Abstract
Internal tandem duplication of the fms-like tyrosine kinase-3 gene (FLT3-ITD) and nucleophosmin-1 (NPM1) mutations have prognostic importance in acute myeloid leukemia (AML) patients with intermediate-risk karyotype at diagnosis, but less is known about their utility to predict outcomes at relapse. We retrospectively analysed outcomes of 70 patients with relapsed, intermediate-risk karyotype AML who received a uniform reinduction regimen, with respect to FLT3-ITD and NPM1 mutation status and first complete remission (CR1) duration. CR1 duration, but not molecular status, was significantly correlated with CR2 rate. On univariate analysis, patients with mutated FLT3-ITD (FLT3+) had significantly worse overall survival (OS) compared with those with neither an NPM1 nor FLT3-ITD mutation (NPM1-/FLT3-). On multivariate analysis, shorter CR1 duration was significantly correlated with inferior OS at relapse (P<0.0001), while FLT3 and NPM1 mutation status and age were not significantly correlated with OS. Patients who subsequently underwent allogeneic stem cell transplant (alloSCT) had a superior OS regardless of CR1 duration, but outcomes were better in patients with CR1 duration>12 months. In intermediate-risk karyotype AML patients receiving reinduction, CR1 duration remains the most important predictor of OS at relapse; FLT3-ITD and NPM1 status are not independent predictors of survival.
Keywords: acute myeloid leukemia, chemotherapy, FLT3 mutation, NPM1 mutation, allogeneic stem cell transplantation, leukemia
Introduction
In patients with acute myeloid leukemia (AML) and intermediate-risk cytogenetics, nucleophosmin-1 (NPM1) mutation status and internal tandem duplication of the fms-like tyrosine kinase-3 gene (FLT3-ITD) can assist in predicting a patient's risk of relapse and overall survival (OS) with frontline induction and consolidation chemotherapy.1, 2, 3, 4 However, there is a paucity of data elucidating the impact of NPM1 and FLT3 status in patients with relapsed disease.
Studies have shown that NPM1 status remains relatively preserved when comparing bone marrow samples from initial diagnosis to first relapse.5, 6 FLT3-ITD status has shown greater variability, with a trend toward increasing mutation levels and the acquisition of new mutations in some patients at relapse.6, 7 Despite this, 75–85% of patients will not show any change in FLT3 status.6
First complete remission (CR1) duration has been demonstrated to be an important predictor of outcome following reinduction therapy.8, 9 However, it is unclear to what extent mutational status influences this, as CR1 duration tends to be shorter in patients with a FLT3-ITD. In addition, some physicians may only offer salvage therapy to those patients whom they suspect are likely to benefit from the treatment, yet it has never been shown conclusively that patients with good-prognosis molecular markers at presentation have a superior response to treatment at relapse. The aim of this study is to clarify whether NPM1 and FLT3 status influence response to salvage therapy at relapse.
Patients and Methods
Patients and treatment
From a database of patients treated at Princess Margaret Hospital between 2002–2010, we identified 97 patients with AML and intermediate-risk cytogenetics based on Southwest Oncology Group criteria10 who relapsed following a documented CR. All patients had been previously treated with a uniform regimen consisting of induction with daunorubicin and cytarabine followed by two cycles of consolidation, as previously reported.11 For those patients given reinduction chemotherapy, treatment consisted of a combination of mitoxantrone, etoposide and high-dose cytarabine (NOVE-HiDAC), as previously described.12
Molecular testing for NPM1 and FLT3-ITD mutation were performed as previously described.11 NPM1 testing was performed at diagnosis, while FLT3-ITD testing was performed at relapse if samples were available (57 reinduced patients). For the remaining patients who did not have samples available for testing at relapse, the initial diagnostic FLT3 result was used. Patients were divided into three molecular subgroups: (1) NPM1 mutation present but no FLT3-ITD (NPM1+/FLT3-); (2) neither mutated NPM1 nor FLT3-ITD present (NPM1-/FLT3-); (3) FLT3-ITD present (FLT3+), regardless of NPM1 status. The sensitivity of the NPM1 assay was ∼1–10%, while FLT3-ITD positivity was defined as a percentage of total FLT3 (mutated+wild-type) of at least 5%.
Statistical methods
The primary outcome of interest was OS after relapse and was calculated as the time from patient relapse date to death or last date of follow-up. CR1 duration was calculated as the time from the date of the first complete response to the date of patient relapse. Differences in reinduction or CR2 rates as well as the association between time to relapse and molecular status were compared using the Fisher's exact test or the Mann–Whitney–Wilcoxon test or Kruskal–Wallis test for continuous variables. The Kaplan–Meier method was used to generate survival curves and estimate OS probabilities. Unadjusted and adjusted hazard ratios (HR) and 95% confidence intervals (CI) were estimated using the Cox proportional hazards model. SAS version 9.2 (SAS Institute Inc., Cary, NC, USA) and the open source statistical software R version 12.2.1 were used to perform all statistical analyses. Two-sided P-values of<0.05 were used to determine statistical significance.
Results
Table 1 summarizes the characteristics of the patients included in the study. Of the 97 patients, 70 (72%) were reinduced with salvage chemotherapy. There were no significant differences between the entire group of relapsed patients and the subset who were reinduced, with the exception that those who were reinduced were younger (P<0.001). Other cytogenetics included +X (1 case) and −12p (1 case). Of the 57 patients who underwent repeat FLT3 testing at relapse, there was a change in 15 cases (26%); 10 changed from negative to positive, and 5 from positive to negative.
Table 1. Patient characteristics for all relapsed patients, and those that received reinduction.
| Overall | Reinduced | |
|---|---|---|
| N | 97 | 70 |
| NPM1+ (%) | 39 (40) | 28 (40) |
| Sex (%) | ||
| Female | 43 (44) | 32 (46) |
| Male | 54 (56) | 38 (54) |
| Age at relapse, median (range) | 55 (31–76) | 52 (31–72) |
| <60 (%) | 63 (65) | 54 (77) |
| ⩾60 (%) | 34 (35) | 16 (23) |
| WBC × 109/l, median (range) | 32 (1–207) | 30 (1–207) |
| Prior disease (%) | ||
| De novo | 85 (88) | 63 (90) |
| Secondary | 12 (12) | 7 (10) |
| Cytogenetics | ||
| Diploid | 89 | 64 |
| Abn Y | 3 | 3 |
| +8 | 3 | 2 |
| Other | 2 | 1 |
| Mutation status (%) | ||
| FLT3-/NPM1+ | 20 (21) | 10 (14) |
| FLT3-/NPM1- | 43 (44) | 31 (44) |
| FLT3+ | 34 (31) | 29 (41) |
| CR1 duration (months) | ||
| <6 | 31 (32) | 20 (29) |
| 6–12 | 37 (38) | 25 (36) |
| ⩾12 | 29 (30) | 25 (36) |
| AlloSCT | ||
| None | 74 (76) | 53 (76) |
| Prior alloSCT in CR1 | 4 (4) | 0 (0) |
| AlloSCT in CR2 | 18 (19) | 17 (24) |
Abbreviations: alloSCT, allogeneic stem cell transplant; CR, complete remission; FLT3, fms-like tyrosine kinase-3; NPM1, nucleophosmin-1; WBC, white blood cell.
The clinical features according to molecular subgroup are summarized in Table 2. The FLT3+ group had a significantly shorter median CR1 duration (7.1 months) than the NPM1-/FLT3- (12.2 months) and NPM1+/FLT3- (11.2 months) groups.
Table 2. Clinical characteristics of molecular subgroups.
| FLT3-/NPM1+ | FLT3-/NPM1- | FLT3+ | P | |
|---|---|---|---|---|
| Total (% of relapsed patients) | 10 (80%) | 31 (68%) | 29 (74%) | NS |
| Age (median) | 52 | 54 | 47 | NS |
| CR1 duration | 0.0018 | |||
| <6 months | 3 | 6 | 11 | |
| 6–12 months | 1 | 9 | 15 | |
| >12 months | 6 | 16 | 3 | |
| CR2 rate (%) | 6 (60%) | 21 (68%) | 15 (52%) | NS |
| AlloSCT in CR2 | 1 | 10 | 6 | |
| CR2 duration, median (months) | 13.8 | 12.2 | 8.1 | 0.007 |
Abbreviations: alloSCT, allogeneic stem cell transplant; CR, complete remission; FLT3, fms-like tyrosine kinase-3; NPM1, nucleophosmin-1; NS, not significant.
Of the 70 evaluable patients who received reinduction chemotherapy, 42 (60%) achieved a CR2. Of these, 17 underwent allogeneic stem cell transplant (alloSCT) in CR2; 6 of these were FLT3+ (Table 2). At a median follow-up of 10 months from relapse (range 2–53 months), 50 of the reinduced patients (71%) have died. The median OS was 8.2 months (range 6.1–11.4 months) for all 97 patients and 9.7 months (range 7.5–14.7 montsh) for the 70 patients who were reinduced.
Analysis of factors influencing CR2 rates
The proportion of patients undergoing reinduction was similar across the three molecular subgroups (P=0.65). As shown in Table 2, among those reinduced, CR2 rates were also not significantly different between molecular subgroups (P=0.29), although CR2 duration was significantly shorter in the FLT3+ subgroup. CR1 duration was, however, a predictor of CR2 rates. Those with a time to relapse<6 months had a CR2 rate of 20%, as compared with 64% for patients with CR1 duration of 6–12 months and 88% for those with CR1 duration⩾12 months (P<0.0001).
Univariate analysis for OS
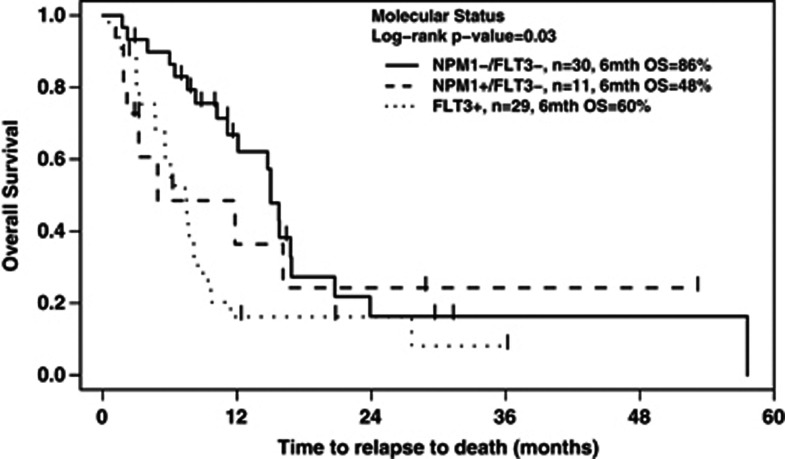
For patients receiving reinduction chemotherapy, there was a significant association between molecular status and OS (log-rank P=0.03), as shown in Figure 1. On pairwise univariate analysis (Table 3), FLT3+ patients had a significantly worse OS than those who were NPM1-/FLT3-. This difference persisted (P=0.022) when restricted to the 64 patients with diploid karyotype. There were no significant differences in OS between the NPM1+/FLT3- cohort and the double-negative group, nor between the FLT3+ group and the NPM1+/FLT3- group. When the patients who underwent alloSCT in CR2 were excluded, there was still a significant association between molecular status and OS (P=0.035), and between the FLT3+ and NPM1-/FLT3- groups (HR: 2.26, P=0.001).
Figure 1.
OS from time of relapse, according to mutation status, for the 70 patients who underwent reinduction.
Table 3. Univariate pairwise comparisons for OS by mutation status and CR1 duration.
| HR | 95% CI | P-value | ||
|---|---|---|---|---|
| Mutation status | 0.03 | |||
| FLT3-/NPM1+ versus FLT3-/NPM1- | 1.21 | 0.48 | 3.03 | 0.68 |
| FLT3+versus FLT3-/NPM1- | 2.16 | 1.17 | 3.97 | 0.014 |
| FLT3+versus FLT3-/NPM1+ | 1.78 | 0.72 | 4.43 | 0.21 |
| CR1 duration | ||||
| 6–12 versus<6 months | 3.22 | 1.57 | 6.62 | 0.001 |
| ⩾12 versus<6 months | 13.96 | 5.59 | 34.91 | <0.0001 |
| ⩾12 versus 6–12 months | 4.33 | 1.96 | 9.56 | 0.0003 |
Abbreviations: CI, confidence interval; CR, complete remission; FLT3, fms-like tyrosine kinase-3; HR, hazard ratio; NPM1, nucleophosmin-1. Bold entries highlight the statistically significant.
When restricting the analysis to only the 57 patients who were retested for FLT3 at the time of relapse, the pairwise association with molecular status was no longer significant (P=0.095), possibly due to a reduction in the sample size. However, the OS of FLT3+ group was still significantly inferior to the NPM1-/FLT3- group (P=0.045). Similarly, when the 20 patients with early relapse (CR1 duration<6 months) were excluded, the association between molecular status and OS was not longer significant (P=0.08).
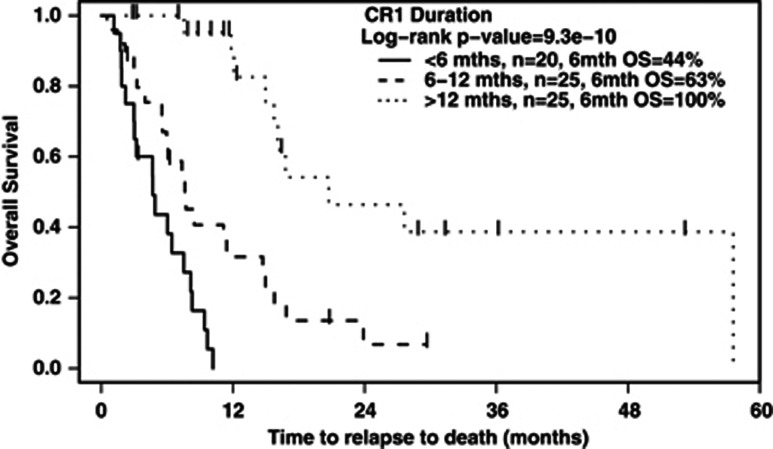
CR1 duration was an important predictor of OS from relapse (see Figure 2). On pairwise comparison, those with CR1 duration of 6 months or less had a significantly worse OS than those with CR1 duration >6 months. Similarly, patients with CR1 duration between 6 and 12 months had a worse OS than patients who relapsed 12 months or more after CR1 (Table 3).
Figure 2.
OS from time of relapse, according to CR1 duration, for the 70 patients who underwent reinduction.
Multivariate analysis for OS
A multivariate Cox proportional hazards model was created, using mutation status, age at relapse and CR1 duration. In this model, CR1 duration was a highly significant predictor of OS (Table 4). After adjusting for CR1 duration, molecular status and age did not have a significant impact on OS. The results were the same when only the 57 patients who were tested for FLT3 at relapse were evaluated (P-value for molecular status 0.58, P=0.46 for FLT3+).
Table 4. Multivariate analysis for OS, incorporating mutation status, age and CR1 duration.
| Variable | HR | 95% CI | P-value | |
|---|---|---|---|---|
| Molecular status | 0.17 | |||
| NPM1-/FLT3- | 1.00 | — | — | — |
| NPM+/FLT3- | 2.44 | 0.86 | 6.95 | 0.094 |
| FLT3+ | 1.57 | 0.80 | 3.06 | 0.19 |
| Age at relapse (continuous) | 1.002 | 0.97 | 1.03 | 0.90 |
| CR1 duration | <0.0001 | |||
| <6 months | 15.29 | 5.67 | 41.23 | <0.001 |
| 6–12 months | 5.41 | 2.17 | 13.50 | 0.0003 |
| ⩾12 months | 1.00 | — | — | — |
Abbreviations: CI, confidence interval; CR, complete remission; FLT3, fms-like tyrosine kinase-3; HR, hazard ratio; NPM1, nucleophosmin-1. Bold entries highlight the statistically significant.
Influence of alloSCT in CR2
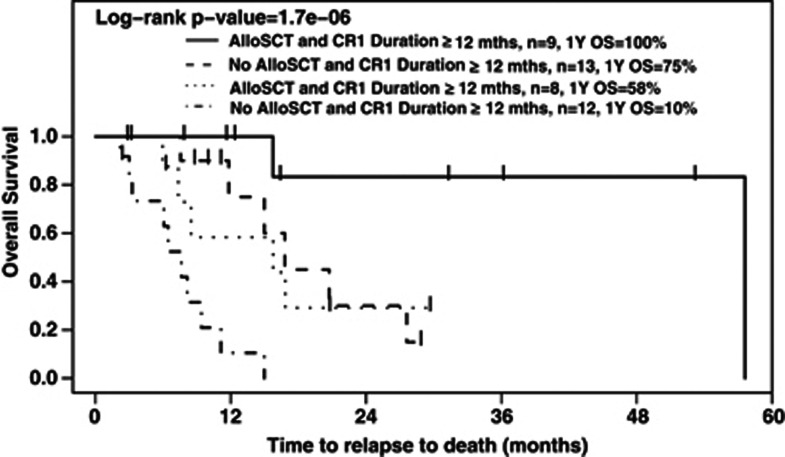
Among patients who achieved a CR2, those who subsequently underwent alloSCT had a significantly superior OS (HR: 3.61 (95% CI: 1.39–9.35), P=0.0053) than those who did not. Two-year OS rates were 56% and 14%, respectively. This difference was significant for the subset of patients who relapsed at <12 months (HR: 6.20 for patients not receiving an alloSCT compared with those who did (95% CI: 1.75–21.99), P=0.005); there was a similar trend seen amongst those who relapsed at ⩾12 months, with those not receiving a transplant having worse OS (HR: 7.29 (95% CI: 0.87–60.86), P=0.067).
Patients who underwent alloSCT after a CR1 duration ⩾12 months had a significantly better OS than those who underwent alloSCT after CR1 duration <12 months (HR: 6.91 (95% CI: 0.81–59.25), P=0.041). Figure 3 shows Kaplan–Meier OS curves for patients, stratified by CR1 duration (⩾ or <12 months) and by whether or not they received a transplant.
Figure 3.
Outcome of the 42 patients who achieved CR2, according to CR1 duration and whether alloSCT was performed in CR2.
Discussion
In our study, neither NPM1 nor FLT3 status predicted for ability to achieve CR2 with salvage chemotherapy. This is in contrast to the frontline setting, where NPM1 positivity is associated with a higher CR rate.11 In the relapsed setting, CR1 duration appears to be the more important predictor of CR2; patients with CR1 duration <6 months are particularly resistant to current salvage regimens, as previously described.13, 14, 15
Our findings on univariate analysis with respect to the adverse impact of FLT3-ITD positivity on OS are consistent with those of Ravandi et al.16 However, as patients with FLT3-ITD mutations tend to have a shorter CR1 duration,11 it is important to clarify the influence of molecular status versus CR1 duration on outcome following relapse. We found that the negative prognostic impact of a FLT3-ITD mutation disappeared after correcting for CR1 duration on multivariate analysis. Patients with CR1 duration <6 or<12 months had a significantly inferior OS compared with those with longer CR1 duration; the inclusion of molecular status in our multivariate model did not independently influence outcome.
Our findings are in contrast to a French GOELAMS study, which found that both FLT3-ITD status and CR1 duration remained important prognostic factors in refractory or relapsed AML.17 However, that study included patients with primary refractory disease and incorporated favorable and adverse cytogenetic risk groups, while our study was restricted to relapsed patients with intermediate-risk karyoptye. In addition, the GOELAMS study used a salvage regimen incorporating gemtuzumab ozogamicin, which may have influenced outcomes. Their study also used only FLT3-ITD results at initial diagnosis; some of these may have changed at relapse, as previously described.6, 7
In contrast to what is seen in the frontline setting, NPM1+/FLT3− status did not predict for a better OS with salvage chemotherapy in our study, as compared with the other subgroups. The reasons for this are unclear; some cases may have acquired additional mutations, which may have rendered the cells more drug resistant. In contrast to our findings, Chevallier et al.8 reported that NPM1+/FLT3- patients with a normal karyotype had a higher CR rate and superior t2-year OS following salvage chemotherapy compared with those in other molecular subgroups. However, this latter report did not include a multivariate analysis, and in the subsequent study from this group,17 NPM1 status was no longer a significant predictor of survival on either univariate or multivariate analysis.
The high frequency of changes in FLT3 status at the time of relapse (26%) is consistent with previous reports,6, 7 and supports the need for retesting all relapsed patients for FLT3 status. This is particularly important with the availability of FLT3 inhibitors,18 currently in clinical trials. We also did not measure FLT3 allele burden, which may also potentially influence results.19 A limitation in our data is that patients did not have cytogenetics repeated at the time of relapse, and thus we cannot exclude the possibility that some patients may have acquired new cytogenetic abnormalities, placing them into a more unfavorable risk group.
In our study, age at relapse was found not to be a prognostic factor on multivariate analysis, in contrast to what is seen at initial presentation. However, as older patients were less likely to receive salvage chemotherapy, we cannot exclude the possibility of a selection bias that excluded higher-risk elderly patients.
The OS in all subgroups remained poor, and novel treatment approaches therefore need to be considered for all relapsed patients. For patients with FLT3-ITD mutations, the addition of the FLT3 inhibitor CEP-701 to chemotherapy at relapse did not result in any survival benefit in a recent randomized study.20 However, more promising results in relapsed patients have been shown with the more potent FLT3 inhibitor, quizartinib21. Further studies with this agent, either alone or in combination with chemotherapy, are warranted.
AlloSCT appears to confer a survival advantage to those achieving a CR2, regardless of CR1 duration. It is our opinion that this should continue to be recommended to all patients who achieve CR2 following relapse. Nevertheless, performing an alloSCT did not completely efface the negative prognostic weight of a shorter CR1 duration, and results remain suboptimal in this subset of patients. Alternative approaches are needed, both to treat relapsed patients with a short CR1 duration but also to provide better frontline therapy for patients identified as being at high risk of early relapse.
The authors declare no conflict of interest.
References
- Kiyoi H, Naoe T, Nakano Y, Yokota S, Minami S, Miyawaki S, et al. Prognostic implication of FLT3 and N-RAS gene mutations in acute myeloid leukemia. Blood. 1999;93:3074–3080. [PubMed] [Google Scholar]
- Fröhling S, Schlenk RF, Breitruck J, Benner A, Kreitmeier S, Tobis K, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Uhn. Blood. 2002;100:4372–4380. doi: 10.1182/blood-2002-05-1440. [DOI] [PubMed] [Google Scholar]
- Schnittger S, Schoch C, Kern W, Mecucci C, Tschulik C, Martelli C. Nucleophosmin gene mutations are predictors of favorable prognosis in acute myelogenous leukemia with a normal karyotype. Blood. 2005;106:3733–3739. doi: 10.1182/blood-2005-06-2248. [DOI] [PubMed] [Google Scholar]
- Verhaak RGW, Goudswaard CS, van Putten W, Bijl MA, Sanders MA, Hugens W, et al. Mutations in nucleophosmin (NPM1) in acute myeloid leukemia (AML): association with other gene abnormalities and previously established gene expression signatures and their favorable prognostic significance. Blood. 2005;106:3747–3754. doi: 10.1182/blood-2005-05-2168. [DOI] [PubMed] [Google Scholar]
- Palmisano M, Grafone T, Ottaviani E, Testoni N, Baccarani M, Martinelli G, et al. NPM1 mutations are more stable than FLT3 mutations during the course of disease in patients with acute myeloid leukaemia. Haematologica. 2007;92:1268–1269. doi: 10.3324/haematol.11202. [DOI] [PubMed] [Google Scholar]
- Schnittger S, Kern W, Tschulik C, Weiss T, Dicker F, Falini BW, et al. Minimal residual disease levels assessed by NPM1 mutaiton-specific RQ-PCR provide important prognostic information in AML. Blood. 2009;114:2220–2231. doi: 10.1182/blood-2009-03-213389. [DOI] [PubMed] [Google Scholar]
- Shih L, Huang C, Wu J, Lin TL, Po Dunn P, Wang PN, et al. Internal tandem duplication of FLT3 in relapsed acute myeloid leukemia: a comparative analysis of bone marrow samples from 108 adult patients at diagnosis and relapse. Blood. 2002;100:2387–2392. doi: 10.1182/blood-2002-01-0195. [DOI] [PubMed] [Google Scholar]
- Chevallier P, Prebet T, Pigneux A, Hunault M, Delaunay J, Perry F, et al. Influence of NPM1 and FLT3-ITD status on outcome in relapsed/refractory AML patients receiving salvage therapy including gemtuzumab ozogamicin. Leukemia. 2010;24:467–469. doi: 10.1038/leu.2009.214. [DOI] [PubMed] [Google Scholar]
- Kurosawa S, Yamaguchi T, Miyawaki S, Uchida N, Sakura T, Kanamori H, et al. Prognostic factors and outcomes of adult patients with acute myeloid leukemia after first relapse. Haematologica. 2010;85:1857–1864. doi: 10.3324/haematol.2010.027516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group study. Blood. 2000;96:4075–4083. [PubMed] [Google Scholar]
- How J, Sykes J, Gupta V, Yee KWL, Schimmer AD, Schuh AC, et al. Influence of FLT3-ITD allele burden and white blood count on outcome in patients with intermediate-risk karyotype acute myeloid leukemia (AML) Cancer. 2012;118:6110–6117. doi: 10.1002/cncr.27683. [DOI] [PubMed] [Google Scholar]
- Brandwein JM, Gupta V, Schuh AC, Schimmer AD, Yee K, Xu W, et al. Predictors of response to reinduction chemotherapy for patients with acute myeloid leukemia who do not achieve complete remission with frontline induction chemotherapy. Am J Hematol. 2007;83:54–58. doi: 10.1002/ajh.21034. [DOI] [PubMed] [Google Scholar]
- Grigg AP, Szer J, Beresford J, Dodds A, Bradstock K, Durrant S, et al. Factors affecting the outcome of allogeneic bone marrow transplantation for adult patients with refractory or relapsed acute leukaemia. Br J Haematol. 1999;107:409–418. doi: 10.1046/j.1365-2141.1999.01713.x. [DOI] [PubMed] [Google Scholar]
- Estey EH. Treatment of relapsed and refractory acute myelogenous leukemia. Leukemia. 2000;14:476–479. doi: 10.1038/sj.leu.2401568. [DOI] [PubMed] [Google Scholar]
- Ferrara F, Palmieri S, Mele G. Prognostic factors and therapeutic options for relapsed or refractory acute myeloid leukemia. Haematologica. 2004;89:998–1008. [PubMed] [Google Scholar]
- Ravandi F, Kantarjian H, Faderl S, Garcia-Manero G, O'Brien S, Koller C, et al. Outcome of patients with FLT3-mutated acute myeloid leukemia in first relapse. Leuk Res. 2010;34:752–756. doi: 10.1016/j.leukres.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier P, Labopin M, Turlure P, Prebet T, Pigneux A, Hunault A, et al. A new Leukemia Prognostic Scoring System for refractory/relapsed adult acute myelogenous leukaemia patients: a GOELAMS study. Leukemia. 2011;25:939–944. doi: 10.1038/leu.2011.25. [DOI] [PubMed] [Google Scholar]
- Schnittger S, Bacher U, Kern W, Alpermann T, Haferlach C, Haferlach T. Prognostic impact of FLT3-ITD load in NPM1 mutated acute myeloid leukemia. Leukemia. 2011;25:1297–1304. doi: 10.1038/leu.2011.97. [DOI] [PubMed] [Google Scholar]
- Levis M, Ravandi F, Wang ES, Baer MR, Alexander Perl A, Coutre S, et al. Results from a randomized trial of salvage chemotherapy followed by lestaurtinib for patients with FLT3 mutant AML in first relapse. Blood. 2011;117:3294–3301. doi: 10.1182/blood-2010-08-301796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes JE, Perl AE, Smith CC, Kovacsovics T, Dombret H, Dohner H, et al. A phase II open-label, AC220 monotherapy efficacy study in patients with refractory/relapsed Flt3-Itd positive acute myeloid leukemia: updated interim results. Blood. 2011;114:2576a. [Google Scholar]