Abstract
Type I collagen is the most abundant protein in human body synthesized in all tissues as the heterotrimer of two α1(I) and one α2(I) polypeptides. Here we show that intact nonmuscle myosin filaments are required for synthesis of heterotrimeric type I collagen. Conserved 5′ stem-loop in collagen α1(I) and α2(I) mRNAs binds RNA binding protein LARP6. LARP6 interacts with nonmuscle myosin through its C-terminal domain and associates collagen mRNAs with the filaments. Dissociation of nonmuscle myosin filaments results in secretion collagen α1(I) homotrimer, in diminished intracellular colocalization of collagen α1(I) and α2(I) polypeptides, which is required for folding of the heterotrimer, and in their increased intracellular degradation. Inhibition of the motor function of myosin has similar collagen specific effects, while disruption of actin filaments has a general effect on protein secretion. Nonmuscle myosin copurifies with polysomes and there is a subset of polysomes involved in myosin dependent translation of collagen mRNAs. These results indicate that association of collagen mRNAs with nonmuscle myosin filaments is necessary to coordinately synthesize collagen α1(I) and α2(I) polypeptides. We postulate that LARP6/myosin dependent mechanism regulates the synthesis of heterotrimeric type I collagen by coordinating translation of collagen mRNAs.
Keywords: type I collagen, LARP6, nonmuscle myosin, translation
Introduction
Type I collagen is the most abundant protein in human body. It is composed of two α1(I) and one α2(I) polypeptides which fold into a triple helix 1. Fibroproliferative disorders are characterized by excessive production of collagen type I by the activation of fibroblasts and myofibroblasts in tissues that normally do not synthesize type I collagen 2; 3; 4; 5 and they are the major causes of mortality and morbidity, associated with 45% of deaths in USA 6. There is no cure for fibrosis and the excessive collagen production is usually irreversible 7. All complications of fibroproliferative disorders are due to excessive collagen production and to develop antifibrotic drugs the molecular mechanism of excessive collagen synthesis must be elucidated. The biosynthesis of type I collagen has multiple steps, but recently it became evident that regulation of stability of collagen mRNAs and their translation is the predominant mechanism for high level of synthesis in multiple cell types 8; 9; 10; 11; 12.
In the 5′ UTR of collagen α1(I), α2(I) and α1(III) mRNAs there is a conserved 5′SL structure (5′SL) 13; 14; 15. We cloned LARP6 as the protein which binds the 5′SL with high affinity and specificity 16. This binding is necessary for high level of expression of type I collagen. We postulated that LARP6 binding serves to prevent premature translation of collagen mRNAs, allowing their subsequent coordinated translation on the membrane of the endoplasmic reticulum (ER) 17. This coordination is evidenced by localization of collagen synthesis into discrete subcellular sites 16. Translation of collagen α1(I) and α2(I) mRNAs in close proximity at these sites may be needed to increase the local concentration of the polypeptides, which favors formation of α1(I)/α2(I)/α1(I) heterotrimers. Heterotrimers of type I collagen are almost exclusively synthesized in all tissues 18, although the homotrimers of α1(I) polypeptides readily form if α2(I) polypeptide is not expressed 19; 20. Folding of collagen triple helix starts with disulfide bonding of two α1(I) and one α2(I) polypeptides at the C-terminal end, with subsequent folding into a triple helix 1. Disulfide bonded collagen polypeptides were found associated with polysomes 21, suggesting that the interchain bonding starts before release of the polypeptides from the polysomes. Folding and posttranslational modifications of collagen polypeptides are in kinetic equilibrium and slow folding results in hypermodifications of the polypeptides. Hypermodified collagen peptides fold into unstable triple helix, resulting in a phenotype of osteogenesis imperfecta 22; 23. Therefore, translational elongation, the rate of modifications and the rate of folding are coordinated. TRAM2 protein, as a part of translocon, associates Ca++ pump Serca2b to the translocons where collagen chains are elongated. It has been proposed that this increases local Ca++ concentration to stimulate collagen specific molecular chaperones to facilitate folding of the heterotrimer 12.
Despite cloning and characterization of LARP6, the mechanism which coordinates synthesis of type I collagen is poorly understood. In this manuscript we describe one key step in synthesis of type I collagen by profibrotic cells; the interaction of collagen mRNAs with filaments composed of nonmuscle myosin.
Results
Nonmuscle myosin copurifies with 5′SL RNA
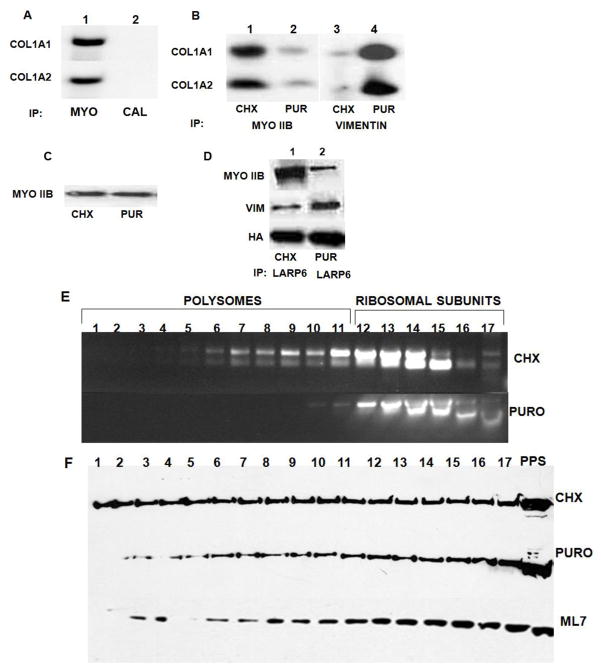
LARP6 was cloned before as the protein which directly binds 5′SL of collagen mRNAs 16, however, other proteins which associate in the complex with LARP6 and 5′SL have been unknown. To identify these proteins we performed tobramycin affinity purifications by attaching tobramycin aptamer to the 5′SL RNA (Fig 1A). Affinity purifications using tobramycin aptamer have been described for purifications of splicing complexes 24; 25. After incubation of the collagen 5′SL/tobramycin aptamer RNA in cytosolic extracts of human lung fibroblasts the bound proteins were pulled-down with tobramycin agarose and eluted with an excess of free tobramycin (Fig 1B, lane 2). As control, inverted 5′SL fused to the aptamer was used (lane 1). The two most prominent proteins specifically pulled down with 5′SL were identified as nonmuscle myosin IIB and vimentin (indicated by arrows in Fig 1B). LARP6 was found as a minor band in these experiments. The identification of nonmuscle myosin IIB as the protein which copurifies with collagen 5′SL was unexpected, as there have been no reports on the role of nonmuscle myosin in synthesis of type I collagen.
Figure 1.

Tobramycin affinity purification of 5′SL associated proteins. A. 5′SL/tobramycin aptamer bait. B. Proteins specifically pulled down with 5′SL bait. Coomassie stained SDS-PAGE gel of proteins pulled down with 5′SL bait (lane 2) or inverted 5′SL bait (CON, lane 1). Lane 3 is size marker. Nonmuscle myosin IIB (MYO IIB) and vimentin (VIM) are indicated by arrows.
Interaction of collagen mRNAs with nonmuscle myosin
Since LARP6 is the only protein which directly binds 5′SL 16, it is likely that nonmuscle myosin had been tethered to the 5′SL by the protein-protein interaction with LARP6. To verify if LARP6 and nonmuscle myosin interact we performed co-immunoprecipitation experiments. LARP6 has 4 domains, the N-terminal domain of unknown function, the La-homology domain found in other LARPs 26, the unique RNA binding domain (RBD) necessary for binding 5′SL 16 and the C-terminal domain of unknown function (Fig 2A). To identify which domain is needed for the interaction with myosin IIB we expressed HA-tagged full size LARP6 and HA-tagged LARP6 lacking the C-terminal domain (ΔC-LARP6) and performed immunoprecipitations with anti-HA antibody. While myosin IIB co-immunoprecipitated with the full size LARP6 (Fig 2B, lane 1), it failed to co-immunoprecipitate with ΔC-LARP6 (lane 2) or the control RNA binding protein RBMS3 27 (lane 3). Myosin IIA was also co-immunoprecipitated with full size LARP6, but not with ΔC-LARP6 (Fig 2C), suggesting that LARP6 interacts with the both major isoforms of nonmuscle myosin through its C-terminal domain. The C-terminal domain of LARP6 is not needed for binding 5′SL 16.
Figure 2.

Interaction of LARP6 with nonmuscle myosin. A. Schematic representation of the domains of LARP6. N-terminal domain (N-TER), La-homology domain (LA), RNA binding domain (RBD) and C-terminal domain (C-TER) are shown with amino-acid numbering on the top. ΔC-LARP6, C-terminal deletion mutant of LARP6. B. Immunoprecipitation of LARP6 and nonmuscle myosin. HA-tagged LARP6 (lane 1), HA-tagged ΔC-LARP6 (lane 2) and HA-tagged RBMS3 (lane 3) were expressed in human lung fibroblasts, immunoprecipitated with anti-HA antibody and the immunoprecipitated material analyzed by western blot with anti-myosin IIB antibody (MYO IIB). HA, western blot with anti-HA antibody as control for immunoprecipitation efficiency of the tagged proteins. C. Experiment as in B, but the western blot was done using anti-myosin IIA antibody (MYO IIA). D. Interaction of LARP6 and nonmuscle myosin does not depend on integrity of RNA. Experiment as in B, but the lysate was treated with RNAse A prior to immunoprecipitation (lane 1) or untreated (lanes 2 and 3). The immunoprecipitate was analyzed with anti-myosin IIB antibody (MYO IIB), anti-fibronectin antibody (FIB), as control for specificity and anti-HA antibody (HA), as control for precipitation efficiency.
We also assessed if the interaction between LARP6 and nonmuscle myosin is dependent on intact RNA by digesting the samples with RNase A prior to analysis (Fig 2D). Immunoprecipitation of LARP6 pulled-down myosin IIB, regardless of RNase A digestion (lanes 1 and 2), suggesting that these proteins form a complex by protein-protein interactions. These interactions were specific, because fibronectin and RBMS3 were not coimmunoprecipitated (lane 3). Collagen α1(I) and α2(I) mRNAs were also found immunoprecipitated with nonmuscle myosin in the 5′SL dependent manner, this is described in Fig 8.
Figure 8.

Association of collagen α1(I) and α2(I) mRNAs with nonmuscle myosin. A. Expression of collagen mRNAs in WT and Δ5′ SL mutant MEFs. RT-PCR with total RNA from WT MEFs (lane 1) and Δ5′ SL mutant MEFs (lane 2) and primers specific for collagen α1(I) mRNA (COL1A1), collagen α2(I) mRNA (COL1A2) and fibronectin mRNA (FIB), as loading control. B. Coprecipitation of collagen mRNAs with LARP6. HA-tagged LARP6 was expressed in WT MEFs (lane 1) and Δ5′SL mutant MEFs (lane 2) and immunoprecipitated with anti-HA antibody. Immunoprecipitate was analyzed by RT-PCR as in A. C. Coprecipitation of collagen mRNAs with nonmuscle myosin. Immunoprecipitation with anti-myosin IIB antibody from extracts of WT MEFs (lane 1) and Δ5′SL mutant MEFs (lane 2). Immunoprecipitate was analyzed by RT-PCR as in A. Bottom panel: control western blot for equal pull down of myosin IIB.
Integrity of nonmuscle myosin filaments is necessary for secretion of type I collagen
To assess the role of nonmuscle myosin in collagen synthesis we disrupted nonmuscle myosin filaments in two different cell types, primary human lung fibroblasts and primary scleroderma skin fibroblasts. These cells are responsible for lung and skin fibrosis. Nonmuscle myosin filaments were disrupted by either treatment of the cells with ML-7 28 or by overexpression of kinase-dead myosin light chain kinase (KD-MLCK mutant, a kind gift of Dr. P. Gallagher, Indiana University) 29; 30.
ML-7 is a specific inhibitor of myosin light chain kinase (MLCK) 28 and inhibition of MLCK leads to disassembly of myosin IIA and IIB filaments 29; 30. Fig 3A shows immunostaining of human lung fibroblasts for myosin IIB without (left panel) and with (right panel) treatment with ML-7. In untreated cells myosin filaments are prominent, while in the ML-7 treated cells the myosin staining is confined around the nucleus. The same effect was seen in scleroderma fibroblasts. The total level on myosin IIB remained unchanged, as judged by western blot (Fig 3B). The levels of collagen α1(I) and α2(I) mRNAs were also not significantly affected by ML-7 (see Fig 8).
Figure 3.
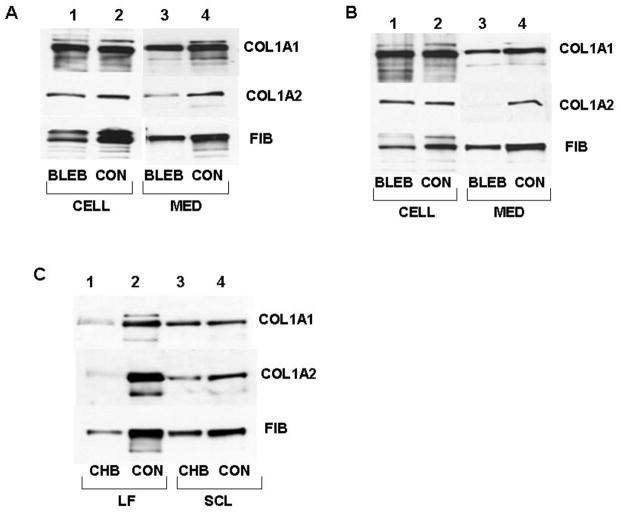
Disruption of nonmuscle myosin by ML-7 results in secretion of homotrimeric type I collagen. A. ML-7 disrupts nonmuscle myosin filaments. Immunostaining of nonmuscle myosin IIB in control (CON) lung fibroblasts and in lung fibroblasts treated with ML-7 (ML-7). B. ML-7 does not change total level of nonmuscle myosin protein. Western blot with anti-myosin IIB antibody (MYO IIB) of control cells (lane 1) and cells treated with ML-7 (lane 2). Fibronectin (FIB), loading control. C. Effect of ML-7 on collagen secretion from lung fibroblasts. Western blot of cellular proteins from control cells (lane 2) and ML-7 treated cells (lane 1) and of medium proteins of control (lane 4) and ML-7 treated cells (lane 3). COL1A1, collagen α1(I) polypeptide, COL1A2, collagen α2(I) polypeptide, FIB, fibronectin as loading control. D. Homotrimeric triple helix in the medium of ML-7 treated cells. The medium samples from C were analyzed under reducing conditions (lanes 1 and 2) and nonreducing conditions (lanes 2 and 3) with antibody specific for collagen α1(I) polypeptide. Migration of collagen α1(I) monomers (COL1A1) and the disulfide bonded chains (S-S BONDED) is indicated. E. Effect of ML-7 on collagen secretion from scleroderma fibroblasts. Experiment as in C, but primary scleroderma skin fibroblasts were used. Putative cellular degradation products of collagen α1(I) and α2(I) polypeptides are indicated by arrows. F. Increased intracellular degradation of collagen polypeptides in ML-7 treated cells. Lung fibroblasts (lanes 1 and 2) and scleroderma fibroblasts (lanes 3 and 4) were treated with epoxomycin alone (lanes 1 and 3) and with epoxomycin and ML-7 (lanes 2 and 4). Intracellular collagen was analyzed by western blot with antibodies against collagen α1(I) polypeptide (COL1A1) and α2(I) polypeptide (COL1A2). Putative degradation products are indicated by arrows.
To assess the effect of ML-7 on collagen protein synthesis and secretion we analyzed the levels of collagen α1(I) and α2(I) polypeptides intracellularly and secreted in the medium using western blot and the chain specific antibodies 12; 15; 31; 32; 33. ML-7 treatment profoundly affected secretion of type I collagen, but the effect differed in the two types of the primary fibroblasts. In lung fibroblasts, the intracellular level of collagen α1 and α2 polypeptides was not significantly affected by ML-7 treatment (Fig 3C, lanes 1 and 2). In the medium collagen α1(I) polypeptide was found in the similar amounts in control and ML-7 treated cells, however, collagen α2(I) polypeptide was secreted only in tracing amounts after ML-7 treatment (lanes 3 and 4). The absence of α2(I) polypeptide in the medium suggests that ML-7 uncoupled secretion of α1(I) and α2(I) chains. α2(I) polypeptide can not be efficiently secreted without folding with α1(I) polypeptide 34. The secretion of fibronectin was not affected, suggesting that the general machinery for protein secretion was intact. Immunostaining for the ER marker protein calnexin did not show a difference in ML-7 treated cells and control cells, suggesting that organization of the ER was also not significantly changed. To assess if the secreted α1(I) polypeptides had been secreted as disulfide bonded homotrimers we performed western blot under nonreducing conditions (Fig 3D). In ML-7 treated cells a similar fraction of disulfide bonded collagen was found as in control cells (compare lanes 3 and 4). However, since α2(I) polypeptide was absent in the cell medium of ML-7 treated cells, the bands must represent disulfide bonded collagen α1(I) chains. The α2(I) antibody could not recognize the polypeptide under nonreducing conditions, so we could not directly tested its presence in the S-S bonded collagen.
Since there are reports that ML-7 can cause apoptosis of epithelial cells 29, we tested if ML-7 caused apoptosis of the primary fibroblasts. Treatment with ML-7 increased the percentage of apoptotic cells from 2.2% to 4.2% in lung fibroblasts and did not have an effect on percentage of apoptotic scleroderma fibroblasts (0.3%). Therefore, we concluded that increased apoptosis is not responsible for the observed effects of ML-7. In scleroderma skin fibroblasts the intracellular level of collagen α1(I) polypeptide was not changed with ML-7 treatment, however, the level of α2(I) polypeptide was reduced (Fig 3E, lanes 1 and 2). In multiple experiments, but only in the ML-7 treated samples, we have noticed the appearance of bands indicated by arrows in Fig 3E. They likely represent degradation products of α1(I) and α2(I) polypeptides (see bellow). In the medium collagen α1(I) polypeptide was barely detectable, while collagen α2(I) polypeptide was absent (lanes 3 and 4). Thus, in scleroderma fibroblasts ML-7 treatment almost completely abolishes collagen secretion. The secretion of fibronectin was not affected.
Although collagen α2(I) polypeptide was not secreted into the medium of lung fibroblasts and both collagen polypeptides were not secreted from scleroderma fibroblasts, their intracellular levels were not increased after ML-7 treatment. To test if they have been subjected to accelerated degradation we treated the cells with proteosome inhibitor epoxomycin in combination with ML-7. The intracellular level of both collagen polypeptides was significantly increased with the combined treatment compared to the epoxomycin treatment alone (Fig 3F). An additional product of approximately 120 kD (indicated by arrow in Fig 3F) was prominently seen in the epoxomycin and ML-7 treated samples (lanes 2 and 4), but not in the epoxomycin alone treated samples (lanes 1 and 2). This is the same degradation product seen in scleroderma fibroblasts without epoxomycin (Fig 3E), but which accumulated to a high level when proteosomal degradation had been inhibited. We concluded from these experiments that the failure of lung fibroblasts to secrete collagen α2(I) polypeptide and scleroderma fibroblasts to secrete both polypeptides after ML-7 treatment is due to their inefficient folding into heterotrimer and accelerated intracellular degradation.
To exclude that nonspecific effects of ML-7 are responsible for the perturbation in collagen synthesis we repeated the analysis after overexpressing the kinase-dead isoform of MLCK (KD-MLCK). This isoform acts as a dominant negative protein for myosin filaments assembly 29; 30. KD-MLCK isoform was constructed as adenovirus for efficient delivery into the primary fibroblasts. Overexpression of KD-MLCK resulted in disassembly of the filaments in lung fibroblasts (Fig 4A, right panel), similar to that seen upon ML-7 treatment. The same effect was seen in scleroderma fibroblasts. In lung fibroblasts, KD-MLCK dramatically reduced the secretion of α2(I) polypeptide, with little effect on secretion of α1(I) polypeptide (Fig 4B, lane 3). In scleroderma fibroblasts it reduced the level of both polypeptides intracellularly (Fig 4C, lane 1), but this time we did not observe the characteristic degradation products seen after the ML-7 treatment. In the medium α1(I) polypeptide was dramatically reduced, while α2(I) polypeptide was absent (lane 3). Thus, a similar effect on collagen synthesis was seen when nonmuscle myosin filaments were disrupted by KD-MLCK.
Figure 4.
Disruption of nonmuscle myosin filaments by dominant negative isoform of MLCK results in secretion of homotrimeric type I collagen. A. Disruption of nonmuscle myosin filaments by overexpression of kinanse-dead isoform of MLCK. Immunostaining of myosin IIB in lung fibroblasts infected with control adenovirus (CON) and with adenovirus expressing kinanse-dead isoform of MLCK (KD). B. Diminished secretion of collagen α2(I) polypeptide from lung fibroblasts overexpressing KD-MLCK. Cellular level (lanes 1 and 2) and medium level (lanes 3 and 4) of collagen α1(I) polypeptide (COL1A1) and α2(I) polypeptide (COL1A2) analyzed for cells transduced with control adenovirus (CON, lanes 2 and 4) and adenovirus expressing kinanse-dead isoform of MLCK (KD, lanes 1 and 3). Fibronectin is shown as loading control. C. The same experiment as in B, but using scleroderma fibroblasts.
To fold into collagen heterotrimer the α1(I) and α2(I) polypeptides must colocalize in the lumen of the ER. Since in lung fibroblasts ML-7 treatment did not affect the cellular level of the individual collagen polypeptides (Fig 3B), we used this cell type to assess if their subcellular colocalization was affected by ML-7. Using the chain specific antibodies for immunostaining we observed a high degree of colocalization of α1(I) and α2(I) polypeptides in control cells (Fig 5A, top panel). This colocalization was confined to the ER, as judged by costaining with the ER marker calnexin. However, in ML-7 treated cells a significant fraction of α2(I) polypeptide (red) was not colocalized with α1(I) polypeptide (green) (Fig 5A, bottom panel). This is consistent with lack of their folding into the heterotrimer.
Figure 5.

Colocalization of collagen α1(I) and α2(I) polypeptides in the cell. A. Collagen α1(I) and α2(I) polypeptides do not colocalize after ML-7 treatment. Immunostaining with collagen α1(I) specific antibody (COL1A1, green) and collagen α2(I) antibody (COL1A2, red) and the overlaid image (MERGE) in control cells (CON, upper panel) and ML-7 treated cells (ML-7, lower panel). B. Co-immunoprecipitation of nonmuscle myosin with LARP6 is decreased after ML-7 treatment. Immunoprecipitation of HA-tagged LARP6 from control lung fibroblasts (lane 1) and from lung fibroblasts treated with ML-7 (lane 2), analyzed by western blot with anti-myosin IIB antibodies (MYO IIB), anti-fibronectin antibody and anti-HA antibody, as control for precipitation efficiency.
The interaction between LARP6 and nonmuscle myosin was also diminished by disruption of the filaments (Fig 5B). ML-7 did not change the level of myosin IIB protein, just its polymerization (Fig 3A), however, significantly less myosin IIB was pulled-down with LARP6 from ML-7 treated cells (lane 2) than from control cells (lane 1). From these experiments we concluded that tethering of collagen mRNAs to the nonmuscle myosin filaments by LARP6 coordinates synthesis of α1(I) and α2(I) polypeptides for productive secretion of the heterotrimeric type I collagen.
Blebbistatin is the inhibitor of the ATPase function of nonmuscle myosin and blocks the motor function of the filaments 35; 36; 37. To assess if the myosin motor is required for collagen synthesis we treated the cells with blebbistatin and analyzed collagen α1(I) and α2(I) polypeptides. Blebbistatin reduced the secretion of α2(I) polypeptide from lung fibroblasts (Fig 6A, lane 3) and abolished its secretion from scleroderma fibroblasts (Fig 6B, lane 3), while minimally affecting the secretion of α1(I) polypeptide in both cell types. The cellular levels of both collagen polypeptides were unchanged. There was a small effect on the expression of fibronectin, which was reduced intracelularly and in the medium. Therefore, we included another loading control for the cellular fractions (tubulin), which showed equal loading. We concluded that the motor function of myosin is involved in coordination of secretion of type I collagen.
Figure 6.
Motor function of nonmusle myosin is required for secretion of type I collagen. A. Effect of blebbistatin on collagen secretion from lung fibroblasts. Western blot of cellular proteins from control cells (lane 2) and blebbistatin treated cells (lane 1) and of medium proteins of control (lane 4) and blebbistatin treated cells (lane 3). COL1A1, collagen α1(I) polypeptide, COL1A2, collagen α2(I) polypeptide, FIB, fibronectin as loading control. B. Effect of blebbistatin on collagen secretion from scleroderma fibroblasts. Experiment as in A, except primary scleroderma skin fibroblasts were used. C. Effect of disrupting actin filaments. Western blot of medium proteins of lung fibroblasts (LF, lanes 1 and 2) and scleroderma fibroblasts (SCL, lanes 3 and 4) of control cells (CON, lanes 2 and 4) and of cells treated with cytochalasin B (CHB, lanes 1 and 3). Western blot was probed with antibodies against collagen α1(I) polypeptide (COL1A1), collagen α2(I) polypeptide (COL1A2) and fibronectin (FIB), as loading control.
It is possible that the effect of disrupting myosin is indirect, due to lack of motility of actin filaments. Therefore, we disrupted actin filaments with cytochalasin B 38 and repeated the experiments (Fig 6C). In lung fibroblasts cytochalasin B diminished the secretion of collagen α1(I) and α2(I) polypeptides, but also had an effect on secretion of fibronectin (lane 1). This suggests that general secretion machinery may have been affected. In scleroderma fibroblasts there was no effect on secretion on collagen α1(I) polypeptide and a small effect on secretion of α2(I) polypeptide and fibronectin. Thus, the effects of disrupting nonmuscle myosin are specific for collagen, while disrupting actin has effects on general protein secretion machinery.
The effect of nonmuscle myosin on collagen synthesis is mediated by the 5′SL
To investigate the role of conserved 5′SL of collagen mRNAs in regulation of their translation we obtained mouse embryonic fibroblasts (MEFs) from mice in which 5′SL was disrupted in the context of the endogenous collagen α1(I) gene (Parsons et al., submitted). The mutation of the 5′SL did not change the coding region of the α1(I) gene (Fig. 7A) and the 5′SL of α2(I) gene was not changed. We then treated the MEFs with ML-7 and estimated the effect on collagen α1(I) polypeptide level intracellularly and in the medium (Fig 7B). In wt MEFs ML-7 treatment did not change the cellular level of α1(I) polypeptide (lanes 1 and 2), however, its secretion into the medium was drastically reduced (lanes 3 and 4). This result is similar to the result obtained with scleroderma fibroblasts (Fig 3E). In Δ5′SL MEFs the treatment with ML-7 did not affect secretion of α1(I) polypeptide; similar amounts were found in the medium of cells treated with ML-7 and in the medium of untreated cells (Fig 7B, lanes 7 and 8). In these experiments we could not measure the expression of collagen α2(I) polypeptide because the antibody poorly recognized the rodent polypeptide. Nevertheless, this result indicates that ML-7 treatment affects secretion of collagen polypeptides only if it is encoded by the mRNA with the 5′SL.
Figure 7.
5′SL regulates myosin dependent collagen secretion. A. Sequence of 5′SL of mouse collagen α1(I) mRNA (WT) and the mutation that was used to create the α1(I) 5′SL knock-in mouse (Δ5′SL). B. Effect of ML-7 on collagen secretion is dependent on 5′SL. Western blot of cellular (lanes 1 and 2) and medium proteins (lanes 3 and 4) from WT MEFs treated with ML-7 (lanes 1 and 3) and untreated control WT MEFs (lanes 2 and 4). COL1A1, collagen α1(I) polypeptide, FIB, fibronectin as loading control. Lanes 5–8, the same experiment with Δ5′SL mutant MEFs.
Involvement of monmuscle myosin in translation of collagen mRNAs
To assess how the mutation of 5′SL in α1(I) mRNA affects association of α1(I) and α2(I) mRNAs with nonmuscle myosin we first analyzed their ability to bind LARP6. Fig 8A shows that wt and Δ5′SL MEFs have comparable steady state level of collagen α2(I) mRNA, but Δ5′SL MEFs have 50% less of collagen α1(I) mRNA (lane 2). This is due to the mutation of 5′SL, which destabilized this mRNA. We then expressed HA-tagged LARP6 in wt and Δ5′SL MEFs and performed immunoprecipitation with anti-HA antibody, followed by RT-PCR analysis of collagen mRNAs in the immunoprecipitated material (Fig 8B). In wt MEFs both collagen mRNAs were pulled down with LARP6 (lane 1), while in Δ5′SL MEFs only collagen α2(I) mRNA was pulled down (lane 2). This was expected since the 5′SL was mutated only in α1(I) mRNA. Fibronectin mRNA was not pulled down in either cell type. This verified that LARP6 interacts with the 5′SL in vivo. When we performed the pull down with anti-myosin IIB antibody from wt MEFs, both collagen mRNAs were found in the immunoprecipitate (Fig 8C, lane 1). In Δ5′SL MEFs collagen α1(I) mRNA was not associated with myosin IIB (lane 2), indicating that intact 5′SL is needed for this association. Unexpectedly, the α2(I) mRNA, which had the wt 5′SL and interacted with LARP6, was also not pulled down with myosin IIB (Fig 8C, lane 2). This suggests that α2(I) mRNA can not associate with nonmuscle myosin independently of α1(I) mRNA and that intact 5′SL on α1(I) mRNA is needed for binding of both mRNAs to the myosin. We could not test if the opposite is true, because MEFs with mutation of collagen α2(I) 5′ SL are not available.
One of the roles of nonmuscle myosin filaments may be to present collagen mRNAs to the ribosomes. Therefore, we assessed if the association of collagen mRNAs with nonmuscle myosin is dependent on the intact polysomes. To show that collagen mRNAs specifically associate with nonmuscle myosin we performed immunoprecipitations with anti-myosin IIB specific antibody and with anti-calnexin antibody and analyzed for pull down of collagen mRNAs (Fig 9A). Only anti-myosin antibody immunoprecipitated collagen α1(I) and α2(I) mRNAs (lane 1). We then immunoprecipitated nonmuscle myosin from lung fibroblasts treated with cycloheximide or puromycin and analyzed if this can change the pull down of collagen mRNAs. As control, the association with vimentin was analyzed in the same samples (Fig 9B). In cycloheximide treated cells both collagen mRNAs were pulled down with nonmuscle myosin IIB (lane 1), but when the cells were treated with puromycin the great majority these mRNAs were absent from the immunoprecipitate (lane 2). The treatment did not change the total level of nonmuscle myosin (Fig 9C), suggesting that dissociation of polysomes decreased the association of collagen mRNAs with nonmuscle myosin. The association of collagen mRNAs with vimentin was increased after dissociation of polysomes (lanes 3 and 4). Puromycin treatment decreased the interaction of LARP6 and nonmuscle myosin and increased its interaction with vimentin (Fig 9D). From these experiments we concluded that association of collagen mRNAs with nonmuscle myosin is favored when polysomes are intact, while collagen mRNAs preferentially bind vimentin filaments when polysomes are dissociated.
Figure 9.
Association of nonmuscle myosin with polysomes. A. Specific immunoprecipitation of collagen mRNAs with nonmuscle myosin. Immunoprecipitation with anti-myosin IIB antibody (lane 1, MYO) and anti-calnexin antibody (lane 2, CAL) and analysis of immunoprecipitate by RT-PCR with collagen α1(I) (COL1A1) and collagen α2(I) (COL2A2) specific primers. B. Distribution of collagen mRNAs between nonmuscle myosin and vimentin. Immunoprecipitation with anti-myosin IIB antibody (lanes 1 and 2) and anti-vimentin antibody (lanes 3 and 4) from lung fibroblasts treated with cycloheximide (CHX) or puromycin (PUR). Immunoprecipitate was analyzed by RT-PCR with collagen α1(I) (COL1A1) and collagen α2(I) (COL2A2) specific primers. C. Level of nonmuscle myosin IIB is similar in cycloheximide and puromycin treated cells. Western blot of nonmuscle myosin IIB (MYO IIB) in cells treated with cycloheximide (CHX) and puromycin (PUR). D. Distribution of LARP6 between nonmuscle myosin and vimentin. Immunoprecipitation of HA-tagged LARP6 from lung fibroblasts treated with cycloheximide (CHX) and puromycin (PUR). Immunoprecipitate was analyzed by western blot with antibodies against nonmuscle myosin IIB (MYO IIB), vimentin (VIM) and HA-tag (HA), as control for immunoprecipitation efficiency. E. Identification of puromycin sensitive polysomes. Polysomes from lung fibroblasts treated with cycloheximide (CHX) and puromycin (PUR) were fractionated on 15–45% continuous sucrose gradient and fractions analyzed for presence of ribosomal RNA. Fraction 1, 45% sucrose, fraction 17, 15% sucrose. F. Nonmuscle myosin copurifies with polysomes. Polysomes were fractionated as in D and the fractions were probed for presence of nonmuscle myosin by western blot. PPS, postpolysomal supernatant. Bottom panel; the cells were treated with ML-7 prior to fractionation of polysomes.
To assess if nonmuscle myosin copurifies with polysomes we fractionated polysomes on sucrose gradients 16 and analyzed for presence of nonmuscle myosin in the fractions by western blot (Fig 9F). To confirm which fractions represent polysomes we compared the distribution of ribosomal RNA of cells treated with puromycin and cycloheximide (Fig 9E). This analysis revealed that fractions 1–11 are puromycin sensitive and represent polysomes, while fractions 12–17 contained ribosomes and ribosomal subunits. Nonmuscle myosin was found in all polysomal fractions, as well as in nonpolysomal fractions and in postpolysomal supernatant (Fig 9F, upper panel). When polysomes were dissociated, some nonmuscle myosin was lost, mostly from the fractions 1–10. Puromycin did not change the total level of nonmuscle myosin, which was comparable to that of cycloheximide treated cells; the subsequent analysis confirmed that it was retained in the insoluble material, removed during preparation of the polysomal lysate. When the cells were treated with ML-7 there was a decrease in the amount of nonmuscle myosin in the heaviest fractions containing polysomes (fractions, 1–11, lower panel). ML-7 did not have an effect on the overall distribution of polysomes. We concluded that a fraction of nonmuscle myosin is associated with polysomes and that there is a correlation between the association of nonmuscle myosin with polysomes and the ability of cells to secrete type I collagen.
Collagen mRNAs can be clearly translated in the absence of nonmuscle myosin filaments. This is indicated by the fact that collagen α1(I) and α2(I) polypeptides were present intracellularly after ML-7 treatment, as well as after overexpression of KD-MLCK (Fig 3). Since polysomes can form on collagen mRNAs without participation of nonmuscle myosin, we surmised that the effect of ML-7 on polysomal profile of collagen mRNAs will be masked by this default translation. Nevertheless, Fig 10 shows that after ML-7 treatment a small fraction of collagen α1(I) and α2(I) mRNAs was shifted into the nonpolysomal fractions 16 and 17. The total level of collagen mRNAs, as the sum of all fractions, was not significantly affected by ML-7 treatment. This indicates that there is a subset of polysomes involved in translation of collagen mRNAs, assembly of which is dependent on the integrity of nonmuscle myosin filaments.
Figure 10.
The effect of ML-7 on distribution of collagen mRNAs in polysomal fractions. A. Distribution of collagen α1(I) mRNA on polysomes. Polysomes were fractionated as in Fig. 9E from control cells (CON) and ML-7 treated cells (ML7) and the fractions were analyzed by RT-PCR for presence of collagen α1(I) mRNA (COL1A1). B. The same experiment as in A, except collagen α2(I) mRNA was analyzed (COL1A2). C. Analysis of GAPDH mRNA in the fractions.
Discussion
In heart fibrosis re-expression of the fetal form of nonmuscle myosin was found only at the sites of focal fibrosis 39. Mice that have the mutated 5′SL in the endogenous collagen α1(I) gene (and from which Δ5′SL MEFs used in this study were derived) develop 50% less liver fibrosis than control mice (Parsons et al., submitted). These findings suggest that the mechanism involving 5′SL and nonmuscle myosin is important for high level of collagen synthesis in vivo. Therefore, the results described here have high relevance for elucidating regulation of collagen expression in fibrosis and for development of antifibrotic drugs.
Nonmuscle myosin has classically been implicated in promoting cell contractility, motility and karyokinesis 40; 41; 42. During the state of high collagen demand, like wound healing or fibrosis, there is activation and migration of fibroblasts to the site of the insult. To enable the motility, activated fibroblasts and myofibroblasts upregulate nonmuscle myosin expression 43; 44. Our results suggest that nonmucle myosin filaments are also a prerequisite for secretion of type I collagen, which commences after arrival of the cells to the wound. Thus, motility and the ability to make type I collagen are integrated processes of collagen producing cells.
Most human tissues synthesize exclusively heterotrimer of type I collagen, although in the absence of α2(I) chains homotrimers of α1(I) chains readily form 19; 20. So, the cells have the ability to fold and secrete homotrimer of type I collagen, but there must be a mechanism that normally prevents this. If the translation of individual collagen chains is random and their registration and folding is not strictly coordinated, the formation of homotrimers would inevitably happen to a significant extent. One way to assure predominant synthesis of the heterotrimer would be to prevent independent translation of α1(I) mRNA and couple it to that of α2(I) mRNA. We have shown previously that LARP6 is the protein which specifically binds 5′stem-loop of collagen α1(I) and α2(I) mRNA 16. The binding of LARP6 is of high affinity to prevent translation, suggesting that one of the roles of LARP6 may be to prevent random translation of collagen mRNAs. There has been no report on the involvement of nonmuscle myosin in translation. Here we show that: 1. LARP6 associates collagen mRNAs with filaments composed of nonmuscle myosin. 2. Disruption of nonmuscle myosin filaments results in either, lack of secretion collagen α2(I) polypeptide or diminished secretion of both α1(I) and α2(I) polypeptides and their increased intracellular degradation. 3. Subcellular colocalization of collagen α1(I) and α2(I) polypeptides is diminished when myosin filaments are disrupted, 4. The function of nonmuscle myosin is dependent on the presence of 5′SL in collagen mRNAs. 5. Nonmuscle myosin associates with polysomes and there is a subset of polysomes involved in translation of collagen mRNAs that is dependent on the integrity of nonmuscle myosin filaments.
In the absence of the filaments collagen polypeptides seem to be synthesized randomly, fail to fold into the heterotrimer and are subjected to intracellular degradation. The secretion of α1(I) polypeptide was observed in lung fibroblasts treated with ML-7 and it was diminished in scleroderma fibroblasts. Since α1(I) polypeptide has the propensity to form homotrimers 19; 20, it seems likely that, in the absence of myosin filaments, lung fibroblasts can compensate and secrete the homotrimers while scleroderma fibroblasts can not. Nevertheless, in both cell types increased intracellular degradation of α1(I) polypeptide also became apparent upon inhibition of the proteosome (Fig 3F), suggesting that even the synthesis of collagen homotrimers is inefficient. Thus, nonmuscle myosin filaments are critical for coordinating translation and folding of collagen polypeptides, possibly representing the mechanism that assures preferential synthesis of the heterotrimer of type I collagen.
Collagen α1(I) and α2(I) polypeptides show strict colocalization in the lumen of the ER, however, without nonmuscle myosin filaments this colocalization can not be maintained (Fig 5A). At the same time collagen polypeptides are subjected to accelerated intracellular degradation (Fig 3F), which prevented their excessive accumulation in the cell. Our results indicate that the function of myosin in collagen synthesis may not be entirely dependent on the integrity of actin filaments. While disruption of nonmuscle myosin specifically affected collagen secretion, disruption of actin filaments in lung fibroblasts diminished secretion of fibronectin as well, and had only a minimal effect on collagen secretion in scleroderma fibroblasts (Fig 6C).
The function of myosin filaments in collagen secretion is dependent on the presence of the 5′SL in collagen mRNA. When the 5′SL was mutated in the endogenous collagen α1(I) gene, the cells secreted α1(I) polypeptides regardless of the integrity of nonmuscle myosin filaments, but when the polypeptides were encoded by mRNA with the 5′SL, their secretion was dependent on nonmuscle myosin filaments (Fig 7B). This clearly indicates that association of collagen mRNAs to the nonmuscle myosin filaments by binding of LARP6 to the 5′SL is needed for proper folding and secretion of type I collagen 11. The additional band seen in lane 4 of Fig 7B probably represents the mature collagen, processed by cleavage of the N-terminal and C-terminal domains of procollagen. LARP6 and nonmucle myosin interact through the C-terminal domain of LARP6 (Fig 2). Nonmuscle myosin does not bind directly collagen mRNAs and, since LARP6 is the only protein which binds 5′SL with high affinity 16, it is almost certain that 5′SL associates collagen mRNAs with nonmuscle myosin through LARP6.
There have been no reports that nonmuscle myosin can regulate translation of the specific mRNAs. Our evidence that nonmuscle myosin participates in translation of collagen mRNAs is indirect and based on the following: 1. Substantional amount of nonmuscle myosin copurifies with polysomes. 2. Disruption of the filaments reduces the amount of nonmuscle myosin found in the polysomal fractions (Fig 9F). 3. Dissociation of polysomes results in redistribution of collagen mRNAs from myosin filaments onto vimentin filaments (Fig 9B). 4. The strict subcellular colocalization of collagen α1(I) and α2(I) polypeptides is diminished if the filaments are disrupted (Fig 5A). 5. There is a subset of polysomes which translate collagen mRNAs in the myosin dependent manner (Fig 10). A dramatic change in polysomal profile of collagen mRNAs upon treatment with ML-7 was not found, because collagen mRNAs can be translated in the absence of 5′ SL (Fig 7B) and nonmuscle myosin filaments (Fig 3). However, this results in the synthesis of homotrimer.
LARP6 can form dimers, raising the possibility that it can organize collagen mRNAs into ribonucleoprotein particles containing multiple collagen mRNAs (manuscript in preparation). Collagen α2(I) mRNA does not bind nonmuscle myosin if the 5′SL is mutated in α1(I) mRNA (Fig 8). This indicates that there is some crosstalk between the two mRNAs through LARP6 and 5′SL and that they may bind as complex to the nonmuscle myosin filaments. Electron microscopy of the isolated polysomes containing collagen mRNAs showed that chain insertion into the ER lumen is coordinated 45. Coordinated translation of the signal peptides of collagen α1(I) and α2(I) chains may commence while these particles are still associated with the nonmuscle myosin filaments. Then, the signal recognition particle may target the nascent chains to the membrane of the ER for cotranslational insertion into the lumen and folding, which starting from the C-terminus of the chains 46. To achieve this, the cell must integrate three processes; 1. the cytoplasmic organization of collagen mRNAs, involving collagen mRNAs, LARP6 and nonmuscle myosin, 16 2. translation elongation events on the membrane of the ER, involving signal recognition particle, translocons, and TRAM2 12 and, 3. protein folding events in the lumen of the ER, involving molecular chaperones and collagen modifying enzymes 47.
In conclusion, we have shown that LARP6 and nonmuscle myosin dependent mechanism is required for synthesis of normal heterotrimeric type I collagen. We postulate that synthesis of type I collagen requires coordination of translation of collagen α1(I) and α2(I) mRNAs. This is initiated by binding of LARP6 to the 5′SL and interaction of LARP6 with nonmuscle myosin. Nonmuscle myosin filaments coordinate translation of collagen α1(I) and α2(I) polypeptides, which favors productive folding of α1(I)-α2(I)-α1(I) heterotrimer of type I collagen. This mechanism seems to be active in fibrosis, raising the possibility that targeting binding of LARP6 to collagen mRNAs or to nonmuscle myosin may lead to specific antifibrotic drugs.
Materials and Methods
Chemicals and cells
ML-7, blebbistatin, cytochalasin B, puromycin and cycloheximide were from purchased from Sigma. ML-7 was used at 40 μM, blebbistatin at 100 μM and cytochalasin B at 20 μM. Cells were incubated with the drugs for 16h before analysis. Puromycin was added at 200 μg/ml and cycloheximide at 100 μg/ml to the cells for two hours.
Human lung fibroblasts were described before 16. Scleroderma fibroblasts were purchased from the European Collection of Cell Cultures (ECACC, cell line BM0070) and were derived from skin of a scleroderma patient. Mouse embryonic fibroblasts were derived from knock-in mice in which 5′SL of collagen α1(I) gene has been mutated (Parsons et al., submitted). Several independent cell isolates were used throughout the study. All cells were cultured in DMEM supplemented with 10% FBS for up to 10 passages.
Plasmid constructs and adenovirus preparation
The HA-tagged LARP6 clone and the deletion mutants were described 16. Adenoviruses were constructed by recloning of the constructs into pAdCMVTRACK vector, followed by recombination with pAdEasy vector and amplification, as described 48. Expression of each construct was verified by western blot. The viruses made express both, the full-size test protein, as well as GFP which is encoded by an independent transcription unit 48. Expression of GFP served as a control for viral transduction.
RT-PCR analysis
Total cellular RNA was isolated using an RNA isolation kit (Sigma). RT-PCRs were done with 100 ng of total RNA or using rTth reverse transcriptase (Boca Scientific, Boca Raton, FL), [32P]dCTP was included in the PCR step to label the products, which were resolved on sequencing gels, as described 8; 31; 49; 50. When RNA from polysomal fractions was analyzed an equal aliquot of each fraction was used. The number of cycles was adjusted to be in the linear range of the reaction. The primers were used for RT-PCR were: h-collagen α1(I), 5′ primer AGAGGCGAAGGCAACAGTCG, 3′ primer, GCAGGGCCAATGTCTAGTCC, h-collagen α2(I), 5′ primer, CTTCGTGCCTAGCAACATGC, 3′ primer TCAACACCATCTCTGCCTCG.
Antibodies
Antibodies were from: anti-HA antibody and anti-GFP antibody from Sigma, anti-MYH10 antibody from University of Iowa hybridoma bank, anti-nucleolin antibody and anti-vimentin antibody from Santa Cruz Biotechnology, anti-collagen α1(I) antibody from Rockland, anti-collagen α2(I) antibody from Cell Signaling, anti-fibronectin antibody and anti-tubulin antibody from BD biosciences and anti-LARP6 antibody from Abnova.
Western blot analysis and immunostaining
Protein concentration was estimated by the Bradford assay with BSA as the standard 51. Western blots of cellular proteins were done using 50 μg of protein. For western blot of secreted proteins, equal number of cells were seeded and serum free medium was added to the cells and incubation continued for three hours. The medium was collected and an aliquot analyzed directly on western blot. Use of serum free medium for collection of secreted proteins is essential, because fetal calf serum contains substantial amounts of collagen and fibronectin 15; 31.
For immunostaining, cells were seeded onto glass coverslips and after treatment the cells were fixed with 4% paraformaldehyde for 30 min at room temperature and permeabilized with 0.5% Triton X-100 in PBS for 10 minutes. After blocking with 10% goat serum/5% BSA in PBS for 1 hr at room temperature, the cells were incubated with primary antibody overnight at 4° C, washed and visualized with Alexafluor 594 or Cy-2 conjugated secondary antibodies. The cells were mounted using VECTASHIELD mounting medium containing DAPI (Vector Laboratories) and images taken by Leica TCS SP2 AOBS laser confocal microscope equipped with a Chameleon Ti:Sapphire multiphoton laser. Optical sections were processed with LCSLite software.
Immunoprecipitation
Cell extracts were prepared in lysis buffer (10 mM KCl, 1.5 mM MgCl2, 10 mM Tris-HCl pH 7.5, 0.5% NP-40, 170 μg/ml phenylmethylsufonyl fluoride) and, after removal of nuclei by centrifugation, the clear lysate was incubated with 1 μg of antibody for 1 hour at 4 °C. 20 μl of washed protein A/G plus-agarose (Santa Cruz Biotechnology) was added, and incubation continued for an additional 3 hours. After washing the beads three times in PBS, immunoprecipitated complexes were dissolved in SDS-PAGE loading dye or RNA was extracted.
In reactions in which RNase A was added after immunoprecipitation, 0.2μg/μl of RNase A was incubated with the A/G plus-agarose beads for 15 minutes at room temperature, followed by washing two times in phosphate-buffered saline.
Tobramacyn affinity pull-downs
Tobramycin affinity pull-downs were done as described 25. A chimeric RNA bait was made by in vitro transcription from a template having collagen α1(I) 5′SL followed by tobramycin aptamer. Control bait had inverted 5′SL and tobramycin aptamer. Cytosolic extracts were prepared from human lung fibroblasts and the baits were incubated in the extract for 30 min on ice. Tobramycin was coupled to agarose beads as described 25, and the tobramycin beads were added to the extract. After 30 min incubation the beads were pelleted, washed five times in and the bound proteins were eluted by 5 mM of free tobramycin. The purified proteins were resolved on SDS-PAGE gel and visualized by Coomassie staining. The proteins specifically purified by 5′SL bait were excised and in-gel digested with trypsin. Eluted peptides were sequenced by LC-coupled ESI tandem MS (LC-MS/MS) on an LTQ XL instrument (Thermo Scientific). The corresponding proteins were identified by searching against all entries in the National Center for Biotechnology Information nonredundant database by using MASCOT (Matrix Science) search engine.
Fractionation of polysomes
Polysomes were fractionated as described 16. 0.5 ml sucrose fractions were collected and total RNA was extracted by phenol/chlorophorm and isopropanol precipitation. Equivalent amounts of each fraction were analyzed by RT-PCR. Total proteins were extracted from sucrose fractions by TCA precipitation and equivalent amounts were analyzed by western blot.
Supplementary Material
Acknowledgments
This work has been supported by grants: NIH R01DK059466 and National Scleroderma Foundation to B.S. and American Heart Association to L.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kivirikko KI. Collagen biosynthesis: a mini-review cluster. Matrix Biol. 1998;16:355–6. doi: 10.1016/s0945-053x(98)90008-7. [DOI] [PubMed] [Google Scholar]
- 2.Friedman SL. Cytokines and fibrogenesis. Semin Liver Dis. 1999;19:129–40. doi: 10.1055/s-2007-1007105. [DOI] [PubMed] [Google Scholar]
- 3.Tsukada S, Parsons CJ, Rippe RA. Mechanisms of liver fibrosis. Clin Chim Acta. 2006;364:33–60. doi: 10.1016/j.cca.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 4.Leask A, Denton CP, Abraham DJ. Insights into the molecular mechanism of chronic fibrosis: the role of connective tissue growth factor in scleroderma. J Invest Dermatol. 2004;122:1–6. doi: 10.1046/j.0022-202X.2003.22133.x. [DOI] [PubMed] [Google Scholar]
- 5.Matsusaka T, Katori H, Homma T, Ichikawa I. Mechanism of cardiac fibrosis by angiotensin. New insight revealed by genetic engineering. Trends Cardiovasc Med. 1999;9:180–4. doi: 10.1016/s1050-1738(00)00018-9. [DOI] [PubMed] [Google Scholar]
- 6.Bitterman PB, Henke CA. Fibroproliferative disorders. Chest. 1991;99:81S–84S. doi: 10.1378/chest.99.3_supplement.81s. [DOI] [PubMed] [Google Scholar]
- 7.Friedman SL, Bansal MB. Reversal of hepatic fibrosis -- fact or fantasy? Hepatology. 2006;43:S82–8. doi: 10.1002/hep.20974. [DOI] [PubMed] [Google Scholar]
- 8.Stefanovic B, Hellerbrand C, Holcik M, Briendl M, Aliebhaber S, Brenner DA. Posttranscriptional regulation of collagen alpha1(I) mRNA in hepatic stellate cells. Mol Cell Biol. 1997;17:5201–9. doi: 10.1128/mcb.17.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eckes B, Mauch C, Huppe G, Krieg T. Differential regulation of transcription and transcript stability of pro-alpha 1(I) collagen and fibronectin in activated fibroblasts derived from patients with systemic scleroderma. Biochem J. 1996;315:549–54. doi: 10.1042/bj3150549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mauch C, Kozlowska E, Eckes B, Krieg T. Altered regulation of collagen metabolism in scleroderma fibroblasts grown within three-dimensional collagen gels. Exp Dermatol. 1992;1:185–90. doi: 10.1111/j.1600-0625.1992.tb00187.x. [DOI] [PubMed] [Google Scholar]
- 11.Stefanovic B, Brenner DA. 5′ stem-loop of collagen alpha 1(I) mRNA inhibits translation in vitro but is required for triple helical collagen synthesis in vivo. J Biol Chem. 2003;278:927–33. doi: 10.1074/jbc.M209175200. [DOI] [PubMed] [Google Scholar]
- 12.Stefanovic B, Stefanovic L, Schnabl B, Bataller R, Brenner DA. TRAM2 protein interacts with endoplasmic reticulum Ca2+ pump Serca2b and is necessary for collagen type I synthesis. Mol Cell Biol. 2004;24:1758–68. doi: 10.1128/MCB.24.4.1758-1768.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindquist JN, Kauschke SG, Stefanovic B, Burchardt ER, Brenner DA. Characterization of the interaction between alphaCP(2) and the 3′-untranslated region of collagen alpha1(I) mRNA. Nucleic Acids Res. 2000;28:4306–16. doi: 10.1093/nar/28.21.4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stefanovic B, Hellerbrand C, Brenner DA. Regulatory role of the conserved stem-loop structure at the 5′ end of collagen alpha1(I) mRNA. Mol Cell Biol. 1999;19:4334–42. doi: 10.1128/mcb.19.6.4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stefanovic B, Lindquist J, Brenner DA. The 5′ stem-loop regulates expression of collagen alpha1(I) mRNA in mouse fibroblasts cultured in a three-dimensional matrix. Nucleic Acids Res. 2000;28:641–7. doi: 10.1093/nar/28.2.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai L, Fritz D, Stefanovic L, Stefanovic B. Binding of LARP6 to the conserved 5′ stem-loop regulates translation of mRNAs encoding type I collagen. J Mol Biol. 2009;395:309–26. doi: 10.1016/j.jmb.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai L, Fritz D, Stefanovic L, Stefanovic B. Coming together: liver fibrosis, collagen mRNAs and the RNA binding protein. Expert Rev Gastroenterol Hepatol. 2009;3:1–3. doi: 10.1586/17474124.3.1.1. [DOI] [PubMed] [Google Scholar]
- 18.Uitto J. Collagen polymorphism: isolation and partial characterization of alpha 1(I)-trimer molecules in normal human skin. Arch Biochem Biophys. 1979;192:371–9. doi: 10.1016/0003-9861(79)90105-x. [DOI] [PubMed] [Google Scholar]
- 19.Malfait F, Symoens S, Coucke P, Nunes L, De Almeida S, De Paepe A. Total absence of the alpha2(I) chain of collagen type I causes a rare form of Ehlers-Danlos syndrome with hypermobility and propensity to cardiac valvular problems. J Med Genet. 2006;43:e36. doi: 10.1136/jmg.2005.038224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sims TJ, Miles CA, Bailey AJ, Camacho NP. Properties of collagen in OIM mouse tissues. Connect Tissue Res. 2003;44(Suppl 1):202–5. [PubMed] [Google Scholar]
- 21.Beck K, Boswell BA, Ridgway CC, Bachinger HP. Triple helix formation of procollagen type I can occur at the rough endoplasmic reticulum membrane. J Biol Chem. 1996;271:21566–73. doi: 10.1074/jbc.271.35.21566. [DOI] [PubMed] [Google Scholar]
- 22.Lamande SR, Bateman JF. Procollagen folding and assembly: the role of endoplasmic reticulum enzymes and molecular chaperones. Semin Cell Dev Biol. 1999;10:455–64. doi: 10.1006/scdb.1999.0317. [DOI] [PubMed] [Google Scholar]
- 23.Pace JM, Wiese M, Drenguis AS, Kuznetsova N, Leikin S, Schwarze U, Chen D, Mooney SH, Unger S, Byers PH. Defective C-propeptides of the proalpha2(I) chain of type I procollagen impede molecular assembly and result in osteogenesis imperfecta. J Biol Chem. 2008;283:16061–7. doi: 10.1074/jbc.M801982200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartmuth K, Urlaub H, Vornlocher HP, Will CL, Gentzel M, Wilm M, Luhrmann R. Protein composition of human prespliceosomes isolated by a tobramycin affinity-selection method. Proc Natl Acad Sci U S A. 2002;99:16719–24. doi: 10.1073/pnas.262483899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartmuth K, Vornlocher HP, Luhrmann R. Tobramycin affinity tag purification of spliceosomes. Methods Mol Biol. 2004;257:47–64. doi: 10.1385/1-59259-750-5:047. [DOI] [PubMed] [Google Scholar]
- 26.Wolin SL, Cedervall T. The La protein. Annu Rev Biochem. 2002;71:375–403. doi: 10.1146/annurev.biochem.71.090501.150003. [DOI] [PubMed] [Google Scholar]
- 27.Fritz D, Stefanovic B. RNA-binding protein RBMS3 is expressed in activated hepatic stellate cells and liver fibrosis and increases expression of transcription factor Prx1. J Mol Biol. 2007;371:585–95. doi: 10.1016/j.jmb.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bain J, McLauchlan H, Elliott M, Cohen P. The specificities of protein kinase inhibitors: an update. Biochem J. 2003;371:199–204. doi: 10.1042/BJ20021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Connell LE, Helfman DM. Myosin light chain kinase plays a role in the regulation of epithelial cell survival. J Cell Sci. 2006;119:2269–81. doi: 10.1242/jcs.02926. [DOI] [PubMed] [Google Scholar]
- 30.Takizawa N, Ikebe R, Ikebe M, Luna EJ. Supervillin slows cell spreading by facilitating myosin II activation at the cell periphery. J Cell Sci. 2007;120:3792–803. doi: 10.1242/jcs.008219. [DOI] [PubMed] [Google Scholar]
- 31.Stefanovic B, Schnabl B, Brenner DA. Inhibition of collagen alpha 1(I) expression by the 5′ stem-loop as a molecular decoy. J Biol Chem. 2002;277:18229–37. doi: 10.1074/jbc.M108065200. [DOI] [PubMed] [Google Scholar]
- 32.Adesida AB, Grady LM, Khan WS, Hardingham TE. The matrix-forming phenotype of cultured human meniscus cells is enhanced after culture with fibroblast growth factor 2 and is further stimulated by hypoxia. Arthritis Res Ther. 2006;8:R61. doi: 10.1186/ar1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rieske P, Krynska B, Azizi SA. Human fibroblast-derived cell lines have characteristics of embryonic stem cells and cells of neuro-ectodermal origin. Differentiation. 2005;73:474–83. doi: 10.1111/j.1432-0436.2005.00050.x. [DOI] [PubMed] [Google Scholar]
- 34.Oliver JE, Thompson EM, Pope FM, Nicholls AC. Mutation in the carboxy-terminal propeptide of the Pro alpha 1(I) chain of type I collagen in a child with severe osteogenesis imperfecta (OI type III): possible implications for protein folding. Hum Mutat. 1996;7:318–26. doi: 10.1002/(SICI)1098-1004(1996)7:4<318::AID-HUMU5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 35.Allingham JS, Smith R, Rayment I. The structural basis of blebbistatin inhibition and specificity for myosin II. Nat Struct Mol Biol. 2005;12:378–9. doi: 10.1038/nsmb908. [DOI] [PubMed] [Google Scholar]
- 36.Kovacs M, Toth J, Hetenyi C, Malnasi-Csizmadia A, Sellers JR. Mechanism of blebbistatin inhibition of myosin II. J Biol Chem. 2004;279:35557–63. doi: 10.1074/jbc.M405319200. [DOI] [PubMed] [Google Scholar]
- 37.Limouze J, Straight AF, Mitchison T, Sellers JR. Specificity of blebbistatin, an inhibitor of myosin II. J Muscle Res Cell Motil. 2004;25:337–41. doi: 10.1007/s10974-004-6060-7. [DOI] [PubMed] [Google Scholar]
- 38.Dancker P, Low I. Complex influence of cytochalasin B on actin polymerization. Z Naturforsch C. 1979;34:555–7. doi: 10.1515/znc-1979-7-811. [DOI] [PubMed] [Google Scholar]
- 39.Pandya K, Kim HS, Smithies O. Fibrosis, not cell size, delineates beta-myosin heavy chain reexpression during cardiac hypertrophy and normal aging in vivo. Proc Natl Acad Sci U S A. 2006;103:16864–9. doi: 10.1073/pnas.0607700103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lofgren M, Ekblad E, Morano I, Arner A. Nonmuscle Myosin motor of smooth muscle. J Gen Physiol. 2003;121:301–10. doi: 10.1085/jgp.200208720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marini M, Bruschi M, Pecci A, Romagnoli R, Musante L, Candiano G, Ghiggeri GM, Balduini C, Seri M, Ravazzolo R. Non-muscle myosin heavy chain IIA and IIB interact and co-localize in living cells: relevance for MYH9-related disease. Int J Mol Med. 2006;17:729–36. [PubMed] [Google Scholar]
- 42.Simerly C, Nowak G, de Lanerolle P, Schatten G. Differential expression and functions of cortical myosin IIA and IIB isotypes during meiotic maturation, fertilization, and mitosis in mouse oocytes and embryos. Mol Biol Cell. 1998;9:2509–25. doi: 10.1091/mbc.9.9.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tangkijvanich P, Tam SP, Yee HF., Jr Wound-induced migration of rat hepatic stellate cells is modulated by endothelin-1 through rho-kinase-mediated alterations in the acto-myosin cytoskeleton. Hepatology. 2001;33:74–80. doi: 10.1053/jhep.2001.20677. [DOI] [PubMed] [Google Scholar]
- 44.Conrad PA, Giuliano KA, Fisher G, Collins K, Matsudaira PT, Taylor DL. Relative distribution of actin, myosin I, and myosin II during the wound healing response of fibroblasts. J Cell Biol. 1993;120:1381–91. doi: 10.1083/jcb.120.6.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Veis A, Leibovich SJ, Evans J, Kirk TZ. Supramolecular assemblies of mRNA direct the coordinated synthesis of type I procollagen chains. Proc Natl Acad Sci U S A. 1985;82:3693–7. doi: 10.1073/pnas.82.11.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khoshnoodi J, Cartailler JP, Alvares K, Veis A, Hudson BG. Molecular recognition in the assembly of collagens: terminal noncollagenous domains are key recognition modules in the formation of triple helical protomers. J Biol Chem. 2006;281:38117–21. doi: 10.1074/jbc.R600025200. [DOI] [PubMed] [Google Scholar]
- 47.Gura T, Hu G, Veis A. Posttranscriptional aspects of the biosynthesis of type 1 collagen pro- alpha chains: the effects of posttranslational modifications on synthesis pauses during elongation of the pro alpha 1 (I) chain. J Cell Biochem. 1996;61:194–215. doi: 10.1002/(sici)1097-4644(19960501)61:2<194::aid-jcb4>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 48.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95:2509–14. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stefanovic L, Brenner DA, Stefanovic B. Direct hepatotoxic effect of KC chemokine in the liver without infiltration of neutrophils. Exp Biol Med (Maywood) 2005;230:573–86. doi: 10.1177/153537020523000809. [DOI] [PubMed] [Google Scholar]
- 50.Stefanovic L, Stefanovic B. Mechanism of Direct Hepatotoxic Effect of KC Chemokine: Sequential Activation of Gene Expression and Progression from Inflammation to Necrosis. J Interf Cytokine Res. 2006;26:760–770. doi: 10.1089/jir.2006.26.760. [DOI] [PubMed] [Google Scholar]
- 51.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.