Abstract
Objective
Mitochondrial DNA deletions (Δ-mtDNA) are implicated in the pathogenesis of multiple sclerosis (MS), Parkinson’s disease (PD), Alzheimer’s disease (AD) and ageing. Given the diffuse nature of inflammation in MS, aim of this study was to determine whether Δ-mtDNA caused respiratory deficient cells in excess of age within choroid plexus (CP) and ongoing mutagenesis or clonal expansion accounted for the respiratory deficiency in MS.
Methods
Respiratory chain complex IV and complex II activity was determined sequentially using histochemistry. Δ-mtDNA were characterized using real time PCR, long range PCR, sequencing and single molecule PCR. Sources of reactive oxygen and nitrogen species (RONS) were explored using immunohistochemistry.
Results
Respiratory deficient cells (lacking complex IV and with intact complex II activity) within CP epithelium were in excess of age in MS, PD and AD. Subunit-I of complex IV was lacking to a greater extent in MS than controls. Percentage of respiratory deficient cells harboring >50% heteroplasmy level of Δ-mtDNA was significantly greater in MS than PD, AD and controls. Long range PCR and sequencing confirmed Δ-mtDNA. Single molecule PCR identified clonally expanded Δ-mtDNA in MS, despite an increase in sources of RONS.
Interpretation
Our findings establish clonal expansion of Δ-mtDNA causing respiratory deficiency in MS and the extraparenchymal intracranial location indicated the potential to involve multiple cell types. Understanding factors that influence clonal expansion of Δ-mtDNA, a molecular link between inflammation and delayed cellular energy failure, may identify potential therapeutic targets for progressive forms of MS as well as other neurodegenerative disorders.
Introduction
Mitochondrial defects have been implicated in the pathogenesis of multiple sclerosis (MS) as well as Parkinson’s disease (PD) and Alzheimer’s disease (AD) and ageing 1–10. Unlike PD and AD, there is extensive inflammation and demyelination in MS 11. The frequent involvement of the central nervous system (CNS) in primary mitochondrial disorders, due to mutations in mitochondrial DNA (mtDNA), highlights the importance of mtDNA for the nervous system 12.
MtDNA, located within the mitochondrial matrix in multiple copies, encodes functionally important subunits of the mitochondrial respiratory chain complexes [with the exception of succinate dehydrogenase (SDH) or complex II] 7. MtDNA is particularly susceptible to damage by reactive oxygen and nitrogen species (RONS) compared with nuclear DNA 13. Following double strand breaks, DNA repair mechanisms are thought to generate deletions of mtDNA (Δ-mtDNA) 14. For a biochemical defect to manifest from Δ-mtDNA, the proportion of Δ-mtDNA as a percentage of total mtDNA copies (heteroplasmy) in single cells needs to exceed a threshold (>50%) 7, 14. In chronically inflamed MS tissue, Δ-mtDNA led respiratory deficiency [lack of cytochrome c oxidase (COX or complex IV) and with intact SDH activity] may be due to ongoing mutagenesis of mtDNA by RONS leading to the accumulation of multiple clones of Δ-mtDNA in single cells. Alternatively, heteroplasmy level of Δ-mtDNA may increase in single cells due to expansion of one clone of mutant mtDNA (clonal expansion of mtDNA mutations), as reported in PD and with ageing 7, 15. In a recent study of mtDNA within cortical neurons in MS, we identified multiple Δ-mtDNA and proposed clonal expansion of Δ-mtDNA as a mechanism that caused respiratory deficiency in MS 16. However, single cell studies to confirm clonal expansion of Δ-mtDNA within neurons in MS were not possible due to technical limitations (due to the size of respiratory deficient neurons in MS). Demyelination, which is now recognized to influence mitochondrial function and dynamics, may influence Δ-mtDNA within neurons 17. Furthermore, whether Δ-mtDNA in MS clonally expand in cells other than neurons is not known.
Key requirements for clonal expansion of Δ-mtDNA include abundance of mitochondria, mtDNA replication, which occurs proportionate to metabolic activity and independent of cell cycle, and persistence of post-mitotic cells allowing sufficient time (years or decades) for clonal expansion to occur 7, 15, 18. In this regard, choroid plexus (CP) is an appealing structure to study Δ-mtDNA at a single cell level 19, 20. Respiratory deficient CP epithelial cells were reported in AD and aged controls 21, 22, although mtDNA was not investigated 23, 24. The intracranial and extraparenchymal location detaches CP from demyelination, whilst involved in the inflammatory response of MS 25. We hypothesized that Δ-mtDNA clonally expand at a single cell level within CP epithelium in MS and result in respiratory deficiency in excess of age. Indeed CP epithelium in MS harbored significantly more respiratory deficient cells than controls and AD. A significantly greater proportion of respiratory deficient cells harbored >50% heteroplasmy level of Δ-mtDNA in MS compared with PD, AD and controls. Using long range PCR and single molecule PCR, more than one clone of Δ-mtDNA was rarely detected within single respiratory deficient cells despite ongoing inflammation and evidence supporting elevated RONS in MS. These results establish clonal expansion of Δ-mtDNA, rather than accumulation of multiple Δ-mtDNA through ongoing mutagenesis in single cells, as a mechanism of respiratory deficiency in MS.
Methods
Autopsy Material
Snap frozen blocks of CP from MS (n=10), PD (n=5), AD (n=5) and control (n=9) cases were obtained from UK PD and MS Tissue Bank, NeuroResource (UK) and Newcastle Brain Tissue Resource (Supplementary Table 1). Tissue banks performed neuropathological characterisation. Newcastle and North Tyneside Local Research Ethics Committee approved the study (05/Q0906/182).
COX/SDH histochemistry
Cryostat sections (10µm thickness) were placed on glass and membrane slides. To assess complex IV (COX) and complex II (SDH) activity histochemical assay was applied sequentially as previously described 16. Nuclei were identified using Hoechst stain and Sections were subjected to Hoechst stain, 50% followed by 100% ethanol for 10min and histoclear prior to mounting.
Immunofluorescent histochemistry
To ascertain the status of subunit-I of complex IV (COX-I) in and inflammatory markers on respiratory deficient cells, immunofluorescent histochemistry was carried out on cryostat sections on glass slides following COX histochemistry, as previously described 26. Respiratory deficient cells were identified by intense complex II 70kDa or porin labelling. Sections on membrane slides were used for real time PCR of respiratory deficient cells with and without COX-I.
Immunohistochemistry
Inflammatory cells and sources of RONS were identified using the Menapath X-Cell Plus HRP Polymer Detection System 26. Cryostat sections were fixed in 4% paraformaldehyde for 30min and either immersed in EDTA (boiling) or 70%, 95% and 100% methanol with reversal. Primary antibodies were applied and detected with Menapath system and DAB (Supplementary Table 2). VIP chromogen was used when immunohistochemistry was preceded by COX histochemistry.
Laser micro-dissection and real-time PCR analysis
Randomly selected single CP epithelial cells (respiratory deficient and with intact complex IV activity) were laser captured from membrane slides following COX/SDH histochemistry and COX histochemistry/immunofluorescent histochemistry for COX-I. DNA was extracted and heteroplasmy levels were determined using real-time PCR (MTND1/MTND4), as previously described 1, 2, 5, 6, 16. All samples including no template and blood controls and deletion standards were run in triplicate.
Long range PCR
A two-step long-range PCR was carried out on the major arc of the mitochondrial genome using the Expand Long Template PCR System to detect Δ-mtDNA, as previously described 16. DNA was extracted from twenty pooled cells and then single cells (respiratory deficient and cells with intact complex IV activity). Control blood DNA was used as wildtype. DNA products were separated using a 0.7% agarose gel containing ethidium bromide and viewed under UV light.
Gel extraction and sequencing
To confirm Δ-mtDNA and assess breakpoints, DNA was extracted from agarose gels using Qiagen gel extraction kit. Cycle conditions for sequencing were as previously reported 16. Sequences, obtain using ABI 3100Genetic Analyser, were compared with revised Cambridge Reference Sequence.
Single molecule PCR
Clonally expanded Δ-mtDNA in single respiratory deficient CP epithelial cells were confirmed using single molecule PCR, as previously described 5. DNA samples were diluted in TE buffer and diluted DNA was added to a mastermix with components according to manufacturer’s guidelines (TaKaRa Mirrus Bio Madison WI). 31 cycles of first stage PCR consisted of 20sec at 95°C and 40sec at 68°C. A second stage reaction mix was added at this point followed by a further 15 cycles at the above conditions. Primers used in the first stage were 3160F32 and 3710R36 or 10161F31 and 10937R31 whilst 3204F34 and 3710R36 or 10195F30 and 10853R35 were used in second stage. PCR products were viewed on a 2% agarose gel containing ethidium bromide and viewed under UV light.
Terminal Deoxynucleotidyl Transferase Mediated dUDP Nick-End Labelling
Following COX histochemistry, Terminal Deoxynucleotidyl Transferase Mediated dUDP Nick-End Labelling (TUNEL) was performed, as per manufacturer’s guidelines (Roche).
Microscopy and quantitation of immunoreactivity and respiratory deficient cells
Respiratory deficient (blue) and cells with intact complex IV activity (brown) in CP epithelium were quantified using COX/SDH histochemistry images captured at x20 magnification and AxioVision 4.8. A mean of 1327±146 cells, with a nucleus stained by Hoechst, were included per case. To determine COX-I status within respiratory deficient cells in MS and controls, combined brightfield and fluorescent images of sequential COX histochemistry and immunofluorescent histochemistry were taken on a Zeiss Axioimager 2. COX-I status (FITC) in twenty respiratory deficient cells, based on intense labelling of complex II 70kDa (rhodamine), was determined as either positive or negative, per case. For sources of RONS, VIP positive cells in stroma were identified in images of sections with sequential COX histochemistry and immunohistochemistry, taking care to exclude cells within blood vessels. Using the same sections, respiratory deficient CP epithelial cells with and without evidence of RONS were analysed. Inflammatory cells with discrete labelling (CD68 and CD3) were imaged and quantitated as cell density. HLA and CD163 immunoreactivity was densitometrically analysed using Image J, keeping threshold constant between cases, as individual cells were difficult to discriminate. Cell densities and densitometric values were determined per area of CP.
Statistics
GraphPad prism 4 was used for all statistics. Nonparametric tests (Kruskal-Wallis test and Mann-Whitney U-test) were used to compare parameters between groups, as data was unevenly distributed. A p value of <0.05 was considered significant.
Results
Respiratory deficient cells were more abundant within choroid plexus epithelium in multiple sclerosis than Parkinson’s disease, Alzheimer’s disease and controls
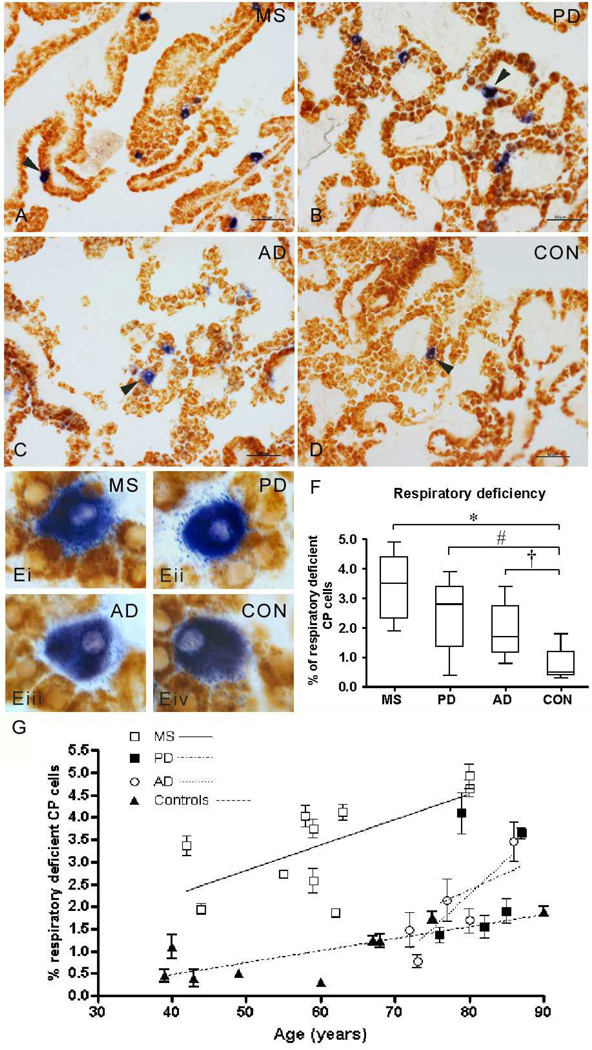
We detected respiratory deficient CP epithelial cells (blue, Fig 1a–d) in MS, PD, AD and controls. Respiratory deficient cells were larger than cells with intact complex IV activity (brown, Fig 1e and Supplementary Table 3), consistent with previously described ‘oncocytic’ nature 21, 22, 27. In MS, PD and AD, a significantly greater percentage of CP epithelial cells were respiratory deficient than controls (Fig 1f). In all four groups, the density of respiratory deficient CP epithelial cells increased with age (Fig 1g). Although the mean age of MS cases and controls were similar, PD and AD cases were significantly older than controls (Supplementary Table 1). Despite the younger age, MS cases contained significantly more respiratory deficient cells than AD. None of the respiratory deficient cells in the four groups was positive for markers of inflammation (HLA and CD68, Supplementary Fig 1) or apoptosis (TUNEL and AIF, not shown).
Figure 1. Respiratory deficient cells in the choroid plexus.
A–E: Sequential complex IV (cytochrome c oxidase or COX) and complex II (succinate dehydrogenase or SDH) histochemical assays identified cells with intact complex IV activity [respiratory efficient cells, stained brown] as well as lacking complex IV but with intact complex II [respiratory deficient, stained blue] within choroid plexus (CP) from multiple sclerosis (MS, a), Parkinson’s disease (PD, b), Alzheimer’s disease (AD, c) and control (CON, d) cases. Respiratory deficient cells (blue) were located in the CP epithelium and were larger than respiratory efficient cells (brown) in all four groups (ei-iv), which is consistent with reports of oncocytic cells in CP. Scale bar; 50µm in a–d and 10µm in e. Cases shown are MS6 (age 59), PD1 (age 76), AD1 (age 72) and CON5 (age 60).
F: The proportion of RD cells as a percentage of all CP epithelial cells was significantly greater in MS (3.39% ± 1.08, *p<0.001), PD (2.45% ± 1.20, #p=0.007) and AD (1.91% ± 0.99, †p=0.02) compared with controls (0.82% ± 0.52, Kruskal Wallis test p<0.001). MS choroid plexus contained significantly greater proportion of respiratory deficient epithelial cells than AD (p=0.027), despite significantly lower mean age of MS cases compared with AD (see Table 1). The difference in the density of respiratory deficient cells between MS and PD CP was not statistically significant. A mean of 1327±146 cells, with a nucleus stained by Hoechst, were included per case.
G: When the density of respiratory deficient cells in the choroid plexus was plotted against age, we observed a significant correlation between the two parameters in MS (p=0.037 and r2=0.439), AD (p=0.049 and r2=0.775) and controls (p=0.037 and r2=0.485). There was a trend towards an increase in the density of respiratory deficient cells with age, which was not statistically significant, in PD, where the age range was narrow (Table 1).
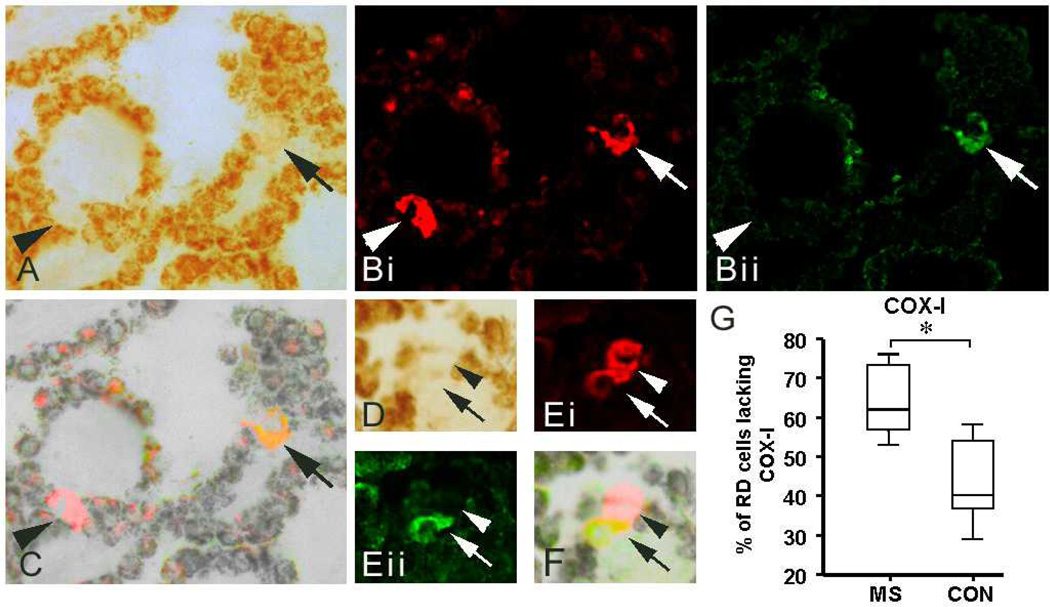
A greater proportion of respiratory deficient cells were devoid of subunit-I of cytochrome c oxidase in multiple sclerosis than Parkinson’s disease, Alzheimer’s disease and controls
Given that complex IV activity may be impaired by the loss of subunits due to high heteroplasmy levels of Δ-mtDNA as well as modifications of respiratory chain complex subunits and inhibitors of respiratory chain such as nitric oxide (NO), the status of mtDNA encoded subunit-I of complex IV (COX-I) was investigated in respiratory deficient CP epithelial cells (Fig 2a–f). Intense complex II 70kDa labeling, following COX histochemistry, identified the respiratory deficient epithelial cells (Fig 2bi). The percentage of respiratory deficient cells lacking COX-I was significantly greater in MS than in controls (Fig 2g). The above observations raised the possibility of Δ-mtDNA led respiratory deficiency in CP epithelial cells.
Figure 2. The status of subunit-I of cytochrome c oxides or complex IV (COX-I) within respiratory deficient cells in multiple sclerosis and controls.
A–F: Subunits of complex II (SDH) and complex IV (COX-I) in respiratory deficient CP epithelial cells were identified by immunofluorescent labeling complex II 70kDa (red) and COX-I (green) following COX histochemistry. As previously reported 26, respiratory chain complex subunits were barely detectable in cells with intact complex IV activity, due to deposits of COX reaction product (brown) interfering with antibody binding. Complex II 70kDa (red), and porin (not shown), was detected in all respiratory deficient cells (bi and ei, arrows and arrowheads), which supports histochemically detected complex II activity in respiratory deficient cells (Fig 1a–e). In contrast to complex II 70kDa, COX-I was detected in a subset of respiratory deficient cells in MS (bii and eii, arrows) and controls. The overlap images of brightfield and immunofluorescent figures identified the respiratory deficient cells and confirmed the presence or absence of COX-I (arrows and arrowheads, respectively) within the respiratory deficient CP epithelial cells (a–f).
G: The percentage of respiratory deficient CP cells lacking COX-I was significantly greater in MS (64.6%±4.7, p<0.001) than controls (43.3%±4.8). 20 respiratory deficient epithelial cells per case were randomly selected to determine the status of COX-I in MS cases (n=10) and controls (n=9).
COX: cytochrome c oxidase. SDH: succinate dehydrogenase.
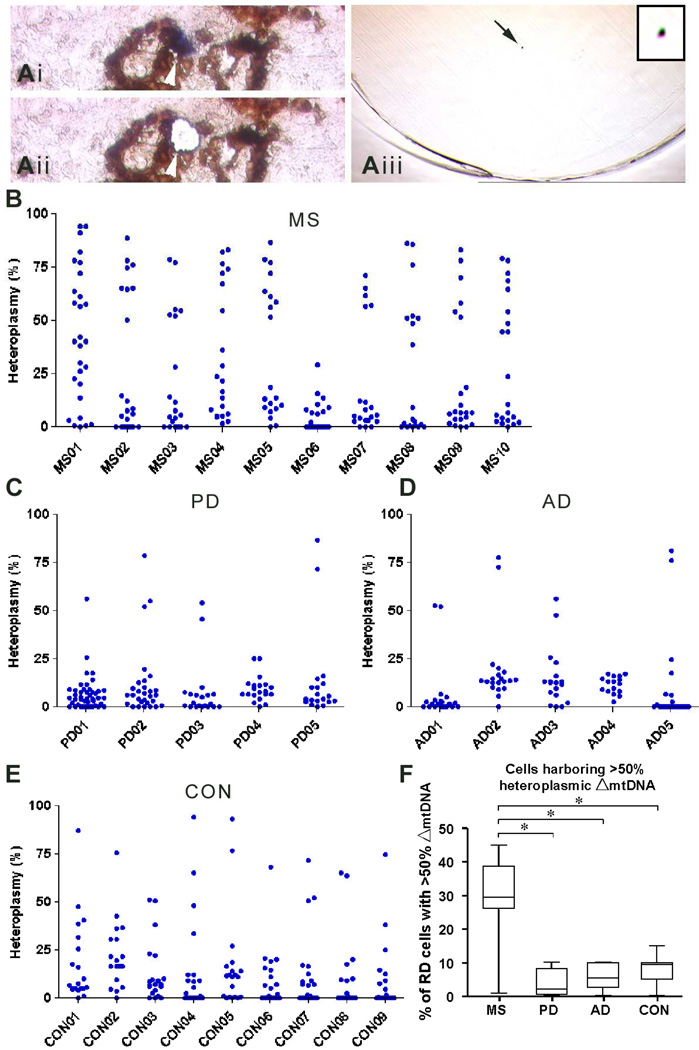
High heteroplasmy levels of mitochondrial DNA deletions were apparent in a greater percentage of respiratory deficient cells within choroid plexus epithelium in multiple sclerosis than Parkinson’s disease, Alzheimer’s disease and controls
To establish whether Δ-mtDNA caused respiratory deficient cells, we laser captured single CP epithelial cells (Fig 3a) from MS, PD, AD and controls and used an established real time PCR assay to determine the heteroplasmy level of Δ-mtDNA at a single cell level 1. In randomly selected respiratory deficient cells, the mean heteroplasmy level of Δ-mtDNA was significantly greater in MS cases (25.23%, p<0.001) than in PD (8.59%), AD (9.24%) and control (11.87%) cases (Fig 3b–e). The proportion of respiratory deficient cells, where the heteroplasmy level of Δ-mtDNA was sufficient to cause a biochemical defect (>50%), was significantly greater in MS (29.27%) than PD (3.78%), AD (6.21%) and controls (7.75%, Fig 3f). None of the CP epithelial cells with intact complex IV activity contained >50% heteroplasmy level of Δ-mtDNA (Supplementary Fig 2a–d). Furthermore, none of the respiratory deficient CP epithelial cells with COX-I in MS contained clonally expanded Δ-mtDNA above the 50% threshold level [mean heteroplasmy level of 17.64% ± 4.43 (n=14) compared with 42.91% ± 8.38 (n=10) in respiratory deficient cells without COX-I (p=0.008)].
Figure 3. Heteroplasmy level of Δ-mtDNA within laser captured respiratory deficient choroid plexus epithelial cells.
A: Complex IV (COX)/complex II (SDH) histochemistry was performed on sections placed on membrane slides for laser capture microdissection (a). Randomly selected respiratory deficient CP epithelial cells (ai, blue) were captured following laser microdissection (aii) into the cap of eppendorf tubes (aiii, arrow and insert indicate a single cell following laser capture). CP epithelial cells with intact complex IV activity (brown) were also laser captured (see Supplementary Fig 2).
B–E: DNA extracted from single respiratory deficient CP epithelial cells (blue) was used for real time PCR to determine the heteroplasmy level of Δ-mtDNA. Approximately 20 randomly selected respiratory deficient cells (range 18–23 cells per case, median cells=20) were analysed per case in all four groups. The mean heteroplasmy level of Δ-mtDNA within respiratory deficient cells in MS cases (25.23% ± 31.22, n=218) was significantly (p<0.001) greater than in PD (8.59% ± 15.86, n=131), AD (9.24% ± 22.76, n=94) and controls (11.87% ± 22.31, n=179). Kruskal-Wallis test p<0.001 and MS versus CON *p<0.001. Respiratory deficient cells harboring greater than 50% heteroplasmy level of Δ-mtDNA, sufficient to account for the biochemical defect, were detected in multiple sclerosis (MS, b), Parkinson’s disease (PD, c), Alzheimer’s disease (AD, d) and controls (CON, e).
F: The percentage of RD cells harboring Δ-mtDNA at heteroplasmy levels of >50% (when calculated per case) was significantly greater in MS (29.3%±4.2, p<0.001) compared with PD (3.8%±1.1), AD (6.2%±0.8) and controls (7.8%±0.5). Kruskal-Wallis test p<0.001.The relative lack of Δ-mtDNA led respiratory deficiency in PD and AD, was despite the cases been significantly older than MS cases (Table 1).
COX: cytochrome c oxidase. SDH: succinate dehydrogenase.
Clonally expanded mitochondrial DNA deletions within respiratory deficient choroid plexus epithelial cells in multiple sclerosis
As the next step, we performed a detailed characterization of Δ-mtDNA within respiratory deficient cells in MS to determine whether high heteroplasmy levels of Δ-mtDNA was due to ongoing mutagenesis and accumulation of multiple Δ-mtDNA within single cells or clonal expansion of Δ-mtDNA. When DNA extracted from twenty pooled respiratory deficient cells (per lane in Fig 4a) was used for long range PCR we detected multiple Δ-mtDNA, which were more notable in MS than PD, AD or controls. Because of the relatively large dimensions of CP epithelial cells (compared with neurons and glia, Supplementary Table 3), we were able to study Δ-mtDNA within single cells in detail (Fig 4b–d), including characterization at a single molecule level (Fig 4e). When DNA extracted from single respiratory deficient cells was used for long-range PCR (one cell per lane in Fig 4b), different sized Δ-mtDNA were detected in different cells and more than one clone of Δ-mtDNA was rarely detected within a single cell. Sequencing of Δ-mtDNA extracted from gels following long-range PCR confirmed the presence of Δ-mtDNA and identified the break points (Fig 4c–d and Table 1). As previously reported in PD and primary mitochondrial disorders, some of the break points of Δ-mtDNA in MS tissue was flanked by either perfect or imperfect repeat sequences 14, which have been implicated in models of Δ-mtDNA formation (Table 1). As a single clone of Δ-mtDNA in respiratory deficient cells judged by long range PCR was suggestive of clonal expansion, we extracted DNA from single respiratory deficient CP epithelial cells, diluted to a single copy of Δ-mtDNA per reaction and performed single molecule PCR on multiple occasions (at least three times per cell). In separate reactions of single mtDNA molecules from single respiratory deficient cells, only one clone of Δ-mtDNA (a hallmark of clonal expansion of Δ-mtDNA) or full-length product was detected (Fig 4e).
Figure 4. Respiratory deficient CP epithelial cells in multiple sclerosis harbored clonally expanded Δ-mtDNA.
A: Multiple Δ-mtDNA were detected when DNA was extracted from multiple respiratory deficient cells (a, 20 respiratory deficient cells pooled per lane) from MS cases (lanes 1–4). In contrast, Δ-mtDNA were infrequent in pooled respiratory deficient cells (n=20 per lane) from PD (lanes 5–6), AD (lanes 7–8) and controls (lanes 9–10). Negative control (meninges from control case) showed full-length amplified product (+).
B: When long-range PCR was performed using single cells (DNA extracted from one respiratory deficient cell per lane) more than one Δ-mtDNA was rarely detected in controls (lanes 1–5) or MS cases (lanes 6–10).
C–D: Sequencing of Δ-mtDNA extracted from gel following long-range PCR of single respiratory deficient cells confirmed the Δ-mtDNA by identifying the breakpoints (c–d). In a proportion of Δ-mtDNA, the sequence that flanked the breakpoints showed perfect or imperfect repeats (see Table 2). The repeat sequence of an imperfect repeat is underlined on the 3’ end from the breakpoint, with the imperfect base shown above the sequence in bold (c). Δ-mtDNA without repeat sequences were also detected within respiratory deficient cells in MS (d).
E: Clonality of Δ-mtDNA in respiratory deficient CP cells was confirmed using single molecule PCR. Long range PCR was performed multiple times from diluted DNA of six individual cells. Three representative positive reactions are presented for each of six respiratory deficient cells from MS. Note that each cell contained only one species of mtDNA molecule: either full length wild type (cells 1 and 6), or mtDNA deletions (cells 2–5). Single species of Δ-mtDNA by single molecule PCR in cells 2–5 indicated a hallmark of clonal expansion of Δ-mtDNA.
Table 1. Breakpoints of mtDNA deletions.
Break point sequences of Δ-mtDNA extracted from long range PCR on single respiratory deficient choroid plexus epithelium cells. Break points of Δ-mtDNA in MS were often flanked by repeat sequences (sequence in brackets, imperfect bases underlined), as reported previously in neurodegenerative diseases and ageing. Base pairs are shown pre- (5’) and post-breakpoint (3’).
| Break points | Repeat sequence | 5’ | 3’ |
|---|---|---|---|
| 6426-15,537 | No repeat sequence | AAAACCCCCTG | CCCCTCCCCACA |
| 6703-15,662 | No repeat sequence | AAAAAAAGAACC | ATCCTCCATATA |
| 7227-15,815 | perfect repeat | GACGTTACTCGG | (ACTA)TACTTCAC |
| 6420-16,033 | no repeat sequence | TC AATATAAAAC | GGGGAAGCAGAT |
| 6459-15,592 | imperfect repeat | CCCTCTTCGTCT | (GATCCGTCCCTAA) |
| 6474-15,733 | imperfect repeat | CCGTCCTAATCA | (CAGCAGTCCT)CA |
| 6455-14,865 | imperfect repeat | ACGCCCCTCTTC | (GTCTGATCC)TCC |
| 6826-13,071 | no repeat sequence | TTTCACCTCCGC | CCTACTCCACTC |
| 6646-15,911 | imperfect repeat | TATTCTTATCCT | (ACCAGGCTT)GTA |
Reactive oxygen and nitrogen species within the choroid plexus in multiple sclerosis
Although inflammation in MS involves the CP, sources of RONS within CP have not been reported 25. We detected and quantitated sources of superoxide [nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and myeloperoxidase (MPO)] and NO within CP using antibodies against NADPH oxidase subunits (NOX1 and p22phox), MPO and inducible NO synthase (iNOS, Fig 5 and Table 2). NOX1 was expressed in stromal cells and diffusely within the epithelium in MS compared with discrete NOX1 positive epithelial cells in PD, AD and controls (Fig 5ai–dii). In contrast, p22phox, MPO and iNOS were rarely detected in the epithelium; percentage of respiratory deficient cells without p22phox, MPO and iNOS were 3.09% ± 0.70, 3.49% ± 1.21 and 3.23% ± 0.94, respectively, similar to the density of respiratory deficient cells detected by COX/SDH histochemistry (Fig1). However, there were significantly more NOX1, MPO and iNOS positive cells within CP stroma in MS than PD, AD and controls (Table 2). The supposed increase in RONS in MS was supported by a significant increase in CD68 positive cells and HLA immunoreactivity (Supplementary Fig 3 and Table 2).
Figure 5. Sources of reactive oxygen and nitrogen species within choroid plexus.
To ascertain the extent of reactive oxygen and nitrogen species (RONS) within CP in MS, PD, AD and controls cryostat sections were subjected to either immunohistochemistry (with VIP as chromogen) or sequential complex IV (COX) histochemistry/immunohistochemistry (with VIP).
A–D: In MS, NOX1 was diffusely expressed within the epithelium (ai) as well as in stromal cells (aii). In contrast, NOX1 was restricted to discrete epithelial cells in PD, AD and controls (bi–ii, ci–ii, di–ii). Several respiratory deficient cells with faint NOX1 expression were apparent in MS (aiii) whereas the majority of respiratory deficient cells in PD (biii), AD (ciii) and controls (diii) were not positive for NOX1 (see Table 2 for quantitative data). P22phox, MPO and iNOS were not detected within epithelium in any of the four groups (e–l). MPO positive cells were present within stroma and occasionally in blood vessels (i–l). Interestingly, respiratory deficient cells in any of the four groups were not positive for p22phox, MPO or iNOS, also reflected by quantitative data; with percentage of respiratory deficient cells negative for RONS sources being similar to respiratory deficient cell density by COX/SDH histochemistry.
Table 2. Quantitation of sources of reactive oxygen and nitrogen species and inflammation in choroid plexus.
The means (±standard deviation) indicate either the density of immunoreactive cells per mm2 of choroid plexus tissue (NOX1, p22phox, MPO, iNOS, CD68 and CD3) or densitometric values of immunoreactivity (HLA and CD163), when threshold was kept constant between cases. Parentheses indicate the number of cases studied and p values in left hand column were derived from Kruskal-Wallis test. P values in remaining columns were when compared with controls using Mann-Whitney U-test.
| MS | PD | AD | CON | |
|---|---|---|---|---|
| NOX1 (p<0.001) |
257.59 ± 114.77 (n=5) (p=0.001) |
94.35 ± 36.73 (n=3) |
164.30 ± 43.84 (n=2) (p=0.057) |
95.54 ± 44.81 (n=5) |
| p22phox (p=0.069) |
34.45 ± 16.53 (n=5) |
26.35 ± 14.72 (n=3) |
21.33 ± 8.17 (n=3) |
20.53 ± 7.78 (n=5) |
| MPO (p=0.004) |
40.64 ± 37.12 (n=5) (p=0.004) |
10.55 ± 7.09 (n=3) |
6.87 ± 6.87 (n=2) |
9.16 ± 7.19 (n=5) |
| iNOS (p<0.001) |
22.23 ± 6.44 (n=5) (p<0.001) |
13.83 ± 2.58 (n=3) |
12.43 ± 3.76 (n=3) |
11.29 ± 5.29 (n=5) |
| CD68 (p=0.004) |
64.81 ± 48.31 (n=5) (p=0.036) |
32.60 ± 6.99 (n=3) |
17.02 ± 14.76 (n=3) (p=0.017) |
34.09 ± 12.57 (n=5) |
| HLA (p<0.001) |
17.51 ± 5.96 (n=5) (p=0.009) |
13.28 ± 3.19 (n=3) |
5.64 ± 3.81 (n=3) |
10.49 ± 2.08 (n=6) |
| CD163 (p=0.245) |
7.22 ± 3.55 (n=5) |
7.23 ± 2.01 (n=3) |
5.60 ± 2.64 (n=3) |
5.36 ± 1.87 (n=5) |
| CD3 (p=0.129) |
51.65 ±19.87 (n=6) |
21.92 ±5.18 (n=3) |
24.46 ± 17.53 (n=3) |
45.58 ± 22.24 (n=5) |
Discussion
Here we establish clonal expansion of Δ-mtDNA as a mechanism of respiratory deficiency in MS, rather than accumulation of multiple Δ-mtDNA within single cells through ongoing mutagenesis. Although we detected multiple Δ-mtDNA when DNA was extracted from multiple CP epithelial cells, single cell analysis using long range PCR and single molecule PCR (a relatively error resistant method 15) showed hallmarks of expansion of one clone of Δ-mtDNA within single respiratory deficient cells (clonal expansion of Δ-mtDNA) in MS. Given that there are many hotpots in the mitochondrial genome for double strand breaks and breakpoints of Δ-mtDNA, often flanked by repeat sequences, the odds of RONS either intrinsic or extrinsic to mitochondria giving rise to the same clone of Δ-mtDNA through ongoing mutagenesis would be remote 14. In MS, RONS from non-mitochondrial sources are likely to have initiated Δ-mtDNA formation within respiratory deficient cells when complex IV activity was intact.
While clonal expansion of mtDNA mutations is well-characterized in a number of disorders 1, 5, 6, factors that drive the rate of clonal expansion are less well known, particularly in inflammatory demyelinating environments. Replication advantage of mtDNA molecules with large deletions has been shown to selectively expand small mtDNA molecules within neurons in transgenic animal models 18. Our observations raise the possibility of a dual role for RONS produced by inflammatory cells in the initiation and subsequent clonal expansion of Δ-mtDNA. Factors that induce mitochondrial biogenesis, such as NO, may influence the rate of clonal expansion through increased rate of mtDNA replication, irrespective of how Δ-mtDNA were formed 28. NO may also clonally expand Δ-mtDNA formed through replication stalling 14. In neurons, demyelination may also influence clonal expansion as axonal mitochondrial content, activity, morphology and dynamics change following demyelination 17, 29. Although mitochondrial respiratory chain defects have been described in animal models of MS, including within the CP, mtDNA has not yet been studied 30, 31. Understanding the extrinsic factors that influence clonal expansion of Δ-mtDNA in inflammatory environments may identify potential therapeutic targets for certain neurodegenerative disorders.
The status of COX-I in respiratory deficient cells highlights the multiple modes of mitochondrial injury, particularly in inflammatory environments. The presence of COX-I in a subset of respiratory deficient cells is consistent with either inhibition, modification of subunits by RONS or modified subunits due to point mutations of mtDNA 32, 33. Furthermore, transcripts of respiratory chain complex subunits may be degraded by ROS and depletion of mtDNA may impact functionally important subunits including COX-I 34. Hence, the respiratory deficiency unexplained by Δ-mtDNA in PD and AD may be due to point mutations and depletion of mtDNA. The lack of clonally expanded Δ-mtDNA in PD and ageing is consistent with the view that dopaminergic cells are differentially susceptible to acquiring Δ-mtDNA 2.
With respect to the function of CP, it is unclear how a low percentage of respiratory deficient cells within CP would directly contribute to disease pathogenesis. Besides producing cerebrospinal fluid (CSF), CP has several other important functions including protection of CNS from circulating factors (blood-CSF barrier) and active transport of methyltetrahydrofolate (active form of folate) 35, 36. Respiratory deficient CP epithelial cells were implicated in the cerebral folate deficiency and elevated CSF protein that are characteristics of Kearns Sayre Syndrome, where 30–40% of CP epithelial cells were defective, 27. In PD, AD and progressive stage of MS, there is no evidence to support a breakdown in blood-CSF barrier. Furthermore, studies that investigated folate in MS did not identify a deficiency in CSF 37. A toxic gain of function in respiratory deficient CP epithelial cells, yet to be recognized, may have consequences for the pathogenesis of neurodegenerative disorders.
Within the CNS, clonal expansion of Δ-mtDNA was previously confirmed by single molecule PCR in relatively non-inflammatory microenvironments such as substantia nigra in PD and with ageing 5. Mechanisms that are independent of inflammation have been contentiously proposed to play a role in the pathogenesis of MS, particularly during the progressive stage 38. In this regard, clonal expansion of Δ-mtDNA in post-mitotic cells becomes an attractive potential molecular link between inflammatory events in MS and delayed cellular energy failure, dysfunction and degeneration. Cellular dysfunction in the CNS is increasingly been considered as a possible contributor to the neurological impairment during the progressive stage of MS 39. The presence of clonally expanded Δ-mtDNA in an extraparenchymal intracranial structure and the diffuse nature of inflammation in MS raise the possibility of multiple CNS cell types such as endothelial cells and glia being metabolically compromised by clonally expanded Δ-mtDNA in MS. Magnetic resonance spectroscopic findings indicate a diffuse metabolic disturbance in MS and we have observed abundant respiratory deficient cells within the white matter in a number of MS cases (unpublished data) 40. Demonstrating Δ-mtDNA in white matter cells, however, remains a technical challenge given the relatively small size. In contrast to cellular dysfunction, degeneration of respiratory deficient cells appears to require ongoing inflammation or an additional stress factor(s), as axonal injury was not observed in chronic burnt out MS lesions without inflammation and morphological evidence of apoptosis or neuronal injury was not detected in previous studies 16, 41. A requirement for an additional factor is further supported by findings in primary mitochondrial disorders 23.
In summary, we convincingly demonstrate that clonal expansion of Δ-mtDNA occurs within the CNS in MS despite ongoing inflammation and RONS. Understanding the dynamics of clonal expansion of Δ-mtDNA in chronic inflammatory demyelinating environments may reveal molecular mechanisms that link acute and chronic inflammation with cellular dysfunction and tissue damage during progressive stage of MS as well as identify potential therapeutic targets to prevent progression of MS.
Supplementary Material
Acknowledgements
We would like to thank the Wellcome Trust and Newcastle upon Tyne NHS Hospitals Charity for funding this study. We thank NeuroResource (UK), UK MS and PD Tissue Bank and Newcastle Brain Tissue Resource for providing snap frozen choroid plexus. (Doug, Hans and Konstantin?)
Reference
- 1.Bender A, Krishnan KJ, Morris CM, et al. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet. 2006;38:515–517. doi: 10.1038/ng1769. [DOI] [PubMed] [Google Scholar]
- 2.Bender A, Schwarzkopf RM, McMillan A, et al. Dopaminergic midbrain neurons are the prime target for mitochondrial DNA deletions. J Neurol. 2008;255:1231–1235. doi: 10.1007/s00415-008-0892-9. [DOI] [PubMed] [Google Scholar]
- 3.Broadwater L, Pandit A, Clements R, et al. Analysis of the mitochondrial proteome in multiple sclerosis cortex. Biochim Biophys Acta. 2011;1812:630–641. doi: 10.1016/j.bbadis.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dutta R, McDonough J, Yin X, et al. Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann Neurol. 2006;59:478–489. doi: 10.1002/ana.20736. [DOI] [PubMed] [Google Scholar]
- 5.Kraytsberg Y, Kudryavtseva E, McKee AC, et al. Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat Genet. 2006;38:518–520. doi: 10.1038/ng1778. [DOI] [PubMed] [Google Scholar]
- 6.Krishnan KJ, Ratnaike TE, Gruyter HL, et al. Mitochondrial DNA deletions cause the biochemical defect observed in Alzheimer's disease. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Larsson NG. Somatic mitochondrial DNA mutations in mammalian aging. Annu Rev Biochem. 2010;79:683–706. doi: 10.1146/annurev-biochem-060408-093701. [DOI] [PubMed] [Google Scholar]
- 8.Onyango I, Khan S, Miller B, et al. Mitochondrial genomic contribution to mitochondrial dysfunction in Alzheimer's disease. J Alzheimers Dis. 2006;9:183–193. doi: 10.3233/jad-2006-9210. [DOI] [PubMed] [Google Scholar]
- 9.Schapira AH, Jenner P. Etiology and pathogenesis of Parkinson's disease. Mov Disord. 2011;26:1049–1055. doi: 10.1002/mds.23732. [DOI] [PubMed] [Google Scholar]
- 10.Witte ME, Geurts JJ, de Vries HE, et al. Mitochondrial dysfunction: a potential link between neuroinflammation and neurodegeneration? Mitochondrion. 2010;10:411–418. doi: 10.1016/j.mito.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 11.Hauser SL, Oksenberg JR. The neurobiology of multiple sclerosis: genes, inflammation, and neurodegeneration. Neuron. 2006;52:61–76. doi: 10.1016/j.neuron.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 12.McFarland R, Taylor RW, Turnbull DM. A neurological perspective on mitochondrial disease. Lancet Neurol. 2010;9:829–840. doi: 10.1016/S1474-4422(10)70116-2. [DOI] [PubMed] [Google Scholar]
- 13.Burney S, Caulfield JL, Niles JC, et al. The chemistry of DNA damage from nitric oxide and peroxynitrite. Mutat Res. 1999;424:37–49. doi: 10.1016/s0027-5107(99)00006-8. [DOI] [PubMed] [Google Scholar]
- 14.Krishnan KJ, Reeve AK, Samuels DC, et al. What causes mitochondrial DNA deletions in human cells? Nat Genet. 2008;40:275–279. doi: 10.1038/ng.f.94. [DOI] [PubMed] [Google Scholar]
- 15.Nicholas A, Kraytsberg Y, Guo X, Khrapko K. On the timing and the extent of clonal expansion of mtDNA deletions: evidence from single-molecule PCR. Exp Neurol. 2009;218:316–319. doi: 10.1016/j.expneurol.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell GR, Ziabreva I, Reeve AK, et al. Mitochondrial DNA deletionsand neurodegeneration in multiple sclerosis. Ann Neurol. 2011;69:481–492. doi: 10.1002/ana.22109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiryu-Seo S, Ohno N, Kidd GJ, et al. Demyelination increases axonal stationary mitochondrial size and the speed of axonal mitochondrial transport. J Neurosci. 2010;30:6658–6666. doi: 10.1523/JNEUROSCI.5265-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukui H, Moraes CT. Mechanisms of formation and accumulation of mitochondrial DNA deletions in aging neurons. Hum Mol Genet. 2009;18:1028–1036. doi: 10.1093/hmg/ddn437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cornford EM, Varesi JB, Hyman S, et al. Mitochondrial content of choroid plexus epithelium. Exp Brain Res. 1997;116:399–405. doi: 10.1007/pl00005768. [DOI] [PubMed] [Google Scholar]
- 20.Rickert CH, Paulus W. Tumors of the choroid plexus. Microsc Res Tech. 2001;52:104–111. doi: 10.1002/1097-0029(20010101)52:1<104::AID-JEMT12>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 21.Cottrell DA, Blakely EL, Johnson MA, et al. Cytochrome c oxidase deficient cells accumulate in the hippocampus and choroid plexus with age. Neurobiol Aging. 2001;22:265–272. doi: 10.1016/s0197-4580(00)00234-7. [DOI] [PubMed] [Google Scholar]
- 22.Cottrell DA, Blakely EL, Johnson MA, et al. Mitochondrial enzyme-deficient hippocampal neurons and choroidal cells in AD. Neurology. 2001;57:260–264. doi: 10.1212/wnl.57.2.260. [DOI] [PubMed] [Google Scholar]
- 23.Betts J, Lightowlers RN, Turnbull DM. Neuropathological aspects of mitochondrial DNA disease. Neurochem Res. 2004;29:505–511. doi: 10.1023/b:nere.0000014821.07269.8d. [DOI] [PubMed] [Google Scholar]
- 24.Cottrell DA, Ince PG, Wardell TM, et al. Accelerated ageing changes in the choroid plexus of a case with multiple mitochondrial DNA deletions. Neuropathol Appl Neurobiol. 2001;27:206–214. doi: 10.1046/j.1365-2990.2001.00315.x. [DOI] [PubMed] [Google Scholar]
- 25.Vercellino M, Votta B, Condello C, et al. Involvement of the choroid plexus in multiple sclerosis autoimmune inflammation: a neuropathological study. J Neuroimmunol. 2008;199:133–141. doi: 10.1016/j.jneuroim.2008.04.035. [DOI] [PubMed] [Google Scholar]
- 26.Mahad DJ, Ziabreva I, Campbell G, et al. Detection of cytochrome c oxidase activity and mitochondrial proteins in single cells. J Neurosci Methods. 2009;184:310–319. doi: 10.1016/j.jneumeth.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 27.Tanji K, Schon EA, DiMauro S, Bonilla E. Kearns-sayre syndrome: oncocytic transformation of choroid plexus epithelium. J Neurol Sci. 2000;178:29–36. doi: 10.1016/s0022-510x(00)00354-3. [DOI] [PubMed] [Google Scholar]
- 28.Nisoli E, Clementi E, Paolucci C, et al. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299:896–899. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- 29.Zambonin JL, Zhao C, Ohno N, et al. Increased mitochondrial content in remyelinated axons: implications for multiple sclerosis. Brain. 2011;134:1901–1913. doi: 10.1093/brain/awr110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Engelhardt B, Wolburg-Buchholz K, Wolburg H. Involvement of the choroid plexus in central nervous system inflammation. Microsc Res Tech. 2001;52:112–129. doi: 10.1002/1097-0029(20010101)52:1<112::AID-JEMT13>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 31.Qi X, Lewin AS, Sun L, et al. Mitochondrial protein nitration primes neurodegeneration in experimental autoimmune encephalomyelitis. J Biol Chem. 2006;281:31950–31962. doi: 10.1074/jbc.M603717200. [DOI] [PubMed] [Google Scholar]
- 32.Haider L, Fischer MT, Frischer JM, et al. Oxidative damage in multiple sclerosis lesions. Brain. 2011;134:1914–1924. doi: 10.1093/brain/awr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pye D, Kyriakouli DS, Taylor GA, et al. Production of transmitochondrial cybrids containing naturally occurring pathogenic mtDNA variants. Nucleic Acids Res. 2006;34:e95. doi: 10.1093/nar/gkl516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei J, Guo H, Kuo PC. Endotoxin-stimulated nitric oxide production inhibits expression of cytochrome c oxidase in ANA-1murine macrophages. J Immunol. 2002;168:4721–4727. doi: 10.4049/jimmunol.168.9.4721. [DOI] [PubMed] [Google Scholar]
- 35.Engelhardt B, Sorokin L. The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol. 2009;31:497–511. doi: 10.1007/s00281-009-0177-0. [DOI] [PubMed] [Google Scholar]
- 36.Spector R, Johanson CE. Choroid plexus failure in the Kearns-Sayre syndrome. Cerebrospinal Fluid Res. 2010;7:14. doi: 10.1186/1743-8454-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nijst TQ, Wevers RA, Schoonderwaldt HC, et al. Vitamin B12 and folate concentrations in serum and cerebrospinal fluid of neurological patients with special reference to multiple sclerosis anddementia. J Neurol Neurosurg Psychiatry. 1990;53:951–954. doi: 10.1136/jnnp.53.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stadelmann C. Multiple sclerosis as a neurodegenerative disease: pathology, mechanisms and therapeutic implications. Curr Opin Neurol. 2011;24:224–229. doi: 10.1097/WCO.0b013e328346056f. [DOI] [PubMed] [Google Scholar]
- 39.Dutta R, Trapp BD. Mechanisms of neuronal dysfunction and degeneration in multiple sclerosis. Prog Neurobiol. 2011;93:1–12. doi: 10.1016/j.pneurobio.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aboul-Enein F, Krssak M, Hoftberger R, et al. Reduced NAA-levels in the NAWM of patients with MS is a feature of progression. A study with quantitative magnetic resonance spectroscopy at 3 Tesla. PLoS One. 2010;5:e11625. doi: 10.1371/journal.pone.0011625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frischer JM, Bramow S, Dal-Bianco A, et al. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain. 2009;132:1175–1189. doi: 10.1093/brain/awp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.