Abstract
The interstitial cells of Cajal (ICCs) are important mediators of gastrointestinal (GI) motility because of their role as pacemakers in the GI tract. In addition to their function, ICCs are also structurally distinct cells most easily identified by their ultra-structural features and expression of the tyrosine kinase receptor c-KIT. ICCs have been described in mammals, rodents, birds, reptiles, and amphibians, but there are no reports at the ultra-structural level of ICCs within the GI tract of an organism from the teleost lineage. We describe the presence of cells in the muscularis of the zebrafish intestine; these cells have similar features to ICCs in other vertebrates. The ICC-like cells are associated with the muscularis, are more electron-dense than surrounding smooth muscle cells, possess long cytoplasmic processes and mitochondria, and are situated opposing enteric nervous structures. In addition, immunofluorescent and immunoelectron-microscopic studies with antibodies targeting the zebrafish ortholog of a putative ICC marker, c-KIT (kita), showed c-kit immunoreactivity in zebrafish ICCs. Taken together, these data represent the first ultra-structural characterization of cells in the muscularis of the zebrafish Danio rerio and suggest that ICC differentiation in vertebrate evolution dates back to the teleost lineage.
Keywords: Interstitial cell of Cajal (ICC), C-kit, Gastrointestinal motility, Ultrastructure, Zebrafish, Danio rerio (Teleostei)
Introduction
The coordination of gastroentero-motility is a dynamic process involving cells derived from all three embryonic germ layers: ectoderm, mesoderm, and endoderm. Although specific signaling differs between species, the overall cell types, soluble factors, and anatomical structures related to gastrointestinal (GI) motility are conserved across many vertebrates. The enteric nervous system regulates the contraction and relaxation of smooth muscle cells during the fed and fasting states, in addition to stimulating or inhibiting the secretion of local gastric enzymes that aid in the breakdown of food products (Huizinga 1999). The aboral contractile-mediated propagation of food contents through the GI tract, or peristalsis, is mechanically mediated by the contraction and relaxation of intestinal smooth muscle cells. Spontaneous contractility of the GI tract is believed to be coordinated by a heterogeneous population of mesenchymal cells known as the interstitial cells of Cajal (ICC; Faussone-Pellegrini and Thuneberg 1999).
ICCs are considered to be the pacemaker cells of the GI tract; they propagate rhythmic slow-waves and mediate neurotransmission (Iino and Horiguchi 2006). ICCs were first described by Ramón y Cajal over 100 years ago by means of methylene blue staining and were originally believed to be a part of the autonomic nervous system (Min and Leabu 2006); however, the modern description of ICCs relies on electron microscopy and immunolabeling with ICC markers, such as the tyrosine kinase receptor c-KIT. Because ICCs fail to develop or are significantly reduced when c-KIT is knocked down or deleted, ICCs are believed to be developmentally dependent on c-KIT signaling, most likely through c-KIT-mediated lineage specification of ICCs from smooth-muscle precursor cells (Klüppel et al. 1998). Molecular and developmental studies of ICCs routinely use c-KIT, and other ICC markers, such as TMEM16A, to identify these cells (Chen et al. 2007). Although ICCs can be subcategorized based on their distribution within the inner and outer smooth muscle layers or their association with the myenteric plexus, most ICCs share common ultrastructural features that distinguish them from surrounding smooth muscle cells. Compared with intestinal smooth muscle cells, ICCs tend to have a more stellate, elongated morphology with a relatively high nucleus to cytoplasm ratio, abundant smooth endoplasmic reticulum and mitochondria, and surface caveolae (Komuro 2006).
To date, the ultrastructural features of ICCs have been characterized, by using transmission electron micrsocopy (TEM), in a wide variety of vertebrates (Komuro et al. 1999) and as far back in evolution as amphibians (Miyamoto-Kikuta and Komuro 2007). Although Rich et al. (2007) have demonstrated kit-like immunoreactivity in the intestine of the zebrafish, to our knowledge, no studies have further characterized ICCs in zebrafish or in any other descendent of the teleost lineage. The aim of the present study was to characterize, at the ultrastructural level, the presence of kit-positive ICCs in the zebrafish Danio rerio.
Materials and methods
For fixation for electron microscopy (EM), adult zebrafish were euthanized with tricane, and tissue was fixed in 4% paraformaldehyde (PFA)/2.5% glutaraldehyde in a sodium cacodylate buffer (pH7.4). To avoid mechanical stretching of the intestine and compromising of the ultrastructure, the intestine was dissected out of a partially dissected fish that had previously been fixed whole in 20 ml fixative. The GI tract was exposed by dissecting away the skin, fat, and muscle surrounding the intestine, without removing the intestine. The fish were fixed in the 4% PFA/2.5% glutaraldehyde solution at 4°C overnight with gentle shaking. The intestine was then dissected and placed in 1 ml fresh fixative for an additional hour. Samples were stored in the sodium cacodylate buffer before EM preparation. Zebrafish are “stomachless” fish with vaguely delineated segments of the intestinal tract. For this study, we focused on the mid-portion of the intestine (see below).
For studies by immuno-electron microscopy (immuno-EM), the same protocol was used with PFA fixation to increase antibody sensitivity. After fixation, the intestines were rinsed with phosphate-buffered saline (PBS) and embedded in 2% Agarose in PBS. Vibratome sections (100 μm) were cut at 4°C (Ted Pella-Pelco 100 Vibratome Sectioning System).
The sections were rinsed in PBS, permeabilized with 0.1% Triton in PBS for 8 min at room temperature (RT), incubated in c-Kit primary antibody (ab16832) in antibody buffer (4% normal goat serum with 0.1% Triton in PBS) overnight at 4°C on a rocker, and washed twice in antibody buffer(7 min each). Next, the sections were incubated in nanofluorogold secondary antibody conjugated to Alexa 488 (anti-Rabbit) in antibody buffer overnight at 4°C on a rocker, washed in the antibody buffer for 10 min followed by four washes in PBS (7 min each), and rinsed five times in double distilled water (DDW;5 min each) followed by silver enhancement, under darkroom conditions, for 7 minutes at RT. Subsequently, the sections were rinsed thoroughly in DDW over a 10-min period and then washed twice in 0.1 M phosphate buffer(5 min each). The sections were post-fixed in 0.2% osmium tetroxide in 0.1 M phosphate buffer for 1 h at RT and washed three times in 0.1 M phosphate buffer (5 min each) followed by three rinses in cold 0.1 M acetate buffer(5 min each). An en-bloc step was included with 0.25% uranyl acetate in 0.1 M acetate buffer, for 1 h at 4°C. Following this step, sections were rinsed three times in cold 0.1 M acetate buffer(5 min each).
After the sections had been washed in cold 0.1 M acetate buffer in a variable-wattage Pelco BioWave Pro microwave oven (Ted Pella, Redding, Calif., USA), the following protocol was used to complete embedding: an ethanol dehydration series (50%, 70%, 90%, 100%), an Embed-812 resin/ethanol infiltration series (1:1, 2:1), followed by 100% resin infiltration. The sections were embedded and polymerized in 100% resin for 16 h in a laboratory oven set at 60°C. Thin sections were cut on a Reichert-Jung Ultracut-E ultramicrotome (50 nm thick) and collected on LuxFilm grids (Ted Pella) at a film thickness of 30 nm. The grids were post-stained with uranyl acetate and lead citrate and examined in a JEOL 1010 (JEOL, Tokyo, Japan) transmission electron microscope operated at 80 kV.
Negative controls (minus primary antibody) in which only the secondary antibody was used were performed in parallel to the experimental sections.
For confocal microscopy imaging, intestines were dissected out of adult zebrafish, embedded in OCT compound, and frozen directly in liquid nitrogen. Sections (10 μm thick) were cut on a cryotome, mounted on charged slides, and fixed for 20 min in ice cold methanol followed by 1 min of ice cold acetone. The sections were rinsed with PBS and then permeabilized with 0.2 % Triton X-100 solution for 1 min, and then they were incubated in a blocking solution consisting of 10% normal goat serum and 1% dimethylsulfoxide in 1× PBS for 1 h. Incubation with primary antibodies, c-kit (abcam: 1:100), and acetylated tubulin (Invitrogen: 1:400) was performed at RT for 1 h, followed by two 5- min washes in PBS. Incubation with secondary antibodies (Invitrogen) and a nuclear counter-stain (Hoechst) was carried out in a dark room at RT for 1 h, followed by two 5-min washes in PBS. For comparison purposes, zebrafish embryos were stained and imaged with confocal microscopy to identify kit-positive cells in the developing tissues, including the intestine. Additionally, intestinal samples from an adult mouse were prepared for standard EM with the same protocol as for the zebrafish and imaged as a comparative guide to ICC morphology. Embryo staining, sample mounting, and image preparation were undertaken with a Zeiss Confocal microscope as described previously (Matsuda and Chitnis 2010).
Results
The intestine of the zebrafish consists of an outer longitudinal muscle layer and an inner circular muscle layer connected to folds of epithelial tissue by a thin layer of connective tissue or sub-mucosa (Fig. 1). The muscularis is innervated by an enteric nervous network including nerve bundles, axon processes containing granular and agranular vesicles, and axonal dilations (or varicosities) associated with smooth muscle cells (Fig. 2). Although scarce in number, structures reminiscent of the myenteric plexus found in other vertebrates lie between the areas of the inner and outer muscle layers. Enteric neurons have large nuclei, often with a visible nucleolus, and an electron-lucent cytoplasm (data not shown).
Fig. 1.

Ultrastructure of the zebrafish intestine. Longitudinal section showing an outer longitudinal muscle (LM) layer and an inner circular smooth muscle (CM) layer, a thin submucosal (SM) layer, and epithelial mucosal cells. Bar 2 μm
Fig. 2.
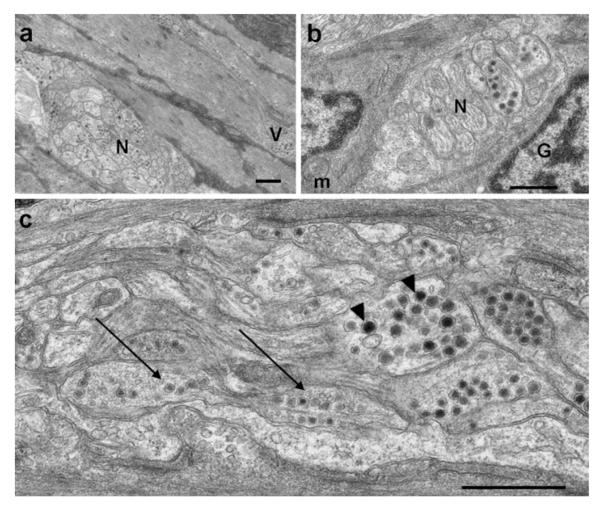
Synaptic vesicles and nerve bundles innervating the muscularis. a Nerve bundles (N) found in the muscularis contain numerous large granular vesicles. Small varicosities (V) are also located throughout the longitudinal smooth and circular smooth muscle. b A glial cell (G) enveloping a nerve bundle (N), opposed to an interstitial cell of Cajal (ICC)-like cell with scant cytoplasm and prominent mitochondria (m). c Nerve bundles containing axons with large granular vesicles (arrowheads) and a mixture of large granular vesicles and small agranular vesicles (long arrows). Bars1 μm
The ultrastructural features of the intestinal smooth muscle cells resembled myoid-like cells similar to those of other vertebrates, with both thin and thick filaments being present. The ICCs in zebrafish were identified by ultrastructural features previously reported in other organisms and were compared with ICCs identified in adult mice (Fig. 3). ICC features included a long stellate appearance with abundant mitochondria, rough endoplasmic reticulum, and a relatively small amount of electron-dense cytoplasm (Fig. 4). ICCs were also found associated with nerve fascicles containing agranular vesicles, which possibly release cholinergic mediators (Fig. 5). Some ICC features described in other vertebrates were not found in zebrafish, including peg-and-socket-like junctions, gap junctions, and surface caveolae. Possible gap junctions were observed, but the ultrastructure was never clear enough to associate them confidently with ICCs. Surface caveolae were not remarkable in ICCs or smooth muscle cells but were found in endothelial cells (data not shown).
Fig. 3.
Morphological comparison between zebrafish (a) and mouse (b) ICCs (stars). Bars 5 μm(a), 2 μm (b)
Fig. 4.

Ultrastructural features of a zebrafish ICC. a ICC associated with the inner circular smooth muscle layer (cm) and a fascicle-like neural structure (f). Inset Higher magnification view of the mitochondrion (m). b High magnification of the cytoplasmic extension (stars ICCs). Bars 2 μm (a and b),500 nm (b, inset in a)
Fig. 5.

Ultrastructural features of a zebrafish ICC situated between the inner and outer muscle layers with cytoplasmic processes that appear to encompass a fascicle-like neural structure (F). Bar500 nm
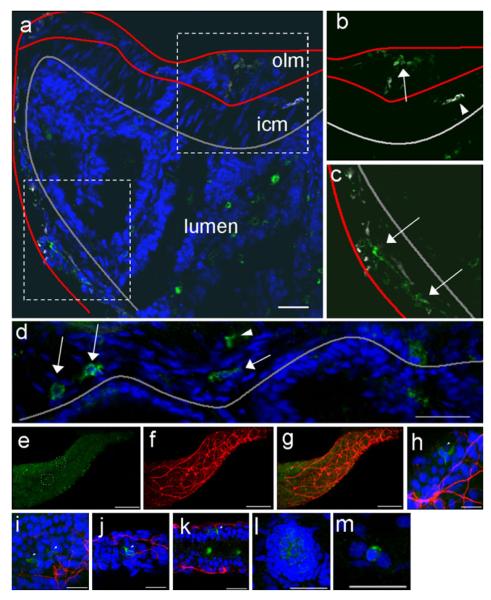
Compared with mice and humans, the identification of distinct myenteric ganglia in zebrafish by conventional light microscopy on sections stained with hematoxylin and eosin was difficult, possibly because of the small size of the zebrafish. However, confocal microscopy immunostaining with antibodies against acetylated-tubulin demonstrated a rich neural network innervating the muscularis. Cells labeled with a c-KIT antibody (termed “kit-positive cells”) were present throughout the muscularis and were possibly associated with the enteric nervous system (Fig. 6a-c). Kit-positive cells were stellate, had a relatively high nucleus to cytoplasm ratio compared with smooth muscle cells, and could be seen in both the adult and larval stages (Fig. 6d-k; Supplemental Movies 1, 2). The degree of kit expression in the intestine of the zebrafish embryo was comparable with that in other cells in which kit is normally expressed, including neuromasts (Fig. 6l) and melanocytes (Fig. 6m).
Fig. 6.
Confocal imaging of kit-positive cells in adult and developing zebrafish. a Longitudinal section of mid-intestine of adult zebrafish showing opposing sides of the epithelial layer of the lumen (outlined in gray). In this slightly angled cross section, the outer longitudinal muscle layer (olm) and inner circular muscle (icm) layer are clearly visible on the top portion of the cross section but are incomplete on the opposing side (muscle layer borders are outlined in red). The dotted boxes are enlarged in b, c to show kit staining (green) in the outer muscle layer (arrows) and in association with acetylated tubulin-stained (white) neural structures (arrowheads). d In a separate cross section of the adult intestine, kit-positive cells are seen both close to the submucosal/mucosal layer (arrows) and within the muscularis (arrowhead). e–g Low and high magnifications of whole-mount projections of the zebrafish intestine during embryonic development (7 days post-fertilization) showing kit-positive cells (green in e) together with a well-developed enteric nervous system network as seen by acetylated tubulin staining (red in f, merged in g). The dotted circled regions shown in e are enlarged and shown at different slices in h–k. The intensity of kit-positive cells in the intestine was similar to the intensity of kit staining in other cells that normally express kit during development, including neuromasts (l) and melanocytes (m). Bars20 μm (a–d, h–m), 10 μm (e–g)
Immuno-EM and confocal microscopy studies revealed that kit-positive cells correlated with ICCs observed by TEM and confocal microscopy with respect to their anatomical location, relative density, and approximate morphology. By immuno-EM, kit-positive cells were identified as cells expressing silver-enhanced nanogold particles on at least four sites of a given cell with at least eight particles per cell. These kit-positive cells possessed long cytoplasmic processes and mitochondria and were mostly observed between the two muscle layers (Fig. 7) or spanning the circular muscle layer (Fig. 8). The limitations of immuno-EM compared with TEM were apparent in the compromised integrity of fine ultrastructural features of organelles, such as mitochondria, which had a less distinct morphology under immuno-EM. Cristae of mitochondria were not visible by immuno-EM (Fig. 7) because the tissues prepared for immuno-EM were only fixed in PFA, and such fine features were only observed in samples also fixed with glutaraldehyde (Fig. 4, inset).
Fig. 7.

Immuno-electron microscopy reveals a kit-positive cell associated with the muscularis. A kit-positive ICC is associated with outer longitudinal smooth muscle layer (arrows silver-enhanced nanogold particles, m mitochondria, LM longitudinal smooth muscle, star ICC). Bar500 nm
Fig. 8.

Immuno-electron microscopy reveals kit-positive cells associated with the muscularis. Kit-positive ICC associated with the border between the circular smooth muscle and the submucosa (arrows silver-enhanced nanogold particles (CM circular smooth muscle, LM longitudinal smooth muscle, star ICC). Bars (solid line)2 μm, (dashed line) 500 nm
Discussion
The aim of the present study was to characterize the ultrastrucutral properties of the ICCs in the zebrafish D. rerio, a descendent of the teleost lineage. Although ICCs have been reported to have various structural features across species and to occur throughout the layer of the GI tract (Rumessen and Vanderwinden 2003), we believe that the overall appearance and location of the cells that we have observed provide the first clear evidence that ICCs are present in zebrafish. Despite the limitations of ICC identification based on ultrastrucutural features alone, our conclusions are novel, because they are strengthened by the correlative immuno-EM and confocal microscopy with the ICC marker c-kit. Taken together, these represent the first convincing data that ICCs are present in the zebrafish D. rerio.
The most remarkable distinctions of the zebrafish ICCs observed here are the lack of abundant surface caveolae and possibly fewer mitochondria. Although unexpected, at least three possible explanations might account for these observations. First, the small size of the zebrafish intestine might have influenced these factors, as ICCs appear to be relatively low in density, making identification of definitive ICCs more challenging than in larger animals such as mice. Second, independent of inter-species variability of ICC morphology, these differences could be attributable, in part, to fixation-related issues as even the most careful dissection of the intestine results in the stretching of the tissue before fixation, an event that could affect the resolution of fine features such as caveolae. Lastly, these structural differences might simply reflect differences between species. In addressing these issues, we have found immuno-EM to be a useful tool to confirm that these cells are truly ICCs despite variability between species.
Although little work has been performed in zebrafish, previous studies examining the ultrastructure of the muscularis in other telesosts have found features similar to those that we describe here (Krementz and Chapman 1975), including axons associated with the myenteric plexus or varicosities containing secretory granules, namely large granular vesicles and agranular vesicles; these axons are believed to be aminergic and cholinergic neurons, respectively (Watson 1981). Perhaps because most ultrastructural studies in lower vertebrates were carried out prior to the availability of well-established criteria regarding ICC features, little attention has been given to the way in which ICCs have evolved across different lineages. Indeed, some researchers have struggled to identify ICCs in lower vertebrates and yet found similar anatomical or functional evidence to support the presence of ICCs involved in intestinal motility. Miyamoto-Kikuta and Komuro (2007) have identified ICCs, based on their morphology in an amphibian model, Xenopus laevis, as myoid-like cells with numerous mitochondria and surface caveolae, often associated with nerve varicosities or nerve bundles.
Although physiological data obtained by analyzing intestinal peristalsis as related to zebrafish ICCs are beyond the range of this study, other researchers have argued that zebra-fish possess ICCs on the basis of functional studies of intestinal peristalsis in developing zebrafish embryos. ICCs are considered the pacemaker cells of the GI tract, responsible for generating the “slow peristaltic wave” or a spontaneous depolarizing stimulus that does not cross the threshold on its own (Hennig et al. 2010). Such waves are thought to determine the frequency of phasic contractions (Horowitz et al. 1999). Using tetrodotoxin (TTX) to diminish neuronal activity, Holmberg et al. (2007) have demonstrated that zebrafish contain ICCs that act in a pacemaker-like fashion as TTX-resistant non-neuronal cells that contribute to the generation and initiation of propagating waves in the intestine of zebrafish embryos. More recent studies with the sox10 mutant zebrafish, colourless, which fails to develop an enteric nervous system, show that colourless and wild-type zebrafish larvae both possess effective propulsive peristalsis (Davuluri et al. 2010). The presence of ICCs would provide an explanation for the peristaltic movements found in zebrafish embryos with genetic or toxin-induced insults to the enteric nervous system. Indeed, we have been able to identify kit-positive cells in the intestine of the zebrafish embryos at similar time points to those in which peristaltic movements are seen, despite their having compromised enteric nervous system development.
The KIT gene, which encodes the tyrosine kinase receptor c-KIT, experienced a duplication during the duplication of the zebrafish genome, effectively resulting in two kit genes: kita (Parichy et al. 1999) and kitb (Mellgren and Johnson 2005), both of which are orthologous to the human KIT (Braasch et al. 2006). The endogenous ligand for c-KIT was also duplicated to produce kitla and kitlb. The interaction of kita and kitla mediates much of melanocyte formation in zebrafish, compared with kitb/kitlb (Hultman et al. 2007). Melanocytes and ICC are developmentally dependent on c-KIT (Klüppel et al. 1998), and genetic alterations in KIT in humans have demonstrated that activating KIT mutations give rise to hyperpigmentation and increased susceptibility to GI stromal tumors (Robson et al. 2004), a mesenchymal neoplasm believed to be derived from ICCs (Kindblom et al. 1998). We hypothesize that ICCs in zebrafish are more likely to be under the control of kita/kitla interactions given other phenotypic associations and previous studies and have observed comparable levels of immunoreactivity in other tissues expected to express kita such as melanocytes (Fig. 6m), neuromasts (Fig. 6l), and renal tubules (Supplemental movie 2).
Interestingly, zebrafish sparse mutants, which harbor a point mutation resulting in a premature stop codon in kita, are viable and can reproduce. In contrast, biallelic loss of c-kit function in mice is embryonically lethal, largely because of defects in hematopoiesis (Edling and Hallberg 2007). Despite a significant decrease in melanocyte number, no apparent hematopoietic or intestinal defects have been reported in sparse mutants. Further studies of zebrafish with kita defects treated with morpholinos targeting kitb might provide additional insights into the role that zebrafish kitorthologs play in the development of ICCs and other kit-dependent lineages.
The appearance of ICCs in the teleost lineage argues that these are evolutionary preserved cells with important functions. The significance of subtle variable ultrastructural features of ICCs across species remains unknown, and further characterization of ICC anatomy might aid in a better understanding of ICC function, including subtypes of ICCs within the alimentary canal (Garcia-Lopez et al. 2009). For example, ICCs of the submucosal plexus are located in the distal-most region of the GI tract and, in mice, are found exclusively in the colon (Vanderwinden et al. 2000). Zebrafish are considered “stomach-less” vertebrates with a simpler GI anatomy at the gross and histological level (Wallace et al. 2005). Despite the simpler anatomy of the zebrafish GI tract, the profiling of molecular and gene expression suggests that the zebrafish GI tract can be subdivided into regions resembling those of other vertebrates, with the distal portion of the zebrafish showing similar expression patterns to that of the human colon (Wang et al. 2010). In the mid-intestine of the adult zebrafish, we have identified subtypes of ICCs at the interface between the inner and outer layers of the muscularis and ICCs between the inner circular muscle layer and the submucosa (Fig. 9). Although these probably correspond to ICC subtypes previously reported in these areas in other organisms, such as mice (Fig. 3), further studies, perhaps in other teleosts, should examine the extent to which ICC subtypes are conserved across evolution.
Fig. 9.
Representation of the zebrafish intestinal tract. a The zebrafish alimentary canal is stomachless and is divided into three general anatomical regions: the anterior-most region or intestinal bulb (red), the mid-intestine (blue), and the distal-most or caudal region (green). The dashed line indicates the primary region of the GI tract that is the focus of this study. b Representation of a longitudinal section in the mid-intestine delineating the main histological sub-regions: the muscularis, which is subdivided into an outer longitudinal muscle (OLM) and an inner circular muscle layer (ICM) with a small space in between containing neuronal cells and ICCs (red), a thin submucosal layer of connective tissue, and a mucosa with epithelial cells extending into the lumen. Additional ICCs have been identified between the submucosal and outer longitudinal muscle layer
Zebrafish are a useful tool in developmental studies and have recently emerged as a model of tumor formation through genetic manipulation and forward genetic screening (Merlino and Khanna 2007). Although ICCs are considered to be mesenchymal cells derived from smooth muscle precursors, neural crest cells are nevertheless believed to contribute to ICC development (Wu et al. 2000). The unique ability to study zebrafish during early embryonic development might shed light on the long-debated role of neural crest cells in ICC development. The ultrastructural demonstration of kit-expressing ICCs in the zebrafish intestine further establishes zebrafish as a new and useful tool for studying genes influencing intestinal motility and ICC development and differentiation.
Supplementary Material
Acknowledgments
Portions of this work were generously funded by the National of Health including NIH DK071588-02; the remaining of this was funded by the Intramural Program of NICHD, NIH.
Footnotes
Electronic supplementary material The online version of this (doi:10.1007/s00441-012-1434-4) contains supplementary which is available to authorized users.
Contributor Information
Evan R. Ball, Section on Endocrinology & Genetics, Program on Developmental Endocrinology & Genetics, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH), Building 10, CRC, Room 1-3330, 10 Center Drive, MSC1103, Bethesda, MD 20892, USA
Miho M. Matsuda, Laboratory of Molecular Genetics, Section on Neural Developmental Dynamics, NICHD, NIH, Bethesda, MD 20892, USA
Louis Dye, National Institute of Child Health and Human Development (NICHD), Microscopy and Imaging Core, Bethesda, MD 20892, USA.
Victoria Hoffmann, Office of Research Services, Division of Veterinary Resources, National Institutes of Health, Bethesda, MD 20892, USA.
Patricia M. Zerfas, Office of Research Services, Division of Veterinary Resources, National Institutes of Health, Bethesda, MD 20892, USA
Eva Szarek, Section on Endocrinology & Genetics, Program on Developmental Endocrinology & Genetics, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH), Building 10, CRC, Room 1-3330, 10 Center Drive, MSC1103, Bethesda, MD 20892, USA.
Adam Rich, Department of Biological Sciences, SUNY Brockport, Brockport, NY 14420, USA.
Ajay B. Chitnis, Program of Differentiation, Section on Neural Developmental Dynamics, NICHD, NIH, Bethesda, MD 20892, USA
Constantine A. Stratakis, Section on Endocrinology & Genetics, Program on Developmental Endocrinology & Genetics, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH), Building 10, CRC, Room 1-3330, 10 Center Drive, MSC1103, Bethesda, MD 20892, USA
References
- Braasch I, Salzburger W, Meyer A. Asymmetric evolution in two fish-specifically duplicated receptor tyrosine kinase paralogons involved in teleost coloration. Mol Biol Evol. 2006;23:1192–1202. doi: 10.1093/molbev/msk003. [DOI] [PubMed] [Google Scholar]
- Chen H, Redelman D, Ro S, Ward SM, Ordög T, Sanders KM. Selective labeling and isolation of functional classes of interstitial cells of Cajal of human and murine small intestine. Am J Physiol Cell Physiol. 2007;292:C497–C507. doi: 10.1152/ajpcell.00147.2006. [DOI] [PubMed] [Google Scholar]
- Davuluri G, Seiler C, Abrams J, Soriano AJ, Pack M. Differential effects of thin and thick filament disruption of zebrafish smooth muscle regulatory proteins. Neurogastroenterol Motil. 2010;22:1100–e285. doi: 10.1111/j.1365-2982.2010.01545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edling CE, Hallberg B. c-Kit—a hematopoietic cell essential receptor tyrosine kinase. Int J Biochem Cell Biol. 2007;39:1995–1998. doi: 10.1016/j.biocel.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Faussone-Pellegrini MS, Thuneberg L. Guide to the identification of interstitial cells of Cajal. Microsc Res Tech. 1999;47:248–266. doi: 10.1002/(SICI)1097-0029(19991115)47:4<248::AID-JEMT4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Garcia-Lopez P, Garcia-Marin V, Martínez-Murillo R, Freire M. Updating old ideas and recent advances regarding the interstitial cells of Cajal. Brain Res Rev. 2009;61:154–169. doi: 10.1016/j.brainresrev.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Hennig GW, Spencer NJ, Jokela-Willis S, Bayguinov PO, Lee HT, Ritchie LA, Ward SM, Smith TK, Sanders KM. ICC-MY coordinate smooth muscle electrical and mechanical activity in the murine small intestine. Neurogastroenterol Motil. 2010;22:e138–e151. doi: 10.1111/j.1365-2982.2009.01448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg A, Olsson C, Hennig GW. TTX-sensitive and TTX-insensitive control of spontaneous gut motility in the developing zebrafish (Danio rerio) larvae. J Exp Biol. 2007;210:1084–1091. doi: 10.1242/jeb.000935. [DOI] [PubMed] [Google Scholar]
- Horowitz B, Ward SM, Sanders KM. Cellular and molecular basis for electrical rhythmicity in gastrointestinal muscles. Annu Rev Physiol. 1999;61:19–43. doi: 10.1146/annurev.physiol.61.1.19. [DOI] [PubMed] [Google Scholar]
- Huizinga JD. Gastrointestinal peristalsis: joint action of enteric nerves, smooth muscle, and interstitial cells of Cajal. Microsc Res Tech. 1999;47:239–247. doi: 10.1002/(SICI)1097-0029(19991115)47:4<239::AID-JEMT3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Hultman KA, Bahary N, Zon LI, Johnson SL. Gene duplication of the zebrafish kit ligand and partitioning of melanocyte development functions to kit ligand a. PLoS Genet. 2007;3:e17. doi: 10.1371/journal.pgen.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino S, Horiguchi K. Interstitial cells of Cajal are involved in neurotransmission in the gastrointestinal tract. Acta Histochem Cytochem. 2006;39:145–153. doi: 10.1267/ahc.06023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998;152:1259–1269. [PMC free article] [PubMed] [Google Scholar]
- Klüppel M, Huizinga JD, Malysz J, Bernstein A. Developmental origin and Kit-dependent development of the interstitial cells of Cajal in the mammalian small intestine. Dev Dyn. 1998;211:60–71. doi: 10.1002/(SICI)1097-0177(199801)211:1<60::AID-AJA6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Komuro T. Structure and organization of interstitial cells of Cajal in the gastrointestinal tract. J Physiol. 2006;576:653–658. doi: 10.1113/jphysiol.2006.116624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro T, Seki K, Horiguchi K. Ultrastructural characterization of the interstitial cells of Cajal. Arch Histol Cytol. 1999;62:295–316. doi: 10.1679/aohc.62.295. [DOI] [PubMed] [Google Scholar]
- Krementz AB, Chapman GB. Ultrastructure of the posterior half of the intestine of the channel catfish, Ictalurus punctatus. J Morphol. 1975;145:441–482. doi: 10.1002/jmor.1051450405. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Chitnis AB. Atoh1a expression must be restricted by Notch signaling for effective morphogenesis of the posterior lateral line primordium in zebrafish. Development. 2010;137:3477–3487. doi: 10.1242/dev.052761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellgren EM, Johnson SL. Kitb, a second zebrafish ortholog of mouse Kit. Dev Genes Evol. 2005;215:470–477. doi: 10.1007/s00427-005-0001-3. [DOI] [PubMed] [Google Scholar]
- Merlino G, Khanna C. Fishing for the origins of cancer. Genes Dev. 2007;21:1275–1279. doi: 10.1101/gad.1563707. [DOI] [PubMed] [Google Scholar]
- Min KW, Leabu M. Interstitial cells of Cajal (ICC) and gastrointestinal stromal tumor (GIST): facts, speculations, and myths. J Cell Mol Med. 2006;10:995–1013. doi: 10.1111/j.1582-4934.2006.tb00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto-Kikuta S, Komuro T. Ultrastructural observations of the tunica muscularis in the small intestine of Xenopus laevis, with special reference to the interstitial cells of Cajal. Cell Tissue Res. 2007;328:271–279. doi: 10.1007/s00441-006-0363-5. [DOI] [PubMed] [Google Scholar]
- Parichy DM, Rawls JF, Pratt SJ, Whitfield TT, Johnson SL. Zebrafish sparse corresponds to an orthologue of c-kit and is required for the morphogenesis of a subpopulation of melanocytes, but is not essential for hematopoiesis or primordial germ cell development. Development. 1999;126:3425–3436. doi: 10.1242/dev.126.15.3425. [DOI] [PubMed] [Google Scholar]
- Rich A, Leddon SA, Hess SL, Gibbons SJ, Miller S, Xu X, Farrugia G. Kit-like immunoreactivity in the zebrafish gastrointestinal tract reveals putative ICC. Dev Dyn. 2007;236:903–911. doi: 10.1002/dvdy.21086. [DOI] [PubMed] [Google Scholar]
- Robson ME, Glogowski E, Sommer G, Antonescu CR, Nafa K, Maki RG, Ellis N, Besmer P, Brennan M, Offit K. Pleomorphic characteristics of a germ-line KIT mutation in a large kindred with gastrointestinal stromal tumors, hyperpigmentation, and dysphagia. Clin Cancer Res. 2004;10:1250–1254. doi: 10.1158/1078-0432.ccr-03-0110. [DOI] [PubMed] [Google Scholar]
- Rumessen JJ, Vanderwinden JM. Interstitial cells in the musculature of the gastrointestinal tract: Cajal and beyond. Int Rev Cytol. 2003;229:115–208. doi: 10.1016/s0074-7696(03)29004-5. [DOI] [PubMed] [Google Scholar]
- Vanderwinden J-M, Rumessen JJ, Bernex F, Schiffmann SN, Panthier J-J. Distribution and ultrastructure of interstitial cells of Cajal in the mouse colon, using antibodies to Kit and Kit W-lacZ mice. Cell Tissue Res. 2000;302:155–170. doi: 10.1007/s004419900170. [DOI] [PubMed] [Google Scholar]
- Wallace KN, Akhter S, Smith EM, Lorent K, Pack M. Intestinal growth and differentiation in zebrafish. Mech Dev. 2005;122:157–173. doi: 10.1016/j.mod.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Wang Z, Du J, Lam SH, Mathavan S, Matsudaira P, Gong Z. Morphological and molecular evidence for functional organization along the rostrocaudal axis of the adult zebrafish intestine. BMC Genomics. 2010;11:392. doi: 10.1186/1471-2164-11-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson HD. The ultrastructure of the innervation of the intestinal wall in the teleosts Myoxocephalus scorpius and Pleuronectes platessa. Cell and Tissue Res. 1981;214:651–658. doi: 10.1007/BF00233504. [DOI] [PubMed] [Google Scholar]
- Wu JJ, Rothman TP, Gershon MD. Development of the interstitial cell of Cajal: origin, kit dependence and neuronal and non-neuronal sources of kit ligand. J Neurosci Res. 2000;59:384–401. doi: 10.1002/(SICI)1097-4547(20000201)59:3<384::AID-JNR13>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.