ABSTRACT
BACKGROUND:
Many chemotherapeutic regimens used to treat colorectal cancer (CRC), including 5-fluorouracil plus leucovorin in combination with irinotecan (FOLFIRI) or oxaliplatin (FOLFOX), are administered on an every-other-week (q2w) dosing schedule. Chemotherapy in combination with a monoclonal antibody (mAb) directed toward the epidermal growth factor receptor (EGFR) has emerged as an effective treatment option. There are currently 2 anti-EGFR mAbs approved by the United States Food and Drug Administration: cetuximab and panitumumab. Mutations of KRAS, a downstream protein in the EGFR pathway, predict resistance to EGFR mAbs. Thus, cetuximab and panitumumab are indicated for patients without a KRAS mutation (KRAS wild-type). Whereas panitumumab is approved on a q2w dosing schedule, cetuximab is approved as a weekly dose. However, only cetuximab is approved with FOLFIRI for frontline metastatic CRC, whereas panitumumab is approved for third-line. Because concomitant therapies are often administered q2w, the weekly dosing of cetuximab results in additional medical office visits.
DESIGN:
Several studies have assessed the safety and efficacy of cetuximab q2w. For this review, a comprehensive literature search of studies evaluating cetuximab q2w dosing was conducted. Safety and efficacy results of these trials and retrospective analyses were summarized and reviewed.
RESULTS:
In general, results with cetuximab q2w were comparable to those obtained with the weekly regimen.
CONCLUSION:
These data suggest that for patients for whom weekly treatment with cetuximab presents a substantial burden to their quality of life, q2w dosing of cetuximab is a viable treatment option with a benefit:risk profile similar to that of the weekly regimen.
Colorectal cancer (CRC) is the third most common cancer in men and the second most common in women worldwide.1 In the United States, an estimated 143,460 new cases of CRC and 51,690 deaths resulting from the disease occurred in 2012.2 CRC has a 5-year relative survival rate of 64% for all stages and 12% for stage IV.2 Outcomes for stage IV or metastatic (mCRC) disease are much worse than those for early-stage CRC.
For decades, standard chemotherapy for mCRC was fluorouracil (5-FU) monotherapy, which results in an overall response rate (ORR) of 10% and a median overall survival (OS) of 10 months.3,4 The ORR improved to 23% with the addition of leucovorin (LV) to 5-FU. Therapeutic outcomes have been further improved by combination regimens that incorporate novel cytotoxic agents with 5-FU, including FOLFIRI (5-FU, LV, and irinotecan) and FOLFOX (5-FU, LV, and oxaliplatin). The oral 5-FU prodrug capecitabine can also be used instead of infusional 5-FU in chemotherapy combinations.5 A phase III noninferiority study demonstrated that capecitabine plus oxaliplatin (XELOX) was noninferior to FOLFOX, with equivalent median progression-free survival (PFS; 4.7 months XELOX vs. 4.8 months FOLFOX).5 The vascular endothelial growth factor inhibitor bevacizumab, when added to any of the therapies previously mentioned, improves clinical outcomes even further in both the frontline and chemorefractory settings.6–10 Initial approval of bevacizumab was based on the results of a trial evaluating irinotecan, bolus 5-FU, and LV plus bevacizumab or placebo, which demonstrated an improvement in median OS (20.3 months vs. 15.6 months; P < .001) for patients who received bevacizumab.7,11 The benefit of bevacizumab when added to other chemotherapeutic regimens used in the first-line treatment of mCRC has been reviewed in detail elsewhere.9 The epidermal growth factor receptor (EGFR) monoclonal antibodies (mAbs) cetuximab and panitumumab are effective treatments for KRAS wild-type (WT) mCRC.12,13 Both cetuximab and panitumumab can be used as monotherapy for the treatment of patients who are unresponsive to irinotecan- or oxaliplatin-based chemotherapy.12,13 Cetuximab is also approved for use in combination with irinotecan for patients with irinotecan-refractory mCRC.12 Recently, cetuximab received approval from the United States Food and Drug Administration (FDA) for use in combination with FOLFIRI as a first-line treatment of mCRC.12
EGFR INHIBITORS IN mCRC
EGFR is an HER family tyrosine kinase receptor that contributes to colon cancer cell proliferation and survival.14 There are currently 2 FDA-approved EGFR inhibitors that have been extensively studied in phase II and III trials: cetuximab and panitumumab. Both of these are mAbs that bind the extracellular domain of EGFR and inhibit downstream signaling. Cetuximab is an immunoglobulin G (IgG1) human–mouse chimeric mAb, whereas panitumumab is an IgG2 human mAb.12,13 These agents competitively inhibit the tyrosine kinase domain of EGFR, thereby preventing dimerization and ligand-induced receptor signaling.
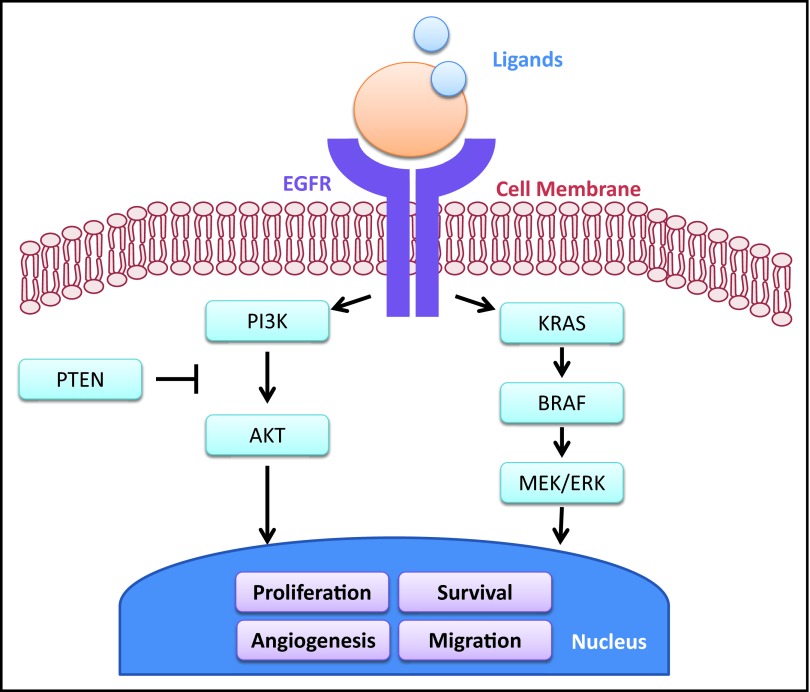
KRAS is an oncogene and a signal transducer modulated by the EGFR pathway (Figure 1).15 Mutations in KRAS, found in approximately 40% of CRC cases, activate the signaling pathway, resulting in cell proliferation, tumor angiogenesis, metastasis, and inhibition of apoptosis.15–17 Further, when KRAS is mutated, the EGFR signaling pathway can be activated in the presence of EGFR inhibition, thus providing a mechanistic basis for the observation that KRAS mutational status predicts resistance to EGFR inhibitors in patients with mCRC.14,18–20 The predictive value of KRAS mutations for resistance to anti-EGFR mAbs has been established in several retrospective analyses and prospective randomized trials.19,21–29
Figure 1.
Overview of the EGFR pathway and downstream signaling pathways, including KRAS. Adapted with permission from Di Fiore F, et al: Molecular determinants of anti-EGFR sensitivity and resistance in metastatic colorectal cancer Br J Cancer 103:1765–1772, 2010.
Mutations in BRAF may also limit the clinical benefits of EGFR inhibitors in the metastatic setting.30,31 BRAF, a member of the RAF kinase family, mediates cellular responses to growth factor signals downstream from KRAS.16 Activating mutations in BRAF have been reported in 5% to 15% of patients with CRC. Shorter PFS and OS were observed among patients with BRAF V600E-mutant mCRC treated with anti-EGFR mAbs.15,30,32 However, several studies have demonstrated that BRAF mutation is a powerful independent marker of poor prognosis and appears to predict outcomes regardless of treatment.33–35
Overview of Approved EGFR Inhibitors
There are several key differences between cetuximab and panitumumab, highlighted in Table 1.36,37 Cetuximab is an IgG1 antibody.12 In addition to inhibiting the dimerization of EGFR to inhibit downstream signaling, cetuximab elicits antibody-dependent, cell-mediated cytotoxicity (ADCC), which has been shown to play a role in the activity of IgG1 antibodies against tumors, although the clinical significance has yet to be fully elucidated.38,39 Cetuximab was also associated with hypersensitivity reactions in about 3% (range, 0%–6%) of patients across 15 clinical trials in patients with mCRC; however, a higher incidence has been noted in smaller retrospective studies conducted in centers within the mid-South region of the United States including an analysis of patients treated at centers in Tennessee and North Carolina (22%) and another study of patients treated at centers in Oklahoma (12.4%).40,41 Risk of development of hypersensitivity to cetuximab is predicted by prior allergies and presence of immunoglobulin E antibodies specific for galactose-α-1,3-galactose.40,42 Panitumumab is an IgG2 antibody, and these antibodies are not associated with the ability to induce ADCC.13 The mean half-life of cetuximab is approximately 4.7 days (range, 2.6–9.6) compared with 7.5 days (range, 3.6–10.9) for panitumumab.12,13 Panitumumab is administered at an approved dose of 6 mg/kg every 14 days as an intravenous infusion,13 whereas cetuximab has an approved weekly schedule of a 400-mg/m2 initial intravenous loading dose followed by 250-mg/m2 weekly infusions.12
Table 1.
Properties of the EGFR inhibitors cetuximab and panitumumab
| Agent | Approved dose | Approved treatment | mAb isotype and origin | Induces ADCC | t1/2 (days) | Approved indications |
|---|---|---|---|---|---|---|
| Cetuximab | 400 mg/m2 followed by 250 mg/m2 IV q1w | Monotherapy; in combination with irinotecan | IgG1; chimeric | Yes | 4.7* | mCRC; SCCHN |
| Panitumumab | 6 mg/kg IV q2w | Monotherapy | IgG2; human | No | 7.5† | mCRC |
ADCC = antibody-dependent cell-mediated cytotoxicity; IgG = immunoglobulin G; IV = intravenous; mAb = monoclonal antibody; mCRC = metastatic colorectal cancer; q1w = once weekly; q2w = every 2 weeks; SCCHN = squamous cell carcinoma of the head and neck; t1/2 = half-life.
Mean t1/2 at recommended dose.12
Elimination t1/2 at recommended dose.13
Panitumumab
Largely based on results from an open-label phase III study of panitumumab compared with best supportive care,43 panitumumab is indicated as a single agent for mCRC treatment in patients with disease progression on or following fluoropyrimidine-, oxaliplatin-, and irinotecan-containing chemotherapy regimens.13 A retrospective analysis of patients treated in this study demonstrated that the efficacy of panitumumab was limited to patients with KRAS WT tumors; as a result, this therapy is not recommended in patients with KRAS mutations.19
In a phase III study comparing panitumumab plus FOLFIRI with FOLFIRI alone in second-line treatment of patients with KRAS WT mCRC, panitumumab plus FOLFIRI demonstrated significant improvement in PFS (hazard ratio [HR], 0.73; 95% confidence interval [CI], 0.59–0.90; P = .004).44 Likewise, the phase III Panitumumab Randomized Trial in Combination With Chemotherapy for Metastatic Colorectal Cancer to Determine Efficacy (PRIME) trial evaluated the addition of panitumumab to FOLFOX4 as a first-line treatment of mCRC. Data from this trial demonstrated that panitumumab plus FOLFOX4 significantly improves PFS (median PFS, 9.6 vs. 8.0 months; HR, 0.80; 95% CI, 0.66–0.97; P = .02), but not OS (median OS, 23.9 vs. 19.7 months; HR, 0.83; 95% CI, 0.67–1.02; P = .07), in patients with mCRC KRAS WT tumors.45
Cetuximab
Cetuximab was approved by the FDA in 2004, in combination with irinotecan in irinotecan-refractory disease and as a single agent in patients intolerant of irinotecan.12 In the pivotal research leading to this approval, the Bowel Oncology With Cetuximab Antibody (BOND) study, patients with mCRC who had been treated with an irinotecan-based regimen were randomized to receive cetuximab alone or cetuximab in combination with irinotecan.46 Combination therapy was associated with a higher ORR than was monotherapy (22.9% vs. 10.8%, P = .007), as well as a longer median time to progression (4.1 vs. 1.5 months, P < .001). The difference in median survival time was not significant (8.6 vs. 6.9 months, P = .48); however, the survival benefit may have been confounded by the crossover on disease progression of 56 (50.1%) patients in the cetuximab monotherapy arm to the cetuximab plus irinotecan arm. Of the patients who crossed over, 39.3% demonstrated stable disease or better and a median time to progression of 1.4 months.46
In the CO.17 study, patients with mCRC who had been treated with fluoropyrimidine, irinotecan, and oxaliplatin were treated with cetuximab or best supportive care.47 Cetuximab was associated with a significant improvement in OS (HR for death, 0.77; 95% CI, 0.64–0.92; P = .005) and in PFS (HR for disease progression or death, 0.68; 95% CI, 0.57–0.80; P < .001). A subanalysis of this study demonstrated that patients with mCRC tumors bearing mutated KRAS did not benefit from cetuximab, whereas patients with KRAS WT mCRC demonstrated benefit (OS, P = .01; PFS, P < .001).48
Cetuximab was approved for and has demonstrated efficacy in the third-line setting; however, there is evidence that supports its use in earlier lines of therapy, including the recent approval for use in combination with FOLFIRI for frontline treatment of KRAS WT mCRC. In the Cetuximab Combined With Irinotecan in First-line Therapy for Metastatic Colorectal Cancer (CRYSTAL) trial, FOLFIRI with or without cetuximab was used as the first-line therapy in patients with KRAS WT mCRC.49 It was determined that the addition of cetuximab to FOLFIRI as first-line therapy improved response rates (57.3% vs. 39.7%; odds ratio, 2.069; P < .001), OS (median 23.5 vs. 20.0 months; HR, 0.796; P = .0093), and PFS (median 9.9 vs. 8.4 months; HR, 0.696; P = .0012) vs. FOLFIRI alone among patients with KRAS WT mCRC. Benefit was not observed among patients with KRAS-mutant mCRC. Also, BRAF tumor mutation was determined to be an indicator of poor prognosis in both treatment arms.
Cetuximab has been evaluated in other settings and with various other combination regimens. The combination of cetuximab and irinotecan has also been evaluated as a second-line treatment in the Erbitux Plus Irinotecan for Metastatic Colorectal Cancer (EPIC) and Monoclonal Antibody Erbitux in a European Pre-license (MABEL) trials.50,51 It has also been evaluated in combination with oxaliplatin-based therapies in first-line trials including the Oxaliplatin and Cetuximab in First-line Treatment of Metastatic Colorectal Cancer (OPUS) trial, the Continuous Chemotherapy Plus Cetuximab or Intermittent Chemotherapy (COIN) trial, and the NORDIC VII trial.52–54 All of these studies, which evaluated the approved weekly dosing of cetuximab, are summarized in Table 2. However, the current weekly dosing of cetuximab may not be convenient or appropriate in all circumstances. There may be situations in which a less frequent dose would be in the best interest of the quality of life of the patient (eg, work productivity, vacation, transportation, and proximity to infusion center). Several smaller clinical studies and retrospective analyses have evaluated the safety and efficacy of cetuximab q2w dosing.
Table 2.
Overview of selected cetuximab q1w dosing studies in which KRAS mutational status was assessed
| Trial name and patient characteristics | Evaluable patients, n | Dose and treatment | KRAS status | ORR, % | Median PFS, mo | Median OS, mo |
|---|---|---|---|---|---|---|
| CRYSTAL: first-line mCRC29 | ||||||
| 599 | FOLFIRI + ctx q1w | Any | 59.3 | 9.9 | 24.9 | |
| 599 | FOLFIRI | Any | 43.2 | 8.7 | 21.0 | |
| 172 | FOLFIRI + ctx q1w | WT | 59.3 | 9.9 | 24.9 | |
| 176 | FOLFIRI | WT | 43.2 | 8.7 | 21.0 | |
| 105 | FOLFIRI + ctx q1w | Mut | 36.2 | 7.6 | 17.5 | |
| 87 | FOLFIRI | Mut | 40.2 | 8.1 | 17.7 | |
| OPUS: first-line mCRC54 | ||||||
| 169 | FOLFOX4 + ctx q1w | Any | 46 | 7.2 | 18.3 | |
| 168 | FOLFOX4 | Any | 36 | 7.2 | 18.0 | |
| 82 | FOLFOX4 + ctx q1w | WT | 57 | 8.3 | 22.8 | |
| 97 | FOLFOX4 | WT | 34 | 7.2 | 18.5 | |
| 77 | FOLFOX4 + ctx q1w | Mut | 34 | 5.5 | 13.4 | |
| 59 | FOLFOX4 | Mut | 53 | 8.6 | 17.5 | |
| COIN: first-line mCRC52 | ||||||
| 362 | Chemotherapy* + ctx q1w | WT | 64 | 8.6 | 17.0 | |
| 367 | Chemotherapy* | WT | 57 | 8.6 | 17.9 | |
| 297 | Chemotherapy* + ctx q1w | Mut | NR | NR | 13.6 | |
| 268 | Chemotherapy* | Mut | NR | NR | 14.8 | |
| NORDIC VII: first-line advanced/ mCRC53 | ||||||
| 185 | FLOX | Any | 41 | 7.9 | 20.4 | |
| 194 | FLOX + ctx q1w | Any | 49 | 8.3 | 19.7 | |
| 97 | FLOX | WT | 47 | 8.7 | NR | |
| 97 | FLOX + ctx q1w | WT | 46 | 7.9 | NR | |
| 58 | FLOX | Mut | 40 | 7.8 | NR | |
| 72 | FLOX + ctx q1w | Mut | 49 | 9.2 | NR | |
| MABEL: second-line. Previously failed irinotecan-based therapy50 | ||||||
| 93 | Irinotecan q1w + ctx q1w | Any | 18.3 | 3.0 | 8.3 | |
| 670 | Irinotecan q2w + ctx q1w | Any | 17.3 | 3.2 | 9.2 | |
| 356 | Irinotecan q3w + ctx q1w | Any | 25.8 | 4.6 | 10.3 | |
| 28 | Irinotecan other + ctx q1w | Any | 21.4 | 2.7 | 7.0 | |
| 1147 | Irinotecan (all) + ctx q1w | Any | 20.1 | 3.2 | 9.2 | |
| EPIC: second-line. Previously failed oxaliplatin and fluoropyrimidine49 | ||||||
| 648 | Irinotecan + ctx q1w | Any | 16.4 | 4.0 | 10.7 | |
| 650 | Irinotecan | Any | 4.2 | 2.6 | 10.0 | |
| BOND: advanced CRC. PD during or within 3 months of irinotecan treatment46 | ||||||
| 218 | Irinotecan + ctx q1w | Any | 22.9 | 4.1† | 8.6 | |
| 111 | Ctx q1w | Any | 10.8 | 1.5† | 6.9 | |
| CO.17: CRC. Previously treated with fluoropyrimidine, irinotecan, and oxaliplatin and no other standard therapy available47,48 | ||||||
| 287 | Ctx q1w | Any | 8 | 1.9 | 6.1 | |
| 285 | BSC | Any | 0 | 1.8 | 4.6 | |
| 117 | Ctx q1w | WT | 12.8 | 3.7 | 9.5 | |
| 113 | BSC | WT | 0 | 1.9 | 4.8 | |
| 81 | Ctx q1w | Mut | 1.2 | 1.8 | 4.6 | |
| 83 | BSC | Mut | 0 | 1.8 | 4.5 | |
BSC = best supportive care; CRC = colorectal cancer; Ctx = cetuximab; FLOX = fluorouracil + leucovorin + oxaliplatin; FOLFIRI = 5-flurouracil + leucovorin + irinotecan; FOLFOX = 5-fluorouracil + leucovorin + oxaliplatin; mCRC = metastatic colorectal cancer; Mut = KRAS mutant; NR = not reported; OS = overall survival; ORR = overall response rate; PD = progressive disease; PFS = progression-free survival; q1w = every week; WT = wild-type.
Oncologists could choose XELOX (capecitabine + oxaliplatin) or FOLFOX.
Reported as time to progression.
REVIEW AND SUMMARY OF CLINICAL STUDIES AND RETROSPECTIVE/POST HOC ANALYSES OF Q2W DOSING OF CETUXIMAB
A comprehensive literature search (including the PubMed database, abstracts from the American Society of Clinical Oncology Annual Meeting, and ClinicalTrials.gov) was conducted to find studies that examined cetuximab q2w dosing in patients with mCRC. It is now known that KRAS mutational status plays a role in the efficacy of cetuximab treatment, but many of these studies were conducted before the initial discovery of this biomarker. Earlier trials did not prospectively assess KRAS mutational status and are thus presented separately in this summary.
Although cetuximab has not been approved for a q2w dosing schedule, there are several key studies supporting the clinical use of this schedule, regardless of whether KRAS mutational status was selected or analyzed (Tables 3, 4). In general, results for q2w dosing of cetuximab are similar to those obtained with the approved weekly regimen (Table 2).
Table 3.
Overview of selected cetuximab q2w dosing studies in which KRAS mutational status was not assessed
| Patient characteristics | Evaluable patients, n | Dose and treatment | ORR, % | Median PFS, mo | Median OS, mo |
|---|---|---|---|---|---|
| First-line mCRC55,56 | 62 | Ctx dose-escalation (400–700 mg/m2) q2w followed by Ctx + FOLFIRI | 42 | 8.4 | NR |
| ≥First-line mCRC57 | 84 | Ctx 500 mg/m2 q2w | 29 | 3 | 9 |
| Second- or third-line, irinotecan-refractory mCRC58 | 126 | Ctx 500 mg/m2 q2w + irinotecan | NR | 14.4* | 86.3%† |
| ≥Second-line mCRC59 | 40 | Ctx 500 mg/m2 q2w + irinotecan | 23 | 3.4* | 8 |
| ≥Third-line, irinotecan-, oxaliplatin-, and 5-FU-refractory mCRC60 | 74 | Ctx 500 mg/m2 q2w + irinotecan | 25 | 5.4 | 8.9 |
| mCRC pts who failed first-line fluoropyrimidine/oxaliplatin regimens61 | 31 | Ctx 500 mg/m2 q2w + irinotecan | 6 | 2.4* | 9.3 |
| Second- and third-line mCRC62 | 18 | Ctx 500 mg/m2 q2w + irinotecan | 11 | 18%‡ | 72%‡ |
5-FU = 5-fluorouracil; Ctx = cetuximab; FOLFIRI = 5-fluorouracil + leucovorin + irinotecan; mCRC = metastatic colorectal cancer; NR = not reported; ORR = overall response rate; OS = overall survival; PFS = progression-free survival; Pts = patients; q2w = every 2 weeks.
Reported as time to progression.
Reported as percent OS at 12 weeks.
PFS and OS expressed as percent at 7 months.
Table 4.
Overview of selected cetuximab q2w dosing studies in which KRAS mutational status was assessed
| Patient characteristics | Patients, n |
Dose and treatment | ORR, % | Median PFS, mo | Median OS, mo | |
|---|---|---|---|---|---|---|
| Total evaluable | KRAS-WT | |||||
| KRAS WT prospectively selected | ||||||
| ≥Third-line, irinotecan-, oxaliplatin-, and 5-FU-refractory mCRC63 | 30 | 30 | Ctx 500 mg/m2 q2w + irinotecan | 30 | 5.3 | 10.8 |
| Second-line mCRC64 | 40 | 40 | Ctx 500 mg/m2 q2w + irinotecan | 45 | 7.1 | 18.5 |
| KRAS WT retrospectively examined | ||||||
| First-line mCRC56 | 48 | 29 | Ctx dose-escalation (400 to 700 mg/m2) q2w followed by Ctx + FOLFIRI | WT, 55 Mut, 32 | WT, 9.4 Mut, 5.6 | NR |
| Failed first-line fluoropyrimidine/ oxaliplatin regimens for mCRC61 | 31 | 8 | Ctx 500 mg/m2 q2w + irinotecan | NR | WT, 2.6* Mut, 1.7* | WT, 14.1 Mut, 5.5 |
5-FU = 5-fluorouracil; Ctx = cetuximab; FOLFIRI = 5-fluorouracil + leucovorin + irinotecan; mCRC = metastatic colorectal cancer; Mut = KRAS mutant; NR = not reported; ORR = overall response rate; OS = overall survival; PFS = progression-free survival; q2w = every 2 weeks; WT = wild-type.
Reported as time to progression.
Frontline
Cetuximab was recently approved in the frontline setting; thus, there are limited data evaluating q2w dosing as a frontline treatment. In a phase I dose-escalation study (n = 62), chemotherapy-naive patients received cetuximab monotherapy for 6 weeks followed by FOLFIRI plus cetuximab until disease progressed or toxicity became unacceptable.55 The primary end point was to find the maximum tolerated dose based on the occurrence of a dose-limiting toxicity (DLT). The standard-dose group received cetuximab 400 mg/m2 followed by weekly doses of 250 mg/m2. If 1 or fewer patients in this standard-dose cohort experienced a DLT, subsequent patients enrolled in the study would receive successively higher doses (500, 600, or 700 mg/m2 q2w) until a DLT occurred. The maximum tolerated dose for the q2w regimen was not reached, but the established optimal q2w dose of cetuximab (and closest pharmacokinetic match to q1w) was 500 mg/m2. There were no notable differences in ORR or PFS across study groups. The ORR reported for FOLFIRI plus cetuximab was comparable to that reported from the CRYSTAL trial (Table 2). KRAS WT was not selected in the primary analysis, but mutation status was assessed in a follow-up paper and biomarker analysis.56 KRAS mutational status and biomarker analysis supported the functional equivalence of q1w and q2w administration of cetuximab.56 This study further confirmed that patients with KRAS WT mCRC were most likely to benefit from cetuximab treatment, even when using a modified q2w schedule.
Although data suggested that cetuximab q2w was generally well tolerated and effective in the clinical trial setting, the role of this regimen in the clinical practice setting remained undetermined. In a retrospective analysis of the safety and efficacy of cetuximab q2w in clinical practice, patient records from pharmacy registries were assessed.57 Patients (n = 91; KRAS mutational status not determined) received cetuximab 500 mg/m2 q2w as monotherapy or combination therapy. For 7 patients, this was the first-line therapy; the remainder of the patients had already received chemotherapy for mCRC. A q2w regimen of cetuximab was active (ORR, 29%) and well tolerated, even in patients in whom previous weekly cetuximab treatments had failed.
Second Line and Beyond
Dosing of cetuximab on a q2w schedule has been more thoroughly evaluated in patients who have received treatment for mCRC. In a phase I pharmacokinetic study (n = 11; KRAS mutational status not determined), patients with mCRC who had been treated with FOLFIRI received irinotecan alone as an internal control followed by cetuximab 500 mg/m2 q2w.65 There was little change in irinotecan pharmacokinetics when the drug was combined with cetuximab in the 11 enrolled patients.
A prospective, multicenter, single-arm study (n = 126; KRAS mutational status not determined) demonstrated that q2w dosing of cetuximab plus irinotecan, in a larger cohort of patients than had been included in previous trials, was generally well tolerated and effective.58 Efficacy and safety were similar to those in historical q1w dosing studies and other q2w studies in which KRAS mutational status was not assessed. The PFS rate was 42.7% (95% CI, 32.8–52.6) at 12 weeks and 22.4% (95% CI, 14.2–30.7) at 24 weeks.
In a phase II single-arm study (n = 40; KRAS mutational status not determined), the safety and efficacy of cetuximab q2w in combination with irinotecan was assessed in chemotherapy-refractory patients with mCRC.59 The ORR was 22.5%, with 2 complete responses and 7 partial responses, and the toxicity compared favorably with that seen with a q1w schedule. Results were similar in both toxicity and efficacy to those obtained with weekly and biweekly administration regimens (Table 2).
Pfeiffer and colleagues60 conducted a noncontrolled study (n = 74; KRAS mutational status not determined) evaluating cetuximab q2w in combination with irinotecan. This study cohort had outcomes similar to those of patients with similar baseline characteristics treated in an identical manner in a previous q1w dosing study. Of note, the q1w data showed a strong correlation between efficacy and rash, whereas the q2w data did not support this finding.
In a phase II study (n = 31; KRAS WT prospectively selected), patients with pretreated mCRC were treated with cetuximab q2w plus irinotecan.63 Efficacy results were similar to or higher than those found in previously reported studies with weekly dosing. The ORR in 30 evaluable patients was 30.0% (95% CI, 14.7–49.4%) and the disease control rate (DCR; stable disease or better) was 76.7% (95% CI, 57.7–90.0%). Median PFS was 5.3 months and median OS was 10.8 months. Safety results included grade 3 skin toxicity in 10% of patients, which is comparable to the rate observed in patients receiving irinotecan plus cetuximab q1w (range, 5.1–13.3%).46,51,60,66
A multicenter, single-arm, open-label phase II study (n = 31; KRAS WT not selected but retrospectively examined) evaluated cetuximab q2w plus irinotecan as second-line therapy for mCRC after failure of a fluoropyrimidine-containing regimen.61 OS and time to progression (TTP) were consistent with those reported previously.46, 51 KRAS and BRAF mutations were detected in 39% and 9%, respectively, of the patients tested. A numerical increase in TTP was observed among patients with nonmutated KRAS and BRAF (2.6 vs. 1.7 months; P = .16), and survival was significantly increased (14.1 vs. 5.5 months; P = .04). The ORR (6%) was lower than previously reported, most likely because of the small sample size and possibly reduced dose intensity.
Kang and colleagues64 conducted a prospective, noncomparative, 2-arm, phase II study (n = 40; KRAS WT prospectively selected). Biweekly cetuximab in combination with irinotecan as second-line treatment showed significant antitumor activity in patients with irinotecan-refractory mCRC and KRAS WT, regardless of EGFR expression status. In 20 patients with EGFR-positive and 20 with EGFR-negative mCRC, ORR was 55% and 35%, median PFS was 8.3 and 4.9 months, and median OS was 17.2 and 18.5 months, respectively.
To determine whether clinical trial data reflected what might occur in clinical practice, a retrospective chart review (n = 50; KRAS WT retrospectively analyzed) was conducted to evaluate clinical records of patients with irinotecan-refractory mCRC who received cetuximab plus irinotecan.62 The review compared the safety and efficacy of 2 cetuximab regimens: 400 mg/m2 followed by 250 mg/m2 q1w (n = 32) and 500 mg/m2 q2w (n = 18). All patients received irinotecan q2w. There was no major difference in efficacy and safety between cetuximab q2w and a weekly regimen, both given in association with irinotecan. For the weekly regimen, DCR was 56.3%, TTP was 28%, OS was 75%, and the skin toxicity rate was 78.1%. For the q2w regimen, DCR was 77.8%, TTP was 18%, OS was 72%, and the skin toxicity rate was 61%.
Taken together, these data support the use of cetuximab q2w. Results from clinical trials and analyses of patients seen in clinical practice suggest that this regimen does not result in decreased efficacy or increased safety concerns compared with the approved q1w dosing schedule.
Ongoing q2w Studies: Overview and Interim Results
Several trials evaluating cetuximab q2w are ongoing. In a phase II trial (n = 152; KRAS WT), patients with mCRC received first-line therapy of FOLFOX4 along with either cetuximab q1w or cetuximab q2w.67 After a median follow-up of 12 months, ORR, PFS, and safety were similar for the q2w and q1w treatment arms. This trial is currently the only randomized study comparing dosing schedules for cetuximab. Results suggest that simplified and standard regimens are equivalent.
In another phase II trial (n = 25; KRAS mutational status not determined), patients were given first-line therapy with oxaliplatin and capecitabine with cetuximab q1w or q2w.68 Twelve of the patients enrolled were treated with 250 mg/m2 q1w cetuximab; the remaining 13 received 500 mg/m2 q2w. The biweekly regimen was active and well tolerated and appeared equal to weekly dosing.
Moving beyond first-line therapy, a phase II study (n = 24; KRAS WT not selected) was designed to evaluate cetuximab q2w plus oxaliplatin and gemcitabine as a salvage therapy.69 Biweekly cetuximab was well tolerated and active in heavily pretreated mCRC patients after a median of 6 cycles of therapy. No meaningful historical controls exist for this treatment regimen (gemcitabine-based) in CRC.
Another phase II study (n = 174; KRAS mutational status determined and retrospectively analyzed) examined cetuximab q2w in combination with irinotecan as a third-line therapy.70 The q2w regimen of cetuximab with irinotecan was as effective and well tolerated as q1w administration. The DCR in KRAS-mutant patients treated with cetuximab q2w was nearly double that reported for those receiving the q1w regimen.
Patients with hepatic metastases from CRC (n = 19; KRAS WT not selected) were treated with capecitabine q2w and cetuximab plus hepatic arterial infusion of oxaliplatin.71 The ORR was 78.9%, disease progression occurred in 15 patients, and OS was not reached at the time the data were presented. The preliminary findings of this study were that combination therapy with hepatic arterial infusion of oxaliplatin with concurrent capecitabine and cetuximab q2w can be safely administered to patients with liver metastases from CRC.
Three additional trials evaluating cetuximab q2w are ongoing and do not yet have data available. Biweekly Cetuximab Combined With FOLFOX-6 in Metastatic Colorectal Cancer (CEBIFOX) is a phase II trial in KRAS WT patients examining cetuximab q2w combined with FOLFOX6 in patients with mCRC.72 This study began in February 2009; the primary completion date was in September 2011, and the final data will be available in September 2014. The phase II trial Study Evaluating Biomarkers in Patients With Colorectal Cancer and Wild Type KRAS Gene Treated With Chemotherapy and Cetuximab (POSIBA) is an evaluation of biomarkers in patients with CRC and KRAS WT treated with chemotherapy (FOLFIRI or FOLFOX6) and cetuximab q2w.73 This study began in January 2011, with expected primary and final completion dates of October and December 2014, respectively. Safety and Efficacy of FOLFOX4 + Weekly Cetuximab vs. FOLFOX + Biweekly Cetuximab by Metastatic Colorectal Cancer (CORE 2) is a randomized phase II trial (KRAS WT not selected) to assess the safety and efficacy of FOLFOX4 in combination with either cetuximab q1w or cetuximab q2w.74 This study began in January 2008; primary data were available in July 2012, and the study completion date was November 2012. Data from these trials will help to provide further evidence supporting cetuximab q2w as a treatment option for some patients.
CONCLUSIONS AND FUTURE DIRECTIONS
The results of clinical trials and retrospective analyses summarized in this review suggest that a biweekly schedule for cetuximab results in similar efficacy rates compared with weekly dosing. In addition, the available evidence shows that biweekly dosing does not increase the incidence or severity of adverse effects over weekly dosing. Thus, the data support this modified therapeutic strategy in a situation in which less frequent dosing becomes necessary.
There are multiple clinical scenarios in which q2w administration of cetuximab may be of particular advantage in routine clinical practice. Currently, cetuximab is approved in combination with chemotherapeutic regimens that are administered on biweekly schedules, including FOLFIRI for first-line therapy and irinotecan for irinotecan-refractory patients. If a patient is determined to be a candidate for a cetuximab-containing regimen, biweekly dosing provides more flexibility for patients with challenging transportation needs, such as older patients with limited social support or patients residing at a significant distance from the cancer treatment center. The alternative regimen also provides more time between treatments to allow for social circumstances that may be of particular importance in the palliative setting, such as family events or vacations. In addition, for patients who experience severe acneiform rash, it is recommended that infusion be delayed by 1 to 2 weeks before dosing is resumed.12 The studies reviewed here support this treatment approach, as they suggest efficacy of q2w dosing. Therefore, biweekly administration of cetuximab may lessen the burden of treatment and improve quality of life during treatment without compromising efficacy or safety.
The studies summarized here provide a more thorough understanding of cetuximab dosing that has direct relevance to situations that are encountered in clinical practice. Ongoing challenges in the understanding of cetuximab include identification of additional biomarkers of resistance beyond KRAS mutation, optimal chemotherapeutic combination partners and regimens, and better management of cetuximab-related toxicity. Addressing these challenges will aid in the improvement of outcomes and quality of life for patients with mCRC.
Acknowledgments
We thank Pamela Tuttle, PhD, and William Fazzone, PhD, for medical editorial assistance with the manuscript.
Footnotes
Editorial assistance was provided by Articulate Science and was funded by Bristol-Myers Squibb Company.
Disclosures of Potential Conflicts of Interest
Dr. Hubbard has served as an advisor and received honoraria from Genomic Health. She has also received research support from Genentech. Dr. Alberts has received research support from the National Cancer Institute.
REFERENCES
- 1. Jemal A, Bray F, Center MM, et al. : Global cancer statistics. CA Cancer J Clin 61:69–90, 2011 [DOI] [PubMed] [Google Scholar]
- 2. Siegel R, Naishadham D, Jemal A: Cancer statistics, 2012. CA Cancer J Clin 62:10–29, 2012 [DOI] [PubMed] [Google Scholar]
- 3. Petrelli N, Douglass HO, Jr, Herrera L, et al. : The modulation of fluorouracil with leucovorin in metastatic colorectal carcinoma: a prospective randomized phase III trial. Gastrointestinal Tumor Study Group. J Clin Oncol 7:1419–1426, 1989 [DOI] [PubMed] [Google Scholar]
- 4. Poon MA, O'Connell MJ, Moertel CG, et al. : Biochemical modulation of fluorouracil: evidence of significant improvement of survival and quality of life in patients with advanced colorectal carcinoma. J Clin Oncol 71407–1418, 1989 [DOI] [PubMed] [Google Scholar]
- 5. Rothenberg ML, Cox JV, Butts C, et al. : Capecitabine plus oxaliplatin (XELOX) versus 5-fluorouracil/folinic acid plus oxaliplatin (FOLFOX-4) as second-line therapy in metastatic colorectal cancer: a randomized phase III noninferiority study. Ann Oncol 19:1720–1726, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Pohl A, Zhang W, Ning Y, et al. : Targeting metastatic colorectal cancer in 2008: a long way from 5-FU. Oncology (Williston Park) 22:456–462, 2008 [PubMed] [Google Scholar]
- 7. Genentech Inc: Avastin (bevacizumab) package insert. Revised March 2013. Available at: http://www.gene.com/gene/products/information/pdf/avastin-prescribing.pdf
- 8. Diaz-Rubio E, Gomez-Espana A, Massuti B, et al. : First-line XELOX plus bevacizumab followed by XELOX plus bevacizumab or single-agent bevacizumab as maintenance therapy in patients with metastatic colorectal cancer: the phase III MACRO TTD study. Oncologist 17:15–25, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Strickler JH, Hurwitz HI: Bevacizumab-based therapies in the first-line treatment of metastatic colorectal cancer. Oncologist 17:513–524, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Van Cutsem E, Rivera F, Berry S, et al. : Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol 20:1842–1847, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Hurwitz HI, Fehrenbacher L, Hainsworth JD, et al. : Bevacizumab in combination with fluorouracil and leucovorin: an active regimen for first-line metastatic colorectal cancer. J Clin Oncol 23:3502–3508, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Bristol-Myers Squibb Company: Erbitux (cetuximab) package insert. Revised February 2013. Available at: http://packageinserts.bms.com/pi/pi_erbitux.pdf
- 13. Amgen Inc.: Vectibix (panitumumab) package insert. Revised March 2013. Available at: http://pi.amgen.com/united_states/vectibix/vectibix_pi.pdf
- 14. Peeters M, Price T, Van Laethem JL: Anti-epidermal growth factor receptor monotherapy in the treatment of metastatic colorectal cancer: where are we today? Oncologist 14:29–39, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Di Fiore F, Sesboue R, Michel P, et al. : Molecular determinants of anti-EGFR sensitivity and resistance in metastatic colorectal cancer. Br J Cancer 103:1765–1772, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deschoolmeester V, Baay M, Specenier P, et al. : A review of the most promising biomarkers in colorectal cancer: one step closer to targeted therapy. Oncologist 15:699–731, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Head and Neck Cancers. Available at: http://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf
- 18. Khambata-Ford S, Garrett CR, Meropol NJ, et al. : Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol 25:3230–3237, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Amado RG, Wolf M, Peeters M, et al. : Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 26:1626–1634, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Allegra CJ, Jessup JM, Somerfield MR, et al. : American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol 27:2091–2096, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Lievre A, Bachet JB, Le Corre D, et al. : KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res 66:3992–3995, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Lievre A, Bachet JB, Boige V, et al. : KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol 26:374–379, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F, et al. : Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res 67:2643–2648, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Di Fiore F, Blanchard F, Charbonnier F, et al. : Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by Cetuximab plus chemotherapy. Br J Cancer 96:1166–1169, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Frattini M, Saletti P, Romagnani E, et al. : PTEN loss of expression predicts cetuximab efficacy in metastatic colorectal cancer patients. Br J Cancer 97:1139–1145, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. De Roock W, Piessevaux H, De Schutter J, et al. : KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol 19:508–515, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Bokemeyer C, Bondarenko I, Makhson A, et al. : Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 27:663–671, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Loupakis F, Ruzzo A, Cremolini C, et al. : KRAS codon 61, 146 and BRAF mutations predict resistance to cetuximab plus irinotecan in KRAS codon 12 and 13 wild-type metastatic colorectal cancer. Br J Cancer 101:715–721, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Van Cutsem E, Kohne CH, Hitre E, et al. : Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 360:1408–1417, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Di Nicolantonio F, Martini M, Molinari F, et al. : Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol 26:5705–5712, 2008 [DOI] [PubMed] [Google Scholar]
- 31. De Roock W, Claes B, Bernasconi D, et al. : Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol 11:753–762, 2010 [DOI] [PubMed] [Google Scholar]
- 32. Laurent-Puig P, Cayre A, Manceau G, et al. : Analysis of PTEN, BRAF, and EGFR status in determining benefit from cetuximab therapy in wild-type KRAS metastatic colon cancer. J Clin Oncol 27:5924–5930, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Yokota T, Ura T, Shibata N, et al. : BRAF mutation is a powerful prognostic factor in advanced and recurrent colorectal cancer. Br J Cancer 104:856–862, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ogino S, Nosho K, Kirkner GJ, et al. : CpG island methylator phenotype, microsatellite instability, BRAF mutation and clinical outcome in colon cancer. Gut 58:90–96, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tol J, Nagtegaal ID, Punt CJ: BRAF mutation in metastatic colorectal cancer. N Engl J Med 361:98–99, 2009 [DOI] [PubMed] [Google Scholar]
- 36. Eng C: The evolving role of monoclonal antibodies in colorectal cancer: early presumptions and impact on clinical trial development. Oncologist 15:73–84, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim R: Cetuximab and panitumumab: are they interchangeable? Lancet Oncol 10:1140–1141, 2009 [DOI] [PubMed] [Google Scholar]
- 38. Saridaki Z, Georgoulias V, Souglakos J: Mechanisms of resistance to anti-EGFR monoclonal antibody treatment in metastatic colorectal cancer. World J Gastroenterol 16:1177–1187, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mellor JD, Brown MP, Irving HR, et al. : A critical review of the role of Fc gamma receptor polymorphisms in the response to monoclonal antibodies in cancer. J Hematol Oncol 6:1–10, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. O'Neil BH, Allen R, Spigel DR, et al. : High incidence of cetuximab-related infusion reactions in Tennessee and North Carolina and the association with atopic history. J Clin Oncol 25:3644–3648, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Hopps S, Medina P, Pant S, et al. : Cetuximab hypersensitivity infusion reactions: Incidence and risk factors. J Oncol Pharm Pract Published online November 7, 2012 [DOI] [PubMed] [Google Scholar]
- 42. Chung CH, Mirakhur B, Chan E, et al. : Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med 358:1109–1117, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Van Cutsem E, Peeters M, Siena S, et al. : Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 25:1658–1664, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Peeters M, Price TJ, Cervantes A, et al. : Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol 28:4706–4713, 2010 [DOI] [PubMed] [Google Scholar]
- 45. Douillard JY, Siena S, Cassidy J, et al. : Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 28:4697–4705, 2010 [DOI] [PubMed] [Google Scholar]
- 46. Cunningham D, Humblet Y, Siena S, et al. : Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351:337–345, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Jonker DJ, O'Callaghan CJ, Karapetis CS, et al. : Cetuximab for the treatment of colorectal cancer. N Engl J Med 357:2040–2048, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Karapetis CS, Khambata-Ford S, Jonker DJ, et al. : K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 359:1757–1765, 2008 [DOI] [PubMed] [Google Scholar]
- 49. Van Cutsem E, Kohne CH, Lang I, et al. : Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 29:2011–2019, 2011 [DOI] [PubMed] [Google Scholar]
- 50. Sobrero AF, Maurel J, Fehrenbacher L, et al. : EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol 26:2311–2319, 2008 [DOI] [PubMed] [Google Scholar]
- 51. Wilke H, Glynne-Jones R, Thaler J, et al. : Cetuximab plus irinotecan in heavily pretreated metastatic colorectal cancer progressing on irinotecan: MABEL Study. J Clin Oncol 26:5335–5343, 2008 [DOI] [PubMed] [Google Scholar]
- 52. Maughan TS, Adams RA, Smith CG, et al. : Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet 377:2103–2114, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tveit KM, Guren T, Glimelius B, et al. : Phase III Trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: The NORDIC-VII study. J Clin Oncol 30:1755–1762, 2012 [DOI] [PubMed] [Google Scholar]
- 54. Bokemeyer C, Bondarenko I, Hartmann JT, et al. : Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol 22:1535–1546, 2011 [DOI] [PubMed] [Google Scholar]
- 55. Tabernero J, Ciardiello F, Rivera F, et al. : Cetuximab administered once every second week to patients with metastatic colorectal cancer: a two-part pharmacokinetic/pharmacodynamic phase I dose-escalation study. Ann Oncol 21:1537–1545, 2010 [DOI] [PubMed] [Google Scholar]
- 56. Tabernero J, Cervantes A, Rivera F, et al. : Pharmacogenomic and pharmacoproteomic studies of cetuximab in metastatic colorectal cancer: biomarker analysis of a phase I dose-escalation study. J Clin Oncol 28:1181–1189, 2010 [DOI] [PubMed] [Google Scholar]
- 57. Bouchahda M, Macarulla T, Liedo G, et al. : Feasibility of cetuximab given with a simplified schedule every 2 weeks in advanced colorectal cancer: a multicenter, retrospective analysis. Med Oncol 28(suppl 1):S253–S258, 2011 [DOI] [PubMed] [Google Scholar]
- 58. Roca JM, Alonso V, Pericay C, et al. : Cetuximab given every 2 weeks plus irinotecan is an active and safe option for previously treated patients with metastatic colorectal cancer. Chemotherapy 56:142–146, 2010 [DOI] [PubMed] [Google Scholar]
- 59. Martin-Martorell P, Rosello S, Rodriguez-Braun E, et al. : Biweekly cetuximab and irinotecan in advanced colorectal cancer patients progressing after at least one previous line of chemotherapy: results of a phase II single institution trial. Br J Cancer 99:455–458, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pfeiffer P, Nielsen D, Bjerregaard J, et al. : Biweekly cetuximab and irinotecan as third-line therapy in patients with advanced colorectal cancer after failure to irinotecan, oxaliplatin and 5-fluorouracil. Ann Oncol 19:1141–1145, 2008 [DOI] [PubMed] [Google Scholar]
- 61. Carneiro BA, Ramanathan RK, Fakih MG, et al. : Phase II study of irinotecan and cetuximab given every 2 weeks as second-line therapy for advanced colorectal cancer. Clin Colorectal Cancer 11:53–59, 2012 [DOI] [PubMed] [Google Scholar]
- 62. Mrabti H, De la Fouchardiere C, Desseigne F, et al. : Irinotecan associated with cetuximab given every 2 weeks versus cetuximab weekly in metastatic colorectal cancer. J Cancer Res Ther 5:272–276, 2009 [DOI] [PubMed] [Google Scholar]
- 63. Shitara K, Yuki S, Yoshida M, et al. : Phase II study of combination chemotherapy with biweekly cetuximab and irinotecan for wild-type KRAS metastatic colorectal cancer refractory to irinotecan, oxaliplatin, and fluoropyrimidines. Invest New Drugs 30:787–793, 2012 [DOI] [PubMed] [Google Scholar]
- 64. Kang MJ, Hong YS, Kim KP, et al. : Biweekly cetuximab plus irinotecan as second-line chemotherapy for patients with irinotecan-refractory and KRAS wild-type metastatic colorectal cancer according to epidermal growth factor receptor expression status. Invest New Drugs 30:1607–1613, 2012 [DOI] [PubMed] [Google Scholar]
- 65. Czejka M, Gruenberger B, Kiss A, et al. : Pharmacokinetics of irinotecan in combination with biweekly cetuximab in patients with advanced colorectal cancer. Anticancer Res 30:2355–2360, 2010 [PubMed] [Google Scholar]
- 66. Tahara M, Shirao K, Boku N, et al. : Multicenter phase II study of cetuximab plus irinotecan in metastatic colorectal carcinoma refractory to irinotecan, oxaliplatin and fluoropyrimidines. Jpn J Clin Oncol 38:762–769, 2008 [DOI] [PubMed] [Google Scholar]
- 67. Ciuleanu T, Nikolic V, Shmueli E, et al. : Cetuximab weekly (q1w) versus every two weeks (q2w) plus FOLFOX4 as first-line therapy in patients (pts) with KRAS wild-type (wt) metastatic colorectal cancer (mCRC). J Clin Oncol 29:762–3S, 2011. (abstr 494) [Google Scholar]
- 68. Yuan Y, Ma H, Cohen DJ, et al. : Activity and tolerance of biweekly CapeOx-cetuximab in 1st line therapy of metastatic colorectal cancer (mCRC): relation to K-ras mutation status. J Clin Oncol 27:(suppl), 2009. (abstr e15018) [Google Scholar]
- 69. De la Cruz S, Rodriguez J, Viudez A, et al. Biweekly cetuximab plus gemcitabine/oxaliplatin for patients with pretreated metastatic colorectal cancer (MCRC). Results from a prospective phase II study. ASCO Gastrointestinal Cancers Symposium, Orlando, FL, January 25–27, 2008. (abstr 377) [Google Scholar]
- 70. Jensen BV, Schou JV, Johannesen HH, et al. : Cetuximab every second week with irinotecan in patients with metastatic colorectal cancer refractory to 5-FU, oxaliplatin, and irinotecan: KRAS mutation status and efficacy. 2010 ASCO Annual Meeting Proceedings. J Clin Oncol 28:762–15S, 2010. (abstr 3573) [Google Scholar]
- 71. Viudez A, Zarate R, Garrido M, et al. : Dose escalation of capecitabine in combination with biweekly cetuximab and hepatic arterial infusion (HAI) of oxaliplatin in patients with liver metastases of colorectal cancer: preliminary clinical results. 2009 ASCO Annual Meeting Proceedings. J Clin Oncol 27:(suppl), 2009. (abstr e15080) [Google Scholar]
- 72. ClinicalTrials.gov: Bi-weekly cetuximab combined with FOLFOX-6 in metastatic colorectal cancer: CEBIFOX. Available at: http://clinicaltrials.gov/ct2/show/NCT01051167 Accessed January 11, 2013
- 73. ClinicalTrials.gov: Study evaluating biomarkers in patients with colorectal cancer and wild type KRAS gene treated with chemotherapy and cetuximab (POSIBA). Available at: http://clinicaltrials.gov/ct2/show/NCT01276379 Accessed January 11, 2013
- 74. Clinicaltrials.gov: Safety and efficacy of FOLFOX4 + weekly cetuximab vs. FOLFOX 4+ biweekly cetuximab by metastatic colorectal cancer (CORE 2). Available at: http://clinicaltrials.gov/ct2/show/NCT00479752 Accessed January 11, 2013