EMBO Reports (2013) 14 6, 534–544 doi:; DOI: 10.1038/embor.2013.51
The term ‘autophagy’ refers to a group of catabolic pathways that deliver cytoplasmic constituents for lysosomal degradation [1]. One of these, macroautophagy, has been shown both to degrade some pathogens and to promote the replication of others [2]. In the case of Chikungunya virus (CHIKV) infection, it had been reported to promote replication in humans and restrict it in mice [3,4]. This discrepancy and its molecular underpinnings have been resolved in a study from the Lecuit group, published in this issue of EMBO reports (Fig 1; [5]), which also provides a strategy to generate a better mouse model to study Chikungunya fever.
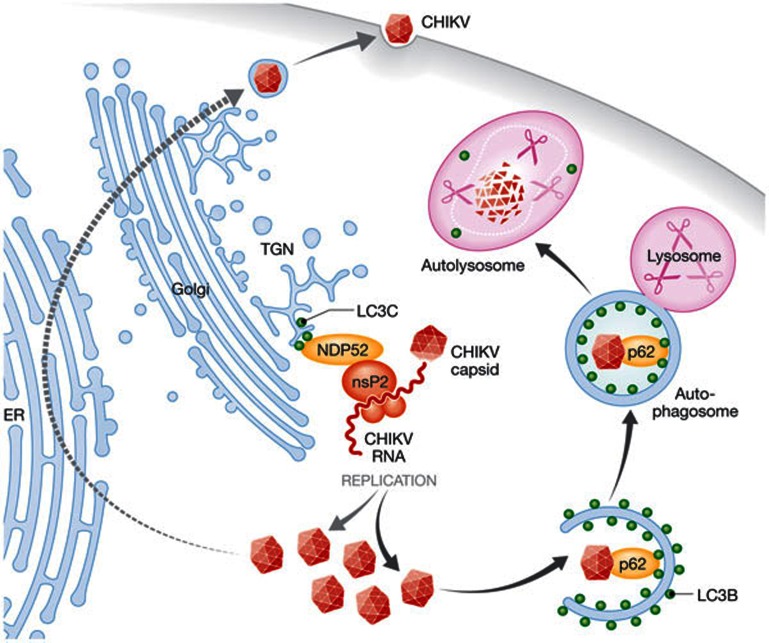
Figure 1.
The molecular machinery of macroautophagy supports and restricts CHIKV replication in human cells. NDP52 recruits nsP2 to the trans-Golgi network by binding to membrane-anchored LC3C. This seems to facilitate the assembly of the CHIKV replication complex at these sites and supports viral replication. Some of the CHIKV capsids produced as a result are ubiquitinated and bind to LC3B on autophagosomal membranes through p62/sequestosome 1. Autophagosomes deliver these capsids for lysosomal degradation, which leads to restriction of viral replication. CHIKV, Chikungunya virus; ER, endoplasmic reticulum; LC3C, light chain protein 3C; NDP52, nuclear dot protein 52; nsP2, non-structural protein 2; TGN, trans Golgi network.
Macroautophagy—the best studied of the autophagic pathways—can engulf cell organelles and protein aggregates into double-membrane-surrounded vesicles, so-called ‘autophagosomes’, which fuse with lysosomes. Substrate selection into these autophagosomes is achieved by binding to the essential autophagy-related protein 8 (ATG8) or its mammalian homologues LC3A–C (light chain protein 3A–C) and GABARAP 1–2 (GABA-receptor associated protein 1–2), which are attached in an ubiquitin-like reaction to the autophagosomal membrane. LC3 binds to LC3-interacting motifs present either directly in cargo proteins, in anchor proteins that also contain an ubiquitin-binding domain to recruit ubiquitinated proteins or through detection of cytosol-exposed glycosylation from damaged vesicles [1,6]. All of the four known macroautophagy anchor proteins—p62/sequestosome 1, NBR1 (neighbour of BRCA1 gene 1), NDP52 (nuclear dot protein 52) and optineurin—can also recruit pathogens to autophagosomes during innate or cell intrinsic immunity [6].
macroautophagy anchor proteins […] recruit pathogens to autophagosomes during innate or cell intrinsic immunity
This innate restriction allows lysosomal targeting of pathogens that have escaped from endosomes into the cytosol or that are contained in damaged vesicles [6]. Among these, neurotropic infections with Sindbis and herpes simplex virus (HSV) have been investigated [7,8]. HSV encodes ICP34.5—a protein that inhibits macroautophagy by binding to its molecular machinery. Mutant HSV lacking this protein is impaired in its ability to cause lethal encephalitis, presumably because it is more efficiently degraded by macroautophagy. Similarly, Sindbis virus capsids are recruited to autophagosomes through p62/sequestosome 1, and Sindbis virus replication is enhanced in macroautophagy-deficient cells, which leads to lethal central nervous system infection. The ubiquitination of Sindbis virus and HSV capsids for recruitment into autophagosomes might be mediated by the SMURF1 (SMAD ubiquitination regulatory factor 1) ubiquitin ligase [9]. In contrast to these anti-viral functions of macroautophagy, its core machinery also seems to support the replication of some viruses and is even stimulated by them. This has been shown for poliovirus, hepatitis C virus (HCV) and human immunodeficiency virus (HIV; [6]). Some viruses seem to manipulate macroautophagy to assemble membrane compartments on which they replicate, as is the case for poliovirus and HCV, whereas others use the autophagic core machinery efficiently to egress from cells after replication, as shown for HIV and suggested for poliovirus. In the latter case, the autophagic membranes are stabilized by the viruses and prevented from fusion with lysosomes. Indeed, several viral proteins that inhibit fusion of autophagosomes with lysosomes have been identified, including the spike complex of Semliki forest virus, the Nef protein of HIV and the M2 protein of influenza A virus [6]. Therefore, the macroautophagy machinery can mediate pro-viral and anti-viral functions, and the fate of the membranes to which it recruits viral components seems to determine whether viral restriction or replication is supported.
Infection with CHIKV—which, as Sindbis and Semliki forest virus, belongs to the alphavirus genus—exemplifies these pro-viral and anti-viral functions. Macroautophagy has been reported to protect mice from lethality by CHIKV infection [4], probably by limiting infection-induced cell death, which promotes viral replication. On the other hand, the macroautophagy machinery promotes viral replication in human kidney epithelial cells [3]. Judith and colleagues demonstrate in primary and immortalized human cells that CHIKV engages the molecular machinery of macroautophagy at different sites (Fig 1). Ubiquitinated viral capsids are degraded by lysosomes after recruitment into autophagosomes through p62/sequestosome 1 binding to LC3B. This protective function of macroautophagy could be observed in both human and mouse cells. By contrast, the viral non-structural protein 2 binds to NDP52 and co-localizes with LC3C on trans-Golgi membranes, promoting viral replication only in human cells. This pro-viral role of the macroautophagy machinery can be transferred to mouse cells by transfection of human NDP52 and LC3C. This study suggests that NDP52 mediates a pro-viral and p62/sequestosome 1 anti-viral effect during CHIKV infection and only the anti-viral effect is conserved in mouse cells. This implies that the mouse model of CHIKV infection could be improved by introducing human NDP52 and LC3C to elicit both the pro-viral and anti-viral functions of macroautophagy in vivo.
macroautophagy […] also seems to support the replication of some viruses and is even stimulated by them
Such a model would be especially useful to interrogate the functions of macroautophagy that are not cell intrinsic and affect the communication of infected cells with components of the immune system during CHIKV infection. Along these lines, the balance of replication and viral capsid degradation could influence the production of pro-inflammatory cytokines as well as antigen presentation [6]. In this respect, viral replication on the cytosolic surface of membranes could engage cytosolic RNA receptors, and viral capsid degradation could co-localize viral RNA with endosomal toll-like receptors (TLRs)—such as TLR7 and TLR8. Both pathways of pathogen-associated molecular pattern recognition and the resulting cytokine production are known to be influenced by macroautophagy [2]. Furthermore, macroautophagy can deliver cytosolic antigens for processing and presentation by major histocompatibility complex class II molecules to amplify CD4+ T-cell stimulation [6]. This antigen presentation could be augmented by enhanced replication and viral capsid degradation, both mediated by the macroautophagy machinery.
[this study] resolves the controversy on the role of macroautophagy in CHIKV infection and suggests a way to improve in vivo models
Thus, the study by Judith and colleagues in this issue of EMBO reports both resolves the controversy on the role of macroautophagy in CHIKV infection and suggests a way to improve in vivo models for this viral infection with relevance to human disease. Notably, such improvements on the in vivo infection model for CHIKV fever would be timely for this re-emerging viral disease, and would allow the investigation of new treatment options required to suppress this disease in its endemic areas and prevent further spreading—such as the outbreak in Italy [10]. More generally, it also argues that pathogens should always be studied in cells of the original host species or the species in which they cause disease, at least in parallel with cells of other origin, because their adaptation to influence cellular pathways has often exquisitely evolved with this particular species.
Footnotes
The author declares that he has no conflict of interest.
References
- Mizushima N et al. (2011) Annu Rev Cell Dev Biol 27: 107–132 [DOI] [PubMed] [Google Scholar]
- Münz C (2009) Annu Rev Immunol 27: 423–429 [DOI] [PubMed] [Google Scholar]
- Krejbich-Trotot P et al. (2011) Virol J 8: 432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubert PE et al. (2012) J Exp Med 209: 1029–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judith D et al. (2013) EMBO Rep [Epub] doi:; DOI: 10.1038/embor.2013.51 [DOI] [Google Scholar]
- Randow F, Münz C (2012) Trends Immunol 33: 475–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvedahl A et al. (2007) Cell Host Microbe 1: 23–35 [DOI] [PubMed] [Google Scholar]
- Orvedahl A et al. (2010) Cell Host Microbe 7: 115–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvedahl A et al. (2011) Nature 480: 113–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt FJ et al. (2012) Lancet 379: 662–671 [DOI] [PubMed] [Google Scholar]