Abstract
Estrogens and estrogen metabolites have important functions in cardiovascular and other physiology, yet the patterns of estrogen synthesis, metabolism and the individual plasma profile of estrogens and estrogen metabolites during human pregnancy as well as in preeclampsia remain undetermined. We performed liquid chromatography mass spectrometry on plasma samples from normotensive pregnant women (normP; n = 8), women with mild (mPE; n = 8) and severe (sPE; n = 8) preeclampsia at labor. Compared to normP, estrone was lower in sPE, whereas plasma level of estradiol-17β was significantly lower in women with mPE and sPE. Estriol was lower in sPE but not in mPE. Although, 2-hydroxyestrone was lower in mPE and sPE, 4-hydroxyestrone was high in sPE. 16-α-hydroxyestrone was higher in mPE but not in sPE. 2-hydroxyestradiol in women with mPE and sPE were lower compared to normP. Compared to 2-methoxyestrone in normP, levels were lower in sPE. 3-methoxyestrone and 4-methoxyestrone were unchanged. 2-methoxyestradiol was lower in mPE and sPE; however, 4-methoxyestradiol was low only in sPE. Compared to normP, 16-keto-estradiol levels were significantly higher in sPE whereas 16-epi-estriol and 17-epi-estriol were lower in women with sPE. Our findings show that preeclampsia is characterized by aberrant synthesis, metabolism and accumulation of estrogens and estrogen metabolites that are likely to be associated with alterations in vascular function. These results underscore the need to investigate the functional vascular and other physiology of estrogens and estrogen metabolites in the pathophysiology of preeclampsia.
Keywords: preeclampsia, estrogens, estrogen metabolites, steroid synthesis, metabolism
Introduction
Preeclampsia is a hypertensive disorder of pregnancy that affects 5% to 8% of pregnancies, thus remaining a significant cause of maternal and fetal morbidity and mortality as well as greater susceptibility and earlier onset of future cardiovascular disease in both mother and baby.1–3Although the etiology of preeclampsia remains elusive, its pathophysiology is characterized by impairment of several of the normal maternal cardiovascular adaptations seen during pregnancy.4, 5
We and others have demonstrated that regulation of normal maternal cardiovascular adaptation during pregnancy is mediated in part by the primary estrogens, estrone, estradiol-17β and estriol, which are synthesized by the uteroplacental unit using circulating steroid precursors from both the maternal and fetal adrenal glands.6, 7 Evidence also supports a role for these primary estrogens in preeclampsia8–10; however, whether primary estrogens can be useful biomarkers for maternal and fetal well-being in adverse pregnancies including preeclampsia has been a subject of controversy. Several studies support evidence that serum and urinary primary estrogens may be useful for screening for adverse pregnancy outcomes8, whereas others contend that measurement of these estrogens is of little value.11 Nevertheless, the levels and plasma profile of circulating primary estrogens in preeclampsia are, at best, unclear and this is further complicated by the lack of information on estrogen metabolites thus hindering our comprehensive understanding of their role(s) in its pathophysiology.
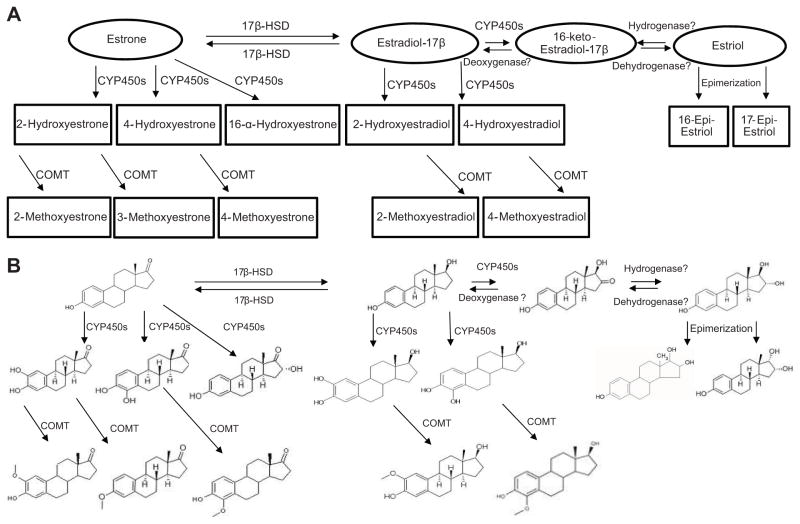
Primary estrogens are converted in the uteroplacental unit by cytochrome P450s (CYP450s) into multiple hydroxylated metabolites defined by the position of hydroxylation such as 2-hydroxyestrone, 4-hydroxyestrone, 16-α-hydroxyestrone, 2-hydroxyestradiol and 4-hydroxyestradiol (Figure 1).12 Hydroxylated primary estrogens especially the catecholestrogens (hydroxylated estrogens at the carbon 2 and 4 positions) undergo enzymatic O-methylation by catechol-O-methyltransferase (COMT) to form methoxyestrogens such as 2-methoxyestrone, 3-methoxyestrone, 4-methoxyestrone, 2-methoxyestradiol and 4-methoxyestradiol (Figure 1).12 Other metabolites of primary estrogens formed from enzymatic pathways include 16-keto-estradiol, 16-epi-estriol and 17-epi-estriol (Figure 1). There is increasing strong support for the concept that regulation of maternal cardiovascular adaptation during pregnancy is partly mediated by estrogen metabolites.13, 14 Estrogen metabolites may also play a role in preeclampsia because pregnant mice deficient in COMT, an enzyme that catalyzes the methylation of catecholestrogens to methoxyestrogens, exhibit a preeclampsia-like phenotype.15 However, the metabolism of estrogens is very complex and necessitates that a plethora of other metabolites of estrogens be properly accounted for to achieve a more comprehensive knowledge of their potential collective contributions to the pathogenesis of preeclampsia.
Figure 1.
Schematic (A) and structural (B) representation of the complexities of the synthesis and multiple mechanisms of metabolism of estrogens and estrogen metabolites. Figure also depicts a working hypothesis that 16-keto-estradiol may be a possible interconversion metabolite between estradiol-17β and estriol through either the sequential actions of CYP450s and a hydrogenase or the sequential actions of a dehydrogenase and a deoxygenase. 17β-HSD (17β-hydroxysteroid dehydrogenase), CYP450s (cytochrome P450) and COMT (catechol-O-methyltransferase).
Therefore, the objective of this study was to compare plasma levels of total primary estrogens and estrogen metabolites in normotensive pregnant women (normP) compared to women with mild (mPE) and severe (sPE) preeclampsia. We hypothesized that preeclampsia, a disease characterized by widespread cardiovascular dysfunction, is associated with aberrant synthesis, metabolism and accumulation of estrogens and estrogen metabolites. In this report, we also present a working hypothesis that 16-keto-estradiol may be a possible interconversion metabolite between estradiol-17β and estriol and may contribute to circulating levels of both primary estrogens (Figure 1).
Methods
Subjects
All subjects signed a written informed consent, and all study-related procedures and protocols were approved by the Institutional Review Boards at the University of Wisconsin and Meriter Hospital, Madison, Wisconsin. The women were recruited at the time of admission to Labor and Delivery.
Inclusion Criteria
The normP control group included pregnant women with no preexisting medical diseases or antenatal complications. Preeclampsia criterion for inclusion was based as described in Creasy and Resnik’s Maternal-Fetal Medicine: Principles and Practice, 6th Edition Chapter 35. Mean (SD) gestational ages at enrollment for normP, mPE and sPE women were 38.9±0.49, 36.6±3.02 and 35.5±3.5 weeks respectively. 24 hour total urine protein was measured in only women that showed ≥1+ on urine dipstick and/or elevated blood pressure on two separate assessments more than 4 hours apart.
Exclusion Criteria
Subjects were excluded if they exhibited any of the following: Lupus, Antiphospholipid Antibody Syndrome and Placental Abruption. In addition, subjects were also excluded if they exhibited Chorioamnionitis or Meconium stained fluids respectively which are signs of antenatal and/or delivery complications.
Sample Collection
Blood samples were collected from normP (n = 8), mPE (n = 8) and sPE (n = 8) subjects via venipuncture of the brachial vein, prepared, centrifuged and plasma aliquots were stored at −80 °C until quantitative measurement.
Liquid chromatography-tandem mass spectrometry
Plasma levels of estrogens and estrogen metabolites were measured by a sensitive, specific and precise high performance liquid chromatography-tandem mass spectrometry method utilizing selected reaction monitoring for measuring the absolute quantities of total (conjugated and unconjugated) estrogens and estrogen metabolites as previously described16 and performed at Primera Analytical Solutions Corp., Princeton, NJ. The unit for the calculated level of estrogens and estrogen metabolites was pg/ml.
Statistical Analysis
Data are presented as Means ± SEM unless noted. The difference in mean values between experimental groups was determined by one-way ANOVA (SigmaPlot 12 Statistical Software) followed by post-hoc Student-Newman- Keuls multiple pairwise comparisons. Level of significance was established a priori at P<0.05.
Results
Characteristics of the Subjects
Compared to normP, sPE but not mPE women had significantly higher body weight and body-mass index (Table 1). Per inclusion criteria, both women with mPE and sPE demonstrated higher than normal systolic and diastolic blood pressures. Moreover, women with sPE had significantly higher systolic and diastolic blood pressure compared to mPE women (Table 1). Per inclusion criteria, compared with normP women who had negative dip stick protein levels, both women with mPE and sPE had higher protein in their urine. In addition, women with sPE had significantly higher proteinuria compared to mPE women. Gestational ages at time of admission were not significantly different amongst the women.
Table 1.
Clinical data and characteristics of the normP, mPE and sPE women
| Characteristic | normP (n=8) | mPE (n=8) | sPE (n=8) |
|---|---|---|---|
| Age (yr.) | 33.6 ±1.8 | 33.0±1.4 | 25.5 ±2.0*† |
| Height (in) | 66.8±1.0 | 66.0±1.4 | 66.0±0.7 |
| Weight (lbs.) | 213.0±17.4 | 200.1±11.7* | 242.8±18.5*† |
| Body mass index | 33.3±2.2 | 32.3±1.8 | 39.1±2.7*† |
| Systolic Blood Pressure (mmHg) | 111.62±2.7 | 149.3± 3.9* | 167.2± 7.0*† |
| Diastolic Blood Pressure (mmHg) | 65.2±2.9 | 95.8±2.9* | 105.0±4.9*† |
| 24 Hour Total Urinary Protein (mg) | 960.3± 398.2 | 4572.2±2499.9† | |
| Gestational Age at Admit (wks.) | 38.9±0.1 | 36.6±1.0 | 35.5±1.2 |
| Gestational Age at Delivery (wks.) | 38.9±0.1 | 37.8±0.4 | 35.4±1.2 |
Values are means ±SEM
Different (P<0.05) compared with normP group.
Different (P<0.05) compared to mPE group.
Plasma Profile of Primary/Classical Estrogens and Estrogen Metabolites in Preeclampsia
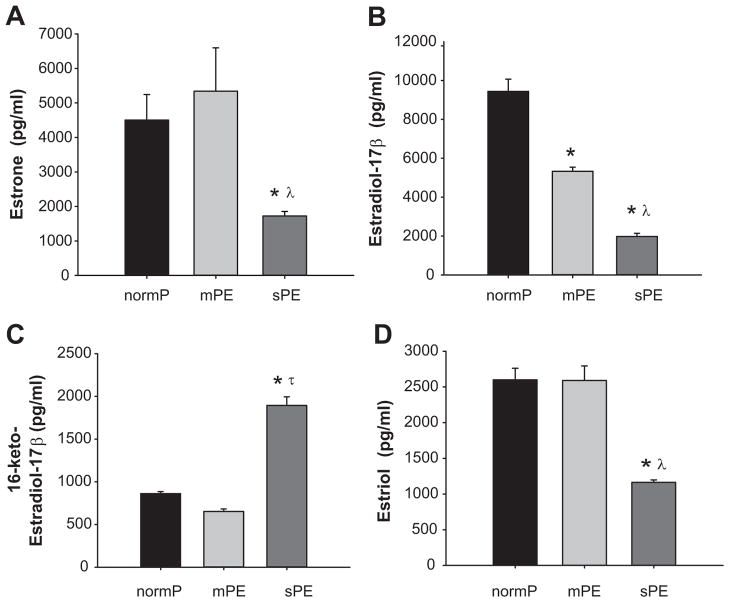
NormP and mPE women had similar levels of plasma estrone (4503 ±740 and 5341 ±1255 pg/ml respectively) (Figure 2A); however, women with sPE had significantly lower level of plasma estrone (1163 ±162 pg/ml) (Figure 2A). Estradiol-17β levels were lower in women with mPE (5326±213 pg/ml) compared to levels in normP (9441±626 pg/ml) (Figure 2B). Moreover, plasma estradiol-17β in women with sPE (1975±162 pg/ml) was lower compared to both normP and mPE (Figure 2B). Levels of 16-keto-estradiol in normP and mPE women (861±22 and 652±229 pg/ml respectively) were not different, whereas levels in sPE women (1894±100 pg/ml) were significantly higher (Figure 2C). Plasma levels of estriol were similar between normP women and women with mPE (2599±162 and 2589±203 pg/ml respectively); however, women with sPE had significantly lower estriol (1163±34 pg/ml) (Figure 2D).
Figure 2. Products of Primary Synthesis.
Plasma levels of estrone, estradiol-17β, 16-keto-estradiol-17β and estriol in normotensive pregnant control women (normP; n=8), women with mild preeclampsia (mPE; n=8), and women with severe preeclampsia (sPE; n=8). *Significantly different (P< 0.001) in levels compared to normP. λ Significantly different (P< 0.001) in levels compared to mPE. τ Significant increase (P< 0.001) in levels compared to normP.
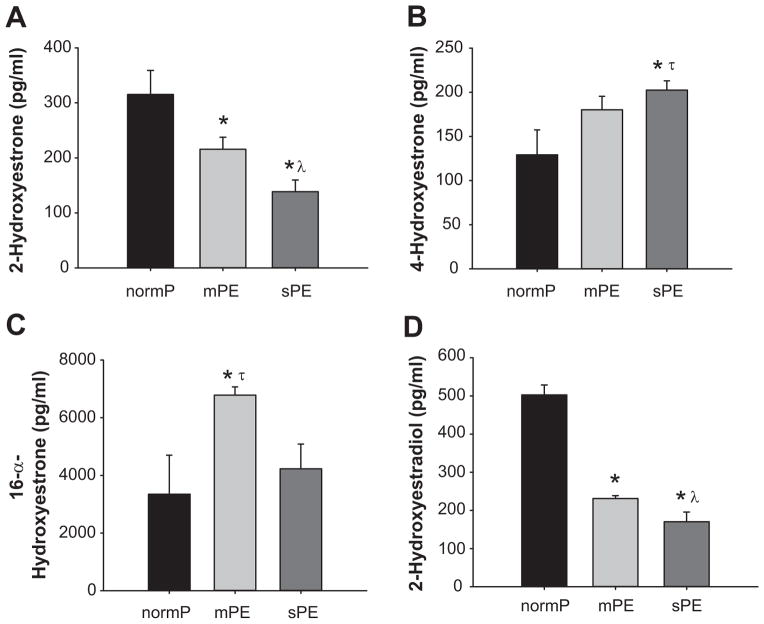
Levels of plasma 2-hydroxyestrone in normP and mPE were 315±43 pg/ml and 215±22 pg/ml respectively (Figure 3A); however, levels in sPE were significantly lower (138±21 pg/ml) (Figure 3A). Compared to normP (129±28 pg/ml), levels of plasma 4-hydroxyestrone were increased in mPE and sPE (180±10 and 202±10 pg/ml respectively) (Figure 3B). Plasma level of 16-α-hydroxyestrone in mPE women (6781±286 pg/ml) was significantly higher compared to normP women (3346±1354 pg/ml) and sPE women (4227±856 pg/ml) (Figure 3C). We observed that plasma level of 2-hydroxyestradiol in women with mPE was significantly lower compared to levels in normP women (Figure 3D). In addition, the levels of plasma 2-hydroxyestradiol in women with sPE (170±25 pg/ml) was significantly lower compared to both normP (502±26 pg/ml) and mPE women (231±7 pg/ml) (Figure 3D).
Figure 3. Products of Hydroxylation.
Plasma levels of 2-hydroyestrone, 4-hydroyestrone, 16-α-hydroxyestrone and 2-hydroxyestradiol-17β, in normotensive pregnant control women (normP; n=8), women with mild preeclampsia (mPE; n=8), and women with severe preeclampsia (sPE; n=8). *Significantly different (P< 0.001) in levels compared to normP. λ Significantly different (P< 0.001) in levels compared to mPE. τ Significant increase (P< 0.001) in levels compared to normP.
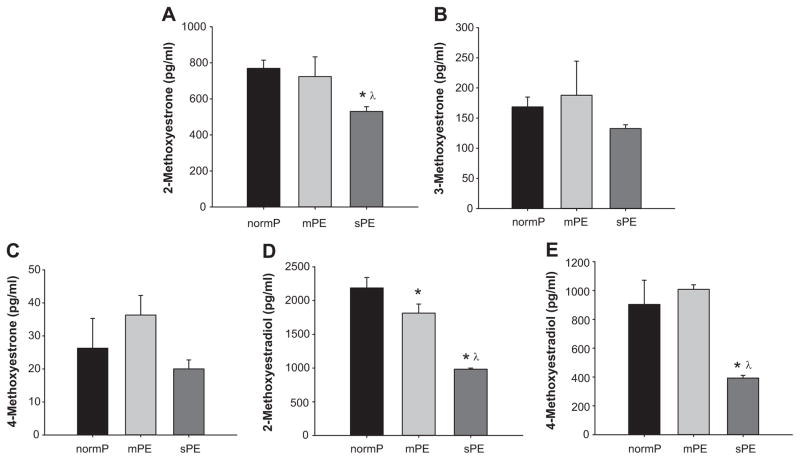
Plasma levels of 2-methoxyestrone were similar between normP women and mPE (769±45 and 723±109 pg/ml respectively); however, women with sPE had significantly lower 2-methoxyestrone (530±26 pg/ml) (Figure 4A). Plasma levels of 3-methoxyestrone (168±16, 187±56 and 132±6 pg/ml respectively) and 4-methoxyestrone (26±9.0, 36±5.9 and 20±2.7 pg/ml respectively) were not significantly different amongst normP, mPE and sPE (Figure 4B and 4C). Plasma levels of 2-methoxyestradiol in women with mPE (1813±133 pg/ml) were significantly lower compared to levels in normP (2186±156 pg/ml) (Figure 4D). In addition, the levels of plasma 2-methoxyestradiol in women with sPE (982±55 pg/ml) were significantly lower compared to both normP and mPE (Figure 4D). NormP and mPE did not have different levels of 4-methoxyestradiol (903±168 and 1008±30 pg/ml respectively) (Figure 4E). However, women with sPE had lower 4-methoxyestradiol (393±18 pg/ml) compared to normP and mPE (Figure 4E).
Figure 4. Products of O-Methylation.
Plasma levels of 2-methoxyestrone, 3-methoxyestrone, 4-methoxyestrone, 2-methoxyestradiol and 4-methoxyestradiol in normotensive pregnant control women (normP; n=8), women with mild preeclampsia (mPE; n=8), and women with severe preeclampsia (sPE; n=8). *Significantly different (P< 0.001) in levels compared to normP. λ Significantly different (P< 0.001) in levels compared to mPE.
The plasma levels of 16-epi-estriol (1104±39 pg/ml) and 17-epi-estriol (189±48 pg/ml) were different in sPE compared to normP (2268±457 and 627±58 pg/ml respectively) and mPE women (2958±124 and 521±16 pg/ml respectively) (Figure 5A and 5B).
Figure 5. Products of Epimerization.
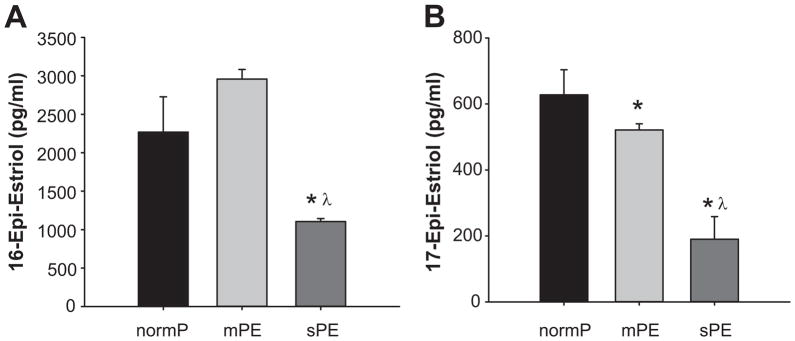
Plasma levels of 16-epi-estriol and 17-epi-estriol in normotensive pregnant control women (normP; n=8), women with mild preeclampsia (mPE; n=8), and women with severe preeclampsia (sPE; n=8). *Significantly different (P< 0.001) in levels compared to normP. λ Significantly different (P< 0.001) in levels compared to mPE.
Discussion
Our findings demonstrate that preeclampsia, especially sPE, is characterized by aberrant synthesis, metabolism and levels of estrogens and estrogen metabolites. Although plasma levels of these estrogens and estrogen metabolites suggest aberrant synthesis and metabolism, they are particularly specific and distinct- therefore, potentially useful in our understanding of the pathophysiology of preeclampsia and exploration of better clinical management and outcomes.
In normal pregnancy, plasma levels of estrone, estradiol-17β and estriol are increased and parallel some of the increases in uteroplacental blood flow as well as development of an extensive uteroplacental vascular bed.6, 7 During this time, the placenta is the main source of these estrogens using circulating steroid precursors from the maternal uterine compartment, adrenal glands as well as fetal adrenal glands.7, 17 Placental biosynthesis of primary estrogens during pregnancy is complex and results from interaction and interdependence of separate maternal, placental and fetal systems that individually do not possess the necessary enzymatic capabilities to make these critical estrogens.18 Because the placenta does not express 17α-hydroxylase, the obligatory synthesis of C19-steroid precursors is not possible for estrogen synthesis.18 Therefore, the primary estrogens are synthesized from C-19 precursors synthesized from the maternal and fetal adrenal glands.19 It is important to note that at or near term, half of placental estrone and estradiol-17β is derived from C-19 precursors from maternal adrenal glands whilst the other half is derived from the fetal adrenal zone.18, 19 By contrast, over ninety percent of placental estriol is derived from C-19 precursors from the fetal adrenal zone whilst only ten percent is derived from maternal sources at or near term.18 Our findings are consistent with studies, albeit controversial, by other investigators that estrone, estradiol-17β and estriol may be low in severe preeclampsia.8, 20 However, it is important to note that only estradiol-17β was found to be low in both mild and severe preelampsia. Estrone and estradiol-17β have been demonstrated to be potent vasodilators in the uterine and systemic vascular beds.21–23 Since severe preeclampsia is characterized partly by impaired uterine and systemic vascular vasodilatory responsiveness, our findings suggest that aberrant activities of uteroplacental aromatase, 17β-hydroxysteroid dehydrogenase, placental sulfatase and/or fetal 16α- and 17α-hydroxylase may be culprits in the pathophysiology of this disease. Low levels of estrone, estradiol-17β and estriol in severe preeclmapsia observed in this study may also be due to reduced circulating steroid precursors from the maternal uterine compartment, adrenal glands as well as fetal adrenal glands. However, previous studies have shown that preeclampsia is not associated with low levels of C-19 steroid precursors including androstenedione, and dehydroepiandrosterone.8, 24
We report herein, for the first time, that the plasma level of 16-keto-estradiol is significantly higher in women with severe preeclampsia. The concomitant higher levels on 16-keto-estradiol-17β with low levels of estradiol-17β and estriol as well as structural and kinetic evaluations led us to the novel hypothesis that this metabolite may be an interconversion metabolite between estradiol-17β and estriol (see Figure 1 schematic).25, 26 Thus, collectively, our observations suggest that elevated levels of 16-keto-estradiol-17β may be used as a predictive biomarker in preeclampsia indicating levels of both estradiol-17β and estriol.
The present findings also show, for the first time, that plasma levels of 2-hydroxyestrone, 4-hydroxyestrone, 16-α-hydroxyestrone and 2-hydroxyestradiol levels are altered in preeclampsia. The low level of both 2-hydroxyestrone and 2-hydroxyestradiol in women with mild and severe preeclampsia suggests low and/or aberrant activity of CYP1A1, CYP1A2 and CYP3A4 which primarily hydroxylate estrogens in the C-2 position to form catecholestrogens.12. We report that levels of 4-hydroxyestrone are only increased in severe preeclampsia compared to normal pregnant women, whereas levels of 16-α-hydroxyestrone are increased in only mild preeclampsia but not in severe preeclampsia. The differential changes in estrogen or estrogen metabolite concentrations between mild versus severe preeclampsia points to the notion that mild and severe preeclampsia may represents a class of related diseases with different etiologies and/or pathophysiologies. Nevertheless, these data suggest that formations of hydroxylated estrogens, which have been demonstrated to possess several uteroplacental vascular effects including vasodilatory activities,27 induction of endothelial cell proliferation,13, 28 generation of prostacyclin29 and synergistic effects with nitric oxide,14 are highly dysregulated in preeclampsia which may contribute to the reduced uterine perfusion in preeclampsia.
Enzymatic O-methylation of catecholestrogens by COMT forms several methoxyestrogens.12 Preeclampsia is also associated with low activity of COMT in human placentas4 and pregnant COMT-deficient mice exhibit a preeclampsia-like phenotype.15 In support of this hypothesis, we show herein that apart from 2-methoxyestradiol, other physiologically relevant COMT-derived metabolites including 2-methoxyestrone and 4-methoxyestradiol are also decreased in severe but not mild preeclampsia. Apart from low activity of COMT, low levels of methoxyestrogens in preeclampsia may also be due to low levels of circulating catecholestrogen precursors as well as the primary estrogens as discussed above. Nevertheless, since methoxyestrogens induce various positive vascular effects via vasoactive and intracellular molecules that have been implicated in preeclampsia such as nitric oxide, prostacyclin, endothelin-1, cyclic nucleotides, HIF-1α and adhesion molecules, our findings suggest that the effects of COMT in preeclampsia could be more critical than previously thought and necessitates further investigation. Many compounds with “catechol” structures are substrates for COMT including the vasoactive catecholamines epinephrine and norepinephrine. We have also previously proposed that this property of COMT also points to the potential relevance of the convergence of the sympathetic catecholamine system and estrogen metabolism systems in the dysregulation of vascular responsiveness in preeclampsia.30
We noted, for the first time, that 16-epi-estriol and 17-epi-estriol are both significantly decreased in severe preeclampsia but only 17-epi-estriol was low both preeclampsia types suggesting that in addition to low estriol levels, epimerization metabolism of estriol may also be aberrant in preeclampsia. 16-epi-estriol has been demonstrated to possess strong anti-inflammatory effects without profound immunosuppressive or glycogenic activities.31, 32 17-epi-estriol possesses negative effects on inflammatory and adhesion by suppressing TNFα-induced and nitric oxide-mediated VCAM-1 expression.33 Thus, since preeclampsia is associated with abnormal increased expression of adhesion molecules and increased levels of pro-inflammatory cytokines that induce alterations in vascular vasodilatory responsiveness,34 our findings suggest the first possibility that low 16-epi-estriol and 17-epi-estriol may potentially contribute to perturbations in inflammatory and impaired adhesion responses in preeclampsia.
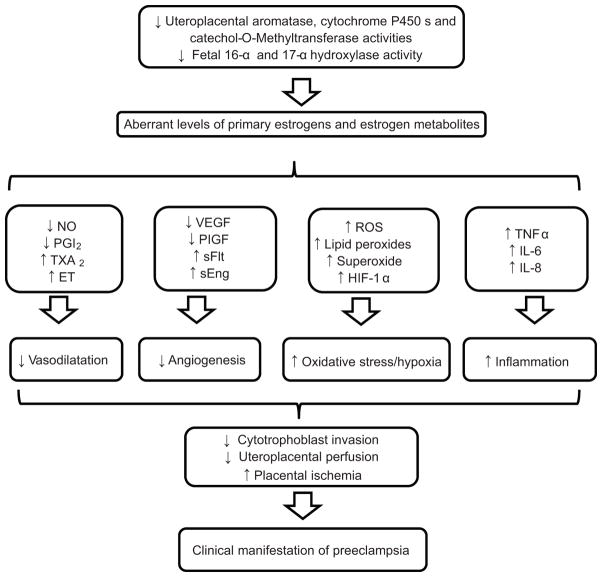
In summary, our findings provide the first complete evidence that preeclampsia is associated with aberrant synthesis, metabolism and distinct levels of 15 estrogens and estrogen metabolites therefore suggesting important roles for a plethora of estrogens and estrogen metabolites in the pathogenesis of preeclampsia. These findings also necessitate the need for prospective longitudinal studies covering early, mid and late trimesters to further assess and validate whether these estrogens and estrogen metabolites can be useful biomarkers in our understanding of the pathophysiology of preeclampsia, early prediction and identification. Therefore, we propose a working hypothesis that reduced activities of uteroplacental aromatase, cytochrome P450s, catechol-O-methyltransferase and fetal 16-α and 17-α hydroxylase activities leading to low levels of primary estrogens and estrogen metabolites may cause low availabilities and activities of several local molecular mediators of numerous normal uteroplacental adaptations during pregnancy thereby triggering some of the pathophysiology and clinical manifestations of preeclampsia (Figure 6).
Figure 6.
Possible connections between preeclampsia and the synthesis and metabolism of estrogens and estrogen metabolites. NO, nitric oxide; PGI2, prostacyclin; TXA2, thromboxane; ET, Endothelin; VEGF, vascular endothelial growth factor; PIGF, placental growth factor; sFlt1, soluble Fms-like tyrosine kinase 1; sEng, soluble endoglin; ROS, reactive oxygen species; HIF-1α, hypoxia-inducible factor 1; TNFα, tumor necrosis factor-alpha; IL-6, interleukin 6; IL-8, interleukin 8
Perspectives.
Evidence is increasing that the multiple and diverse pathways by which primary estrogens are converted to multiple metabolites may contribute to numerous cardiovascular functions attributed to these estrogens in several vascular beds. The connection between estrogen metabolites and preeclampsia was first noted when the activity COMT was shown to be lower in the placentas of patients with severe preeclampsia and that pregnant mice deficient in COMT exhibit a preeclampsia-like phenotype. However, the diverse pathways that extensively convert estrogens to multiple metabolites are extremely complex. This necessitates the importance to investigate a plethora of other functional estrogen metabolites to achieve a more comprehensive knowledge of their contributions, if any, in preeclampsia and other hypertensive diseases. We show herein that aberrant synthesis and metabolism of estrogens and estrogen metabolites in preeclampsia which may suggests a connection and provides insight into several previously unknown and underappreciated critical links between the clinical pathophysiology of the disease with the cardiovascular and other functional physiology of these estrogens and estrogen metabolites.
Novelty and Significance.
What is New?
Preeclampsia is characterized by distinct aberrant synthesis and metabolism of estrogens and estrogen metabolites including estrone, estradiol-17β, 16-keto-estradiol, estriol, 2-hydroxyestrone, 4-hydroxyestrone, 16-α-hydroxyestrone, 2-hydroxyestradiol, 2-methoxyestrone, 3-methoxyestrone, 4-methoxyestrone, 2-methoxyestradiol, 4-methoxyestradiol, 16-epi-estriol and 17-epi-estriol.
What is Relevant?
Preeclampsia is a hypertensive disorder of pregnancy with an elusive etiology and affects 5% to 8% of pregnancies, thus remaining a significant cause of maternal and fetal morbidity and mortality as well as greater susceptibility and earlier onset of future cardiovascular disease in both mother and baby
Summary
Our findings demonstrate that preeclampsia is characterized by specific and distinct aberrant synthesis, metabolism and plasma accumulation of 15 individual estrogens and estrogen metabolites and necessitates the need to investigate the functional vascular and other physiology of these estrogens and estrogen metabolites in the pathophysiology of preeclampsia
Acknowledgments
We are indebted to the patients and staff of the Meriter Hospital Birthing Center and University of Wisconsin division of Maternal Fetal Medicine who participated in this study.
Sources of Funding
This work was supported by National Institutes of Health grants HL49210, HD38843, HL87144 (to RR Magness), R25-GM083252 (to M L Carnes) and T32-HD041921-07 (to IM Bird).
Footnotes
Conflict(s) of Interest/Disclosure(s) Statement
None
References
- 1.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ. The fetal origins of coronary heart disease. Acta Paediatr Suppl. 1997;422:78–82. doi: 10.1111/j.1651-2227.1997.tb18351.x. [DOI] [PubMed] [Google Scholar]
- 3.Cockell AP, Poston L. Flow-mediated vasodilatation is enhanced in normal pregnancy but reduced in preeclampsia. Hypertension. 1997;30:247–251. doi: 10.1161/01.hyp.30.2.247. [DOI] [PubMed] [Google Scholar]
- 4.Pipkin FB. Risk factors for preeclampsia. N Engl J Med. 2001;344:925–926. doi: 10.1056/NEJM200103223441209. [DOI] [PubMed] [Google Scholar]
- 5.Zamudio S, Palmer SK, Dahms TE, Berman JC, Young DA, Moore LG. Alterations in uteroplacental blood flow precede hypertension in preeclampsia at high altitude. J Appl Physiol. 1995;79:15–22. doi: 10.1152/jappl.1995.79.1.15. [DOI] [PubMed] [Google Scholar]
- 6.Magness RR. Maternal cardiovascular and other physiologic responses to the endocrinology of pregnancy. In: Bazer FW, editor. The endocrinology of pregnancy. Totowa, NJ: Humana Press Inc; 1998. [Google Scholar]
- 7.Albrecht ED, Pepe GJ. Placental steroid hormone biosynthesis in primate pregnancy. Endocr Rev. 1990;11:124–150. doi: 10.1210/edrv-11-1-124. [DOI] [PubMed] [Google Scholar]
- 8.Hertig A, Liere P, Chabbert-Buffet N, Fort J, Pianos A, Eychenne B, Cambourg A, Schumacher M, Berkane N, Lefevre G, Uzan S, Rondeau E, Rozenberg P, Rafestin-Oblin ME. Steroid profiling in preeclamptic women: Evidence for aromatase deficiency. Am J Obstet Gynecol. 2010;203:477. e471–479. doi: 10.1016/j.ajog.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Shute E. The influence of estrogens on genuine pre-eclampsia and eclampsia. The American Journal of Surgery. 1943;59:478–483. [Google Scholar]
- 10.Zeisler H, Jirecek S, Hohlagschwandtner M, Knöfler M, Tempfer C, Livingston JC. Concentrations of estrogens in patients with preeclampsia. Wien Klin Wochenschr. 2002;114:458–461. [PubMed] [Google Scholar]
- 11.Duenhoelter JH, Whalley PJ, MacDonald PC. An analysis of the utility of plasma immunoreactive estrogen measurements in determining delivery time of gravidas with a fetus considered at high risk. Am J Obstet Gynecol. 1976;125:889–898. doi: 10.1016/0002-9378(76)90484-1. [DOI] [PubMed] [Google Scholar]
- 12.Zhu BT, Conney AH. Functional role of estrogen metabolism in target cells: Review and perspectives. Carcinogenesis. 1998;19:1–27. doi: 10.1093/carcin/19.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Jobe SO, Ramadoss J, Koch JM, Jiang Y, Zheng J, Magness RR. Estradiol-17beta and its cytochrome p450- and catechol-o-methyltransferase-derived metabolites stimulate proliferation in uterine artery endothelial cells: Role of estrogen receptor-alpha versus estrogen receptor-beta. Hypertension. 2010;55:1005–1011. doi: 10.1161/HYPERTENSIONAHA.109.146399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubey RK, Tofovic SP, Jackson EK. Cardiovascular pharmacology of estradiol metabolites. J Pharmacol Exp Ther. 2004;308:403–409. doi: 10.1124/jpet.103.058057. [DOI] [PubMed] [Google Scholar]
- 15.Kanasaki K, Palmsten K, Sugimoto H, Ahmad S, Hamano Y, Xie L, Parry S, Augustin HG, Gattone VH, Folkman J, Strauss JF, Kalluri R. Deficiency in catechol-o-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature. 2008;453:1117–1121. doi: 10.1038/nature06951. [DOI] [PubMed] [Google Scholar]
- 16.Xu X, Roman JM, Issaq HJ, Keefer LK, Veenstra TD, Ziegler RG. Quantitative measurement of endogenous estrogens and estrogen metabolites in human serum by liquid chromatography-tandem mass spectrometry. Anal Chem. 2007;79:7813–7821. doi: 10.1021/ac070494j. [DOI] [PubMed] [Google Scholar]
- 17.Siiteri PK, MacDonald PC. Placental estrogen biosynthesis during human pregnancy. J Clin Endocrinol Metab. 1966;26:751–761. doi: 10.1210/jcem-26-7-751. [DOI] [PubMed] [Google Scholar]
- 18.Kowalczyk TD, Cabaniss ML, Cusmano L. Association of low unconjugated estriol in the second trimester and adverse pregnancy outcome. Obstet Gynecol. 1998;91:396–400. doi: 10.1016/s0029-7844(97)00677-7. [DOI] [PubMed] [Google Scholar]
- 19.Turnipseed MR, Bentley K, Reynolds JW. Serum dehydroepiandrosterone sulfate in premature infants and infants with intrauterine growth retardation. J Clin Endocrinol Metab. 1976;43:1219–1225. doi: 10.1210/jcem-43-6-1219. [DOI] [PubMed] [Google Scholar]
- 20.Stamilio DM, Sehdev HM, Morgan MA, Propert K, Macones GA. Can antenatal clinical and biochemical markers predict the development of severe preeclampsia? Am J Obstet Gynecol. 2000;182:589–594. doi: 10.1067/mob.2000.103890. [DOI] [PubMed] [Google Scholar]
- 21.Rosenfeld CR, Rivera R. Circulatory responses to systemic infusions of estrone and estradiol-17alpha in nonpregnant, oophorectomized ewes. Am J Obstet Gynecol. 1978;132:442–448. doi: 10.1016/0002-9378(78)90782-2. [DOI] [PubMed] [Google Scholar]
- 22.Magness RR, Rosenfeld CR. Local and systemic estradiol-17 beta: Effects on uterine and systemic vasodilation. Am J Physiol. 1989;256:E536–542. doi: 10.1152/ajpendo.1989.256.4.E536. [DOI] [PubMed] [Google Scholar]
- 23.Magness RR, Phernetton TM, Gibson TC, Chen DB. Uterine blood flow responses to ici 182 780 in ovariectomized oestradiol-17beta-treated, intact follicular and pregnant sheep. J Physiol. 2005;565:71–83. doi: 10.1113/jphysiol.2005.086439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller NR, Garry D, Cohen HW, Figueroa R. Serum androgen markers in preeclampsia. J Reprod Med. 2003;48:225–229. [PubMed] [Google Scholar]
- 25.Lee AJ, Cai MX, Thomas PE, Conney AH, Zhu BT. Characterization of the oxidative metabolites of 17beta-estradiol and estrone formed by 15 selectively expressed human cytochrome p450 isoforms. Endocrinology. 2003;144:3382–3398. doi: 10.1210/en.2003-0192. [DOI] [PubMed] [Google Scholar]
- 26.Blomquist CH, Kotts CE, Hakanson EY. Inhibiton of human placental 17 beta-hydroxysteroid dehydrogenase by steroids and nonsteroidal alcohols: Aspects of inhibitor structure and binding specificity. Arch Biochem Biophys. 1978;186:35–41. doi: 10.1016/0003-9861(78)90460-5. [DOI] [PubMed] [Google Scholar]
- 27.Rosenfeld CR, Jackson GM. Induction and inhibition of uterine vasodilation by catechol estrogen in oophorectomized, nonpregnant ewes. Endocrinology. 1982;110:1333–1339. doi: 10.1210/endo-110-4-1333. [DOI] [PubMed] [Google Scholar]
- 28.Lippert C, Seeger H, Mueck AO, Lippert TH. The effects of a-ring and d-ring metabolites of estradiol on the proliferation of vascular endothelial cells. Life Sci. 2000;67:1653–1658. doi: 10.1016/s0024-3205(00)00747-5. [DOI] [PubMed] [Google Scholar]
- 29.Seeger H, Mueck AO, Lippert TH. Effect of estradiol metabolites on prostacyclin synthesis in human endothelial cell cultures. Life Sci. 1999;65:PL167–170. doi: 10.1016/s0024-3205(99)00383-5. [DOI] [PubMed] [Google Scholar]
- 30.Jobe SO, Fling SN, Ramadoss J, Magness RR. A novel role for an endothelial adrenergic receptor system in mediating catecholestradiol-induced proliferation of uterine artery endothelial cells. Hypertension. 2011;58:874–881. doi: 10.1161/HYPERTENSIONAHA.111.178046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller E, Bates R, Bjorndahl J, Allen D, Burgio D, Bouma C, Stoll J, Latman N. 16-epiestriol, a novel anti-inflammatory nonglycogenic steroid, does not inhibit ifn-gamma production by murine splenocytes. J Interferon Cytokine Res. 1998;18:921–925. doi: 10.1089/jir.1998.18.921. [DOI] [PubMed] [Google Scholar]
- 32.Latman NS, Kishore V, Bruot BC. 16-epiestriol: An anti-inflammatory steroid without glycogenic activity. J Pharm Sci. 1994;83:874–877. doi: 10.1002/jps.2600830623. [DOI] [PubMed] [Google Scholar]
- 33.Mukherjee TK, Nathan L, Dinh H, Reddy ST, Chaudhuri G. 17-epiestriol, an estrogen metabolite, is more potent than estradiol in inhibiting vascular cell adhesion molecule 1 (vcam-1) mrna expression. J Biol Chem. 2003;278:11746–11752. doi: 10.1074/jbc.M207800200. [DOI] [PubMed] [Google Scholar]
- 34.Schiessl B. Inflammatory response in preeclampsia. Mol Aspects Med. 2007;28:210–219. doi: 10.1016/j.mam.2007.04.004. [DOI] [PubMed] [Google Scholar]