Abstract
Idiopathic intracranial hypertension (IIH) is a condition of unknown etiology often encountered in neurologic practice. It produces non-localizing symptoms and signs of raised intracranial pressure and, when left untreated, can result in severe irreversible visual loss. It most commonly occurs in obese women of childbearing age, but it can also occur in children, men, non-obese adults, and older adults. While it is frequently associated with obesity, it can be associated with other conditions, such as obstructive sleep apnea and transverse cerebral venous sinus stenoses. Recent identification of subgroups at high risk for irreversible visual loss, including black patients, men, and patients with fulminant forms of IIH, help guide the optimal management and follow-up. Ongoing studies of venous anatomy and physiology in IIH patients, as well as a recently begun randomized clinical treatment trial, should provide more insights into this common yet poorly understood syndrome of isolated intracranial hypertension.
Keywords: Idiopathic Intracranial Hypertension, Venous Sinus Stenosis, Ventriculoperitoneal Shunting, Lumboperitoneal Shunting, Optic Nerve Sheath Fenestration, Venous Sinus Stenting
Introduction
Idiopathic intracranial hypertension (IIH) is a syndrome in which there is increased intracranial pressure (ICP) of unknown etiology. Previously known as “pseudotumor cerebri” or “benign intracranial hypertension”, this condition most frequently occurs in obese women of childbearing age. It is defined by the revised modified Dandy criteria,1 including: 1) symptoms and signs of raised ICP (headache, nausea, vomiting, pulsatile tinnitus, transient visual obscurations, papilledema); 2) no localizing signs, except for sixth nerve palsy; 3) no cause for raised ICP identified on neuroimaging (brain MRI); 4) cerebrospinal fluid (CSF) opening pressure of greater than 25 cmH2O, with normal CSF composition; and 5) no alternate explanation for the raised ICP. While it is well known that untreated IIH can result in severe irreversible visual loss, major controversies remain regarding its pathophysiology, natural history, and treatment.
What’s in a name? “Idiopathic intracranial hypertension”
Idiopathic intracranial hypertension, by its very name, assumes that no underlying cause for raised ICP has been identified. IIH is a diagnosis of exclusion that can only be made in patients who satisfy the updated modified Dandy criteria.1 Brain imaging studies, including a brain MRI, often with additional specific venous sequences (such as CT or MR venography), and a lumbar puncture are therefore required in the evaluation of patients with suspected IIH. Numerous studies have emphasized that a head CT is not sufficient to exclude other causes of raised ICP, such as cerebral venous thrombosis.2 By definition, IIH includes all patients with isolated raised ICP that is not related to an intracranial process, cerebral venous thrombosis, or a meningeal process. Somewhat ironically, patients who develop a syndrome of raised ICP secondary to specific medications or who are found to have a cerebral venous sinus stenosis (not thrombosis) are still conventionally classified as having IIH. Therefore, although not always perfect, the term “IIH” is the preferred designation for this disorder in the English literature. The term “pseudotumor cerebri” should not be used, as it often includes patients with other causes of raised ICP and is thus confusing. The term “benign intracranial hypertension” erroneously suggests that this disorder is benign, whereas up to 31% of patients with IIH irreversibly lose vision.3 It has been suggested that the terms “primary” and “secondary” intracranial hypertension might be considered to describe either 1) the young obese women with isolated raised ICP and no obvious precipitating factors, or 2) patients with isolated raised ICP associated with factors such as endocrine disorders, anemia, obstructive sleep apnea, medications, or cerebral venous sinus stenoses.4,5 At this point, the preferred term “idiopathic intracranial hypertension” emphasizes our general lack of understanding of the pathophysiology of this disorder.
Update on the epidemiology of IIH
In the United States, the annual incidence of IIH has been reported to be 0.9 per 100,000 in Iowa and 1.07 per 100,000 in Louisiana.6 IIH is more common in women and obese individuals.6–9 Among women aged 20–44 years who are 20% or more above ideal body weight, the incidence increases to approximately 19/100,000, about 20 times the incidence in the general population and similar to the incidence of multiple sclerosis.6,10 Obese women with IIH might have a preferential accumulation of fat in the lower body relative to other obese women in the same age range.11 In a recent multicenter case-control study of newly-diagnosed women with IIH compared to women who were newly-diagnosed with other neuro-ophthalmologic disorders, it was confirmed that higher body mass index is associated with a greater risk of IIH.12 Interestingly, this study showed that even non-obese patients were at greater risk for IIH if they had a recent moderate weight gain. Thus, it is likely that the prevalence of IIH in the developed world will rise in parallel with that of obesity.10
Despite a high predilection for obese young women, IIH can occur in children, older adults, and in non-obese persons of either sex.13,14 IIH does occur in childhood, but no large epidemiologic studies in this age group have been reported to date. The disorder is rare in pre-pubertal children and has distinct characteristics from the adult form, including no apparent predilection for obese girls.13,15 In older teenagers with IIH, however, the rate of obesity is similar to that in the adult IIH population.13–15 In a recent large series, the prevalence of IIH in men was approximately 10%,9 thereby confirming that IIH in men is rare.16–19 Affected men have a similar body mass index when compared with affected women, but are, on average, about a decade older than women at the time of presentation.9 Ethnic background is not thought to affect the incidence of IIH,8,20 although few studies have addressed this question. Familial cases are occasionally encountered, but there is little evidence to support a genetic predisposition.21
Clinical features of IIH
Headache
IIH typically presents with symptoms and signs of raised ICP. Headache, the most common symptom at presentation,18,22 is less likely to be reported by men than by women.9 The headache of IIH is typically holocranial, worse after waking, and exacerbated by maneuvers that increase ICP. If it is predominantly due to raised ICP, it can improve dramatically following a lumbar puncture or a CSF diversion procedure. However, many patients have a coexisting primary headache disorder, such as migraine or tension headache,23 and hence the headache does not always respond to treatments that decrease ICP.
Papilledema and visual loss
Papilledema is the most common sign at presentation and is indistinguishable from papilledema occurring with other causes of raised ICP (Fig. 1). In IIH patients, the severity of papilledema does not correlate with age, race, or body mass index.24 It is usually symmetric, but can be asymmetric, unilateral, or even absent.25–28 Some studies have suggested that, in patients with asymmetric or absent papilledema, anatomic compartmentalization of the subarachnoid space around the optic nerve might stop the CSF pressure gradient from reaching the retrolaminar portion of the optic nerve.29,30 Papilledema can be associated with peripapillary hemorrhages, exudates, and macular edema.
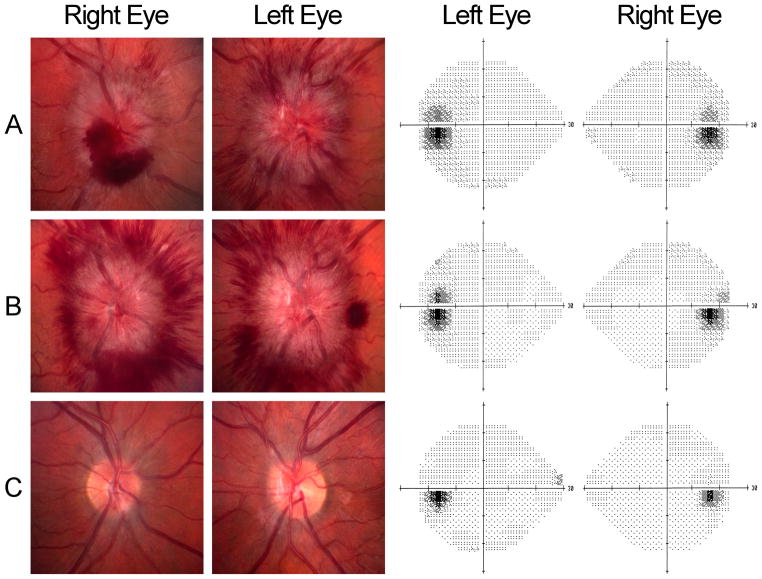
Figure 1. Papilledema and subtle visual field changes in an obese young woman with IIH.
A: At presentation, the patient had severe headache, bilateral papilledema (elevated optic nerves, peripapillary hemorrhages, and dilated retinal veins), and enlargement of the physiologic blind spots on 24-2 Humphrey visual fields. Following a diagnostic lumbar puncture, her headache improved for several days. She had refused to take acetazolamide, but attempted to lose weight.
B: The patient’s headaches and papilledema worsened, despite one lumbar puncture and attempts at weight loss, but her visual fields remained stable. Since she reported dramatic, but transient, improvement in symptoms following serial lumbar punctures, she was referred for a CSF shunting procedure.
C: Three months following lumbo-peritoneal shunting, her symptoms and signs had resolved. The optic nerves remained mildly elevated, with mild gliosis and pallor, but her visual fields were full.
Papilledema often produces brief episodes of monocular or binocular visual loss, called transient visual obscurations (TVOs).18,22 TVOs are often precipitated by postural changes and Valsalva-like maneuvers, with vision rapidly recovering back to baseline between episodes. TVOs are thought to occur due to transient ischemia of the swollen optic nerve head and, although dramatic, are not a sign of impending permanent visual loss. Rarely, IIH patients with papilledema can report gaze-evoked amaurosis, which is transient visual loss precipitated by movement of the eye into an eccentric gaze position.31
Untreated papilledema can result in progressive irreversible visual loss and secondary optic atrophy in up to 31% of patients.16,18,32–34 However, sustained visual loss is less commonly reported than TVOs at the time of presentation.18 Irreversible visual loss is more likely to occur in men,9 black patients,8 patients with a fulminant onset of IIH,35 and patients with coexisting systemic hypertension16 or anemia.36 In most IIH patients, papilledema produces insidious and slowly progressive visual loss. Enlargement of the physiologic blind spot (Fig. 1) is the earliest visual field defect to occur and is not of great concern, since it is simply a refractive scotoma resulting from elevation of the peripapillary retina. Other visual field defects develop with time, including nasal (especially inferonasal) defects, arcuate defects, and severe visual field constriction (Fig. 2).18,37 These visual field defects may not be recognized by patients and may only be evident on formal perimetry. Central visual field defects and decreased visual acuity usually occur in IIH patients with longstanding severe papilledema,18,37 but can sometimes develop rapidly and be present early in the course of the disease.35 In these cases, emergent intervention is required to prevent irreversible visual loss and optic atrophy (Fig. 2). Central visual loss can also result from macular edema, macular exudates, or a hyperopic shift resulting from flattening of the globe or papillomacular choroidal folds,18,38–40 in which case the prognosis for visual recovery is relatively good. Rarely, acute visual loss might result from anterior ischemic optic neuropathy,41 choroidal infarction,42 or hemorrhage from peripapillary choroidal neovascular membranes,43 particularly in the setting of severe or chronic papilledema. Not uncommonly, non-physiologic visual field constriction occurs in patients who have coexisting organic visual loss from papilledema,44 sometimes making management decisions difficult. Patients with raised ICP and visual loss should have obvious papilledema, unless they have already developed severe secondary optic atrophy. In patients with raised ICP without papilledema, another cause for visual loss or non-organic visual loss should be considered.27 Visual loss without papilledema that is directly due to raised ICP has been reported, but remains exceedingly rare.28,45
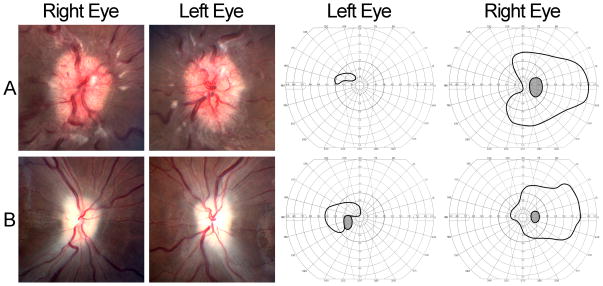
Figure 2. Fulminant onset IIH, with severe headaches, bilateral papilledema, and visual loss.
A: At the time of presentation, the patient had severe papilledema, with exudates, decreased visual acuity in both eyes, and severely constricted Goldmann visual fields in both eyes. Her headaches dramatically improved for a few hours after the diagnostic lumbar puncture. She worsened over the next 24 hours and received a lumbar drain, followed by an urgent CSF shunting procedure.
B: Despite shunting, the patient developed secondary optic atrophy in both eyes and had persistent severe visual field loss.
Tinnitus
Pulsatile (pulse-synchronous) tinnitus is a common symptom of IIH,18,22 but may not be volunteered by patients unless they are specifically asked about it. It can be unilateral or bilateral, intermittent or continuous.46,47 It is often worse at night and can be so loud that it is audible to adjacent people.47 It usually resolves with digital pressure over the ipsilateral internal jugular vein, suggesting that it is venous in origin.48 Indeed, pulsatile tinnitus probably arises due to turbulent blood flow through transverse cerebral venous sinus stenoses.
Diplopia and other symptoms
Binocular horizontal diplopia occurs in IIH patients who have unilateral or bilateral sixth nerve palsies. Vertical diplopia, due to third nerve palsy, fourth nerve palsy, or skew deviation, has been rarely reported in patients with high CSF opening pressure and usually improves dramatically once the ICP is lowered.49–53 Other focal neurologic symptoms, such as facial weakness54 and hearing loss,46,55 can occur, but are atypical and should prompt a thorough search for an alternative diagnosis. It is also not uncommon to have a diagnosis of IIH made when papilledema is detected on a routine eye examination in an asymptomatic patient.56
Course of IIH
The course of IIH is somewhat unpredictable in any given individual. Most IIH patients do well with observation and medical management (hence the previous term “benign intracranial hypertension”). However, many suffer from persistent headaches (that might not be due to raised ICP), with reduced quality of life and depression.12,57 When IIH is associated with an identified triggering factor, such as anemia or untreated obstructive sleep apnea, or a specific medication, treatment of the triggering factor or discontinuation of the offending medication often results in rapid improvement.36,58,59
The principal morbidity of IIH is visual loss due to papilledema. Most patients have visual field defects at presentation18 and up to 31% will develop irreversible visual loss.8,16,18,20,32–34 Thus, all IIH patients must be monitored during careful follow-up with formal automated perimetry, with intervention whenever deterioration of visual fields is documented. Progression of visual field abnormalities is usually slow and in general, reversible, if appropriately treated. Although the syndrome of raised ICP might resolve within a few months, it has been suggested that the disorder is often chronic unless a major intervention occurs.60 Recurrence is unpredictable, although recent weight gain has been noted to be a definite precipitant in many cases.10,12,61 However, there is a small subset of patients with IIH who have an acute onset of symptoms and signs of raised ICP (less than 4 weeks between onset of initial symptoms and severe visual loss), with rapid worsening of visual loss over a few days, who still fit the criteria for IIH, with normal brain MRI and venography. In a recent study of 16 such “fulminant IIH” cases, all were women, all were obese, and the first CSF opening pressure was, on average, 54 cmH2O.35 Surgical treatment was required due to ongoing visual loss in all patients, but 50% remained legally blind and the visual fields remained severely impaired in all cases. Prompt recognition and early aggressive treatment of patients with fulminant IIH is therefore essential.35,62
No consistent correlations have been found between visual outcome and age at diagnosis, body mass index, medication use, CSF opening pressure, duration or type of symptoms, and chronicity of papilledema. A significant association was found between visual loss and the occurrence of systemic hypertension in one study,16 but not others. Anemia was associated with a worse visual outcome in two studies.36,63 A recent study showed that black patients with IIH were 3 times more likely than non-black patients to have severe visual loss in at least one eye and were nearly 5 times more likely to be blind in both eyes.8 The difference was shown to not be the result of racial differences in diagnosis, treatment, or access to care. IIH in black patients appears to be a more aggressive disease and, therefore, needs closer follow-up and a lower threshold for early aggressive intervention. A second study from the same patient database showed that men were more likely to have worse visual acuity and visual fields at presentation and follow-up, and were less likely to report headaches, than women.9 The relative risk of severe visual loss for men compared with women was at least 2.1. Similar to blacks, men with IIH should be followed closely, because they may not reliably experience or report other symptoms of raised ICP. Men with IIH may also need more aggressive treatment. In a recent study comparing French and white American IIH patients,20 American patients had worse visual outcomes, despite receiving more aggressive treatment. The differences in outcome could not be explained by differences in known risk factors.20
Is brain imaging always normal in IIH?
By definition, brain imaging must exclude other causes of raised ICP, such as mass lesions, hydrocephalus, and cerebral venous thrombosis, in order to make the diagnosis of IIH.1 However, imaging abnormalities associated with raised ICP are commonly observed in IIH patients. These include empty sella or flattening of the pituitary, tight subarachnoid spaces, flattening of the posterior globe, protrusion of the optic nerve head, enhancement of the prelaminar portion of the optic nerve head, distension of the optic nerve sheath, and vertical tortuosity of the optic nerve (Fig. 3).64,65 Some patients with presumed IIH have a Chiari I malformation.66 However, the diagnosis of IIH should be made with caution in these patients, whose Chiari I malformation might be the cause of raised ICP and might contraindicate a lumbar puncture.
Figure 3. Imaging findings in IIH.
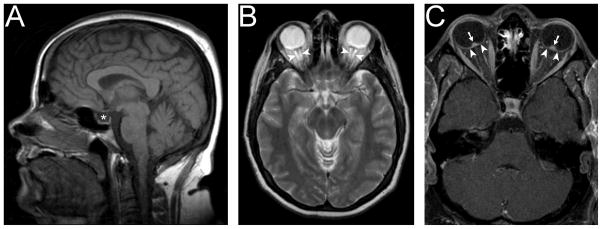
A: Empty sella (*) on sagittal T1-weighted imaging.
B: Dilated optic nerve sheaths (arrowheads) on axial T2-weighted imaging.
C: Posterior globe flattening (arrowheads) and enhancement of protruding optic nerve heads (arrows) on axial T1-weighted imaging with contrast.
Imaging of the cerebral venous system with MR or CT venography should be routinely obtained to exclude cerebral venous thrombosis in patients with presumed IIH, especially in men, non-obese patients, children, older patients, and those with an atypical or fulminant presentation.2,67 Whether dedicated venous imaging must be systematically obtained in all IIH patients remains debated. In most cases, routine brain MRI is sufficient to exclude cerebral venous thrombosis.
Numerous studies published in the last decade have shown that changes at the level of the transverse cerebral venous sinuses (not thrombosis) are common in typical IIH patients.68,69 With older time-of-flight MR venography techniques, these changes were often dismissed as flow-related artifacts.70 However, more recent studies using either direct retrograde cerebral venography, contrast-enhanced MR venography, or contrast-enhanced CT venography have confirmed the presence of true stenoses in the transverse venous sinuses (Fig. 4).68,69,71,72 These stenoses can be bilateral or, in those with a dominant transverse sinus, unilateral (Fig. 4). While patients without IIH rarely have transverse sinus stenoses, the origin and functional significance of these stenoses remains uncertain.
Figure 4. Transverse cerebral venous sinus stenosis in an IIH patient, as seen on contrast-enhanced MR venography. There is a long narrow stenosis of the right transverse venous sinus (arrowheads) and a hypoplastic left transverse venous sinus.
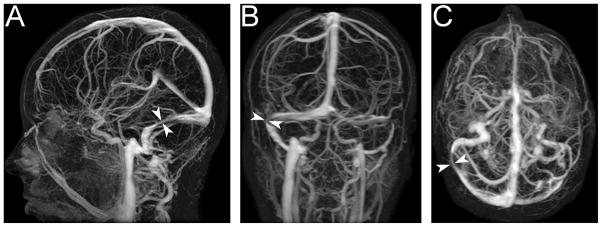
A: Sagittal plane.
B: Coronal plane.
C: Axial plane.
Other imaging studies are generally not required in patients with suspected IIH. However, in those with an atypical presentation or raised CSF protein, cerebral angiography (looking for a dural fistula)73 and spine MRI (to exclude a spinal tumor)74 should be considered, since both conditions can occasionally give rise to isolated raised ICP.
Update on associated conditions
Although the pathogenesis of IIH remains unknown, obesity and female gender are clearly associated with it.9,12 The likely role of sex hormones in its pathogenesis is highlighted by the predilection for post-pubertal pre-menopausal women,6 as well as the absence of a gender preference before puberty.15,75,76 It has recently been shown that men with IIH are more likely than matched controls to have symptoms associated with testosterone deficiency and obstructive sleep apnea.77
Many other factors have been proposed to cause, precipitate, or worsen IIH, including various medications (such as tetracycline and its derivatives, cyclosporine, lithium, nalidixic acid, nitrofurantoin, oral contraceptives, levonorgestrel, danaxol, and tamoxifen), endocrine abnormalities (such as corticosteroid withdrawal, anabolic steroids, excessive growth hormone, and thyroid disease), vitamin A excess78,79 or deficiency, and systemic conditions (such as pregnancy, menstrual irregularities, polycystic ovarian syndrome, anemia, and obstructive sleep apnea).61,80 With some implicated medications, test-retest data in single cases provides compelling evidence for causation. However, in the few case-control studies published to date, no definite association was found between any of these conditions and IIH patients, as compared with age- and sex-matched controls.12,22,33,61 Several factors (such as pregnancy, oral contraceptives, and menstrual irregularities) are very common in young women and, thus, are expected to be common in IIH patients. Studies evaluating the prevalence of obstructive sleep apnea in IIH are ongoing.
Update on the pathophysiology of IIH
The pathophysiologic mechanisms underlying the raised ICP in IIH remain unclear, but must account for the remarkable predilection for obese young women. Theories include increased brain water content, excess CSF production, reduced CSF absorption, and increased cerebral venous pressure.10,61,81 Underlying endocrine dysfunction related to sex hormones and adipose tissue as an actively secreting endocrine organ has also been proposed.82
A great controversy exists regarding the role of cerebral venous hypertension in the etiology of IIH.83 Older studies suggested that increased ICP might be a direct result of increased intra-abdominal pressure, via elevation of the diaphragm, raised pleural pressure, decreased cerebral venous return, and, consequently, sustained cerebral venous hypertension.84 However, this theory requires that central rather than lower body obesity be present in order to increase intra-abdominal pressure,11 and does not account for the preponderance of women with the disorder.
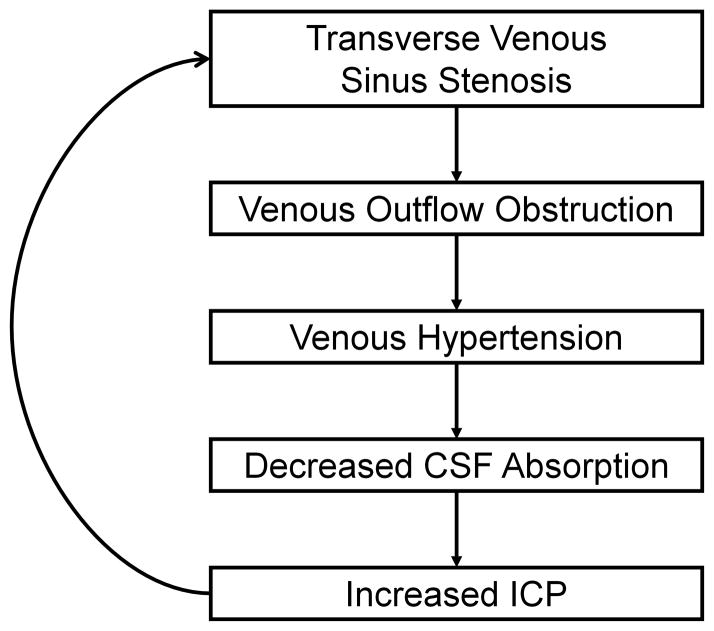
Even more interesting are the numerous recent reports discussing the role of transverse venous sinus stenoses in the pathophysiology of IIH.71,83,85–91 Since CSF drains passively into the venous sinuses, a stenosis of a dominant transverse sinus or stenoses of both transverse sinuses could reduce venous outflow, resulting in cerebral venous hypertension and impaired CSF resorption (Fig 5). Studies using direct retrograde cerebral venography with manometry have demonstrated pressure gradients across these stenoses, with proximal cerebral venous hypertension.71 The origin of these stenoses has been debated, with some anatomic studies suggesting that they might be related to the presence of trabeculae, septae, or large arachnoid granulations in the transverse sinuses.92,93 However, it has also been suggested that patients with increased intracranial pressure may develop transverse sinus stenoses due to external compression, since the stenoses can resolve once CSF pressure is normalized by a lumbar puncture or a CSF shunting procedure.85,94–98 However, even if these venous sinus stenoses are due to raised CSF pressure, it is likely that their presence will still result in decreased CSF absorption and, thus, further elevation of ICP, if there is cerebral venous hypertension due to a pressure gradient across them (Fig. 5), suggesting a possible role for venous sinus stenting in the treatment of IIH. Also of interest is the observation that transverse venous sinus stenoses can occur in patients without IIH, suggesting that they may have no functional significance in some patients.
Figure 5.
Schematic summary of the presumed role of transverse venous sinus stenosis in IIH.
Treatment
The two goals of treatment in IIH are to alleviate unpleasant symptoms (such as headache) and to preserve vision.10,81 General recommendations include weight loss (most successful when under the direction of a nutritionist), evaluation and treatment of potential contributing factors (including medication use, anemia, and obstructive sleep apnea), and even conventional headache management.
The treatment of raised ICP itself begins with the diagnostic lumbar puncture, which is often effective in transiently improving symptoms and signs. Occasional patients can have a lasting clinical remission following a single lumbar puncture,97 obviating the need for further medical or surgical treatment. While lumbar punctures can be useful as a temporizing measure in emergency situations,35 they are poorly tolerated by many patients, especially when technically difficult.
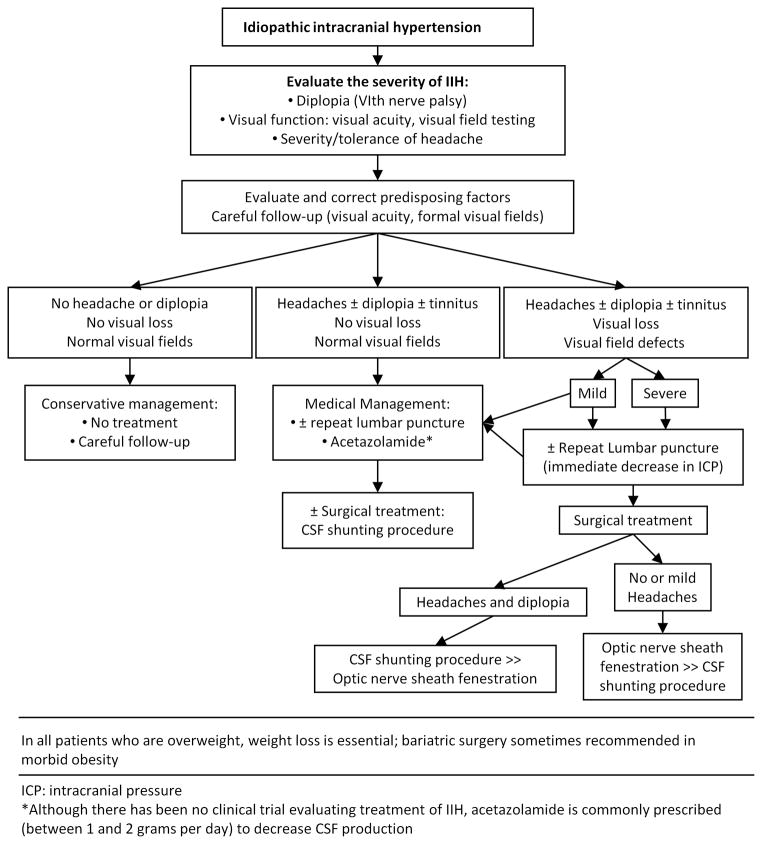
Patients with persistent symptoms and signs can be treated using medical and surgical approaches, although there have been no randomized controlled trials prospectively assessing and comparing these treatments.99 As some patients with papilledema never develop visual field defects, whereas others develop rapid visual loss, a combination of ophthalmologic examination and quantitative perimetry is required to guide management decisions. A suggested treatment algorithm, based on the severity of symptoms and signs, is given in Fig. 6.100 It is important to note that patients with severe symptoms, significant visual loss, or a fulminant onset of disease require more aggressive management from the outset, in order to prevent irreversible visual loss.35
Figure 6.
Algorithm summarizing our suggested approach to IIH management, including conservative, medical, and surgical treatments (reprinted with permission from Biousse and Newman, 2009).100
Weight loss
As obesity and weight gain are well known to be associated with IIH,12 weight loss is routinely advised for obese IIH patients. Only a modest degree of weight loss (about 5–10% of total body weight) is usually required for improvement in symptoms and signs.101–104 A recent study of 25 obese women with IIH showed that weight loss effectively reduces not only headaches and papilledema, but also ICP in IIH patients (Michael Burdon, personal communication). However, weight loss is not an effective short-term treatment and, thus, other treatments must be initiated in parallel for most patients. Bariatric surgery can be considered in morbidly obese IIH patients in whom attempts at weight loss have been unsuccessful.105,106
Drug treatments
Carbonic anhydrase inhibitors, such as acetazolamide, are the main medical treatment prescribed for IIH, although no trial data are currently available to confirm their effectiveness.99 They are thought to exert their therapeutic effect by decreasing CSF production, although they also have a mild diuretic effect. Doses of 1–2g daily, given in divided doses, can be effective, although many patients cannot tolerate high doses due to intolerable side-effects, such as paresthesias, altered taste sensation, and lethargy. The preliminary results of the IIH Pilot Trial from Birmingham, UK, in which 50 patients were randomized to receive acetazolamide or no acetazolamide, emphasize the difficulties of performing such a study due to side-effects (Timothy Matthews, personal communication). The findings of another small trial suggest that acetazolamide can be safely used in pregnant IIH patients.107 A randomized clinical trial of acetazolamide versus placebo for IIH has just begun in North America (see below).108
Excessive analgesic use is discouraged, given the high likelihood of rebound headaches, so prophylactic headache treatments are often required in those patients with prominent headache. Of these, topiramate is the most commonly used, since it is a weak carbonic anhydrase inhibitor, is effective in treating primary headache disorders (such as migraine and chronic daily headache), and often brings about some weight loss as a side-effect. A randomized open-label study has shown that topiramate and acetazolamide have similar beneficial effects at 12 months in IIH patients,109 but further controlled studies are required to confirm these findings.
Steroids have been used as a treatment for IIH in the past, but are associated with significant long-term side-effects, such as weight gain, which are undesirable in this patient population. High-dose intravenous steroids are still occasionally used in patients with rapidly progressive visual loss from fulminant IIH while a more definitive treatment is awaited.35,62
A randomized controlled treatment trial for IIH
Beginning in 2009, the National Eye Institute of the National Institute of Health has sponsored the Neuro-Ophthalmology Research Disease Investigator Consortium (NORDIC) to proceed with a multicenter, double-blind, placebo-controlled clinical trial, called the IIHTT (the Idiopathic Intracranial Hypertension Treatment Trial), comparing the efficacy of acetazolamide and placebo in the treatment of IIH patients.108 Patients must satisfy the modified Dandy criteria for diagnosis of IIH and have mild to moderate disease (mean deviations on automated perimetry ranging from −2 to −5 dB). Patients will be randomized to receive either acetazolamide at increasing doses, as tolerated, or placebo. All patients will also be treated with a low-sodium diet and will participate in a standardized weight loss program. Patients will be monitored with automated perimetry, which is the primary outcome measure at 6 months. Secondary outcome measures will include changes in CSF pressure, papilledema, and quality of life.
Surgical procedures
Surgery is required in patients with a fulminant onset of disease or when other treatments have failed to prevent progressive visual loss. The choice of procedure depends on local resources, as well as the patient’s symptoms and signs (Fig. 6). In patients with papilledema who have severe visual loss, but minimal or no headache, optic nerve sheath fenestration (ONSF) is often advised, while in those with visual loss, papilledema, and headache, a CSF diversion procedure, such as ventriculo- or lumbo-peritoneal shunting, is preferred. Aggressive management with CSF shunting is usually required to prevent catastrophic visual loss in those with acute and rapidly progressive visual loss.35 In contrast, CSF shunting should not be routinely offered for the treatment of isolated headache, given the high rate of complications and the fact that many IIH patients have a coexisting primary headache disorder that does not improve with lowering of ICP.
CSF shunting produces a rapid reduction in ICP, often with a subsequent improvement in symptoms and signs.110–114 Complications are common, however, with many patients developing infections, shunt obstruction, or migration of the shunt tubing, and, therefore, revisions are often required.111–114 While ventriculo-peritoneal shunting is more technically challenging and often requires a stereotactic approach, as IIH patients do not have enlarged ventricles, it may be preferred due to its lower complication rate.115,116
In ONSF, the dural sheath surrounding the retrolaminar portion of the optic nerve is fenestrated, creating a fistula between the subarachnoid space and orbital cavity. Consequently, there is a reduction in pressure on the optic nerve, leading to reduced papilledema and improved visual function in that eye.117–121 Although the papilledema and visual function on the contralateral side can sometimes improve, most patients with bilateral papilledema require sequential bilateral ONSF.118 The papilledema tends not to recur, although the mechanism for this remains uncertain. While a fluid collection can be seen adjacent to the operated site in the initial weeks following ONSF, suggesting a persistent fistula, fibrous tissue formation is observed after a few months, suggesting that scarring at the ONSF site prevents transmission of the CSF pressure gradient to the retrolaminar portion of the nerve.122 ONSF is generally ineffective in reducing ICP, so it will not usually be effective in treating any other symptoms and signs of raised ICP.123,124 Furthermore, some patients might develop transient or permanent visual loss125–127 or continue to lose vision following ONSF,128 so potential risks of the procedure need to be weighed against benefits.
Modeling studies have suggested that stenting of transverse venous sinus stenoses will reduce cerebral venous pressure, leading to increased CSF absorption, reduced ICP, and improved symptoms and signs, even in cases where the stenoses are secondary to raised ICP.91 Supporting this notion are the findings of several case studies and small series, in which patients have undergone endovascular stenting of the stenoses, with subsequent normalization of ICP and resolution of symptoms and signs.87,89,129–133 However, venous sinus stenting can result in serious complications, such as in stent thrombosis and subdural hemorrhage,89,131 and patients can develop recurrent stenoses immediately proximal to the stent.87,89 Furthermore, prospective controlled studies are lacking, and the indications for this treatment remain poorly defined, and therefore it is currently not recommended for routine clinical practice.
Conclusion
Many questions remain unanswered about IIH. Its association with female gender and obesity is striking, and the common finding of transverse venous sinus stenoses intriguing. Recent large studies have indicated that IIH can also occur in men, non-obese adults, older adults, and in pre-pubertal children. Identification of subgroups at high risk for irreversible visual loss, such as black patients, men, and patients with fulminant IIH, help determine management approaches and follow-up criteria. Ongoing studies of venous anatomy and physiology in IIH patients, as well as a recently begun clinical trial, are promising and should provide more insight into this common yet poorly understood syndrome of isolated intracranial hypertension.
Footnotes
Disclosure: This work was supported in part by a departmental grant (Department of Ophthalmology) from Research to Prevent Blindness, Inc, New York, New York; core grant P30-EY06360 (Department of Ophthalmology) from the National Institutes of Health/National Eye Institute; and by grants KL2-RR025009 (BBB) and NIH grant UL1-RR025008 (BBB/VB).
References
- 1.Friedman DI, Jacobson DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology. 2002;59:1492–1495. doi: 10.1212/01.wnl.0000029570.69134.1b. [DOI] [PubMed] [Google Scholar]
- 2.Biousse V, Ameri A, Bousser MG. Isolated intracranial hypertension as the only sign of cerebral venous thrombosis. Neurology. 1999;53:1537–1542. doi: 10.1212/wnl.53.7.1537. [DOI] [PubMed] [Google Scholar]
- 3.Digre KB, Corbett JJ. Idiopathic intracranial hypertension (pseudotumor cerebri): a reappraisal. Neurologist. 2001;7:2–67. [Google Scholar]
- 4.Friedman DI, Jacobson DM. Idiopathic intracranial hypertension. J Neuroophthalmol. 2004;24:138–145. doi: 10.1097/00041327-200406000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Digre KB. Three current controversies in idiopathic intracranial hypertension. Neuroophthalmology. 2009;33:93–99. [Google Scholar]
- 6.Durcan FJ, Corbett JJ, Wall M. The incidence of pseudotumor cerebri: population studies in Iowa and Louisiana. Arch Neurol. 1988;45:875–877. doi: 10.1001/archneur.1988.00520320065016. [DOI] [PubMed] [Google Scholar]
- 7.Radhakrishnan K, Ahlskog JE, Cross SA, Kurland LT, O’Fallon WM. Idiopathic intracranial hypertension (pseudotumor cerebri): descriptive epidemiology in Rochester, Minn, 1976 to 1990. Arch Neurol. 1993;50:78–80. doi: 10.1001/archneur.1993.00540010072020. [DOI] [PubMed] [Google Scholar]
- 8.Bruce BB, Preechawat P, Newman NJ, Lynn MJ, Biousse V. Racial differences in idiopathic intracranial hypertension. Neurology. 2008;70:861–867. doi: 10.1212/01.wnl.0000304746.92913.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruce BB, Kedar S, Van Stavern GP, Monaghan D, Acierno MD, Braswell RA, Preechawat P, Corbett JJ, Newman NJ, Biousse V. Idiopathic intracranial hypertension in men. Neurology. 2009;72:304–309. doi: 10.1212/01.wnl.0000333254.84120.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ball AK, Clarke CE. Idiopathic intracranial hypertension. Lancet Neurol. 2006;5:433–442. doi: 10.1016/S1474-4422(06)70442-2. [DOI] [PubMed] [Google Scholar]
- 11.Kesler A, Kliper E, Shenkerman G, Stern N. Idiopathic intracranial hypertension is associated with lower body adiposity. Ophthalmology. 2010;117:169–174. doi: 10.1016/j.ophtha.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 12.Daniels AB, Liu GT, Volpe NJ, Galetta SL, Moster ML, Newman NJ, Biousse V, Lee AG, Wall M, Kardon R, Acierno MD, Corbett JJ, Maguire MG, Balcer LJ. Profiles of obesity, weight gain, and quality of life in idiopathic intracranial hypertension (pseudotumor cerebri) Am J Ophthalmol. 2007;143:635–641. doi: 10.1016/j.ajo.2006.12.040. [DOI] [PubMed] [Google Scholar]
- 13.Rangwala LM, Liu GT. Pediatric idiopathic intracranial hypertension. Surv Ophthalmol. 2007;52:597–617. doi: 10.1016/j.survophthal.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 14.Bruce BB, Kedar S, Van Stavern GP, Corbett JJ, Newman NJ, Biousse V. Atypical idiopathic intracranial hypertension: normal BMI and older patients. Neurology. 2010 doi: 10.1212/WNL.0b013e3181e0f838. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cinciripini GS, Donahue S, Borchert MS. Idiopathic intracranial hypertension in prepubertal pediatric patients: characteristics, treatment, and outcome. Am J Ophthalmol. 1999;127:178–182. doi: 10.1016/s0002-9394(98)00386-9. [DOI] [PubMed] [Google Scholar]
- 16.Corbett JJ, Savino PJ, Thompson HS, Kansu T, Schatz NJ, Orr LS, Hopson D. Visual loss in pseudotumor cerebri: follow-up of 57 patients from five to 41 years and a profile of 14 patients with permanent severe visual loss. Arch Neurol. 1982;39:461–474. doi: 10.1001/archneur.1982.00510200003001. [DOI] [PubMed] [Google Scholar]
- 17.Digre KB, Corbett JJ. Pseudotumor cerebri in men. Arch Neurol. 1988;45:866–872. doi: 10.1001/archneur.1988.00520320056015. [DOI] [PubMed] [Google Scholar]
- 18.Wall M, George D. Idiopathic intracranial hypertension: a prospective study of 50 patients. Brain. 1991;114:155–180. [PubMed] [Google Scholar]
- 19.Kesler A, Goldhammer Y, Gadoth N. Do men with pseudotumor cerebri share the same characteristics as women? A retrospective review of 141 cases. J Neuroophthalmol. 2001;21:15–7. doi: 10.1097/00041327-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Mrejen S, Vignal C, Bruce BB, Gineys R, Audren F, Preechawat P, Gaudric A, Gout O, Newman NJ, Vighetto A, Bousser MG, Biousse V. Idiopathic intracranial hypertension: a comparison between French and North-American white patients. Rev Neurol (Paris) 2009;165:542–548. doi: 10.1016/j.neurol.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corbett JJ. Familial idiopathic intracranial hypertension. J Neuroophthalmol. 2008;28:337–347. doi: 10.1097/WNO.0b013e31818f12a2. [DOI] [PubMed] [Google Scholar]
- 22.Giuseffi V, Wall M, Siegel PZ, Rojas PB. Symptoms and disease associations in idiopathic intracranial hypertension (pseudotumor cerebri): a case-control study. Neurology. 1991;41:239–244. doi: 10.1212/wnl.41.2_part_1.239. [DOI] [PubMed] [Google Scholar]
- 23.Friedman DI, Rausch EA. Headache diagnoses in patients with treated idiopathic intracranial hypertension. Neurology. 2002;58:1551–1553. doi: 10.1212/wnl.58.10.1551. [DOI] [PubMed] [Google Scholar]
- 24.Mansour AM, Zatorski J. Analysis of variables for papilledema in pseudotumor cerebri. Ann Ophthalmol. 1994;26:172–174. [Google Scholar]
- 25.Marcelis J, Silberstein SD. Idiopathic intracranial hypertension without papilledema. Arch Neurol. 1991;48:392–399. doi: 10.1001/archneur.1991.00530160060014. [DOI] [PubMed] [Google Scholar]
- 26.Lepore FE. Unilateral and highly asymmetric papilledema in pseudotumor cerebri. Neurology. 1992;42:676–678. doi: 10.1212/wnl.42.3.676. [DOI] [PubMed] [Google Scholar]
- 27.Digre KB, Nakamoto BK, Warner JE, Langeberg WJ, Baggaley SK, Katz BJ. A comparison of idiopathic intracranial hypertension with and without papilledema. Headache. 2009;49:185–193. doi: 10.1111/j.1526-4610.2008.01324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thurtell MJ, Newman NJ, Biousse V. Visual loss without papilledema in idiopathic intracranial hypertension. J Neuroophthalmol. 2010;30:96–98. doi: 10.1097/WNO.0b013e3181c5d0bc. [DOI] [PubMed] [Google Scholar]
- 29.Killer HE, Laeng HR, Flammer J, Groscurth P. Architecture of arachnoid trabeculae, pillars, and septa in the subarachnoid space of the human optic nerve: anatomy and clinical considerations. Br J Ophthalmol. 2003;87:777–781. doi: 10.1136/bjo.87.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Killer HE, Jaggi GP, Flammer J, Miller NR, Huber AR, Mironov A. Cerebrospinal fluid dynamics between the intracranial and the subarachnoid space of the optic nerve: is it always bidirectional? Brain. 2007;130:514–520. doi: 10.1093/brain/awl324. [DOI] [PubMed] [Google Scholar]
- 31.O’Duffy D, James B, Elston J. Idiopathic intracranial hypertension presenting with gaze-evoked amaurosis. Acta Ophthalmol Scand. 1998;76:119–120. doi: 10.1034/j.1600-0420.1998.760125.x. [DOI] [PubMed] [Google Scholar]
- 32.Orcutt JC, Page NG, Sanders MD. Factors affecting visual loss in benign intracranial hypertension. Ophthalmology. 1984;91:1303–1312. doi: 10.1016/s0161-6420(84)34149-5. [DOI] [PubMed] [Google Scholar]
- 33.Radhakrishnan K, Thacker AK, Bohlaga NH, Maloo JC, Gerryo SE. Epidemiology of idiopathic intracranial hypertension: a prospective and case-control study. J Neurol Sci. 1993;116:18–28. doi: 10.1016/0022-510x(93)90084-c. [DOI] [PubMed] [Google Scholar]
- 34.Rowe FJ, Sarkies NJ. Assessment of visual function in idiopathic intracranial hypertension: a prospective study. Eye. 1998;12:111–1118. doi: 10.1038/eye.1998.18. [DOI] [PubMed] [Google Scholar]
- 35.Thambisetty M, Lavin PJ, Newman NJ, Biousse V. Fulminant idiopathic intracranial hypertension. Neurology. 2007;68:229–232. doi: 10.1212/01.wnl.0000251312.19452.ec. [DOI] [PubMed] [Google Scholar]
- 36.Biousse V, Rucker JC, Vignal C, Crassard I, Katz BJ, Newman NJ. Anemia and papilledema. Am J Ophthalmol. 2003;135:437–446. doi: 10.1016/s0002-9394(02)02062-7. [DOI] [PubMed] [Google Scholar]
- 37.Wall M, George D. Visual loss in pseudotumor cerebri: incidence and defects related to visual field strategy. Arch Neurol. 1987;44:170–175. doi: 10.1001/archneur.1987.00520140040015. [DOI] [PubMed] [Google Scholar]
- 38.Gittinger JW, Jr, Asdourian GK. Macular abnormalities in papilledema from pseudotumor cerebri. Ophthalmology. 1989;96:192–194. doi: 10.1016/s0161-6420(89)32915-0. [DOI] [PubMed] [Google Scholar]
- 39.Warner JE, Katz BJ. Metamorphopsia as an initial complaint of idiopathic intracranial hypertension. Arch Ophthalmol. 2005;123:1003–1006. doi: 10.1001/archopht.123.7.1003. [DOI] [PubMed] [Google Scholar]
- 40.Lavinsky J, Lavinsky D, Lavinsky F, Frutuoso A. Acquired choroidal folds: a sign of idiopathic intracranial hypertension. Graefes Arch Clin Exp Ophthalmol. 2007;245:883–888. doi: 10.1007/s00417-006-0455-7. [DOI] [PubMed] [Google Scholar]
- 41.Green GJ, Lessell S, Loewenstein JI. Ischemic optic neuropathy in chronic papilledema. Arch Ophthalmol. 1980;98:502–504. doi: 10.1001/archopht.1980.01020030498013. [DOI] [PubMed] [Google Scholar]
- 42.Lamirel C, Bruce BB, Newman NJ, Biousse V. Choroidal infarction in fulminant idiopathic intracranial hypertension. J Neuroophthalmol. 2010 doi: 10.1097/WNO.0b013e3181c25581. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sathornsumetee B, Webb A, Hill DL, Newman NJ, Biousse V. Subretinal hemorrhage from a peripapillary choroidal neovascular membrane in papilledema caused by idiopathic intracranial hypertension. J Neuroophthalmol. 2006;26:197–199. doi: 10.1097/01.wno.0000235583.10546.0a. [DOI] [PubMed] [Google Scholar]
- 44.Ney JJ, Volpe NJ, Liu GT, Balcer LJ, Moster ML, Galetta SL. Functional visual loss in idiopathic intracranial hypertension. Ophthalmology. 2009;116:1808–1813. doi: 10.1016/j.ophtha.2009.03.056. [DOI] [PubMed] [Google Scholar]
- 45.Golnik KC, Devoto TM, Kersten RC, Kulwin D. Visual loss in idiopathic intracranial hypertension after resolution of papilledema. Ophthal Plast Reconstr Surg. 1999;15:442–444. doi: 10.1097/00002341-199911000-00015. [DOI] [PubMed] [Google Scholar]
- 46.Sismanis A. Otologic manifestations of benign intracranial hypertension syndrome: diagnosis and management. Laryngoscope. 1987;97:1–17. doi: 10.1288/00005537-198708001-00001. [DOI] [PubMed] [Google Scholar]
- 47.Biousse V, Newman NJ, Lessell S. Audible pulsatile tinnitus in idiopathic intracranial hypertension. Neurology. 1998;50:1185–1186. doi: 10.1212/wnl.50.4.1185. [DOI] [PubMed] [Google Scholar]
- 48.Sismanis A. Pulsatile tinnitus. Otolaryngol Clin North Am. 2003;36:389–402. doi: 10.1016/s0030-6665(02)00169-x. [DOI] [PubMed] [Google Scholar]
- 49.Baker RS, Buncic JR. Vertical ocular motility disturbance in pseudotumor cerebri. J Clin Neuroophthalmol. 1985;5:41–44. [PubMed] [Google Scholar]
- 50.Friedman DI, Forman S, Levi L, Lavin PJ, Donahue S. Unusual ocular motility disturbances with increased intracranial pressure. Neurology. 1998;50:1893–1896. doi: 10.1212/wnl.50.6.1893. [DOI] [PubMed] [Google Scholar]
- 51.Patton N, Beatty S, Lloyd IC. Bilateral sixth and fourth cranial nerve palsies in idiopathic intracranial hypertension. J R Soc Med. 2000;93:80–81. doi: 10.1177/014107680009300210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bruce BB, Newman NJ, Biousse V. Ophthalmoparesis in idiopathic intracranial hypertension. Am J Ophthalmol. 2006;142:878–880. doi: 10.1016/j.ajo.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 53.Tan H. Bilateral oculomotor palsy secondary to pseudotumor cerebri. Pediatr Neurol. 2010;42:141–142. doi: 10.1016/j.pediatrneurol.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 54.Capobianco DJ, Brazis PW, Cheshire WP. Idiopathic intracranial hypertension and seventh nerve palsy. Headache. 1997;37:286–288. doi: 10.1046/j.1526-4610.1997.3705286.x. [DOI] [PubMed] [Google Scholar]
- 55.Dorman PJ, Campbell MJ, Maw AR. Hearing loss as a false localising sign in raised intracranial pressure. J Neurol Neurosurg Psychiatry. 1995;58:516. doi: 10.1136/jnnp.58.4.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Galvin JA, Van Stavern GP. Clinical characterization of idiopathic intracranial hypertension at the Detroit Medical Center. J Neurol Sci. 2004;223:157–160. doi: 10.1016/j.jns.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 57.Kleinschmidt JJ, Digre KB, Hanover R. Idiopathic intracranial hypertension: relationship to depression, anxiety, and quality of life. Neurology. 2000;54:319–324. doi: 10.1016/s0002-9394(00)00507-9. [DOI] [PubMed] [Google Scholar]
- 58.Kesler A, Goldhammer Y, Hadayer A, Pianka P. The outcome of pseudotumor cerebri induced by tetracycline therapy. Acta Neurol Scand. 2004;110:408–411. doi: 10.1111/j.1600-0404.2004.00327.x. [DOI] [PubMed] [Google Scholar]
- 59.Friedman DI. Medication-induced intracranial hypertension in dermatology. Am J Clin Dermatol. 2005;6:29–37. doi: 10.2165/00128071-200506010-00004. [DOI] [PubMed] [Google Scholar]
- 60.Shah VA, Kardon RH, Lee AG, Corbett JJ, Wall M. Long-term follow-up of idiopathic intracranial hypertension: the Iowa experience. Neurology. 2008;70:634–640. doi: 10.1212/01.wnl.0000299893.43918.a8. [DOI] [PubMed] [Google Scholar]
- 61.Ireland B, Corbett JJ, Wallace RB. The search for causes of idiopathic intracranial hypertension: a preliminary case-control study. Arch Neurol. 1990;47:315–320. doi: 10.1001/archneur.1990.00530030091021. [DOI] [PubMed] [Google Scholar]
- 62.Liu GT, Glaser JS, Schatz NJ. High-dose methylprednisolone and acetazolamide for visual loss in pseudotumor cerebri. Am J Ophthalmol. 1994;118:88–96. doi: 10.1016/s0002-9394(14)72847-8. [DOI] [PubMed] [Google Scholar]
- 63.Mollan SP, Ball AK, Sinclair AJ, Madill SA, Clarke CE, Jacks AS, Burdon MA, Matthews TD. Idiopathic intracranial hypertension associated with iron deficiency anaemia: a lesson for management. Eur Neurol. 2009;62:105–108. doi: 10.1159/000222781. [DOI] [PubMed] [Google Scholar]
- 64.Brodsky MC, Vaphiades M. Magnetic resonance imaging in pseudotumor cerebri. Ophthalmology. 1998;105:1686–1693. doi: 10.1016/S0161-6420(98)99039-X. [DOI] [PubMed] [Google Scholar]
- 65.Agid R, Farb RI, Willinsky RA, Mikulis DJ, Tomlinson G. Idiopathic intracranial hypertension: the validity of cross-sectional neuroimaging signs. Neuroradiology. 2006;48:521–7. doi: 10.1007/s00234-006-0095-y. [DOI] [PubMed] [Google Scholar]
- 66.Banik R, Lin D, Miller NR. Prevalence of Chiari I malformation and cerebellar ectopia in patients with pseudotumor cerebri. J Neurol Sci. 2006;247:71–75. doi: 10.1016/j.jns.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 67.Lin A, Foroozan R, Danesh-Meyer HV, De Salvo G, Savino PJ, Sergott RC. Occurrence of cerebral venous sinus thrombosis in patients with presumed idiopathic intracranial hypertension. Ophthalmology. 2006;113:2281–2284. doi: 10.1016/j.ophtha.2006.05.065. [DOI] [PubMed] [Google Scholar]
- 68.Farb RI, Vanek I, Scott JN, Mikulis DJ, Willinsky RA, Tomlinson G, terBrugge KG. Idiopathic intracranial hypertension: the prevalence and morphology of sinovenous stenosis. Neurology. 2003;60:1418–1424. doi: 10.1212/01.wnl.0000066683.34093.e2. [DOI] [PubMed] [Google Scholar]
- 69.Fera F, Bono F, Messina D, Gallo O, Lanza PL, Auteri W, Nicoletti G, Santoro G, Quattrone A. Comparison of different MR venography techniques for detecting transverse sinus stenosis in idiopathic intracranial hypertension. J Neurol. 2005;252:1021–1025. doi: 10.1007/s00415-005-0710-6. [DOI] [PubMed] [Google Scholar]
- 70.Ayanzen RH, Bird CR, Keller PJ, McCully FJ, Theobald MR, Heiserman JE. Cerebral MR venography: normal anatomy and potential diagnostic pitfalls. Am J Neuroradiol. 2000;21:74–78. [PMC free article] [PubMed] [Google Scholar]
- 71.King JO, Mitchell PJ, Thomson KR, Tress BM. Cerebral venography and manometry in idiopathic intracranial hypertension. Neurology. 1995;45:2224–2228. doi: 10.1212/wnl.45.12.2224. [DOI] [PubMed] [Google Scholar]
- 72.Higgins JN, Tipper G, Varley M, Pickard JD. Transverse sinus stenoses in benign intracranial hypertension demonstrated on CT venography. Br J Neurosurg. 2005;19:137–140. doi: 10.1080/02688690500145563. [DOI] [PubMed] [Google Scholar]
- 73.Cognard C, Casasco A, Toevi M, Houdart E, Chiras J, Merland JJ. Dural arteriovenous fistulas as a cause of intracranial hypertension due to impairment of cranial venous outflow. J Neurol Neurosurg Psychiatry. 1998;65:308–316. doi: 10.1136/jnnp.65.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Porter A, Lyons MK, Wingerchuk DM, Bosch EP. Spinal cord astrocytoma presenting as “idiopathic” intracranial hypertension. Clin Neurol Neurosurg. 2006;108:787–789. doi: 10.1016/j.clineuro.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 75.Lessell S. Pediatric pseudotumor cerebri (idiopathic intracranial hypertension) Surv Ophthalmol. 1992;37:155–166. doi: 10.1016/0039-6257(92)90134-f. [DOI] [PubMed] [Google Scholar]
- 76.Balcer LJ, Liu GT, Forman S, Pun K, Volpe NJ, Galetta SL, Maguire MG. Idiopathic intracranial hypertension: relation of age and obesity in children. Neurology. 1999;52:870–872. doi: 10.1212/wnl.52.4.870. [DOI] [PubMed] [Google Scholar]
- 77.Fraser JA, Bruce BB, Rucker J, Fraser LA, Atkins EJ, Newman NJ, Biousse V. Risk factors for idiopathic intracranial hypertension in men: a case-control study. J Neurol Sci. 2010;290:86–89. doi: 10.1016/j.jns.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Warner JE, Bernstein PS, Yemelyanov A, Alder SC, Farnsworth ST, Digre KB. Vitamin A in the cerebrospinal fluid of patients with and without idiopathic intracranial hypertension. Ann Neurol. 2002;52:647–650. doi: 10.1002/ana.10377. [DOI] [PubMed] [Google Scholar]
- 79.Warner JE, Larson AJ, Bhosale P, Digre KB, Henley C, Alder SC, Katz BJ, Bernstein PS. Retinol-binding protein and retinol analysis in cerebrospinal fluid and serum of patients with and without idiopathic intracranial hypertension. J Neuroophthalmol. 2007;27:258–262. doi: 10.1097/WNO.0b013e31815b9af0. [DOI] [PubMed] [Google Scholar]
- 80.Digre KB, Varner MW, Corbett JJ. Pseudotumor cerebri and pregnancy. Neurology. 1984;34:721–729. doi: 10.1212/wnl.34.6.721. [DOI] [PubMed] [Google Scholar]
- 81.Randhawa S, Van Stavern GP. Idiopathic intracranial hypertension (pseudotumor cerebri) Curr Opin Ophthalmol. 2008;19:445–453. doi: 10.1097/ICU.0b013e3283112d3e. [DOI] [PubMed] [Google Scholar]
- 82.Ooi LY, Walker BR, Bodkin PA, Whittle IR. Idiopathic intracranial hypertension: can studies of obesity provide the key to understanding pathogenesis? Br J Neurosurg. 2008;22:187–194. doi: 10.1080/02688690701827340. [DOI] [PubMed] [Google Scholar]
- 83.Friedman DI. Cerebral venous pressure, intra-abdominal pressure, and dural venous sinus stenting in idiopathic intracranial hypertension. J Neuroophthalmol. 2006;2:61–64. doi: 10.1097/01.wno.0000204663.33559.1e. [DOI] [PubMed] [Google Scholar]
- 84.Sugerman HJ, DeMaria EJ, Felton WL, 3rd, Nakatsuka M, Sismanis A. Increased intra-abdominal pressure and cardiac filling pressures in obesity-associated pseudotumor cerebri. Neurology. 1997;49:507–511. doi: 10.1212/wnl.49.2.507. [DOI] [PubMed] [Google Scholar]
- 85.King JO, Mitchell PJ, Thomson KR, Tress BM. Manometry combined with cervical puncture in idiopathic intracranial hypertension. Neurology. 2002;58:26–30. doi: 10.1212/wnl.58.1.26. [DOI] [PubMed] [Google Scholar]
- 86.Corbett JJ, Digre K. Idiopathic intracranial hypertension: an answer to, “the chicken or the egg? Neurology. 2002;58:5–6. doi: 10.1212/wnl.58.1.5. [DOI] [PubMed] [Google Scholar]
- 87.Owler BK, Parker G, Halmagyi GM, Dunne VG, Grinnell V, McDowell D, Besser M. Pseudotumor cerebri syndrome: venous sinus obstruction and its treatment with stent placement. J Neurosurg. 2003;98:1045–1055. doi: 10.3171/jns.2003.98.5.1045. [DOI] [PubMed] [Google Scholar]
- 88.Bateman GA. Idiopathic intracranial hypertension: priapism of the brain? Med Hypotheses. 2004;63:549–552. doi: 10.1016/j.mehy.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 89.Owler BK, Parker G, Halmagyi GM, Johnston IH, Besser M, Pickard JD, Higgins JN. Cranial venous outflow obstruction and pseudotumor cerebri syndrome. Adv Tech Stand Neurosurg. 2005;30:107–174. doi: 10.1007/3-211-27208-9_4. [DOI] [PubMed] [Google Scholar]
- 90.Bateman GA. Stenoses in idiopathic intracranial hypertension: to stent or not to stent? Am J Neuroradiol. 2008;29:215. doi: 10.3174/ajnr.A0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bateman GA, Stevens SA, Stimpson J. A mathematical model of idiopathic intracranial hypertension incorporating increased arterial inflow and variable venous outflow collapsibility. J Neurosurg. 2009;110:446–456. doi: 10.3171/2008.6.17609. [DOI] [PubMed] [Google Scholar]
- 92.Subramaniam RM, Tress BM, King JO, Eizenberg N, Mitchell PJ. Transverse sinus septum: a new aetiology of idiopathic intracranial hypertension? Australas Radiol. 2004;48:114–116. doi: 10.1111/j.1440-1673.2004.01269.x. [DOI] [PubMed] [Google Scholar]
- 93.Strydom MA, Briers N, Bosman MC, Steyn S. The anatomical basis of venographic filling defects of the transverse sinus. Clin Anat. 2010;23:153–159. doi: 10.1002/ca.20911. [DOI] [PubMed] [Google Scholar]
- 94.Baryshnik DB, Farb RI. Changes in the appearance of venous sinuses after treatment of disordered intracranial pressure. Neurology. 2004;62:1445–1446. doi: 10.1212/01.wnl.0000120750.40453.64. [DOI] [PubMed] [Google Scholar]
- 95.Higgins JN, Pickard JD. Lateral sinus stenoses in idiopathic intracranial hypertension resolving after CSF diversion. Neurology. 2004;62:1907–1908. doi: 10.1212/01.wnl.0000125285.44539.d7. [DOI] [PubMed] [Google Scholar]
- 96.McGonigal A, Bone I, Teasdale E. Resolution of transverse sinus stenosis in idiopathic intracranial hypertension after L-P shunt. Neurology. 2004;62:514–515. doi: 10.1212/wnl.62.3.514. [DOI] [PubMed] [Google Scholar]
- 97.De Simone R, Marano E, Fiorillo C, Briganti F, Di Salle F, Volpe A, Bonavita V. Sudden re-opening of collapsed transverse sinuses and longstanding clinical remission after a single lumbar puncture in a case of idiopathic intracranial hypertension: pathogenetic implications. Neurol Sci. 2005;25:342–344. doi: 10.1007/s10072-004-0368-3. [DOI] [PubMed] [Google Scholar]
- 98.Stienen A, Weinzierl M, Ludolph A, Tibussek D, Hausler M. Obstruction of cerebral venous sinus secondary to idiopathic intracranial hypertension. Eur J Neurol. 2008;15:1416–1418. doi: 10.1111/j.1468-1331.2008.02340.x. [DOI] [PubMed] [Google Scholar]
- 99.Lueck C, McIlwaine G. Interventions for idiopathic intracranial hypertension. Cochrane Database Syst Rev. 2005;3:CD003434. doi: 10.1002/14651858.CD003434.pub2. [DOI] [PubMed] [Google Scholar]
- 100.Biousse V, Newman NJ. Neuro-ophthalmology illustrated. Thieme; New York: 2009. [Google Scholar]
- 101.Newborg B. Pseudotumor cerebri treated by rice reduction diet. Arch Intern Med. 1974;133:802–807. [PubMed] [Google Scholar]
- 102.Johnson LN, Krohel GB, Madsen RW, March GA., Jr The role of weight loss and acetazolamide in the treatment of idiopathic intracranial hypertension (pseudotumor cerebri) Ophthalmology. 1998;105:2313–2317. doi: 10.1016/S0161-6420(98)91234-9. [DOI] [PubMed] [Google Scholar]
- 103.Kupersmith MJ, Gamell L, Turbin R, Peck V, Spiegel P, Wall M. Effects of weight loss on the course of idiopathic intracranial hypertension in women. Neurology. 1998;50:1094–1098. doi: 10.1212/wnl.50.4.1094. [DOI] [PubMed] [Google Scholar]
- 104.Wong R, Madill SA, Pandey P, Riordan-Eva P. Idiopathic intracranial hypertension: the association between weight loss and the requirement for systemic treatment. BMC Ophthalmol. 2007;7:15. doi: 10.1186/1471-2415-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sugerman HJ, Felton WL, 3rd, Sismanis A, Kellum JM, DeMaria EJ, Sugerman EL. Gastric surgery for pseudotumor cerebri associated with severe obesity. Ann Surg. 1999;229:634–640. doi: 10.1097/00000658-199905000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fridley J, Foroozan R, Sherman V, Brandt ML, Yoshor D. Bariatric surgery for the treatment of idiopathic intracranial hypertension. J Neurosurg. 2010 doi: 10.3171/2009.12.JNS09953. [DOI] [PubMed] [Google Scholar]
- 107.Lee AG, Pless M, Falardeau J, Capozzoli T, Wall M, Kardon RH. The use of acetazolamide in idiopathic intracranial hypertension during pregnancy. Am J Ophthalmol. 2005;139:855–859. doi: 10.1016/j.ajo.2004.12.091. [DOI] [PubMed] [Google Scholar]
- 108.http://clinicaltrials.gov/ct2/show/NCT01003639
- 109.Celebisoy N, Gökçay F, Sirin H, Akyürekli O. Treatment of idiopathic intracranial hypertension: topiramate vs acetazolamide, an open-label study. Acta Neurol Scand. 2007;116:322–327. doi: 10.1111/j.1600-0404.2007.00905.x. [DOI] [PubMed] [Google Scholar]
- 110.Johnston I, Besser M, Morgan MK. Cerebrospinal fluid diversion in the treatment of benign intracranial hypertension. J Neurosurg. 1988;69:195–202. doi: 10.3171/jns.1988.69.2.0195. [DOI] [PubMed] [Google Scholar]
- 111.Rosenberg ML, Corbett JJ, Smith C, Goodwin J, Sergott R, Savino P, Schatz N. Cerebrospinal fluid diversion procedures in pseudotumor cerebri. Neurology. 1993;43:1071–1072. doi: 10.1212/wnl.43.6.1071. [DOI] [PubMed] [Google Scholar]
- 112.Eggenberger ER, Miller NR, Vitale S. Lumboperitoneal shunt for the treatment of pseudotumor cerebri. Neurology. 1996;46:1524–1530. doi: 10.1212/wnl.46.6.1524. [DOI] [PubMed] [Google Scholar]
- 113.Burgett RA, Purvin VA, Kawasaki A. Lumboperitoneal shunting for pseudotumor cerebri. Neurology. 1997;49:734–739. doi: 10.1212/wnl.49.3.734. [DOI] [PubMed] [Google Scholar]
- 114.Maher CO, Garrity JA, Meyer FB. Refractory idiopathic intracranial hypertension treated with stereotactically planned ventriculoperitoneal shunt placement. Neurosurg Focus. 2001;10:E1. doi: 10.3171/foc.2001.10.2.2. [DOI] [PubMed] [Google Scholar]
- 115.Bynke G, Zemack G, Bynke H, Romner B. Ventriculoperitoneal shunting for idiopathic intracranial hypertension. Neurology. 2004;63:1314–1316. doi: 10.1212/01.wnl.0000140699.43019.48. [DOI] [PubMed] [Google Scholar]
- 116.McGirt MJ, Woodworth G, Thomas G, Miller N, Williams M, Rigamonti D. Cerebrospinal fluid shunt placement for pseudotumor cerebri-associated intractable headache: predictors of treatment response and an analysis of long-term outcomes. J Neurosurg. 2004;101:627–632. doi: 10.3171/jns.2004.101.4.0627. [DOI] [PubMed] [Google Scholar]
- 117.Corbett JJ, Nerad JA, Tse DT, Anderson RL. Results of optic nerve sheath fenestration for pseudotumor cerebri: the lateral orbitotomy approach. Arch Ophthalmol. 1988;106:1391–1397. doi: 10.1001/archopht.1988.01060140555022. [DOI] [PubMed] [Google Scholar]
- 118.Sergott RC, Savino PJ, Bosley TM. Modified optic nerve sheath decompression provides long-term visual improvement for pseudotumor cerebri. Arch Ophthalmol. 1988;106:1384–1390. doi: 10.1001/archopht.1988.01060140548021. [DOI] [PubMed] [Google Scholar]
- 119.Berman EL, Wirtschafter JD. Improvement of optic nerve head appearance after surgery for pseudotumor cerebri. JAMA. 1992;267:1130. [PubMed] [Google Scholar]
- 120.Kelman SE, Heaps R, Wolf A, Elman MJ. Optic nerve decompression surgery improves visual function in patients with pseudotumor cerebri. Neurosurgery. 1992;30:391–395. doi: 10.1227/00006123-199203000-00013. [DOI] [PubMed] [Google Scholar]
- 121.Chandrasekaran S, McCluskey P, Minassian D, Assaad N. Visual outcomes for optic nerve sheath fenestration in pseudotumour cerebri and related conditions. Clin Experiment Ophthalmol. 2006;34:661–665. doi: 10.1111/j.1442-9071.2006.01301.x. [DOI] [PubMed] [Google Scholar]
- 122.Yazici Z, Yazici B, Tuncel E. Findings of magnetic resonance imaging after optic nerve sheath decompression in patients with idiopathic intracranial hypertension. Am J Ophthalmol. 2007;144:429–435. doi: 10.1016/j.ajo.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 123.Kaye AH, Galbraith JE, King J. Intracranial pressure following optic nerve decompression for benign intracranial hypertension. J Neurosurg. 1981;55:453–456. doi: 10.3171/jns.1981.55.3.0453. [DOI] [PubMed] [Google Scholar]
- 124.Jacobson EE, Johnston IH, McCluskey P. The effects of optic nerve sheath decompression on cerebrospinal fluid dynamics. Neuroophthalmology. 1996;16:290. [Google Scholar]
- 125.Brodsky MC, Rettele GA. Protracted postsurgical blindness with visual recovery following optic nerve sheath fenestration. Arch Ophthalmol. 1998;116:107–109. [PubMed] [Google Scholar]
- 126.Flynn WJ, Westfall CT, Weisman JS. Transient blindness after optic nerve sheath fenestration. Am J Ophthalmol. 1994;117:678–679. doi: 10.1016/s0002-9394(14)70085-6. [DOI] [PubMed] [Google Scholar]
- 127.Mauriello JA, Jr, Shaderowfsky P, Gizzi M, Frohman L. Management of visual loss after optic nerve sheath decompression in patients with pseudotumor cerebri. Ophthalmology. 1995;102:441–445. doi: 10.1016/s0161-6420(95)31002-0. [DOI] [PubMed] [Google Scholar]
- 128.Wilkes BN, Siatkowski RM. Progressive optic neuropathy in idiopathic intracranial hypertension after optic nerve sheath fenestration. J Neuroophthalmol. 2009;29:281–283. doi: 10.1097/WNO.0b013e3181c2530b. [DOI] [PubMed] [Google Scholar]
- 129.Kollar C, Parker G, Johnston I. Endovascular treatment of cranial venous sinus obstruction resulting in pseudotumor syndrome: report of three cases. J Neurosurg. 2001;94:646–651. doi: 10.3171/jns.2001.94.4.0646. [DOI] [PubMed] [Google Scholar]
- 130.Higgins JN, Owler BK, Cousins C, Pickard JD. Venous sinus stenting for refractory benign intracranial hypertension. Lancet. 2002;359:228–230. doi: 10.1016/S0140-6736(02)07440-8. [DOI] [PubMed] [Google Scholar]
- 131.Higgins JN, Cousins C, Owler BK, Sarkies N, Pickard JD. Idiopathic intracranial hypertension: 12 cases treated by venous sinus stenting. J Neurol Neurosurg Psychiatry. 2003;74:1662–1666. doi: 10.1136/jnnp.74.12.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Donnet A, Metellus P, Levrier O, et al. Endovascular treatment of idiopathic intracranial hypertension: clinical and radiologic outcome of 10 consecutive patients. Neurology. 2008;70:641–647. doi: 10.1212/01.wnl.0000299894.30700.d2. [DOI] [PubMed] [Google Scholar]
- 133.Bussiere M, Falero R, Nicolle D, Proulx A, Patel V, Pelz D. Unilateral transverse sinus stenting of patients with idiopathic intracranial hypertension. Am J Neuroradiol. 2009 doi: 10.3174/ajnr.A1890. [DOI] [PMC free article] [PubMed] [Google Scholar]