CASE REPORT
Dr. Won: This is the case of a 72-year-old woman who presented to her primary care physician with persistent left upper quadrant abdominal pain. Abdominal ultrasound revealed a pancreatic mass. Subsequent CT imaging confirmed a 2.4 cm pancreatic head mass with multiple small volume liver lesions, lymphadenopathy, and a peritoneal implant. Biopsy of one of the liver lesions confirmed metastatic adenocarcinoma, consistent with a pancreatic primary.
Dr. Abou-Alfa: Dr. O'Reilly, would you expect this patient to be jaundiced on presentation?
Dr. O'Reilly: Pancreatic head tumors classically present with painless jaundice. However, it is not uncommon for patients, especially those with uncinate tumors or small masses, not to present with jaundice.1 The presence of a large exophytic pancreatic head mass would raise the suspicion of a neuroendocrine tumor.2
Dr. Won: The patient's past medical history was significant for hypertension, dyslipidemia, and hypothyroidism. She was a former smoker, with a 55-pack-a-year smoking history. Her family history was notable for a brother with a leukemia of unknown type diagnosed at age 50 and a half-sister with uterine cancer.
Dr. Abou-Alfa: What, if any, risk factor does this patient have for developing pancreatic cancer?
Dr. O'Reilly: Smoking is a known epidemiological risk factor for pancreatic cancer; however, the relative risk contribution of cancer is much less than in lung cancer. Smoking confers a 2.5–3 times higher risk of pancreatic cancer but is associated with 30× higher risk of lung cancer.3 Prospective data show that patients with a family history of pancreatic cancer have lower risk of developing pancreatic cancer than smokers.4
Dr. Abou-Alfa: Dr. Shamseddine, what are the data regarding smoking and the risk of cancer in Lebanon?
Dr. Shamseddine: Smoking prevalence rates among men are in the range of 50%–60%. In women, the prevalence has increased from 28% in the 1960s to 35% in 1992.5 Based on our Tumor Registry at the American University of Beirut Medical Cancer, the most common cancers in men are lung (28.5 per 100,000 cases) and bladder cancer (28 per 100,000 cases), which can be attributed to smoking habits.6 In women, the most common cancer is breast (69.15 per 100,000), followed by colorectal (10.75 per 100,000).6 Based on the 2003 Registry data, pancreatic cancer accounts for only 1.5% of cancers.7 Over the past 30 years, the frequency of lung cancer has increased in both sexes; however, colorectal has decreased, contrary to what is seen in Western countries. We postulate that may be the result of dietary differences and the Mediterranean diet. We continue to collect data in the Tumor Registry about other malignancies and their risk factors, including pancreatic cancer.
Dr. Yu: I would add that in patients who stop smoking for more than 10 years, their risk of developing pancreatic cancer drops to the same level as the general population.8,9 Age is also a risk factor for pancreatic cancer, with each decade of life leading to increased risk.10 In regard to family history, there is a half-sister with uterine cancer; if there was a strong history of colon cancer, one may consider a familial Hereditary Nonpolyposis Colorectal Cancer (HNPCC) or Lynch syndrome; however, that is not present in this patient's history.11
Dr. O'Reilly: The presence of leukemia in a first-degree relative with pancreatic cancer makes one consider whether there is a potential link of a p53 mutation. Li-Fraumeni syndrome is an autosomal dominant disorder characterized by p53 gene mutation in which patients develop multiple malignancies including sarcomas, breast cancer, and leukemias, with an early age of onset of cancer.12
Dr. Abou-Alfa: If this patient had a daughter who was very concerned for her risk of pancreatic cancer, would you recommend screening for pancreatic cancer?
Dr. O'Reilly: This is an area of continued research. There is no proven screening intervention that alters the natural history of pancreatic cancer. There is indirect evidence that in selected high-risk families, such as in BRCA carriers, and possibly HNPCC and hereditary pancreatitis patients, there may be a benefit of surveillance.13,14 Other groups of patients who may benefit from surveillance are those with intraductal papillary mucinous neoplasms (IPMNs) seen on radiographic imaging.15 There is no consensus on what screening test should be used or how often surveillance should be done. In this individual, with no clearly defined genetic syndrome, the patient should undergo recommendations for the general population, eg, avoid or stop smoking. It is important to recognize that surveillance measures in nonselected populations can lead to unnecessary evaluation stemming from the high false positive rate, and they are of uncertain utility.14
Dr. Won: The patient's review of symptoms was notable for intermittent mild back pain relieved with NSAIDS and was otherwise negative. She remained physically active and able to complete all her activities of daily living. On physical examination, she was a robust woman with vitality, who appeared younger than her stated age of 72. White blood cell count (WBC) was 15.3 K/mcL with an absolute neutrophil count (ANC) of 9.9 K/mcL and monocyte count of 0.9 K/mcL, hemoglobin 12.7 g/dl, and platelets 372,000 mcL. A basic metabolic panel and liver function tests were normal with a bilirubin of 0.2 mg/dl. Tumor markers included a cancer antigen (CA) 19–9 that was elevated to 609 units/mL and a carcinoembryonic antigen (CEA) of 10.8 ng/dl.
Dr. Abou-Alfa: Is further staging evaluation necessary prior to treatment?
Dr. Naghy: I am concerned about the patient's back pain symptoms, which could represent bony metastatic disease. Is there a role of bone scan in complete staging?
Dr. Yu: Back pain is very common in pancreatic cancer and usually results from retroperitoneal extension of the tumor, and rarely from metastatic disease. Our practice at MSKCC is that once metastatic disease is established, I do not do further staging in the absence of symptoms. In the setting of adenocarcinomas, bone scans are not indicated in the absence of symptoms. If the patient had back pain symptoms that were concerning, a bone scan would not be the ideal modality for evaluation. In that setting, an MRI of the spine would be the better imaging tool.
Dr. Won: Dr. Haydar, can you please comment on the CT scan?
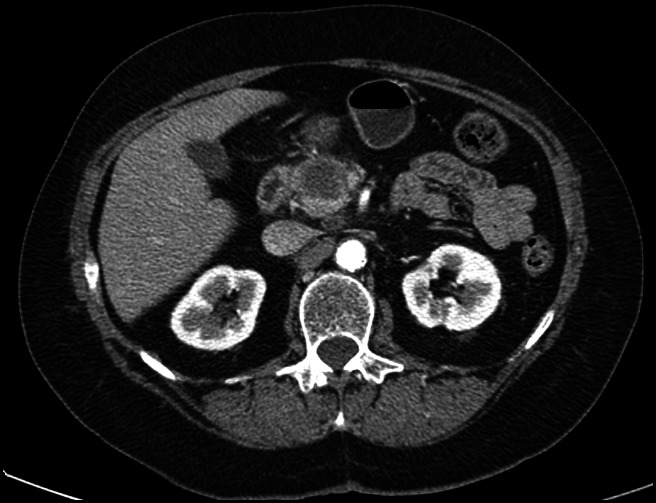
Dr. Haydar: The images we are looking at form the portal venous phase, with a hypo-attenuating lesion in the pancreas, in between the duodenum and the SMA (Figure 1). There are few lymph nodes around the left renal vein. In the liver, multiple small hypo-attenuating lesions are seen in segment 4A, segment 3, and segment 2, suspicious for metastasis.
Figure 1.
Baseline CT abdomen with contrast.
Dr. Abou-Alfa: Dr. Shamseddine, what would be your treatment recommendations for this patient?
Dr. Shamseddine: FOLFIRINOX is to be considered in this fit patient. The FOLFIRINOX data are level 1 evidence for advanced pancreatic cancer. The ACCORD/PRODIGE study randomized phase III study for patients with untreated metastatic pancreatic cancer to FOLFIRINOX vs. gemcitabine.16 All patients had an ECOG 0–1 performance status. The results showed a tripling of the response rate (31.6% compared to 9.4%) and doubling of median survival with 11.1 months in the FOLFIRINOX arm compared to 6.4 months in the gemcitabine arm. This study showed the biggest increment improvement in the treatment of pancreatic cancer to date.
Dr. Yu: This patient presented to the clinic in September 2010, shortly after the FOLFIRINOX data were presented at ASCO 2010.17 At that point, there was still significant debate about whether to use this regimen, especially in view of the significant toxicity. However, the data were very compelling, and despite the patient's advanced age, she was very fit and functional, so we decided to go ahead with treatment with FOLFIRINOX.
Dr. Won: There were significantly more grade 3 and 4 toxicities in the FOLFIRINOX group. These included febrile neutropenia, thrombocytopenia, diarrhea, sensory neuropathy, and elevation in liver enzymes. Despite this, the subgroup analysis showed that even older patients ≥65 years old did better in the FOLFIRINOX arm compared to gemcitabine.
Dr. Abou-Alfa: Can our colleagues at the American University of Beirut and the National Guard Hospital in Riyadh tell us about their experience with FOLFIRINOX?
Dr. Mukherjee: Given the patient's good performance status and the data from the ACCORD study, we would also recommend this regimen for this patient, and we are already using it at the American University of Beirut.
Dr. El-Olayan: At the National Guard Hospital in Riyadh, we still favor single-agent gemcitabine or gemcitabine in combination with a platinum compound if the performance status of the patient allows. Unfortunately, the majority of our patients in Riyadh present late with very advanced disease and are frail, thus are less likely to tolerate FOLFIRINOX.
Dr. Saltz: FOLFIRINOX is a regimen that we have in our toolbox to use against pancreatic cancer; however, it is not for all patients, as we have discussed. We all agree that it should be reserved to patients with a good performance status. The data are compelling to support its use. Chronological age should not, however, determine the use of aggressive regimens; rather, the functional age should be considered.
Dr. Naghy: I still feel that gemcitabine should be used. The toxicity of FOLFIRINOX is worse than gemcitabine, and shouldn't I worry about quality of life?
Dr. O'Reilly: In this study, quality-of-life measures were measured and were equivalent in the two groups.16 Poorly controlled pancreatic cancer is the biggest degradation for quality of life. For many, the balance is in favor of better disease control when we consider the symptomatology of advanced pancreatic cancer.
Dr. Abou-Alfa: Dr. Yu, would you give FOLFIRINOX? Would you give the full dose regimen as reported in the ACCORD/PRODIGE study?
Dr. Yu: This needs to be carefully considered. If you look carefully at the ACCORD/PRODIGE study data, the median dose of FOLFIRINOXreceived was approximately 80%. Especially in a patient who is 72 years old, I would start at 80% of the reported dose.
Dr. O'Reilly: Our MSKCC experience was presented at ASCO 2012.18 We did a retrospective study starting in 2010, identifying patients with stage III or IV pancreatic cancer with an ECOG 0–1 who received FOLFIRINOX and evaluated their outcomes. Baseline characteristics were very similar to the trial, with the exception that the MSKCC review included 29 stage III patients. The outcomes were very similar to what was reported in the ACCORD study. The median overall survival was equivalent at 11.1 months. Patients were treated with an 80% dose reduction compared to the parent regimen. Our toxicity rates were slightly less than the original trial. Growth factor was used in almost 80% of patients. Dose reductions were made in 52% of stage IV patients and in 47% of stage III patients. Furthermore, 44% of patients were able to receive second-line treatment with gemcitabine-based treatment. Our experience at MSKCC shows that FOLFIRINOX is an active regimen with acceptable toxicity in selected patients treated at 80% dose intensity. Based on each individual patient's clinical and functional status, you can justify the use of single-agent therapy, doublet, or three drugs as in FOLFIRINOX.
Dr. Won: The patient was started on FOLFIRINOX with an 80% dose reduction. G-CSF was given with each cycle. The patient tolerated treatment very well, and the dose was escalated to 90% of the parent dosage for cycle 4. She developed nausea and vomiting, and the dosage was reduced back to 80%. She required one hospitalization for diarrhea. The patient developed anemia and thrombocytopenia requiring dose reductions, with subsequent recovery of her blood counts to hemoglobin of 11–12 g/dl and platelet count ranging from 128,000 to 289,000/ mcL. After 6 cycles of treatment, she had a great response to treatment with significant decrease in the primary mass, hepatic lesions, and lymph nodes. Her CA19–9 level dropped from 609 to 47 units/ml. After 12 cycles, the oxaliplatin was stopped for sensory neuropathy, and she was maintained on FOLFIRI with continued response.
Nineteen months into treatment, the patient developed herpes zoster. She was treated with acyclovir with resolution of the infection. When she returned for treatment, her CBC showed a WBC 10 K/mcL, Hgb 9.6 g/dl, and platelets 61,000 mcL. Treatment was held for the thrombocytopenia. It was felt that the recent viral infection and medication contributed to the thrombocytopenia. However, her platelet count did not recover, and a few weeks later, she was found to have hemoglobin 6 g/dl and platelet of 12,000 mcL without clinical bleeding. The patient was admitted to the hospital for evaluation. She received red blood cell and platelet transfusions. Workup revealed no signs of hemolysis (Table 1).
Table 1.
Laboratory data during hospitalization
| Variable | Reference range, adults | On admission | Day 2, post-transfusions |
|---|---|---|---|
| Hematocrit (%) | 34–46 | 18.2 | 27.4 |
| Hemoglobin (g/dl) | 11.5–16 | 7.4 | 9.5 |
| Platelet (mcL) | 160,000–400,00 | 12,000 | 53,000 |
| White blood cell count (K/mcL) | 4–11 | 7.4 | 6.6 |
| Differential count (%) | |||
| Neutrophils | 38–80 | 21 | |
| Lymphocytes | 12–48 | 52 | |
| Monocytes | 0–12 | 24 | |
| Eosinophils | 0–7 | 1 | |
| Blasts | 0 | 0 | |
| Reticulocyte count | 1.1 | ||
| Haptoglobin (mg/dl) | 30–200 | 74 | |
| Fibrinogen (mg/dl) | 190–387 | 346 | |
| Urea nitrogen (mg/dl) | 6–20 | 20 | |
| Creatinine (mg/dl) | 0.6–1.3 | 1.1 | |
| Total bilirubin (mg/dl) | 0–1 | 0.6 | |
| Asparate aminotransferase (units/L) | 10–27 | 29 | |
| Alanine aminotransferase (units/L) | 5–37 | 22 | |
| Lactate dehydrogenase (units/L) | 12–246 | 562 | |
| Iron (mcg/dl) | 34–165 | 311 | |
| Ferritin (ng/ml) | 6–200 | 646 | |
| Vitamin B12 (pg/ml) | 211–9111 | >1600 | |
| Thyroid-stimulating hormone (mcUnits/ml) | 0.55–4.78 | 4.75 |
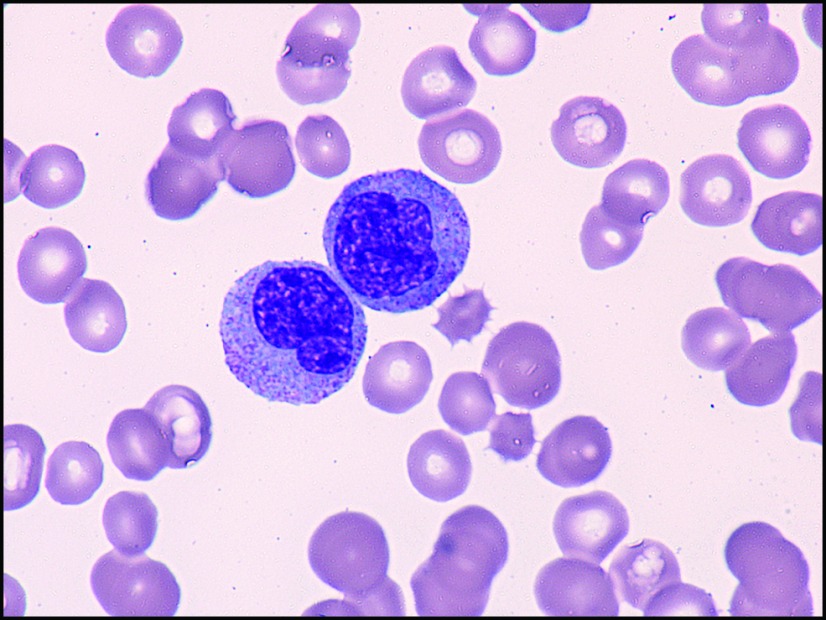
A blood smear showed red cell dysplasia, rare blasts, left shift of myeloid cells, and a paucity of platelets (Figure 2). The hematology service was consulted; the anemia and thrombocytopenia were felt to be due to extensive chemotherapy. The left shift and rare blasts could be seen in a patient who has received recent growth factors. The patient was discharged from the hospital after repeat blood counts remained stable after transfusion with hemoglobin 9.5 g/dl and platelet 53,000 mcL.
Figure 2.
Peripheral blood smear: high-power view (100×) of monocytes.
Dr. Saltz: The fact that she was so anemic with a reticulocyte count of 1.1 suggests a compromised bone marrow from prolonged chemotherapy. The other question that arises is how much of the thrombocytopenia is from sequestration from hepatic fibrosis given the long duration of time she was on irinotecan-based treatment.
Dr. Abou-Alfa: This brings up an important point. She has now been on almost 2 years of therapy, but where do you draw the limit of how much FOLFIRINOX to give?
Dr. Yu: In general, we are very reluctant to stop chemotherapy in patients with pancreatic cancer. We maintain patients on FOLFIRI after stopping oxaliplatin for neuropathy. In a phase II trial that compared FOLFIRI alternating with gemcitabine vs. gemcitabine alone in untreated metastatic pancreatic cancer, the response rate was much higher in the FOLFIRI group with a doubling of 6-month PFS compared to gemcitabine alone.19 We discuss these data to show that some data support keeping patients on FOLFIRI after FOLFIRINOX.
Dr. Saltz: In colon cancer, with the addition of oxaliplatin, there was a beginning of discussion regarding chemotherapy holidays. The OPTIMOX study in colon cancer is a very important study and for me defines the management of metastatic disease.20 The issues surrounding pancreatic cancer are the same ones addressed in the colon cancer 10 years ago. The issue in pancreatic cancer is how far to push the oxaliplatin. We all know that neuropathy is an important detriment to the quality of life and not very well assessed in the formal quality-of-life assessments.
Dr. O'Reilly: This is a dilemma that we did not have in the previous era of pancreatic cancer: what to do with our patients who are doing well on long duration of treatment. We are starting to explore treatment breaks in a carefully selected subset of patients with well-controlled disease.
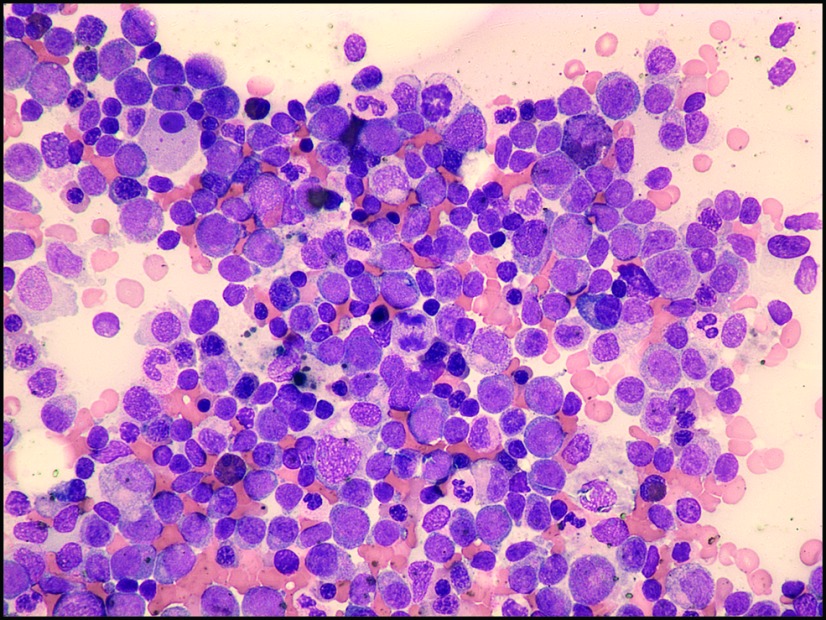
Dr. Won: Unfortunately repeat blood work one week later showed persistent cytopenias, WBC 7.3 K/mcL, hemoglobin 7.5 g/dl, and platelet 10,000 mcL. Differential counts revealed absolute neutrophil count (ANC) 1 K/mcL with elevated monocytes at 45%. The patient had a bone marrow biopsy and aspirate performed. This revealed a mildly hypercellular marrow with 10% blasts, 27% monocytes, erythroid dysplasia, and decreased megakaryocytes (Figure 3). Based on the multilineage dysplasia seen, blast count of less than 20%, and overproduction of mature monocytic cells, a diagnosis of myelodysplasia/chronic monomyelocytic leukemia was made.
Figure 3.
Bone marrow aspirate is relatively hypercellular with monocytic and dysplastic myeloid elements (40×).
Dr. Abou-Alfa: What do you think about this diagnosis of myelodysplasia/chronic myelomonocytic leukemia in a patient with long duration of treatment for pancreatic cancer?
Dr. Yu: In retrospect, the patient has a family history of leukemia and presented with mild leukocytosis. Even prior to her pancreatic cancer diagnosis, she has had a long-standing history of mild leukocytosis that was not previously evaluated. The other aspect is that she has been on ongoing chemotherapy for a long time, and whether this played a role in the evolution of any underlying bone marrow disorder she may have had.
Dr. Saltz: I would be very careful about making any assumptions about association. In the colorectal cancer experience, an enormous number of patients have been treated so far with FOLFOX and/or FOLFIRI with no increased incidence of MDS or leukemia.
Dr. Won: Therapy-related MDS accounts for 10%–20% of cases of MDS or acute myelogenous leukemia (AML). It is classically associated with exposure to alkylating agents or radiation with an average latency period of 5–7 years and associated with chromosome 5 and 7 abnormalities.21 Exposure to topoisomerase II inhibitors (etoposide, doxorubicin, epirubicin) has been associated with a shorter latency period of 1–3 years after chemotherapy.22 In this case, patients often present with overt leukemia and rarely with MDS. Cytogenetic abnormalities associated with topoisomerase inhibitors are 11q26, t(9,11), or 21q22.22 There have been very few case reports (total of 6 cases) describing MDS/AML as therapy-related effects after exposure to oxaliplatin.23–25
Dr. Saltz: It is very important to recognize the very small number of case reports of therapy-related MDS/AML in a five-year period and consider the many thousands of patients treated with similar regimens in the same time period. This incidence is similar to the incidence of MDS/leukemia in the general population, so one must be very skeptical to make an association for therapy-related MDS.
Dr. Temraz: I agree with Dr. Saltz. I do not think that this is a therapy-related MDS. If it were, the prognosis is dismal, regardless of treatment. The only curative approach would be an allogeneic bone marrow transplant, which the patient is not a candidate for given her stage IV pancreatic cancer.
Dr. Makanjoula: If we go back to the original debate about FOLFIRINOX vs. gemcitabine-based treatment to balance the toxicity and morbidity effects, has the decision to use FOLFIRINOX been worth the complications? I do not think this is a therapy-related MDS, but I ask this question in regard to the chemotherapy toxicity, hospitalization for diarrhea, and development of neuropathy she experienced.
Dr. Yu: Over two years and prior to the MDS diagnosis, the patient was hospitalized only once for the diarrhea. She has been living her life and traveling and came to the clinic once every two weeks. I think FOLFIRINOX controlled this patient's disease, thereby allowing her to have an excellent quality of life. If the MDS had never developed, she would have continued with her lifestyle. Even now, although she does require transfusions, she still feels very well.
Dr. Won: The patient's bone marrow cytogenetic analysis did not show a del 5q, del 7q or monosomy 5, 7, or del 20q. She was started on treatment for her myelodysplastic syndrome with decitabine, a DNA hypomethylating agent. Repeat bone marrow analyses after three cycles of treatment show an improvement in her blast count. She remains pancytopenic and is transfusion dependent. In regard to the pancreatic cancer, the most recent CT scan shows that our patient continues to have stable disease three months off treatment.
Dr. Abou-Alfa: The paradigm for the management of advanced pancreatic cancer is changing with new treatment regimens like FOLFIRINOX leading to longer durations of response and disease control. Treatment for advanced pancreatic cancer requires a thoughtful consideration of a patient's functional status and overall health. This case illustrates the challenges of managing the longer term toxicities of metastatic pancreatic cancer. Our patient likely had an underlying subclinical myelodysplasia prior to her pancreatic cancer and did not develop a therapy-related myelodysplasia. Her prolonged chemotherapy for pancreatic cancer may have accelerated bone marrow failure.
Acknowledgments
This case was presented at the MSKCC/American Univesity of Beirut/National Guard Hospital, Riyadh case conference in August 2012. The conference is supported by an endowment gift of Mrs. Mamdouha El-Sayed Bobst and the Bobst Foundation.
REFERENCES
- 1. Porta M, Fabregat X, Malats N, et al. : Exocrine pancreatic cancer: symptoms at presentation and their relation to tumour site and stage. Clin Transl Oncol 7:189–197, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Furukawa H, Mukai K, Kosuge T, et al. : Nonfunctioning islet cell tumors of the pancreas: clinical, imaging and pathological aspects in 16 patients. Jpn J Clin Oncol 28:255–261, 1998 [DOI] [PubMed] [Google Scholar]
- 3. U.S. Department of Health and Human Services How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease: a report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2010 [PubMed] [Google Scholar]
- 4. Hassan MM, Bondy ML, Wolff RA, et al. : Risk factors for pancreatic cancer: case-control study. Am J Gastroenterol 102:2696–2707, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shamseddine A, Sibai A, Gehchan N, et al. : Postwar Lebanon: findings from the first national population-based registry, 1998. Ann Epidemiol 14:663–668, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Shamseddine A, Musallam KM: Cancer epidemiology in Lebanon. Middle East J Cancer 1:41–44, 2010 [Google Scholar]
- 7. National Cancer Registry: Cancer in Lebanon 2003. Available from: http://www.moph.gov.lb/Publications/Pages/NationalCancerRegistry2003.aspx
- 8. Fuchs CS, Colditz GA, Stampfer MJ, et al. : A prospective study of cigarette smoking and the risk of pancreatic cancer. Arch Intern Med 156:2255–2260, 1996 [PubMed] [Google Scholar]
- 9. Lynch SM, Vrieling A, Lubin JH, et al. : Cigarette smoking and pancreatic cancer: a pooled analysis from the pancreatic cancer cohort consortium. Am J Epidemiol 170:403–413, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Howlader N, Noone AM, Krapcho M, et al. (eds): SEER cancer statistics review, 1975–2009. National Cancer Institute; Bethesda, MD, available from: http://seer.cancer.gov/csr/1975_2009_pops09/ [Google Scholar]
- 11. Kastrinos F, Mukherjee B, Tayob N, et al. : Risk of pancreatic cancer in families with Lynch syndrome. JAMA 302:1790–1795, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li FP, Fraumeni JF, Jr, Mulvihill JJ, et al. : A cancer family syndrome in twenty-four kindreds. Cancer Res 48:5358–5362, 1988 [PubMed] [Google Scholar]
- 13. Canto MI, Hruban RH, Fishman EK, et al. : Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology 142:796–804, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Verna EC, Hwang C, Stevens PD, et al. : Pancreatic cancer screening in a prospective cohort of high-risk patients: a comprehensive strategy of imaging and genetics. Clin Cancer Res 16:5028–5037, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Tanaka M, Chari S, Adsay V, et al. : International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology 6:17–32, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Conroy T, Desseigne F, Ychou M, et al. : FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364:1817–1825, 2011 [DOI] [PubMed] [Google Scholar]
- 17. Conroy T, Desseigne F, Ychou M, et al. : Randomized phase III trial comparing FOLFIRINOX (F: 5FU/leucovorin [LV], irinotecan [I], and oxaliplatin [O]) versus gemcitabine (G) as first-line treatment for metastatic pancreatic adenocarcinoma (MPA): preplanned interim analysis results of the PRODIGE 4/ACCORD 11 trial. Presented at the American Society of Clinical Oncology Annual Meeting, Chicago, IL, June 4–8, 2010 [Google Scholar]
- 18. Lowery M, Yu K, O'Reilly EM, et al. : Activity of front-line FOLFIRINOX (FFX) in stage III/IV pancreatic adenocarcinoma (PC) at Memorial Sloan-Kettering Cancer Center (MSKCC). J Clin Oncol 28:15s, 2010. (suppl; abstr 4152) [Google Scholar]
- 19. Trouilloud I, Dupont-Gossard AC, Artru P, et al. : FOLFIRI. 3 (CPT-11 plus folinic acid pluse 5-FU) alternating with gemcitabine or gemcitabine (G) alone in patients (pts) with previously untreated metastatic pancreatic adenocarcinoma (MPA): results of the randomized multicenter AGEO phase II trial FIRGEM. J Clin Oncol 30, 2012. (suppl; abstr 4018) [Google Scholar]
- 20. Tournigand C, Cervantes A, Figer A, et al. : OPTIMOX1: A randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-go fashion in advanced colorectal cancer: a GERCOR study. J Clin Oncol 24:394–400, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Pedersen-Bjergaard J, Rowley JD: The balanced and the unbalanced chromosome aberrations of acute myeloid leukemia may develop in different ways and may contribute differently to malignant transformation. Blood 83:2780–2786, 1994 [PubMed] [Google Scholar]
- 22. Pui C-H, Relling MV: Topoisomerase II inhibitor-related acute myeloid leukemia. Br J Haematol 109:13–23, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Carneiro BA, Kaminer L, Eldibany M, et al. : Oxaliplatin-related acute myelogenous leukemia. Oncologist 11:261–262, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Merrouche Y, Mugneret F, Cahn JY: Secondary acute promyelocytic leukemia following irinotecan and oxaliplatin for advanced colon cancer. Ann Oncol 17:1025–1026, 000 [DOI] [PubMed] [Google Scholar]
- 25. Damodaran S, Bellavia T, Sait SN, et al. : Acute myeloid leukemia secondary to oxaliplatin treatment for esophageal cancer. Clin Colorectal Cancer 11:151–154, 2012 [DOI] [PubMed] [Google Scholar]