) | |
| Lung | 1998 | (32.1) | 0.90 (0.72–1.13)* | 0.91 (0.72–1.17) | 4069 | (65.3) | 1.14 (1.08–1.21)* | 1.05 (0.81–1.36) | |
| Breast | 2331 | (34.8) | 1.15 (0.91–1.45)* | 1.17 (0.92–1.50) | 3922 | (58.6) | 0.78 (0.74–0.82)* | 0.74 (0.56–0.96)* | |
| Skin | 98 | (46.0) | 1.68 (1.18–2.40)* | 1.85 (1.26–2.72)* | 141 | (66.2) | 1.15 (0.87–1.54) | 1.02 (0.66–1.56) | |
| Soft tissue | 455 | (45.0) | 1.49 (1.15–1.92)* | 1.66 (1.25–2.19)* | 519 | (51.3) | 0.61 (0.54–0.69)* | 0.64 (0.47–0.86)* | |
| Genitourinary | 523 | (36.6) | 1.08 (0.86–1.39) | 1.13 (0.87–1.46) | 948 | (66.3) | 1.17 (1.05–1.31)* | 1.10 (0.83–1.46) | |
| Gynecologic | 478 | (32.8) | 0.99 (0.77–1.28) | 1.11 (0.84–1.46) | 967 | (66.3) | 1.17 (1.05–1.31)* | 1.10 (0.82–1.48) | |
| Lymphoma | 1445 | (47.1) | 1.76 (1.39–2.22)* | 1.85 (1.44–2.39)* | 1856 | (60.5) | 0.89 (0.82–0.96)* | 0.85 (0.65–1.12) | |
| Brain | 100 | (43.9) | 1.46 (1.02–2.10)* | 1.40 (0.93–2.09) | 158 | (69.3) | 1.33 (1.00–1.77)* | 1.57 (1.00–2.46)* | |
| Disease characteristics | |||||||||
| Sepsis | 1668 | (54.0) | 1.80 (1.64–1.97)* | 1.86 (1.69–2.05)* | 2006 | (65.0) | 1.11 (1.02–1.20)* | 1.02 (0.92–1.14) | |
| Pneumonia | 998 | (48.9) | 1.61 (1.44–1.79)* | 1.65 (1.47–1.85)* | 1371 | (67.2) | 1.23 (1.11–1.35)* | 1.24 (1.09–1.40)* | |
| Intensive care use | 1059 | (65.1) | 2.38 (2.09–2.71)* | 2.63 (2.29–3.03)* | 1171 | (72.0) | 1.55 (1.39–1.74)* | 1.64 (1.40–1.92)* | |
| Hypotension | 38 | (40.4) | 0.89 (0.56–1.42) | 0.98 (0.59–1.6) | 66 | (70.2) | 1.39 (0.89–2.16) | 1.41 (0.81–2.47) | |
| Mechanical ventilation | 271 | (69.7) | 1.78 (1.37–2.31)* | 1.81 (1.37–2.40)* | 276 | (71.0) | 1.45 (1.16–1.80)* | 1.04 (0.77–1.41) | |
Reflects the percentage of patients who received guideline-concordant care.
p<0.05
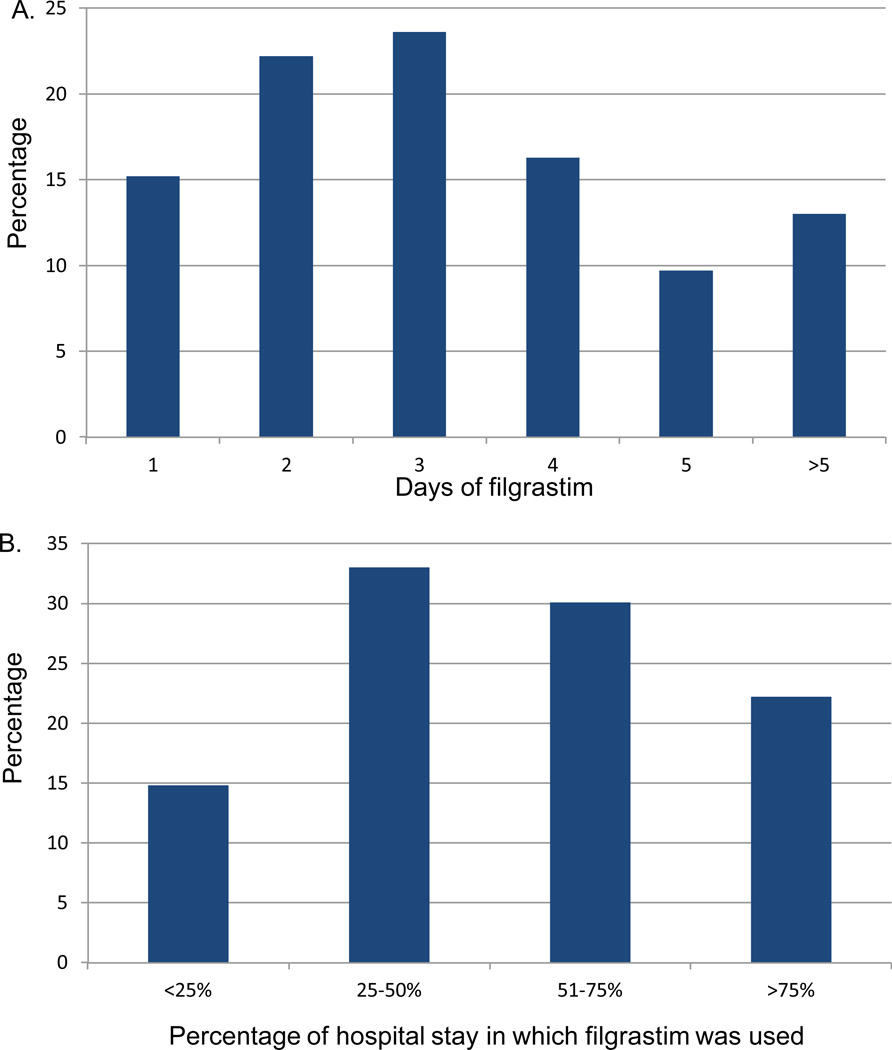
Despite recommendations against empiric use, GCSF was given to 15,880 (62.9%) patients and only decreased with time from 72.5% in 2000 to 55.0% in 2010 (p<0.0001) (Figure 1C). GCSF was utilized in 62.1% of low-risk and 65.9% of high-risk patients. Among patients who received filgrastim, 15.2% received one day of treatment and 22.2% 2 days, while 13.0% received the agent for >5 days (Figure 2A). In the cohort that received 1–2 days of filgrastim, 33.8% had a hospital stay of <3 days while 27.4% were hospitalized for >5 days. Among patients who received filgrastim, 14.8% received the drug for <25% of the days of their hospitalization, 33.0% on 25–50% of the hospitalization, 30.1% on 51–75%, and 22.2% of patients received GCSF on >75% of the days in which they were hospitalized (Figure 2B). Patients treated at teaching hospitals (OR=0.71; 95 CI, 0.63–0.80) and those at large hospitals (OR=0.80; 95% CI, 0.67–0.95) were less likely to receive GCSF (Table 3). Use of GCSF was higher in patients with pneumonia and those admitted to the ICU. Among the 12,184 low-risk patients who received filgrastim a total of 40,080 daily doses were administered at a cost of $9,355,874. The 3570 high-risk patients who received filgrastim received a total of 14,351 daily doses at a cost of $3,349,954.
Figure 2.
Use of filgrastim in patients with febrile neutropenia. A. Number of days of filgrastim use. B. Percentage of hospital days in which filgrastim was used in patients who received the drug.
The effect of adherence to guideline-based treatment recommendations on adverse outcomes was examined (Table 4). Among low-risk patients, use of guideline-based antibiotics reduced the risk of non-routine discharge by 23% (OR=0.77; 95% CI, 0.65–0.92) and reduced in-hospital mortality by 37% (OR=0.65; 95% CI, 0.42–0.95). In contrast, use of empiric vancomycin and GCSF did not improve outcomes. In general, among low-risk patients adverse outcomes were more common in older patients, Medicare beneficiaries, and those with more comorbid diseases. For high-risk patients with FN there was no association between use of guideline-based antibiotics and improved outcomes. Likewise, use of vancomycin and GCSF did not positively influence outcomes.
Table 4.
Effect of treatment patterns on outcome for low and high-risk patients with febrile neutropenia.
| Low-risk patients | High-risk patients | ||||
|---|---|---|---|---|---|
| Non-routine discharge (n=882) |
Death (n=273) | Non-routine discharge (n=619) |
Death (n=555) | ||
| Age | |||||
| <60 years | Referent | Referent | Referent | Referent | |
| ≥60 years | 2.16 (0.88–1.27) | 2.71 (1.62–4.54)* | 1.53 (1.17–2.00)* | 1.61 (1.15–2.25)* | |
| Gender | |||||
| Male | Referent | Referent | Referent | Referent | |
| Female | 1.05 (0.88–1.27) | 0.98 (0.66–1.47) | 1.22 (0.99–1.51) | 0.98 (0.74–1.28) | |
| Year of diagnosis | |||||
| 2000–2003 | Referent | Referent | Referent | Referent | |
| 2004–2006 | 1.07 (0.87–1.31) | 0.73 (0.46–1.16) | 1.01 (0.78–1.31) | 0.70 (0.50–0.97)* | |
| 2007–2010 | 0.95 (0.78–1.17) | 0.61 (0.37–1.01) | 0.96 (0.74–1.23) | 0.66 (0.48–0.90)* | |
| Race | |||||
| White | Referent | Referent | Referent | Referent | |
| Black | 1.25 (0.96–1.63) | 1.32 (0.70–2.52) | 1.01 (0.75–1.38) | 1.16 (0.77–1.74) | |
| Other | 0.92 (0.74–1.14) | 1.08 (0.59–1.98) | 0.72 (0.55–0.95)* | 0.99 (0.70–1.39) | |
| Marital status | |||||
| Married | Referent | Referent | Referent | Referent | |
| Single | 2.31 (1.83–2.92)* | 0.86 (0.45–1.62) | 1.60 (1.20–2.13)* | 1.17 (0.81–1.70) | |
| Unknown | 1.84 (1.54–2.20)* | 0.84 (0.55–1.28) | 1.58 (1.29–1.94)* | 0.94 (0.72–1.24) | |
| Insurance status | |||||
| Medicare | Referent | Referent | Referent | Referent | |
| Medicaid | 0.57 (0.40–0.80)* | 1.47 (0.71–3.07) | 0.54 (0.36–0.82)* | 0.65 (0.38–1.11) | |
| Commercial | 0.50 (0.40–0.61)* | 0.65 (0.41–1.03) | 0.56 (0.44–0.71)* | 0.73 (0.54–0.99)* | |
| Uninsured | 0.34 (0.16–0.71)* | 1.82 (0.59–5.61) | 0.07 (0.01–0.51)* | 0.85 (0.35–2.11) | |
| Unknown | 0.48 (0.25–0.92)* | 1.22 (0.36–4.21) | 0.37 (0.16–0.87)* | 0.95 (0.41–2.20) | |
| Location | |||||
| Metropolitan | Referent | Referent | Referent | Referent | |
| Non-metropolitan | 0.87 (0.66–1.15) | 0.89 (0.30–2.66) | 1.01 (0.76–1.34) | 1.07 (0.72–1.60) | |
| Teaching status | |||||
| Non-teaching | Referent | Referent | Referent | Referent | |
| Teaching | 0.96 (0.78–1.19) | 0.94 (0.40–2.20) | 0.98 (0.78–1.23) | 0.91 (0.67–1.25) | |
| Hospital size | |||||
| <400 beds | Referent | Referent | Referent | Referent | |
| 400–600 beds | 1.13 (0.90–1.41) | 1.22 (0.50–3.01) | 1.07 (0.84–1.36) | 0.80 (0.57–1.13) | |
| >600 beds | 0.99 (0.73–1.33) | 1.21 (0.37–3.93) | 1.01 (0.73–1.38) | 0.88 (0.57–1.34) | |
| Hospital location | |||||
| Eastern | Referent | Referent | Referent | Referent | |
| Midwest | 0.82 (0.62–1.08) | 0.64 (0.19–2.20) | 1.24 (0.91–1.70) | 0.57 (0.36–0.88)* | |
| Southern | 0.61 (0.47–0.80)* | 0.83 (0.28–2.46) | 0.95 (0.70–1.27) | 0.73 (0.49–1.10) | |
| West | 0.69 (0.50–0.96)* | 0.92 (0.23–3.63) | 0.95 (0.65–1.37) | 0.70 (0.43–1.14) | |
| Comorbidity | |||||
| <2 | Referent | Referent | Referent | Referent | |
| 2 | 1.50 (1.07–2.10)* | 1.90 (0.84–4.27) | 1.47 (0.92–2.34) | 1.50 (0.85–2.66) | |
| >2 | 1.89 (1.42–2.52)* | 2.26 (1.11–4.60)* | 1.81 (1.20–2.73)* | 1.49 (0.90–2.45) | |
| Physician specialty | |||||
| Medical oncology | Referent | Referent | Referent | Referent | |
| Internal medicine | 1.81 (1.43–2.28)* | 1.18 (0.46–3.03) | 1.80 (1.40–2.32)* | 0.98 (0.70–1.37) | |
| Family practice | 1.74 (1.24–2.43)* | 1.15 (0.28–4.75) | 1.91 (1.31–2.79)* | 1.07 (0.64–1.80) | |
| Hospitalist | 2.31 (1.55–3.45)* | 0.54 (0.05–6.40) | 1.40 (0.88–2.22) | 0.77 (0.40–1.46) | |
| Other | 1.30 (0.87–1.96) | 1.11 (0.24–5.26) | 1.41 (0.88–2.27) | 1.00 (0.52–1.92) | |
| Unknown | 1.33 (0.75–2.37) | 2.53 (0.42–15.11) | 1.81 (0.99–3.30* | 0.91 (0.35–2.35) | |
| Physician volume | |||||
| Low | Referent | Referent | Referent | Referent | |
| Intermediate | 0.95 (0.77–1.18) | 0.78 (0.32–1.90) | 1.07 (0.85–1.35) | 0.86 (0.62–1.18) | |
| High | 1.06 (0.80–1.40) | 0.76 (0.25–2.26) | 1.10 (0.82–1.49) | 0.74 (0.49–1.11) | |
| Hospital volume | |||||
| Low | Referent | Referent | Referent | Referent | |
| Intermediate | 0.86 (0.69–1.07) | 0.78 (0.43–2.48) | 0.79 (0.63–1.00) | 1.20 (0.87–1.66) | |
| High | 0.59 (0.45–0.79)* | 0.68 (0.22–2.15) | 0.74 (0.55–1.00)* | 1.14 (0.76–1.72) | |
| Primary tumor site | |||||
| Colorectal | 0.78 (0.44–1.39) | 2.83 (0.86–9.34) | 1.25 (0.61–2.55) | 1.48 (0.61–3.58) | |
| Other gastrointestinal | 0.66 (0.36–1.20) | 2.47 (0.72–8.45) | 1.12 (0.54–2.32) | 1.14 (0.47–2.78) | |
| Head and neck | 0.88 (0.47–1.63) | 0.84 (0.21–3.29) | 0.77 (0.35–1.68) | 0.85 (0.34–2.12) | |
| Lung | 0.61 (0.35–1.07) | 3.76 (1.17–12.06)* | 1.03 (0.52–2.04) | 1.82 (0.78–4.24) | |
| Breast | 0.34 (0.19–0.61)* | 0.56 (0.16–1.90) | 0.73 (0.35–1.49) | 0.89 (0.37–2.18) | |
| Skin | 1.07 (.47–2.47) | 0.49 (0.04–6.65) | 1.73 (0.52–5.74) | 1.69 (0.38–7.59) | |
| Soft tissue | 0.36 (0.17–0.78)* | 0.74 (0.13–4.21) | 1.34 (0.57–3.14) | 1.55 (0.53–4.54) | |
| Genitourinary | 0.79 (0.44–1.43) | 0.58 (0.15–2.30) | 1.21 (0.59–2.48) | 0.97 (0.39–2.43) | |
| Gynecologic | 0.73 (0.39–1.35) | 2.55 (0.69–9.50) | 0.89 (0.40–1.95) | 1.58 (0.59–4.23) | |
| Lymphoma | 0.75 (0.42–1.35) | 0.35 (0.09–1.36) | 0.82 (0.40–1.68) | 0.72 (0.29–1.75) | |
| Brain | 3.70 (1.74–7.90)* | 1.82 (0.20–16.32) | 3.77 (1.44–9.86)* | 0.78 (0.16–3.70) | |
| Treatment | |||||
| Guideline-based antibiotics | 0.77 (0.65–0.92)* | 0.63 (0.42–0.95)* | 1.02 (0.80–1.31) | 0.80 (0.59–1.09) | |
| Vancomycin | 1.39 (1.17–1.65)* | 1.70 (1.12–2.59)* | 1.69 (1.38–2.08)* | 0.98 (0.76–1.27) | |
| Granulocyte colony stimulating factor | 1.15 (0.97–1.35) | 1.64 (1.08–2.50)* | 1.09 (0.90–1.34) | 0.89 (0.69–1.16) | |
| Disease characteristics | |||||
| Sepsis | - | - | 0.94 (0.76–1.16) | 3.15 (2.30–4.30)* | |
| Pneumonia | - | - | 1.15 (0.92–1.43) | 1.46 (1.10–1.95)* | |
| Intensive care use | - | - | 1.59 (1.29–1.96)* | 2.76 (2.06–3.70)* | |
| Hypotension | - | - | 1.02 (0.50–2.08) | 3.11 (1.32–7.31)* | |
| Mechanical ventilation | - | - | 1.14 (0.82–1.57) | 27.38 (13.35–56.16)* | |
p<0.05
Discussion
We noted substantial variability in the allocation of guideline-based care for cancer patients with febrile neutropenia. The use of appropriate empiric antibiotic therapy is high and increasing, with over 80% of patients admitted with FN receiving guideline-concordant antibiotics in 2010. However, use of vancomycin and granulocyte colony stimulating factors also remains common despite guideline recommendations against routine use.
Prior studies of practice patterns for the treatment and prevention of neutropenia have suggested that recommendations by clinicians are often poorly aligned with guideline-based care.19–21 In a survey of over 1200 members of the American Society of Clinical Oncology (ASCO) addressing the management of low-risk patients with FN, Freifeld and colleagues noted that the majority of respondents recommended non-guideline concordant antibiotics and that 48% adjunctively used growth factors for low-risk patients.21 While guideline-based antibiotics were correctly given to nearly three quarters of the patients in our cohort, we identified widespread overuse of empiric vancomycin and GCSF.
The use of therapeutic granulocyte colony stimulating factors for high-risk patients with established FN remains an area of controversy. Trials and meta-analyses have suggested that therapeutic GCSF use is associated with small (one day), but statistically significant, reductions in length of stay and time to neutrophil recovery but has no effect on mortality.13–17 Despite the lack of convincing data, some consensus guidelines suggest that GCSFs can be “considered” in higher risk patients with profound or prolonged FN or FN associated with severe infectious complications (i.e., pneumonia, hypotension, sepsis).9,12 However, the most recent rendition of the Infectious Disease Society of America guidelines for FN continue to recommend against therapeutic GCSF for all patients with FN given the cost and adverse effects of the drugs.6 We noted that use of GCSF remains common, but perhaps more concerning was the pattern of use of GCSF in patients who received the drug. Over a third of subjects received only one or two days of filgrastim, a dose unlikely to lead to any meaningful clinical benefit.9 We previously noted similar findings in patients receiving erythropoiesis-stimulating agents; misuse was common with nearly a quarter of patients receiving only one week of therapy.34
While patient characteristics, such as age, race, and insurance status influenced patterns of care for FN, we noted that physician and hospital factors also impacted treatment choices. Overall, FN case volume had the strongest association with guideline-adherence. Patients treated at high-FN volume hospitals were more likely to receive guideline-based antibiotics and vancomycin and less likely to receive GCSF, while patients managed by high-FN volume physicians were more likely to receive appropriate antibiotics and less likely to receive vancomycin. While the association between volume and treatment and outcome has received the most attention for surgical procedures, there is growing recognition that volume impacts care for common medical conditions as well.35,36 Physician specialty was also associated with treatment choice; hospitalists were more likely to use guideline-based antibiotics, yet also more likely to treat with vancomycin. Prior work has suggested that care by hospitalists is associated with reduced cost for common medical conditions.37
Among lower risk FN patients, prompt administration of guideline-based antibiotics was associated with reduced in-hospital mortality. The demonstration that guideline-adherence improves outcomes is not only important for clinical care but also suggests that antibiotic choice can be used as a quality metric for FN. For many diseases it has been difficult to correlate adherence to a process measure with outcome. A large study examining the well-accepted practice of perioperative antibiotic use found that adherence to individual measures had no association with infection and adherence to an all-or-none composite measure had only a modest association with infection rates.38 We were unable to demonstrate an association between guideline-based antibiotic use and outcomes for higher risk patients, however 82.7% of high-risk patients received guideline-based antibiotics and 50.8% vancomycin. Although patients who received vancomycin and GCSF may have had more severe underlying disease, we noted that both interventions were associated with increased cost, but neither was associated with improved outcome in any of the subsets of patients we analyzed. However, it should be noted that without these interventions it is always possible that these patients may have done worse.
While our study benefits from the inclusion of a large cohort of patients with FN, we recognize a number of important limitations. Current ICD-9 coding lacks a specific code for febrile neutropenia. Prior studies have used a number of classification schema including selecting patients with neutropenia or fever or infection or various combinations. Although these selection criteria demonstrated good validity, a priori we chose a restrictive definition to include only cancer patients specifically admitted with a primary diagnosis of neutropenia. Although this may have limited our sample, we believe it allowed us to accurately capture those patients whose primary diagnosis was FN and to measure initial decision-making.1–3,26 Likewise, using administrative data it is difficult to use previously developed risk stratification schema for FN. We analyzed a series of factors that predicted poor outcome (death) that were reliably identifiable from administrative data and that have been used as components in other risk stratification schema, and used the presence of these factors as a surrogate for high-risk FN. Using administrative data, we were unable to capture several confounding factors, most notably the tumor characteristics and absolute neutrophil count (ANC), which impact both prognosis and treatment.5–7 As these factors were most likely to influence use of vancomycin and GCSF use, we performed sensitivity analyses and noted that even among the lowest risk patients (younger, little comorbidity, short lengths of stay) use of vancomycin and GCSF were substantial. As with any study of administrative data we can report associations but defining causality requires further randomized controlled trials. Finally, while we examined a large sample of hospitals from across the U.S. patterns of care may differ at other facilities.
The variability in practice patterns for FN suggests that initiatives to improve outcomes and reduce medical expenditures are urgently needed. For many inpatient conditions, formalized order writing protocols have led to improved outcomes.39,40 The American College of Chest Physicians guidelines for the prevention of venous thromboembolic disease now specifically call on hospitals to put formalized guidelines in place to guide physicians.41 Similarly, computerized alerts to guide best practice have been shown to increase use of evidence-based treatments in some settings.42 There has also been an increase in public reporting and pay-for-performance initiatives to improve quality.43 ASCO’s Quality Oncology Practice Initiative (QOPI) has gained increased interest and includes some measures of symptom and toxicity measurements.44 These initiatives provide further opportunities to promote more effective and less costly care for cancer patients with febrile neutropenia.
Acknowledgments
Dr. Hershman is the recipient of a grant from the National Cancer Institute (NCI R01CA134964).
Footnotes
There are no conflicts of interest or disclosures.
References
- 1.Caggiano V, Weiss RV, Rickert TS, Linde-Zwirble WT. Incidence, cost, and mortality of neutropenia hospitalization associated with chemotherapy. Cancer. 2005;103:1916–1924. doi: 10.1002/cncr.20983. [DOI] [PubMed] [Google Scholar]
- 2.Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106:2258–2266. doi: 10.1002/cncr.21847. [DOI] [PubMed] [Google Scholar]
- 3.Weycker D, Malin J, Edelsberg J, Glass A, Gokhale M, Oster G. Cost of neutropenic complications of chemotherapy. Ann Oncol. 2008;19:454–460. doi: 10.1093/annonc/mdm525. [DOI] [PubMed] [Google Scholar]
- 4.Elting LS, Lu C, Escalante CP, et al. Outcomes and cost of outpatient or inpatient management of 712 patients with febrile neutropenia. J Clin Oncol. 2008;26:606–611. doi: 10.1200/JCO.2007.13.8222. [DOI] [PubMed] [Google Scholar]
- 5.Lyman GH, Kuderer NM. Epidemiology of febrile neutropenia. Support Cancer Ther. 2003;1:23–35. doi: 10.3816/SCT.2003.n.002. [DOI] [PubMed] [Google Scholar]
- 6.Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2011;52:e56–e93. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 7.NCCN CPGiO. Prevention and treatment of cancer-related infections. 2009 2009. [Google Scholar]
- 8.Aapro MS, Bohlius J, Cameron DA, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011;47:8–32. doi: 10.1016/j.ejca.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 9.NCCN CPGiO. Myeloid growth factors. 2010;v.1 2010. [Google Scholar]
- 10.Hughes WT, Armstrong D, Bodey GP, et al. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis. 2002;34:730–751. doi: 10.1086/339215. [DOI] [PubMed] [Google Scholar]
- 11.Ozer H, Armitage JO, Bennett CL, et al. 2000 update of recommendations for the use of hematopoietic colony-stimulating factors: evidence-based, clinical practice guidelines. American Society of Clinical Oncology Growth Factors Expert Panel. J Clin Oncol. 2000;18:3558–3585. doi: 10.1200/JCO.2000.18.20.3558. [DOI] [PubMed] [Google Scholar]
- 12.Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187–3205. doi: 10.1200/JCO.2006.06.4451. [DOI] [PubMed] [Google Scholar]
- 13.Berghmans T, Paesmans M, Lafitte JJ, et al. Therapeutic use of granulocyte and granulocyte-macrophage colony-stimulating factors in febrile neutropenic cancer patients. A systematic review of the literature with meta-analysis. Support Care Cancer. 2002;10:181–188. doi: 10.1007/s00520-001-0312-5. [DOI] [PubMed] [Google Scholar]
- 14.Clark OA, Lyman GH, Castro AA, Clark LG, Djulbegovic B. Colony-stimulating factors for chemotherapy-induced febrile neutropenia: a meta-analysis of randomized controlled trials. J Clin Oncol. 2005;23:4198–4214. doi: 10.1200/JCO.2005.05.645. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Carbonero R, Mayordomo JI, Tornamira MV, et al. Granulocyte colony-stimulating factor in the treatment of high-risk febrile neutropenia: a multicenter randomized trial. J Natl Cancer Inst. 2001;93:31–38. doi: 10.1093/jnci/93.1.31. [DOI] [PubMed] [Google Scholar]
- 16.Maher DW, Lieschke GJ, Green M, et al. Filgrastim in patients with chemotherapy-induced febrile neutropenia. A double-blind, placebo-controlled trial. Ann Intern Med. 1994;121:492–501. doi: 10.7326/0003-4819-121-7-199410010-00004. [DOI] [PubMed] [Google Scholar]
- 17.Vellenga E, Uyl-de Groot CA, de Wit R, et al. Randomized placebo-controlled trial of granulocyte-macrophage colony-stimulating factor in patients with chemotherapy-related febrile neutropenia. J Clin Oncol. 1996;14:619–627. doi: 10.1200/JCO.1996.14.2.619. [DOI] [PubMed] [Google Scholar]
- 18.Paul M, Borok S, Fraser A, Vidal L, Leibovici L. Empirical antibiotics against Gram-positive infections for febrile neutropenia: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2005;55:436–444. doi: 10.1093/jac/dki028. [DOI] [PubMed] [Google Scholar]
- 19.Abernethy AP, Barbour SY, Uronis H, et al. Quality management of potential chemotherapy-induced neutropenic complications: evaluation of practice in an academic medical center. Support Care Cancer. 2009;17:735–744. doi: 10.1007/s00520-008-0562-6. [DOI] [PubMed] [Google Scholar]
- 20.Danova M, Rosti G, De Placido S, Bencardino K, Venturini M. Use of granulocyte colony-stimulating factor: a survey among Italian medical oncologists. Oncol Rep. 2005;14:1405–1412. [PubMed] [Google Scholar]
- 21.Freifeld A, Sankaranarayanan J, Ullrich F, Sun J. Clinical practice patterns of managing low-risk adult febrile neutropenia during cancer chemotherapy in the USA. Support Care Cancer. 2008;16:181–191. doi: 10.1007/s00520-007-0308-x. [DOI] [PubMed] [Google Scholar]
- 22.Lindenauer PK, Pekow PS, Lahti MC, Lee Y, Benjamin EM, Rothberg MB. Association of corticosteroid dose and route of administration with risk of treatment failure in acute exacerbation of chronic obstructive pulmonary disease. Jama. 2010;303:2359–2367. doi: 10.1001/jama.2010.796. [DOI] [PubMed] [Google Scholar]
- 23.Rothberg MB, Pekow PS, Lahti M, Brody O, Skiest DJ, Lindenauer PK. Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. Jama. 2010;303:2035–2042. doi: 10.1001/jama.2010.672. [DOI] [PubMed] [Google Scholar]
- 24.Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353:349–361. doi: 10.1056/NEJMoa041895. [DOI] [PubMed] [Google Scholar]
- 25.Logan AC, Yank V, Stafford RS. Off-label use of recombinant factor VIIa in U.S. hospitals: analysis of hospital records. Ann Intern Med. 2011;154:516–522. doi: 10.7326/0003-4819-154-8-201104190-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen-Hardee S, Chrischilles EA, Voelker MD, et al. Population-based assessment of hospitalizations for neutropenia from chemotherapy in older adults with non-Hodgkin's lymphoma (United States) Cancer Causes Control. 2006;17:647–654. doi: 10.1007/s10552-005-0502-4. [DOI] [PubMed] [Google Scholar]
- 27.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 28.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 29.Hollenbeck BK, Wei Y, Birkmeyer JD. Volume, process of care, and operative mortality for cystectomy for bladder cancer. Urology. 2007;69:871–875. doi: 10.1016/j.urology.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Birkmeyer JD, Sun Y, Wong SL, Stukel TA. Hospital volume and late survival after cancer surgery. Ann Surg. 2007;245:777–783. doi: 10.1097/01.sla.0000252402.33814.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aapro MS, Cameron DA, Pettengell R, et al. EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphomas and solid tumours. Eur J Cancer. 2006;42:2433–2453. doi: 10.1016/j.ejca.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 32.NCCN. Prevention and treatment of cancer related infections. 2009;v.2 2009. [Google Scholar]
- 33.NCCN. Myeloid growth factors. 2010;v.1 2010. [Google Scholar]
- 34.Wright JD, Neugut AI, Wilde ET, et al. Physician characteristics and variability of erythropoiesis-stimulating agent use among Medicare patients with cancer. J Clin Oncol. 2011;29:3408–3418. doi: 10.1200/JCO.2010.34.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ross JS, Normand SL, Wang Y, et al. Hospital volume and 30-day mortality for three common medical conditions. N Engl J Med. 2010;362:1110–1118. doi: 10.1056/NEJMsa0907130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–2127. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 37.Lindenauer PK, Rothberg MB, Pekow PS, Kenwood C, Benjamin EM, Auerbach AD. Outcomes of care by hospitalists, general internists, and family physicians. N Engl J Med. 2007;357:2589–2600. doi: 10.1056/NEJMsa067735. [DOI] [PubMed] [Google Scholar]
- 38.Stulberg JJ, Delaney CP, Neuhauser DV, Aron DC, Fu P, Koroukian SM. Adherence to surgical care improvement project measures and the association with postoperative infections. Jama. 2010;303:2479–2485. doi: 10.1001/jama.2010.841. [DOI] [PubMed] [Google Scholar]
- 39.Corbelli JC, Janicke DM, Cziraky MJ, Hoy TA, Corbelli JA. Acute coronary syndrome emergency treatment strategies: Improved treatment and reduced mortality in patients with acute coronary syndrome using guideline-based critical care pathways. Am Heart J. 2009;157:61–68. doi: 10.1016/j.ahj.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 40.Aziz EF, Pratap B, De Benedetti Zunino ME, et al. Success in implementing a hospital-wide evidence-based clinical pathways system for the management of cardiac patients: the ACAP program experience. Crit Pathw Cardiol. 2011;10:22–28. doi: 10.1097/HPC.0b013e3182053331. [DOI] [PubMed] [Google Scholar]
- 41.Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. (8th Edition) 2008;133:381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 42.Kucher N, Koo S, Quiroz R, et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med. 2005;352:969–977. doi: 10.1056/NEJMoa041533. [DOI] [PubMed] [Google Scholar]
- 43.Lindenauer PK, Remus D, Roman S, et al. Public reporting and pay for performance in hospital quality improvement. N Engl J Med. 2007;356:486–496. doi: 10.1056/NEJMsa064964. [DOI] [PubMed] [Google Scholar]
- 44.Campion FX, Larson LR, Kadlubek PJ, Earle CC, Neuss MN. Advancing performance measurement in oncology: quality oncology practice initiative participation and quality outcomes. J Oncol Pract. 2011;7:31s–35s. doi: 10.1200/JOP.2011.000313. [DOI] [PMC free article] [PubMed] [Google Scholar]