Abstract
Purpose
Sphingosine kinase is an oncogene that is up-regulated in several solid tumors. The product of the sphingosine kinase activity, sphingosine-1-phosphate is a potent mitogen involved in diverse cell processes such as cell survival and migration. Current standard therapy in the treatment of glioblastoma multiforme (GBM) is a combination of surgery, radiation, and chemotherapy using the drug temozolomide (TMZ). However, virtually all tumors become resistant to TMZ. Therefore, new drug targets are necessary. In this study, we investigated whether inhibiting sphingosine kinase could induce cell death in TMZ-resistant GBM cells.
Methods
To study TMZ resistance in vitro, we have generated TMZ-resistant cell lines from established GBM cells. We used a potent inhibitor of sphingosine kinase to study its effect on colony formation and cell growth in GBM cells with a limited dilution and WST assay. Moreover, cell death was determined by measuring caspase-3 activity using flow cytometry.
Results
A sphingosine kinase inhibitor reduced cell colony formation and activated caspase-3 in both TMZ-sensitive and resistant GBM cells.
Conclusion
Addition of a sphingosine kinase inhibitor to the standard chemotherapy regimen against GBM may be beneficial.
Keywords: Glioblastoma, Sphingosine, Sphingosine kinase, Inhibitor
Introduction
Glioblastoma multiforme (GBM) is among the most aggressive solid tumors [1]. In recent years, little significant progress in new therapies has been made. One of the most significant advancements in treating GBM has been the introduction of the chemotherapeutic drug temozolomide (TMZ) which has been shown to provide a survival benefit for GBM patients when used in combination with surgery and radiation. While the initial treatment of many GBM with TMZ shows dramatic results, virtually all tumors recur and become resistant to this drug. Thus, the identification of new drug targets is essential for developing effective therapies against GBM.
Sphingosine-1-phosphate (S1P) has emerged as a potent lipid signaling molecule that plays a role in many biological processes such as cell survival, mitogenesis and cell migration [2]. S1P has a central role in T cell trafficking and maturation which was discovered by studies involving the immunosuppressant drug FTY720. FTY720 was developed as an analog of myriocin. Further investigations on the action of FTY720 revealed that the drug is phosphorylated in vivo and acts as an agonist to the S1P1 receptor and thereby altering the T cell biology. Currently, FTY720 is tested in clinical trials as an immunosuppressant in organ transplant rejection and multiple sclerosis [3]. Moreover, the enzyme sphingosine kinase 1 (Sphk1) that produces S1P, has been implicated in cancer as many tumors show an increased expression when compared to the matching normal tissues [4]. Recently, Paugh et al. [5] demonstrated that the proinflammatory cytokine IL-1 which is secreted by multiple GBM cell lines can regulate Sphk1 expression. Sphk1 is considered an oncogene due to its ability to induce colony formation in soft agar [6]. In vitro, S1P has been shown to be a strong signal for cell proliferation in different cell types [7].
Of relevance to our study, Van Brocklyn et al. [8] have shown a correlation between Sphk1 expression in GBM tissues and poor patient prognosis. Moreover, Anelli et al. [9] have demonstrated that hypoxia-inducible factor (HIF) controls Sphk1 expression in glioblastoma cells. Using microarray analysis to compare the gene expression profile of GBM cells sensitive and resistant to TMZ, we observed an increase in Sphk1 gene expression. Therefore, to study the effect of Sphk1 on GBMs we have used a recently published and commercially available inhibitor of Sphk1, 2-(p-hydroxyanilino)-4-(p-chlorophenyl)thiazole (referred to as SKI = sphk1 inhibitor) [4]. In the present paper, we demonstrate that SKI inhibits colony formation of both TMZ sensitive and resistant cells as measured in a limiting dilution assay. Moreover, it induced apoptotic cell death in both TMZ sensitive and resistant cell lines.
Methods
Drug
Temozolomide (Schering-Plough, Kenilworth, NJ) and O6-benzylguanine (O6-BG) (kindly supplied by Dr. Robert C. Moschel, National Cancer Institute, Bethesda, MD) were dissolved in dimethylsulfoxide and freshly prepared each time before use. SKI was purchased from Calbiochem and also dissolved in dimethylsulfoxide.
Cell lines
U251 and D54MG cells were grown in Improved MEM Zinc Option (Richter’s Modification) Medium (Invitrogen) supplemented with 10% FCS (Invitrogen).
Laboratory generated resistance to TMZ
Cells were initially cultured in the presence of 10 µM of TMZ and 25 µM O6-BG. After every three passages, the concentration of TMZ was increased 5–10 µM. The resulting resistant cell lines are denoted as O6-BG and TMZ resistance (OTR) and the sensitive lines as P (parental).
Drug treatments
All treatments of U251 cells with TMZ were done in the presence of 20 µM O6-BG to pharmacologically ablate the DNA repair enzyme, methylguanine-methyltransferase (MGMT). Treatment of D54MG cells with TMZ did not include O6-BG as these cells do not express detectable amounts of MGMT. Treatment of cells with SKI was done in medium containing 10% charcoal-stripped FBS (Invitrogen) due to the finding that serum contains a significant amount of S1P, the product of Sphk1 [10]. Only the long-term limiting dilution assays with SKI were performed in medium containing normal FBS.
Real-time quantitative RT-PCR
RNA was isolated from cells using RNeasy kit (Qiagen) and 1–1.5 µg was converted to cDNA using the Superscript First Strand Synthesis System from Invitrogen. TaqMan Gene Expression Assays for Sphk1 (cat# Hs01116528_m1) and GAPDH (cat# Hs99999905_m1) were purchased from Applied Biosystems. The PCR reactions were run on an 7900HT Real-Time PCR System (Applied Biosystems) with the following program: 50°C, 2 min; 95°C, 10 min; 95°C, 15 s (40 cycles); 60°C, 1 min.
Limiting dilution assay
The limiting dilution assay was executed exactly as described previously [11]. Briefly, cells were harvested in exponential growth and treated at a density of 106 cells/ml with SKI (concentrations, 3, 5, 10, 20, 30, 40, 60 µM). Serial fivefold dilutions (with medium containing SKI at the respective concentration) were made from each original tube, and 100 µl were plated per well in a 96-well flat-bottomed tissue culture plate (6 wells/dilution, 8 dilutions/original tube). The plates were incubated at 37°C in a humidified atmosphere of 5% CO2 for 12 days, and the wells were examined for colony formation (>30 cells), using an inverted microscope. Each well was scored as positive or negative for the presence of colonies.
Cell proliferation assay
Cell proliferation was estimated by measuring metabolic activity performed using the Cell Counting Kit 8 (Dojindo) which uses a WST compound. Cells were plated at 5,000–10,000 cells per well in a 96-well plate. After 24 h, medium was aspirated and cells incubated with SKI for 48 h. After incubation, WST-8 reagent was added and cells incubated for an additional 1–2 h at 37°C. Absorbance was read at 450 nm.
Measurement of caspase-3 activity by flow cytometry
U251P and U251-OTR cells were treated with 100 µM TMZ or 20 µM of SKI. After 72 h, the activation status of caspase-3 was measured on BD FACSCalibur using the caspase-3, Active Form kit (BD Pharmingen) according to the manufacturer’s instructions.
Result
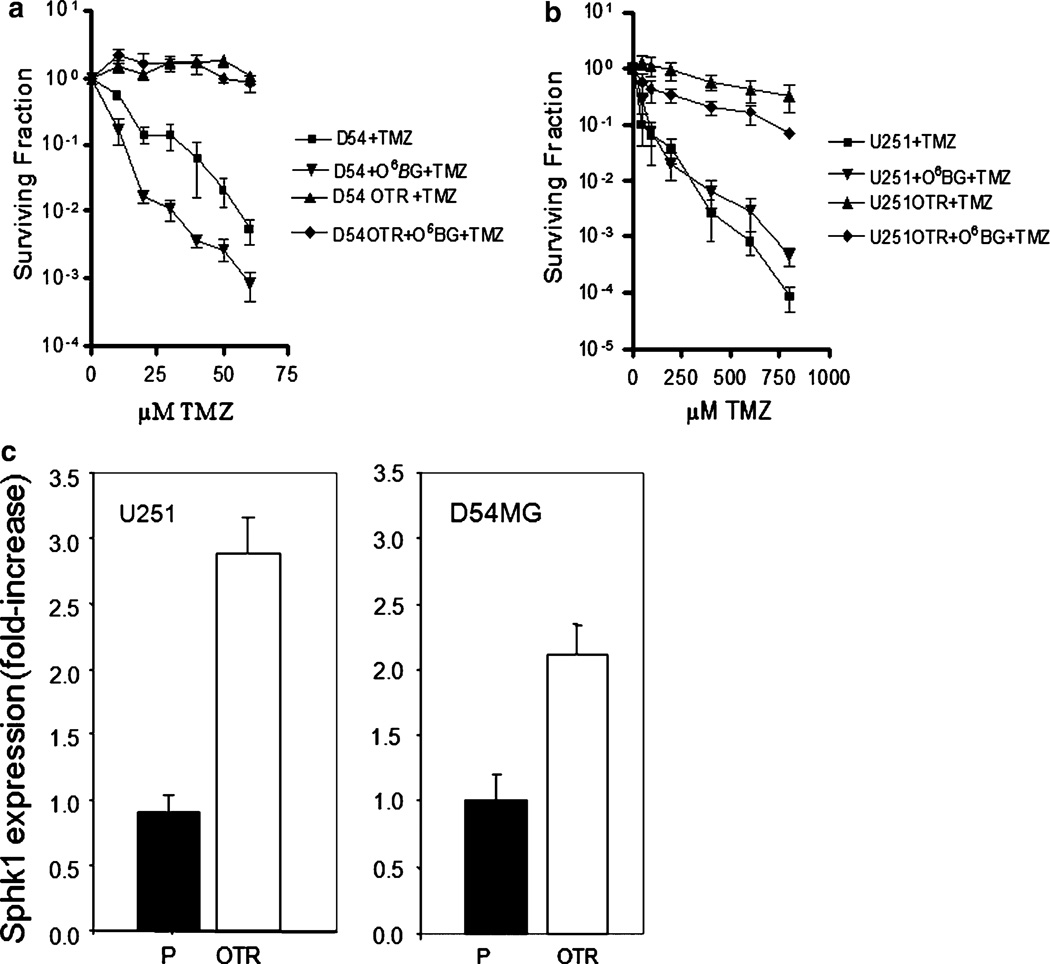
In order to study resistance mechanisms and design new drug targets in GBM, we have generated cell lines that are resistant to TMZ in the presence of O6-BG. The presence of the O6-BG precluded resistance caused by elevation of MGMT expression. Both the parental and OTR cells of the GBM cell lines U251 and D54MG are DNA mismatch repair proficient (data not shown). As shown in Fig. 1a, b, TMZ had relatively little effect on colony formation in the OTR lines even in the presence of O6-BG.
Fig. 1.
Limiting dilution assay shows gain of resistance to TMZ in OTR cell lines of a D54MG cells and b U251 cells. Data are the mean ± SD of three different experiments and are expressed as survival fractions. c Real-time RT-PCR displays up-regulation of Sphk1 gene expression in the OTR lines of U251 and D54MG compared to the respective parental line. Data are the mean ± SD of duplicate determinations and are expressed as fold increase compared with parental cell line
A microarray analysis comparing U251P and U251-OTR revealed a 4.8-fold up-regulation of Sphk1 gene expression. Sphk2 gene expression was not altered. Other genes involved in sphingolipid metabolism and signaling that were changed more than twofold in U251-OTR versus U251P were the S1P receptor isoform 1 (3.2-fold increase) and the LAG1 (longevity assurance gene) homolog ceramide synthase 6 (twofold decrease). To confirm the result with the Sphk1 gene, we measured the Sphk1 RNA levels by real-time RT-PCR in the TMZ sensitive and resistant U251 and D54MG cells. In both instances, we observed an increase in the Sphk1 RNA expression in the OTR cells relative to the parental cells (Fig. 1c): approximately threefold for U251-OTR and twofold for D54MG-OTR.
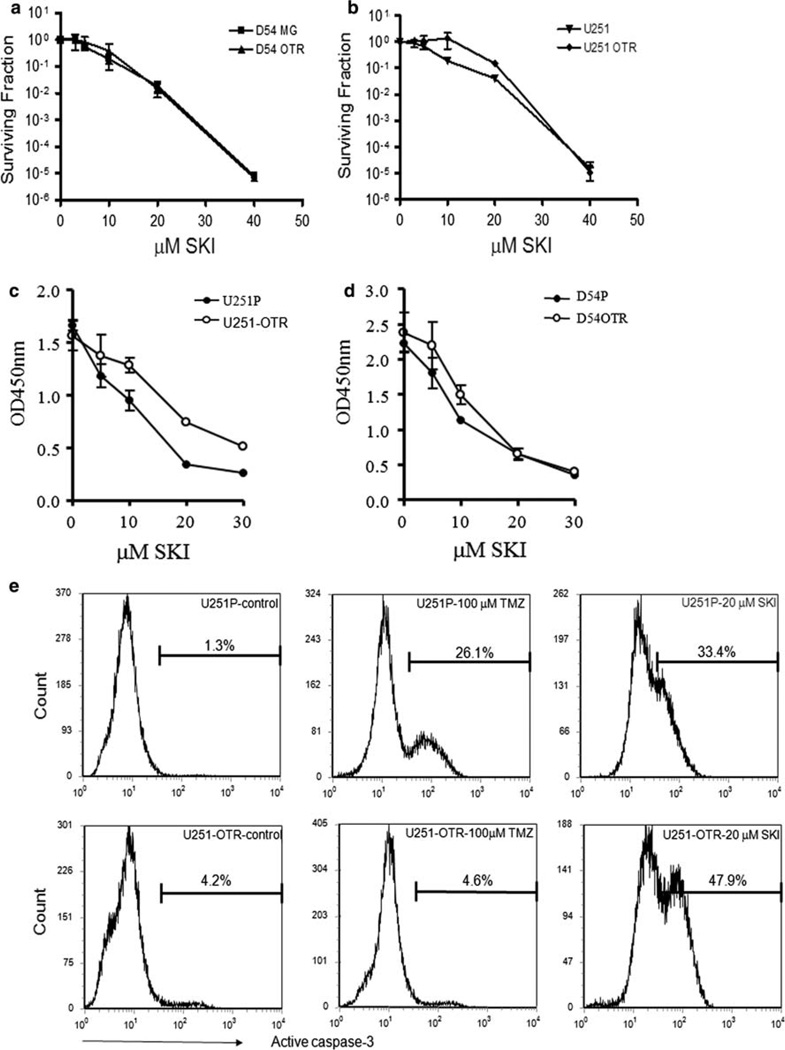
To determine the effect of inhibition of Sphk1 on U251 and D54MG cells, we treated the cells with Sphk1 inhibitor, SKI, and measured colony formation. We observed that SKI inhibited colony formation in both the P and OTR lines of U251 and D54MG to a similar extent (Fig. 2a, b). The calculated LD10’s for SKI in U251P, U251-OTR, D54MG, and D54MG-OTR were 11.2 ± 0.7, 12.9 ± 2.0, 10.3 ± 1.8, and 10.0 ± 2.7 µM, respectively. To examine the short term effects of SKI, we carried out cell proliferation assays as shown in Fig. 2c, d. After 48 h incubation, SKI significantly inhibited cell growth in all the cell lines.
Fig. 2.
SKI reduces cell growth and colony formation and induces cell death. Treatment of D54MG (a) and U251 (b) cells with SKI inhibits colony formation in both the parental and OTR lines as measured with a limiting dilution assay. Data are the mean ± SD of three experiments performed on different days and are expressed as survival fractions. Effect of SKI on cell proliferation in U251 (c) and D54MG (d) cells were measured as described under “Methods”. Data are the mean ± SD of quadruplicate determinations and are displayed as absorption measured at OD 450 nm. Similar results were obtained in two additional experiments. e Caspase-3 activation in U251 cells upon SKI treatment was measured using flow cytometry. Similar results were obtained in two additional experiments
Temozolomide has been found to cause cell-cycle inhibition and cell death in glioma cells [12, 13]. TMZ causes the methylation of DNA at N7 guanine, N3 adenine and O6 guanine, whereby the latter methylation is critical for TMZ’s cytotoxicity. Methylated O6 guanine incorrectly pairs with thymine which triggers the mismatch repair system. The repair attempts fail leading to the reinsertion of thymine. The repetitive and futile attempts to repair the mismatch leads ultimately to DNA strand breaks, growth arrest and cell death [14]. When U251P cells were treated with 100 µM TMZ, we observed a significant proportion of the cells in apoptosis as quantified by caspase-3 activation (Fig. 2e). In contrast, U251-OTR cells showed no caspase-3 activation following TMZ treatment (Fig. 2e). In contrast, when U251P or OTR cells were treated with SKI, we observed caspase-3 activation in both the TMZ sensitive and resistant cells. A similar result was observed for the D54MG cells (data not shown).
Discussion
Our initial undertaking was to study the role of Sphk1 in TMZ-mediated resistance. The impetus for this was the finding that U251-OTR and D54MG-OTR cells expressed higher amounts of Sphk1 RNA. However, when we inhibited Sphk1, we found a similar cytotoxic effect on the parental and TMZ-resistant cells.
Sphingosine kinase 1 is a well-known lipid mediator in cell survival and apoptosis resistance. For example, melanoma cells that were resistant to cell death induction by CD95 antigen showed a higher expression of this lipid enzyme [15]. Down-regulation of Sphk1 rendered resistant melanoma cells susceptible to CD95-induced cell death.
Glioblastoma multiforme are hypoxic in the center of the tumor. Consequently, HIFs are stabilized which in turn up-regulate many of their target genes such as vascular endothelial growth factor (VEGF) that lead to tumor cell survival and tumor progression [16]. Recently, it was published that hypoxia induces Sphk1 expression and activity [9]. It is therefore conceivable that expression of Sphk1 may contribute to an aggressive and drug-resistant tumor phenotype. For example, it has been shown that S1P increases invasiveness of GBM cells [17].
Sphingosine-1-phosphate, the product of Sphk1 is as potent a mediator of angiogenesis as VEGF [18, 19]. S1P exerts its angiogenic properties primarily through its specific receptor S1P1 which was also significantly upregulated in U251OTR cells [2]. Moreover, it has previously been shown that there is a crosstalk between S1P and well-known pro-angiogenic growth factors such as VEGF, EGF, PDGF, bFGF and pro-angiogenic cytokines such as IL-6 and IL-8 leading to a positive signaling loop [20]. Specifically, Sphk1 is a downstream target of all the above mentioned growth factors.
Anti-VEGF antibodies such as bevacizumab have shown promising results in clinical trials for the treatment of recurrent GBM [21]. It is possible that a limiting factor for the efficacy of anti-VEGF antibody treatment may be that S1P produced by Sphk1 replaces VEGFs role in angiogenesis. Recently, Visentin et al. [19] have demonstrated that using antibodies against S1P can reduce or even diminish tumors in mouse models. Furthermore, the inhibition of tumor growth is likely mediated by the anti-angiogenic and anti-tumorigenic properties of the anti-S1P antibody [19]. It will be important to determine if inhibition of Sphk1 has an effect on GBM mouse xenografts since inhibitors were observed to have an antitumor effect in a murine adenocarcinoma [22]. A likely explanation for sphingosine kinase inhibitors to reduce cell proliferation and induce cell death may be the increase in the levels of sphingosine and/or its subsequent conversion to ceramide when Sphk1 activity is blocked. Both sphingosine and ceramide have been strongly implicated in apoptotic cell death in many cell types [2].
In conclusion, it becomes increasingly clear that any treatment of a cancer as complex in its biology as GBM will require the targeting of multiple targets. Our results indicate that Sphk1 may prove to be one of these.
Acknowledgment
This work was supported by NIH Grant 5P50CA108786.
Abbreviations
- GBM
Glioblastoma multiforme
- TMZ
Temozolomide
- S1P
Sphingosine-1-phosphate
- Sphk1
Sphingosine kinase 1
- SK1
Sphk1 inhibitor
- O6-BG
O6-Benzylguanine
- MGMT
Methylguanine-methyltransferase
Contributor Information
Meryem Bektas, Email: meryem_bektas@med.unc.edu, Department of Pathology, Duke University Medical Center, Durham, NC, USA.
Stewart P. Johnson, Department of Surgery, Duke University Medical Center, Durham, NC, USA
William E. Poe, Department of Pathology, Duke University Medical Center, Durham, NC, USA
Darell D. Bigner, Department of Pathology, Duke University Medical Center, Durham, NC, USA
Henry S. Friedman, Department of Surgery, Duke University Medical Center, Durham, NC, USA Department of Pediatrics, Duke University Medical Center, Durham, NC, USA; Department of Medicine, Duke University Medical Center, Durham, NC, USA.
References
- 1.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 2.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 3.Horga A, Montalban X. FTY720 (fingolimod) for relapsing multiple sclerosis. Expert Rev Neurother. 2008;8:699–714. doi: 10.1586/14737175.8.5.699. [DOI] [PubMed] [Google Scholar]
- 4.French KJ, Schrecengost RS, Lee BD, Zhuang Y, Smith SN, Eberly JL, Yun JK, Smith CD. Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Res. 2003;63:5962–5969. [PubMed] [Google Scholar]
- 5.Paugh BS, Bryan L, Paugh SW, Wilczynska KM, Alvarez SM, Singh SK, Kapitonov D, Rokita H, Wright S, Griswold-Prenner I, Milstien S, Spiegel S, Kordula T. Interleukin-1 regulates the expression of sphingosine kinase 1 in glioblastoma cells. J Biol Chem. 2009;284:3408–3417. doi: 10.1074/jbc.M807170200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xia P, Gamble JR, Wang L, Pitson SM, Moretti PA, Wattenberg BW, D’Andrea RJ, Vadas MA. An oncogenic role of sphingosine kinase. Curr Biol. 2000;10:1527–1530. doi: 10.1016/s0960-9822(00)00834-4. [DOI] [PubMed] [Google Scholar]
- 7.Olivera A, Kohama T, Edsall L, Nava V, Cuvillier O, Poulton S, Spiegel S. Sphingosine kinase expression increases intracellular sphingosine-1-phosphate and promotes cell growth and survival. J Cell Biol. 1999;147:545–558. doi: 10.1083/jcb.147.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Brocklyn JR, Jackson CA, Pearl DK, Kotur MS, Snyder PJ, Prior TW. Sphingosine kinase-1 expression correlates with poor survival of patients with glioblastoma multiforme: roles of sphingosine kinase isoforms in growth of glioblastoma cell lines. J Neuropathol Exp Neurol. 2005;64:695–705. doi: 10.1097/01.jnen.0000175329.59092.2c. [DOI] [PubMed] [Google Scholar]
- 9.Anelli V, Gault CR, Cheng AB, Obeid LM. Sphingosine kinase 1 is up-regulated during hypoxia in U87MG glioma cells. Role of hypoxia-inducible factors 1 and 2. J Biol Chem. 2008;283:3365–3375. doi: 10.1074/jbc.M708241200. [DOI] [PubMed] [Google Scholar]
- 10.Edsall L, Vann L, Milstien S, Spiegel S. Enzymatic method for measurement of sphingosine 1-phosphate. Methods Enzymol. 2000;312:9–16. doi: 10.1016/s0076-6879(00)12895-2. [DOI] [PubMed] [Google Scholar]
- 11.Friedman HS, Colvin OM, Kaufmann SH, Ludeman SM, Bullock N, Bigner DD, Griffith OW. Cyclophosphamide resistance in medulloblastoma. Cancer Res. 1992;52:5373–5378. [PubMed] [Google Scholar]
- 12.Kanzawa T, Bedwell J, Kondo Y, Kondo S, Germano IM. Inhibition of DNA repair for sensitizing resistant glioma cells to temozolomide. J Neurosurg. 2003;99:1047–1052. doi: 10.3171/jns.2003.99.6.1047. [DOI] [PubMed] [Google Scholar]
- 13.Roos WP, Batista LF, Naumann SC, Wick W, Weller M, Menck CF, Kaina B. Apoptosis in malignant glioma cells triggered by the temozolomide-induced DNA lesion O6-methylguanine. Oncogene. 2007;26:186–197. doi: 10.1038/sj.onc.1209785. [DOI] [PubMed] [Google Scholar]
- 14.Friedman HS, Kerby T, Calvert H. Temozolomide and treatment of malignant glioma. Clin Cancer Res. 2000;6:2585–2597. [PubMed] [Google Scholar]
- 15.Bektas M, Jolly PS, Muller C, Eberle J, Spiegel S, Geilen CC. Sphingosine kinase activity counteracts ceramide-mediated cell death in human melanoma cells: role of Bcl-2 expression. Oncogene. 2005;24:178–187. doi: 10.1038/sj.onc.1208019. [DOI] [PubMed] [Google Scholar]
- 16.Fischer I, Gagner JP, Law M, Newcomb EW, Zagzag D. Angiogenesis in gliomas: biology and molecular pathophysiology. Brain Pathol. 2005;15:297–310. doi: 10.1111/j.1750-3639.2005.tb00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Brocklyn JR, Young N, Roof R. Sphingosine-1-phosphate stimulates motility and invasiveness of human glioblastoma multiforme cells. Cancer Lett. 2003;199:53–60. doi: 10.1016/s0304-3835(03)00334-3. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, Rosenfeldt HM, Nava VE, Chae SS, Lee MJ, Liu CH, Hla T, Spiegel S, Proia RL. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest. 2000;106:951–961. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Visentin B, Vekich JA, Sibbald BJ, Cavalli AL, Moreno KM, Matteo RG, Garland WA, Lu Y, Yu S, Hall HS, Kundra V, Mills GB, Sabbadini RA. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell. 2006;9:225–238. doi: 10.1016/j.ccr.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 20.Shu X, Wu W, Mosteller RD, Broek D. Sphingosine kinase mediates vascular endothelial growth factor-induced activation of ras and mitogen-activated protein kinases. Mol Cell Biol. 2002;22:7758–7768. doi: 10.1128/MCB.22.22.7758-7768.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vredenburgh JJ, Desjardins A, Herndon JE, II, Marcello J, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S, Sampson J, Wagner M, Bailey L, Bigner DD, Friedman AH, Friedman HS. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 22.French KJ, Upson JJ, Keller SN, Zhuang Y, Yun JK, Smith CD. Antitumor activity of sphingosine kinase inhibitors. J Pharmacol Exp Ther. 2006;318:596–603. doi: 10.1124/jpet.106.101345. [DOI] [PubMed] [Google Scholar]