Abstract
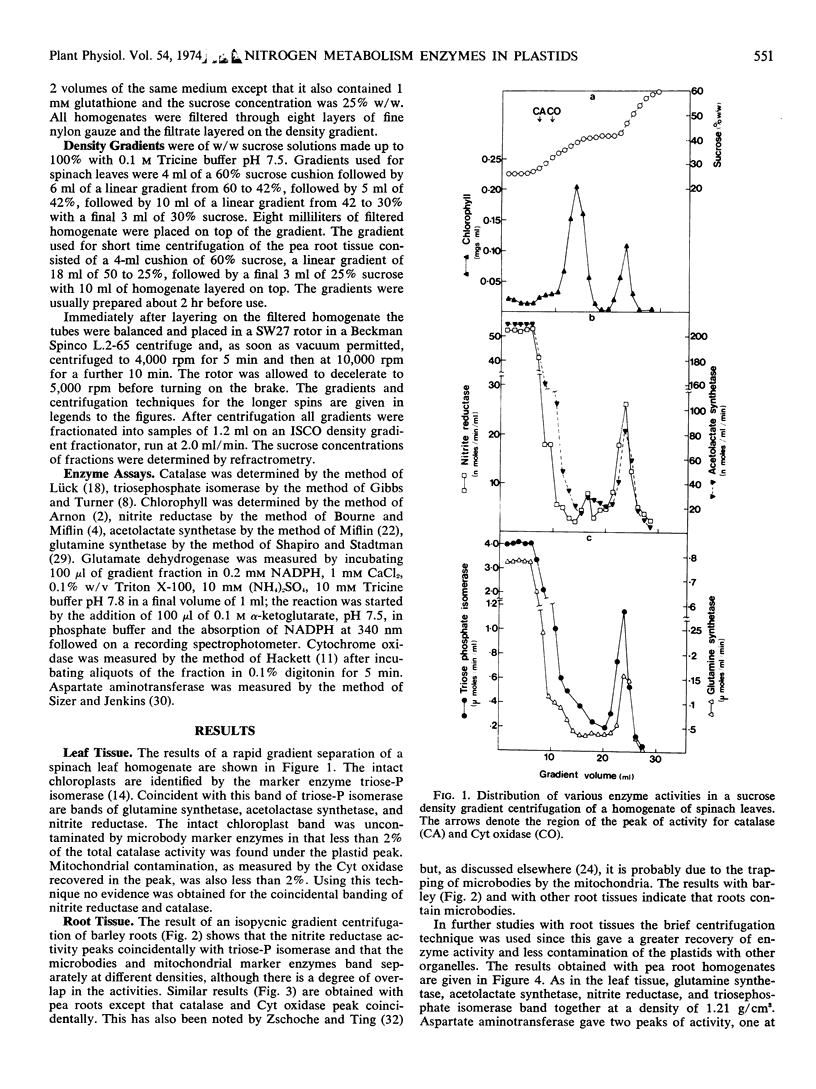
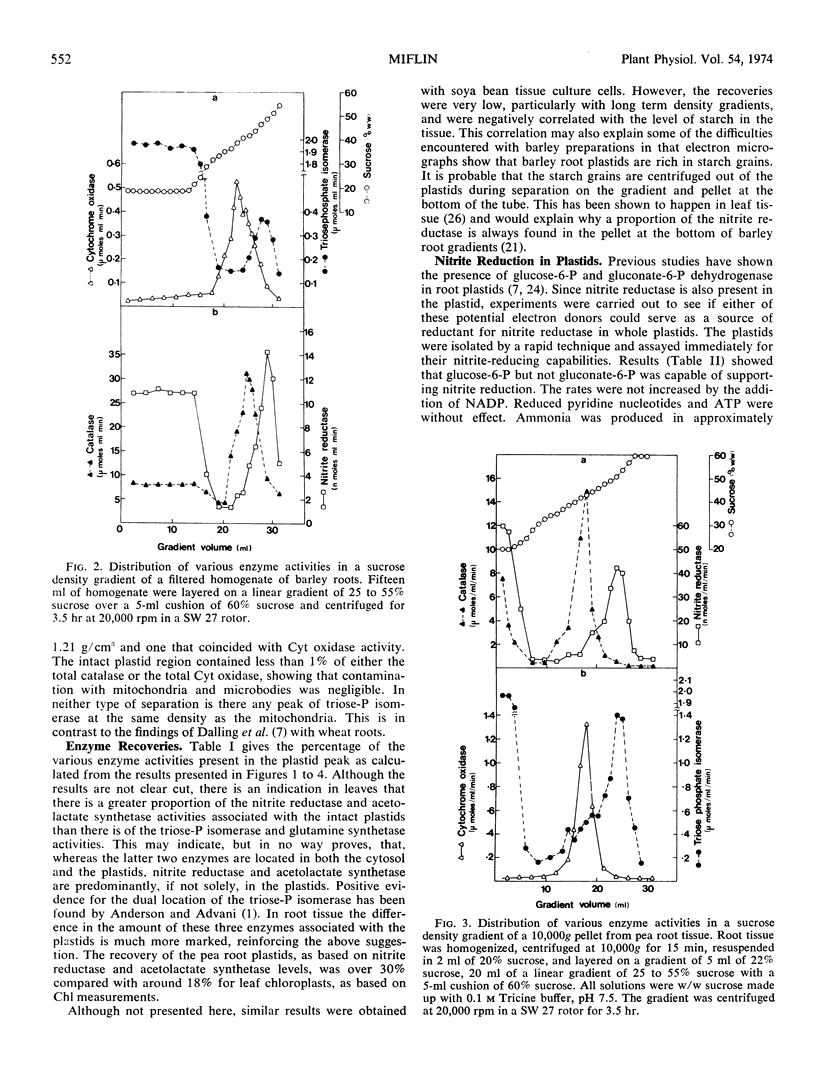
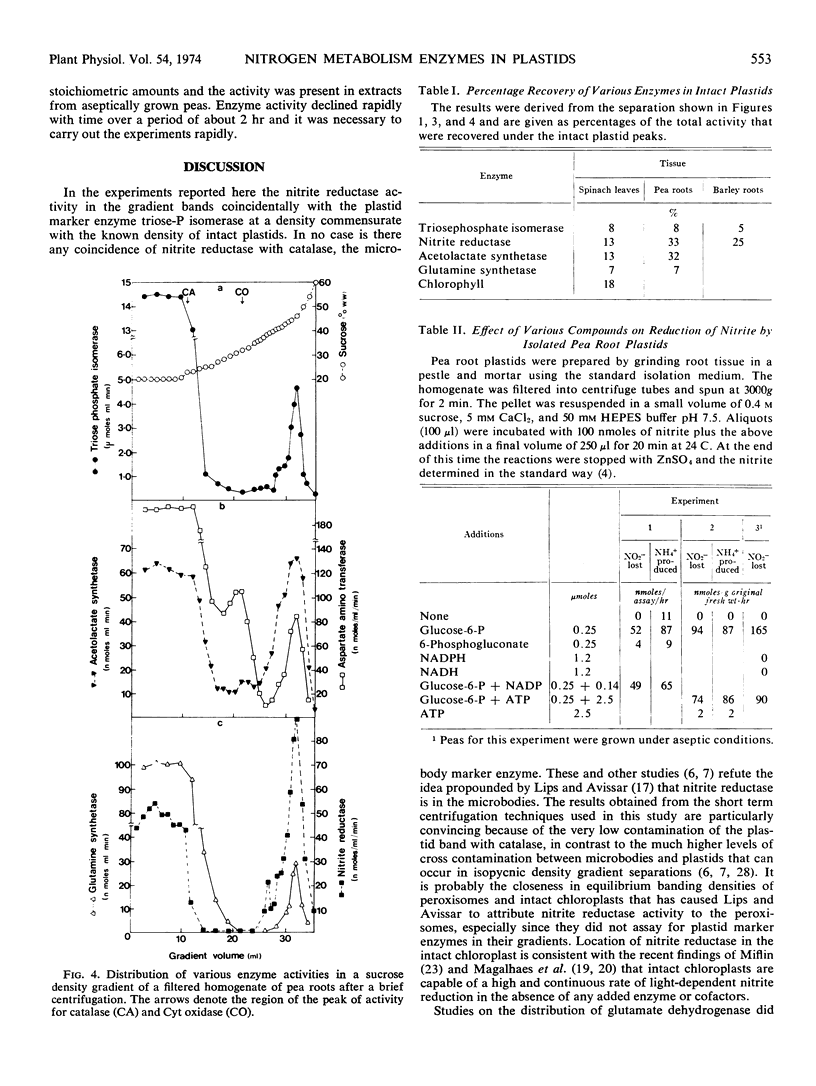
Density gradient separation of plastids from leaf and root tissue was carried out. The distribution in the gradients of the activity of the following enzymes was determined: nitrite reductase, glutamine synthetase, acetolactate synthetase, aspartate aminotransferase, catalase, cytochrome oxidase, and triosephosphate isomerase. The distribution of chlorophyll was followed in gradients from leaf tissue. The presence of plastids that have retained their stroma enzymes was denoted by a peak of triosephosphate isomerase activity. Coincidental with this peak were bands of nitrite reductase, acetolactate synthetase, glutamine synthetase, and aspartate aminotransferase activity. The results suggest that most, if not all, the nitrite reductase and acetolactate synthetase activity of the cell is in the plastids. The plastids were found to contain only part of the total glutamine synthetase, aspartate aminotransferase, and triosephosphate dehydrogenase activity in the cell. Some evidence was obtained for low levels of glutamate dehydrogenase activity in chloroplasts.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. E., Advani V. R. Chloroplast and cytoplasmic enzymes: three distinct isoenzymes associated with the reductive pentose phosphate cycle. Plant Physiol. 1970 May;45(5):583–585. doi: 10.1104/pp.45.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTT V. S., BEEVERS H. The regulation of pathways of glucose catabolism in maize roots. Biochem J. 1961 Jul;80:21–27. doi: 10.1042/bj0800021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair G. E., Ellis R. J. Protein synthesis in chloroplasts. I. Light-driven synthesis of the large subunit of fraction I protein by isolated pea chloroplasts. Biochim Biophys Acta. 1973 Aug 24;319(2):223–234. doi: 10.1016/0005-2787(73)90013-0. [DOI] [PubMed] [Google Scholar]
- Dalling M. J., Tolbert N. E., Hageman R. H. Intracellular location of nitrate reductase and nitrite reductase. I. Spinach and tobacco leaves. Biochim Biophys Acta. 1972 Dec 14;283(3):505–512. doi: 10.1016/0005-2728(72)90266-6. [DOI] [PubMed] [Google Scholar]
- Dalling M. J., Tolbert N. E., Hageman R. H. Intracellular location of nitrate reductase and nitrite reductase. II. Wheat roots. Biochim Biophys Acta. 1972 Dec 14;283(3):513–519. doi: 10.1016/0005-2728(72)90267-8. [DOI] [PubMed] [Google Scholar]
- Givan C. V., Givan A. L., Leech R. M. Photoreduction of alpha-Ketoglutarate to Glutamate by Vicia faba Chloroplasts. Plant Physiol. 1970 May;45(5):624–630. doi: 10.1104/pp.45.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk P. R., Leech R. M. Amino Acid Biosynthesis by Isolated Chloroplasts during Photosynthesis. Plant Physiol. 1972 Aug;50(2):228–234. doi: 10.1104/pp.50.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R. M., Kirk P. R. An NADP-dependent L-glutamate dehydrogenase from chloroplasts of Vicia faba L. Biochem Biophys Res Commun. 1968 Aug 21;32(4):685–690. doi: 10.1016/0006-291x(68)90293-3. [DOI] [PubMed] [Google Scholar]
- Lips S. H., Avissar Y. Plant-leaf microbodies as the intracellular site of nitrate reductase and nitrite reductase. Eur J Biochem. 1972 Aug 18;29(1):20–24. doi: 10.1111/j.1432-1033.1972.tb01952.x. [DOI] [PubMed] [Google Scholar]
- Magalhaes A. C., Neyra C. A., Hageman R. H. Nitrite assimilation and amino nitrogen synthesis in isolated spinach chloroplasts. Plant Physiol. 1974 Mar;53(3):411–415. doi: 10.1104/pp.53.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miflin B. J., Beevers H. Isolation of intact plastids from a range of plant tissues. Plant Physiol. 1974 Jun;53(6):870–874. doi: 10.1104/pp.53.6.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miflin B. J. Cooperative feedback control of barley acetohydroxyacid synthetase by leucine, isoleucine, and valine. Arch Biochem Biophys. 1971 Oct;146(2):542–550. doi: 10.1016/0003-9861(71)90159-7. [DOI] [PubMed] [Google Scholar]
- O'Neal D., Joy K. W. Localisation of glutamine synthetase in chloroplasts. Nat New Biol. 1973 Nov 14;246(150):61–62. doi: 10.1038/newbio246061a0. [DOI] [PubMed] [Google Scholar]
- Randall D. D., Tolbert N. E. 3-Phosphoglycerate Phosphatase in Plants: III. Activity Associated with Starch Particles. Plant Physiol. 1971 Oct;48(4):488–492. doi: 10.1104/pp.48.4.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarius K. A., Stocking C. R. Intracellular localization of enzymes in leaves and chloroplast membrane permeability to compounds involved in amino acid syntheses. Z Naturforsch B. 1969 Sep;24(9):1170–1179. doi: 10.1515/znb-1969-0915. [DOI] [PubMed] [Google Scholar]
- Schnarrenberger C., Oeser A., Tolbert N. E. Isolation of Plastids from Sunflower Cotyledons during Germination. Plant Physiol. 1972 Jul;50(1):55–59. doi: 10.1104/pp.50.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAGNER R. P., BERGQUIST A. Synthesis of valine and isoleucine in the presence of a particulate cell fraction of Neurospora. Proc Natl Acad Sci U S A. 1963 Jun;49:892–897. doi: 10.1073/pnas.49.6.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zschoche W. C., Ting I. P. Malate Dehydrogenases of Pisum sativum: Tissue Distribution and Properties of the Particulate Forms. Plant Physiol. 1973 Jun;51(6):1076–1081. doi: 10.1104/pp.51.6.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]



