Abstract
We present a unique case of a patient with deafness and blindness secondary to carcinomatous meningitis from colorectal adenocarcinoma with accompanying radiologic and pathologic images and a brief review of the relevant literature.
Keywords: meningeal carcinomatosis, severe visual loss, severe hearing loss
INTRODUCTION
Multiple cranial nerve palsies often reveal a meningeal process that may be infectious, inflammatory, or neoplastic in origin. Even when there is a known history of malignancy, the rarity with which a given primary cancer causes meningeal carcinomatosis can influence diagnostic decision-making and lead to delays in diagnosis. We present a unique case of a patient with deafness and blindness secondary to carcinomatous meningitis from colorectal adenocarcinoma.
CASE REPORT
A 65 year-old man with a history of hypertension presented with six weeks of progressive balance difficulties, four weeks of acute hearing loss in both ears, and two weeks of acute vision loss in the right eye prior to our initial evaluation. His course was also complicated by delirium, which developed after he was started on high dose oral steroids for presumed giant cell arteritis at an outside facility.
His visual acuity was “hand motion” in the right eye and 20/25 in the left eye. He had a right relative afferent pupil defect, full extraocular movements, and profound hearing loss in both ears. Visual fields were very constricted by confrontation in both eyes. Fundus examination showed bilateral optic nerve head edema, suggesting bilateral optic neuropathies. Communication with the patient was made very difficult by his poor vision, deafness and altered mental status. His neurologic and general examinations were otherwise unremarkable. Erythrocyte sedimentation rate (ESR) was 82 mm/h and C-reactive protein (CRP) was 18.3 (normal <0.9). Lumbar puncture showed a CSF opening pressure of 28 cm H2O with 1 red cell, and 138 white cells, which were 85% macrophages and 15% lymphocytes. CSF protein was >300 mg/dL; glucose was 27 mg/dL. CSF VDRL was 1:8 and serum RPR was negative. CSF cytology was negative. Anti-nucleolar antinuclear (ANA) antibodies were positive at 1:160. ANCA was negative. MRI of the brain with contrast was normal.
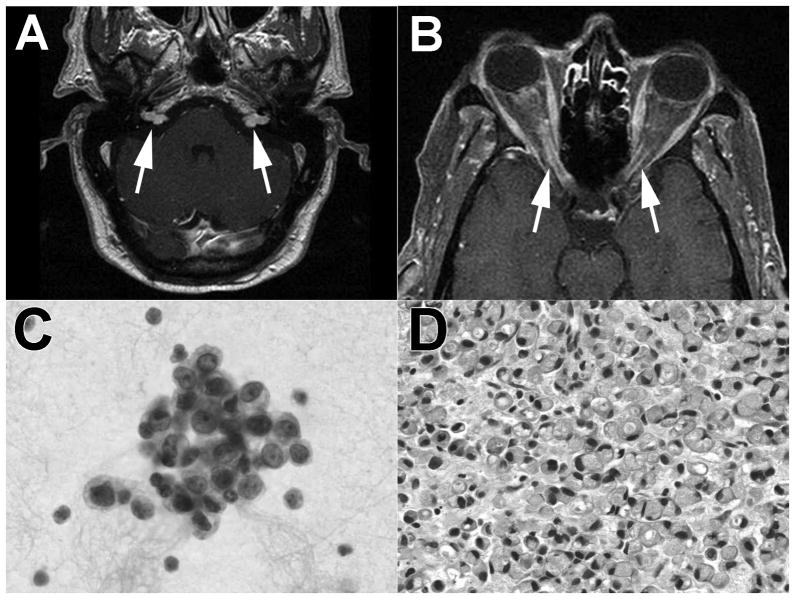
A meningeal process (infectious, inflammatory, or neoplastic) was suspected given the multiple cranial nerve involvement and CSF findings. The CSF profile and positive VDRL raised suspicion for neurosyphilis and tuberculosis (TB) for which the patient was treated with penicillin and four drug TB therapy. The initial differential diagnosis also included vasculitic processes such as giant cell arteritis (GCA) and Wegeners granulomatosis given his age and biologic inflammatory syndrome. Cytology was repeated on two subsequent lumbar punctures, and both samples showed rare atypical epitheloid cells (Figure 1C). Carcinoembryonic antigen (CEA) was 55.9 (nl 0–5.0). CT of the abdomen suggested that the patient had had previous colorectal surgery but there was no mass lesion. Upon further interview of distant family members, we discovered that the patient had a history of previous rectal cancer diagnosed 2 years previously, treated with chemotherapy, surgery, and radiation; however, the exact type of cancer and staging were unknown. Repeat neuroimaging showed bilateral enhancement of the proximal optic nerves, left nodular enhancement of the trigeminal nerve, and bilateral enhancing cerebellopontine angle mass lesions (Figure 1A&B). Biopsy of the right cerebellopontine angle lesion showed signet ring adenocarcinoma (Figure 1D), immunohistochemically positive for cytokeratin and histochemically positive for mucicarmine, suggestive of gastrointestinal origin. The patient’s clinical status deteriorated rapidly to death following whole brain radiation and before chemotherapy could be initiated.
Figure 1.
Rectal meningeal carcinomatosis. A. Bilateral, nodular enhancement in the cerebellopontine angles (MRI T1 post-contrast administration). B. Bilateral enhancement of the proximal optic nerves (MRI T1 post-contrast administration). C. CSF cytology with rare, atypical epitheloid cells. D. Cerebellopontine angle biopsy showing signet ring adenocarcinoma (H&E section).
DISCUSSION
We present an unusual case of meningeal carcinomatosis from rectal carcinoma. Meningeal processes can be caused by infectious, inflammatory, and neoplastic processes, but differentiation can be difficult. As in this case, the typical CSF profile of meningeal carcinomatosis is that of elevated opening pressure, low glucose, and elevated protein. However, this CSF profile is also seen in various inflammatory and infectious etiologies. Contrast-enhanced MRI is about 70% sensitive for leptomeningeal metastases, but differentiation from alternate etiologies is also difficult with this technique.1 Positive CSF cytology remains the gold-standard diagnostic technique; however, it is positive in only 50–70% of cases on the initial lumbar puncture. The sensitivity increases to nearly 100% after three attempts, and thus, as in this case, vigilance in repeating the lumbar puncture is necessary when initial results are negative.1, 2 Sensitivity can be further increased by obtaining at least 10.5 mL of CSF for cytological analysis and immediately processing the sample.2
Breast, small cell lung cancer, and melanoma are the most common solid tumors reported to cause meningeal carcinomatosis, while other tumors such as head & neck, cervical, ovarian, renal, and bladder cancer have been reported to do so with much less frequency.1, 3 Colorectal carcinoma is particularly unlikely to metastasize to the meninges, occurring in only 0.019% of patients with this primary cancer. It is the responsible tumor in only 0.56% of cases of meningeal carcinomatosis.4
Reviewing the English literature after 1960, we identified ten cases of meningeal carcinomatosis where colorectal cancer was the underlying neoplasm.5–10 Some presentations had only mild symptoms, such as mild, isolated upper extremity paresthesias,6 while others had significant alterations of consciousness.10 In some of these cases, the presentation of meningeal carcinomatosis occurred over a year after initial diagnosis,5 while in others it was the only clue to an underlying, occult neoplasm.10 We identified a single report of blindness8 and a single report of acute hearing loss5 from carcinomatous meningitis related to colorectal cancer.
In Wasserstrom et al.’s review of 90 cases of leptomeningeal metastasis from solid tumors, blindness only occurred in three (3%) while some degree of hearing loss was reported in 7 (8%).11 Our literature review identified eleven additional cases of blindness from meningeal carcinomatosis12–20 related to a variety of mechanisms, including papilledema from increased intracranial pressure,12 direct infiltration of the optic nerve/sheath complex,17 treatment related toxicity,15 and retinal vascular occlusions.19 Responsible neoplasms included breast,15, 16, 19 lung,12–14 ovarian,17 gastric,18 and endometroid sarcoma.13 While bilateral hearing loss is a more frequent symptom of meningeal carcinomatosis as seen in Wasserstrom et al.’s series, it is exceptional that it occurs in isolation, 21–24 and the dramatic, simultaneous presentation of blindness and deafness seen in our patient has not, to our knowledge, been reported previously in association with meningeal carcinomatosis from any cause.
Prognosis in this setting is extremely poor. In the absence of treatment, median survival is a mere 4–6 weeks. Aggressive therapy with radiation to symptomatic sites combined with intrathecal chemotherapy prolong median survival to 3–6 months, but fixed neurologic deficits rarely improve. Because radiation combined with the most commonly used chemotheaputics for meningeal carcinomatosis (methotrexate, Ara-C, and thiotepa) can result in significant neurological and systemic toxicity, it is important to balance the patient’s functional status, degree of fixed neurologic dysfunction, and natural history of the underlying malignancy to help guide the decision between therapy and supportive care.25
Footnotes
Disclosure:
This work was supported in part by a departmental grant (Department of Ophthalmology) from Research to Prevent Blindness, Inc, New York, New York, by core grant P30-EY06360 (Department of Ophthalmology) from the National Institutes of Health/National Eye Institute, and PHS Grants UL1-RR025008 (Drs. Biousse and Bruce) & KL2-RR025009 (Dr. Bruce) from the Clinical and Translational Science Award Program, National Institutes of Health/National Center for Research Resources. Dr. Bruce was a recipient of the American Academy of Neurology Practice Research Fellowship. Dr. Newman is a recipient of a Research to Prevent Blindness Lew R. Wasserman Merit Award. Dr. Tehrani has nothing to disclose.
References
- 1.Pavlidis N. The diagnostic and therapeutic management of leptomeningeal carcinomatosis. Ann Oncol. 2004;15(Suppl 4):iv285–iv291. doi: 10.1093/annonc/mdh941. [DOI] [PubMed] [Google Scholar]
- 2.Glantz MJ, Cole BF, Glantz LK, et al. Cerebrospinal fluid cytology in patients with cancer: minimizing false-negative results. Cancer. 1998 Feb 15;82(4):733–739. doi: 10.1002/(sici)1097-0142(19980215)82:4<733::aid-cncr17>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 3.Jayson GC, Howell A. Carcinomatous meningitis in solid tumours. Ann Oncol. 1996 Oct;7(8):773–786. doi: 10.1093/oxfordjournals.annonc.a010755. [DOI] [PubMed] [Google Scholar]
- 4.Giglio P, Weinberg JS, Forman AD, Wolff R, Groves MD. Neoplastic meningitis in patients with adenocarcinoma of the gastrointestinal tract. Cancer. 2005 Jun 1;103(11):2355–2362. doi: 10.1002/cncr.21082. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki T, Sakaguchi H, Yamamoto S, Hisa Y. Sudden hearing loss due to meningeal carcinomatosis from rectal carcinoma. Auris Nasus Larynx. 2006 Sep;33(3):315–319. doi: 10.1016/j.anl.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Sartore-Bianchi A, Pedrazzoli P, Ponchio L, Lanza A, Pavesi L. Meningeal carcinomatosis in rectal cancer. Clin Oncol (R Coll Radiol) 2002 Feb;14(1):82. doi: 10.1053/clon.2001.0017. [DOI] [PubMed] [Google Scholar]
- 7.Nagashima T, Muroi K, Kunitama M, et al. Colon cancer with meningeal carcinomatosis and myelodysplastic syndrome in a patient who underwent intensive chemotherapy for acute myelogenous leukemia: a case report. Jpn J Clin Oncol. 2001 May;31(5):221–225. doi: 10.1093/jjco/hye041. [DOI] [PubMed] [Google Scholar]
- 8.McFadzean R, Brosnahan D, Doyle D, Going J, Hadley D, Lee W. A diagnostic quartet in leptomeningeal infiltration of the optic nerve sheath. J Neuroophthalmol. 1994 Sep;14(3):175–182. doi: 10.3109/01658109409024045. [DOI] [PubMed] [Google Scholar]
- 9.Kato H, Emura S, Takashima T, Ohmori K, Sunaga T. Gadolinium-enhanced magnetic resonance imaging of meningeal carcinomatosis in colon cancer. Tohoku J Exp Med. 1995 Jun;176(2):121–126. doi: 10.1620/tjem.176.121. [DOI] [PubMed] [Google Scholar]
- 10.Hawkins D, Brown D. Meningeal carcinomatosis. Can Med Assoc J. 1963 Feb 2;88:225–228. [PMC free article] [PubMed] [Google Scholar]
- 11.Wasserstrom WR, Glass JP, Posner JB. Diagnosis and treatment of leptomeningeal metastases from solid tumors: experience with 90 patients. Cancer. 1982 Feb 15;49(4):759–772. doi: 10.1002/1097-0142(19820215)49:4<759::aid-cncr2820490427>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 12.Altrocchi PA, Reinhardt PH, Eckman PB. Blindness and meningeal carcinomatosis. Arch Ophthalmol. 1972 Nov;88(5):508–512. doi: 10.1001/archopht.1972.01000030510007. [DOI] [PubMed] [Google Scholar]
- 13.Altrocchi PH, Eckman PB. Meningeal carcinomatosis and blindness. J Neurol Neurosurg Psychiatry. 1973 Apr;36(2):206–210. doi: 10.1136/jnnp.36.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Appen RE, de Venecia G, Selliken JH, Giles LT. Meningeal carcinomatosis with blindness. Am J Ophthalmol. 1978 Nov;86(5):661–665. doi: 10.1016/0002-9394(78)90186-1. [DOI] [PubMed] [Google Scholar]
- 15.Boogerd W, Moffie D, Smets LA. Early blindness and coma during intrathecal chemotherapy for meningeal carcinomatosis. Cancer. 1990 Feb 1;65(3):452–457. doi: 10.1002/1097-0142(19900201)65:3<452::aid-cncr2820650313>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 16.Cantillo R, Jain J, Singhakowinta A, Vaitkevicius VK. Blindness as initial manifestation of meningeal carcinomatosis in breast cancer. Cancer. 1979 Aug;44(2):755–757. doi: 10.1002/1097-0142(197908)44:2<755::aid-cncr2820440249>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 17.Levy J, Marcus M, Shelef I, Lifshitz T. Acute bilateral blindness in meningeal carcinomatosis. Eye. 2004 Feb;18(2):206–207. doi: 10.1038/sj.eye.6700582. discussion 207–208. [DOI] [PubMed] [Google Scholar]
- 18.McCrary JA, 3rd, Patrinely JR, Font RL. Progressive blindness caused by metastatic occult signet-ring cell gastric carcinoma. Arch Ophthalmol. 1986 Mar;104(3):410–413. doi: 10.1001/archopht.1986.01050150110039. [DOI] [PubMed] [Google Scholar]
- 19.Schaible ER, Golnik KC. Combined obstruction of the central retinal artery and vein associated with meningeal carcinomatosis. Arch Ophthalmol. 1993 Nov;111(11):1467–1468. doi: 10.1001/archopht.1993.01090110025011. [DOI] [PubMed] [Google Scholar]
- 20.Teare JP, Whitehead M, Rake MO, Coker RJ. Rapid onset of blindness due to meningeal carcinomatosis from an oesophageal adenocarcinoma. Postgrad Med J. 1991 Oct;67(792):909–911. doi: 10.1136/pgmj.67.792.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagemakers M, Verhagen W, Borne B, Venderink D, Wauters C, Strobbe L. Bilateral profound hearing loss due to meningeal carcinomatosis. J Clin Neurosci. 2005 Apr;12(3):315–318. doi: 10.1016/j.jocn.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Uppal HS, Ayshford CA, Wilson F. Sudden onset bilateral sensorineural hearing loss: a manifestation of occult breast carcinoma. J Laryngol Otol. 2001 Nov;115(11):907–910. doi: 10.1258/0022215011909323. [DOI] [PubMed] [Google Scholar]
- 23.Testoni S, Pirodda A, Pastore Trossello M, Minguzzi E, D’Alessandro R. Meningeal carcinomatosis causing isolated bilateral symmetric progressive hearing loss. Neurol Sci. 2005 Feb;25(6):345–347. doi: 10.1007/s10072-004-0369-2. [DOI] [PubMed] [Google Scholar]
- 24.Lai TH, Chen C, Yen DJ, Yu HY, Yiu CH, Kwan SY. Isolated acute hearing loss as the presenting symptom of leptomeningeal carcinomatosis. J Chin Med Assoc. 2006 Oct;69(10):496–498. doi: 10.1016/S1726-4901(09)70316-8. [DOI] [PubMed] [Google Scholar]
- 25.Grossman SA, Krabak MJ. Leptomeningeal carcinomatosis. Cancer Treat Rev. 1999 Apr;25(2):103–119. doi: 10.1053/ctrv.1999.0119. [DOI] [PubMed] [Google Scholar]