Abstract
Background
Klinefelter syndrome (KS) with the karyotype 47,XXY is one of the commonest types of congenital chromosomal disorder in males, with an incidence of 0.1% to 0.2% of newborn male infants. It causes hypogonadism and infertility. Until now, however, only about one-quarter of all persons with KS received the diagnosis during their lifetimes.
Methods
Selective review of the literature.
Results
KS is caused by aneuploidy of the sex chromosomes. Small, firm testes, the manifestations of androgen deficiency (sparse development of male-pattern body hair, greater than average height, lack of libido, erectile dysfunction) and, in more than 90% of affected men, azoospermia are its main features in adults. Affected boys may have verbalization difficulties and problems with learning and socialization. KS is often accompanied by other disturbances such as gynecomastia, varicose veins, thrombosis, osteoporosis, the metabolic syndrome, type 2 diabetes, and epilepsy. The most important therapeutic measure is testosterone supplementation, which should be initiated if the testosterone concentration drops below 12 nmol/L and should be given as directed in the guidelines for the treatment of hypogonadism. This recommendation is made even though there have not been any randomized controlled trials documenting the efficacy of testosterone therapy in adolescents or young adults. In some cases, viable sperm can be obtained from individual testicular tubules by biopsy, so that these patients are able to become fathers.
Conclusion
The diagnosis of KS would be less frequently missed if doctors were more aware of, and attentive to, its key manifestations, particularly the small, firm testes, erectile dysfunction, and the comorbidities mentioned above. If the diagnosis were made more often, patients would more often be able to receive early treatment, which would improve their quality of life.
Klinefelter syndrome 47,XXY was first described 70 years ago (1). With an incidence of 0.1% to 0.2% of male neonates (i.e. 1 to 2 per 1000), it is one of the commonest congenital chromosome disorders resulting in hypogonadism and genetically-determined infertility (2, 3).
Klinefelter syndrome is associated with a significantly higher morbidity rate compared to the male population as a whole. The main associated disorders are varicose veins, thrombosis, embolism, type 2 diabetes, bone fractures, epilepsy, and other neurological and mental disorders (4– 6) (Table 1). This leads to a life expectancy 11.5 years below that of the male population as a whole (6).
Table 1. Comorbidities in Klinefelter syndrome: prevalence and mortality.
| Incidence | Reference | Mortality | Reference | |
| % | (SMR*) | |||
| Gynecomastia | 38 | (2) | – | – |
| Breast cancer | 0.3 | (e7) | 29 | (e7) |
| Thrombosis | 4.7 | (5) | 8 | (4) |
| Pulmonary embolism | 2.3 | (5) | 6 | (4) |
| Metabolic syndrome | 44 | (14) | – | – |
| Type 2 diabetes | 10 | (14) | 6 | (4) |
| Osteopenia | 40 | (18) | – | – |
| Osteoporosis | 10 | (18) | – | – |
| Hip fracture | ? | – | 39 | (4) |
| Maldescended testes | 27 | (2) | – | – |
| Mediastinal tumors | 0.4 | (5) | – | – |
| Epilepsy | 5.5 | (5) | 7 | (4) |
| Mental retardation | 4.2 | (5) | – | – |
| Delayed verbal ‧development | 40 | (19) | – | – |
| Language disorder | 70 to 80 | (21) | – | – |
| Legasthenia | 50 to 70 | (21) | – | – |
| Learning difficulties | 75 | (19) | – | – |
*SMR: standardized mortality rate, i.e. actual vs. predicted deaths (4)
As a result of this increased morbidity, Klinefelter syndrome patients require doctor and hospital treatment disproportionately often. However, a survey completed by 290 practising primary care physicians, internal medicine specialists, and urologists showed that two-thirds of primary care physicians and internal medicine specialists had had not knowingly seen any cases of Klinefelter syndrome in recent years, although their theoretical knowledge of it was good; urologists fared a little better regarding diagnosis of Klinefelter syndrome (7). On the basis of patient registries in Denmark, it is suspected that only 25% of all Klinefelter syndrome patients are diagnosed during their lifetimes (8). Nevertheless, suspected Klinefelter syndrome is easy to diagnose if physicians know the syndrome and thorough clinical examination is performed. Because knowledge of Klinefelter syndrome can only be derived from diagnosed cases, it is not known whether other cases remain undetected because they have a different range of symptoms. Furthermore, the known symptoms are not exclusive: Only one-quarter of patients in whom Klinefelter syndrome was suspected following clinical examination in a specialized facility actually showed a corresponding karyotype (9).
This article aims to attract greater medical attention to this important syndrome, in order to provide more patients with appropriate treatment. It is based on a selective search of the literature using a regular PubMed search over a 40-year period of clinical and scientific consideration of Klinefelter syndrome.
Pathophysiology
The disease pattern is caused by congenital aneuploidy of the sex chromosomes. 80% of patients have the 47,XXY karyotype. The remaining 20% have either mosaic 47,XXY/46,XY (i.e. different karyotypes in different cells), supernumerary X chromosome aneuploidy (48,XXY; 49,XXXXY), one or several additional Y chromosomes (e.g. 48,XXYY), or structurally abnormal additional X chromosomes (10, 11). These numerical chromosome abnormalities are the result of nondisjunction in maternal oogenesis in approximately two-thirds of cases, and in paternal spermatogenesis in the remaining third.
Although primordial germ cells (stem cells) are present in the testes of patients with Klinefelter syndrome, they degenerate unusually quickly, with the result that by puberty there are few or no remaining germ cells and very few remaining seminiferous tubules with complete spermatogenesis (12). The hyperplastic Leydig cells are unable to produce sufficient testosterone. This leads to testosterone deficiency, which can be modified by androgen receptor polymorphism and the severity of which varies between individual patients (13).
Ultimately almost all organ systems are associated with an elevated risk of morbidity and mortality in Klinefelter syndrome (4, 5) (Table 1). Gynecomastia, which is common in Klinefelter syndrome (occurring in 38% of our patients), is accompanied by a slightly higher incidence of breast cancer than in men with normal karyotype, who in total account for barely 1% of all breast cancers. In addition to increased frequency of breast cancer, mediastinal nonseminomatous germ cell tumors are also more common, most commonly between the ages of 15 and 30 years. Osteoporosis results in an increased incidence of bone fractures; femoral fractures are associated with a high mortality rate. Patients with Klinefelter syndrome suffer from vascular diseases, particularly varicose veins and thromboembolisms, most commonly pulmonary embolisms. Central obesity with reduced glucose tolerance is often observed and can lead to metabolic syndrome and type 2 diabetes (14) (Figure 1). Epilepsy and other neurological and mental disorders occur significantly more frequently in Klinefelter syndrome patients.
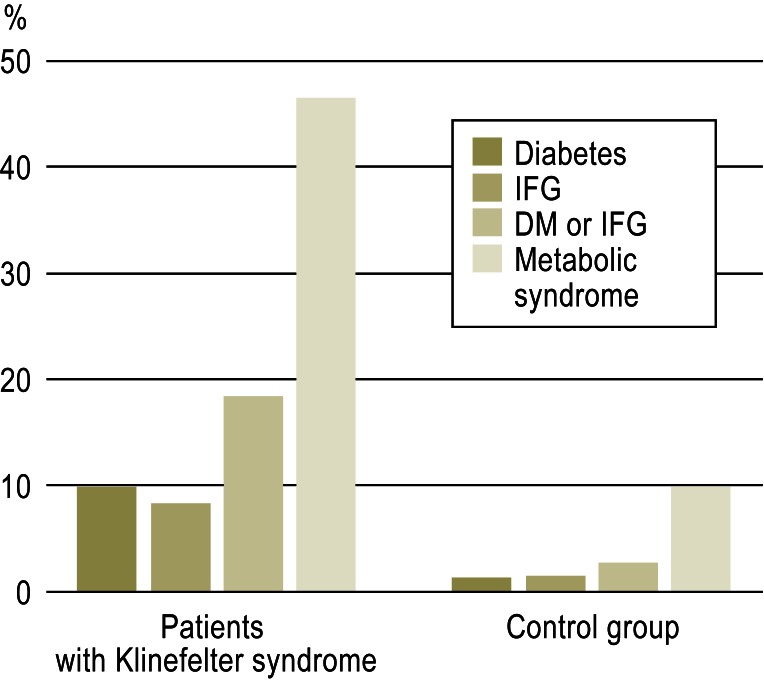
Figure 1.
Prevalence of type 2 diabetes mellitus (DM), impaired fasting glucose (IFG), or metabolic syndrome in 70 patients with Klinefelter syndrome and 70 age-matched controls (from [14]). Reproduced with the kind permission of John Wiley & Sons
Whether testosterone deficiency alone is responsible for this increased morbidity and mortality is doubtful, as a historical analysis of singers castrated before puberty, i.e. men with extremely low testosterone levels, showed the same life expectancy as for eugonadal singers (15). One risk factor may be the usually reduced arterial diameter of patients with Klinefelter syndrome, which leads to reduced perfusion of organ systems (16).
Disease pattern
Leading symptoms are signs of testosterone deficiency; very small, firm testes; and infertility (Box). Klinefelter syndrome patients can be reliably distinguished from eugonadal men by their small testes, which is always grounds for suspecting Klinefelter syndrome (Figure 2). They often display symmetrical gynecomastia with significantly palpable glands, and tall stature with long legs and a short trunk. However, unlike other forms of prepubertal-onset hypogonadism such as Kallman syndrome, in Klinefelter syndrome arm span does not exceed height. The severity of testosterone deficiency can vary greatly; thus the clinical phenotype can range from almost eugonadism to severe hypogonadism. While most patients pass through puberty with only mild symptoms and achieve normal penis size, approximately 70% of patients complain of falling libido and potency from the age of 25 years (17). Testosterone deficiency can also cause mild anemia. Beard growth and other secondary sexual hair growth are often sparse. Doctor’s certificates are often issued for lumbar pain and other musculoskeletal complaints resulting from the onset of osteopenia and osteoporosis (3, 18).
Box. Symptoms and parameters leading to diagnosis of Klinefelter syndrome*.
-
Clinical symptoms
Tall stature (with long legs)
Gynecomastia
Small, firm testes
-
Signs of hypogonadism
Sparse beard growth
Loss of libido
Erectile dysfunction
Osteoporosis
Mild anemia
Infertility
-
Psychosocial problems
Limited verbal development
Attention deficit
Learning difficulties
Legasthenia
Social maladjustment
Professional development below family level
-
Laboratory parameters
Azoospermia >90%
Oligoasthenoteratozoospermia <10%
Testosterone ↓
LH ↑
FSH ↑ ↑ ↑
Barr bodies 82% positive
47, XXY karyotype or mosaic 100%
RBC count ↓
Bone density ↓
*Modified according to (2, 3, 9)
LH: luteinizing hormone; FSH: follicle-stimulating hormone, RBC: red blood cell
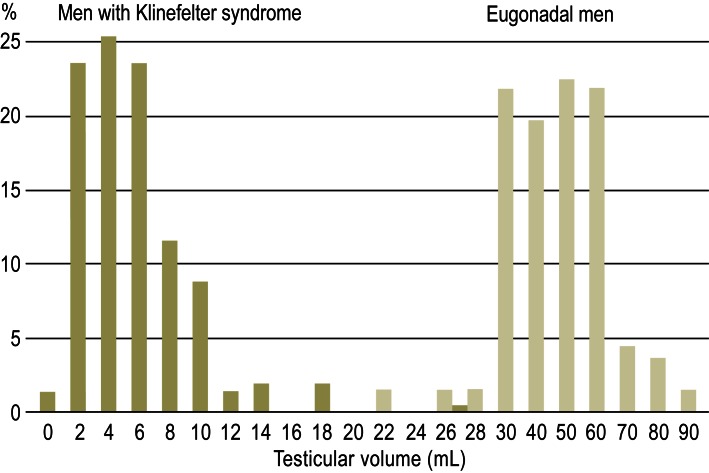
Figure 2.
Distribution of bilateral testicular volumes (both sides combined) in 160 patients with Klinefelter syndrome and 137 eugonadal men (modified according to [2])
There are no symptoms that reliably indicate Klinefelter syndrome in prepubertal patients. Maldescended testes is present in one-quarter of cases, significantly more frequently than in boys in the population as a whole. Intellectual capacity may develop completely normally, but mild impairment of cognitive development is found in 4.2% of cases. Boys with Klinefelter syndrome may suffer from verbal and cognitive deficiencies (19) (Table 1) and often develop learning difficulties (75%). There may be attention deficit, and development of social skills is problematic. Klinefelter syndrome patients often achieve lower performance and professional status than their relatives (19– 21). Evidence of learning disabilities has also been found in mice with Klinefelter syndrome, which means the phenomenon can be researched experimentally (22).
Many Klinefelter syndrome patients consult their doctors—and obtain a diagnosis—as a result of difficulties in becoming fathers. Azoospermia is present in more than 90% of cases, while in less than 10% of cases some or all sperm have reduced motility and morphology (2). Histological analysis of the testes reveals hyalinizing fibrosis of the seminiferous tubules, aspermatogenesis or focal spermatogenesis only, and hyperplasia of the Leydig cells. Spontaneous fatherhood in patients with Klinefelter syndrome is extremely rare.
Diagnosis
Suspected Klinefelter syndrome can be diagnosed as a result of thorough physical examination (paying attention to signs of testosterone deficiency and, in particular, testicle size) (Figure 2) or of chance observation during treatment of concomitant diseases (Table 1). Testicular volume is measured by ultrasound examination, which usually also shows testicular hypoechogenicity. Smear testing can be used to look for Barr bodies in the buccal epithelium, corresponding to the inactive supernumerary X chromosome. Although no longer frequently used, this low-cost screening procedure has high specificity (95%) and adequate sensitivity (82%) (9). Diagnosis is finally confirmed cytogenetically, by karyotyping (Box).
Laboratory tests can reveal mild anemia as a result of testosterone deficiency. A marked increase in serum FSH levels is particularly characteristic of Klinefelter syndrome. Testosterone levels, which should always be determined in the morning due to fluctuations during the course of a day, may be either within normal range or abnormally low. For borderline cases in particular, it is recommended that sex hormone-binding globulin (SHBG) levels be determined and used with the total testosterone level to calculate free testosterone, which is a more sensitive indicator than total testosterone. Elevated LH values correlate with low testosterone but often remain elevated even after the testosterone level has risen to within normal range (Figure 3).
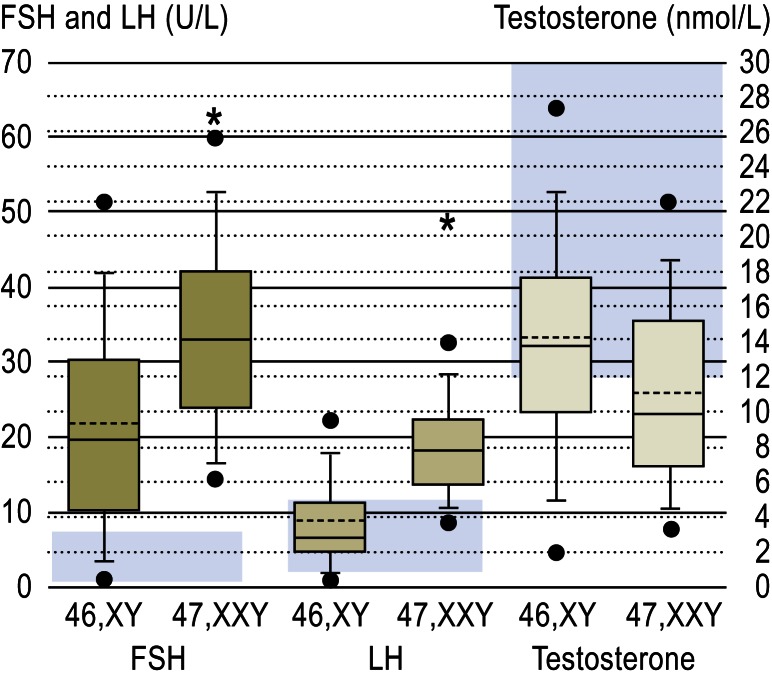
Figure 3.
FSH, LH, and testosterone levels in 228 patients with Klinefelter syndrome and 224 infertile men with normal karyotypes, with normal values shown in the background (blue fields). Boxes show interquartile range (IQR) with 5th and 95th percentiles. Continuous = median, broken lines = mean, solid circles = values not between 5th and 95th percentiles. Stars indicate significant differences (from [3])
LH: luteinizing hormone; FSH: follicle-stimulating hormone
Bone density measurement by DXA (18), or inexpensively by phalanx osteosonography, is indicated in order to determine the extent and progression of any osteoporosis (3).
Semen analysis must be performed according to WHO guidelines. Azoospermia can only be reliably diagnosed following thorough examination of the sediment of centrifuged semen (23).
Treatment
In adults with Klinefelter syndrome, testosterone substitution should be used liberally, as this is generally thought to increase quality of life and prevent long-term effects. In hypogonadal men in general, rather than Klinefelter syndrome patients specifically, testosterone substitution has been shown to reduce the mortality rate from 5.4 to 3.7 per 100 patient years (24). In particular, it can achieve increased overall masculinity with corresponding muscle strength and bone density and thicker secondary hair growth, including beard growth, and normal red blood cell count. Loss of libido and erectile dysfunction, as well as depressive moods and lack of drive, are reduced or eliminated. Because testosterone reduces insulin resistance and leads to an increase in muscle mass over fat, the tendency towards metabolic syndrome is reduced. Although there are no specific guidelines for Klinefelter syndrome, guidelines on the treatment of testosterone deficiency in general can also be used for Klinefelter syndrome patients (25). However, to date there have been no randomized controlled trials on testosterone therapy in Klinefelter syndrome.
Testosterone deficiency should be treated only with natural testosterone in preparations that induce physiological serum levels. Of the wide variety of pharmaceutical forms available (3, 26), the commonest are intramuscular depot injections and transdermal gels. Complete substitution, which requires only four injections per year and achieves normal serum levels, involves intramuscular oil-based depot injections of 1000 mg testosterone undecanoate; however, this requires initial saturation, so the second injection is given six weeks after the first, and subsequent injections at 12-week intervals (27). This form of depot substitution has replaced the previously standard injections of 250 mg testosterone enanthate at two- to three-week intervals; the latter lead to major fluctuations in patients’ general condition as a result of fluctuating testosterone kinetics (26). There are various testosterone gels on the market, varying in testosterone concentration and composition. The higher a gel’s testosterone concentration, the smaller the quantity that must be applied once daily to the abdomen or upper arm to achieve normal serum levels (28, 29).
Drug selection should take account of the patient’s preferences. In patients in whom endogenous production remains relatively high, however, low-dose, short-acting drugs should be preferred, possibly administered at prolonged intervals.
Essentially, testosterone therapy should be started when clinical symptoms so require and serum levels fall below the normal values of 12 nmol/L total testosterone and/or 250 pmol/L free testosterone (30, 31). This occurs at very different ages in individual Klinefelter syndrome patients and thanks to regular annual checkups should not go unnoticed by physicians. In most patients it occurs between the ages of 20 and 30 years.
More recent research indicates that there are threshold values for various symptoms and groups of symptoms of testosterone deficiency; for example, loss of libido and drive occurs when levels drop below 15 nmol/L, while hot flashes and erectile dysfunction do not occur until levels fall below 8 nmol/L. Although these figures were obtained in patients with late-onset hypogonadism, they can be applied to other forms of hypogonadism (30, 32). In the future, pharmacokinetic issues that take account of androgen receptor polymorphism will also play a role (27).
Testosterone therapy must be accompanied by regular checkups that include inquiries into wellbeing, strength, and sexual function and examination of general condition, red blood cell count (excessively high doses lead to a risk of polycythemia), bone density, prostate, and PSA (3, 33) (Table 2). It must be considered lifelong substitution. In Klinefelter syndrome patients over the age of 50 years, guidelines on the treatment of late-onset hypogonadism should be followed (34); measurement of PSA levels and digital prostate examination are as important as for other men.
Table 2.
| Clinical parameters | Laboratory parameters |
| General wellbeing | Serum testosterone levels |
| Drive and mood | (Serum LH and FSH) |
| Libido and sexual activity (erections, ejaculations, intercourse) | RBC/hemoglobin/Hc/lipids/fibrinolysis |
| Body weight/BMI | Ejaculate volume |
| Bodily proportions | Prostate size and consistency |
| Hair growth | PSA (over 50 years) |
| Sebum production | Bone density |
| Voice |
Testosterone-dependent parameters for monitoring of substitution therapy*
*Modified according to (33)LH: luteinizing hormone; FSH: follicle-stimulating hormone; PSA: prostate-specific antigen; Hc: hematocrit
It is difficult to decide whether testosterone substitution should begin at puberty, as there are only a small number of studies on this question, and no controlled trials. Some researchers’ experience supports substitution as early as possible, as this achieves better physical, psychological, and scholastic development and social integration (35, 36). In individual cases it has been observed that criminal tendencies disappear in adolescent patients with Klinefelter syndrome receiving testosterone substitution (case studies in [35, 37]). Remaining endogenous production should not be suppressed, so intermittent treatment with short-acting testosterone preparations should be considered. Depot substitution would also suppress any remaining spermatogenesis, so later fertility must be taken into account even at this early age (38, 39). The decision must always be made on an individual basis until evidence-based recommendations can be given. Psychological support and speech therapy are also advisable.
Testosterone substitution has almost no effect on gynecomastia, and antiestrogens and aromatase inhibitors are only beneficial in isolated cases. However, no controlled trials are available. If gynecomastia is cosmetically problematic, mastectomy should be performed by surgeons with experience in this area.
Treatment for infertility has changed drastically in the last 15 years, although spontaneous pregnancy in partners of Klinefelter syndrome patients remains extremely rare. Also, to date only a few pregnancies have been reported from sperm taken from the semen of Klinefelter syndrome patients via ICSI (intracytoplasmic sperm injection). However, pregnancy is achieved more frequently using TESE (testicular sperm extraction) followed by ICSI treatment (2, 40). Individual seminiferous tubules may contain sperm that can be obtained by biopsy and introduced directly into the egg cell (ICSI). This achieves a higher success rate if microTESE, which in recent years has become established, is used in preference to conventional TESE, as shown by comparative reviews from 2004 (2) and 2011 (40). Pregnancy and live birth were achieved in approximately half the Klinefelter syndrome patients in whom TESE was attempted and whose data have been published to date (40).
Some groups have reported positive outcomes following hCG and/or antiestrogen pretreatment (38, e1). However, these trials were not controlled, and it remains questionable whether such pretreatment really has an effect, as controlled, double-blind trials in other types of male fertility disorders have shown no positive effects (e2). There is also evidence that sperm are more likely to be found in the testes of younger Klinefelter syndrome patients than those of older patients (39, e3, e4). Cryopreservation of testicular biopsy material of pubertal boys with Klinefelter syndrome is therefore being considered (e5).
In any event, if patients with Klinefelter syndrome wish to have children the initial step is to interrupt testosterone substitution, as testosterone suppresses residual spermatogenesis. In patients already receiving testosterone substitution, it must be interrupted for at least three to six months to allow remaining spermatogenesis to recover. The few spermatogonia produced in the testes of patients with Klinefelter syndrome seem to have a 46,XY chromosome set, unlike somatic cells (11, e6). This explains why most children of fathers with Klinefelter syndrome conceived in this way to date have normal karyotypes (2, 11, 40). However, it should be explained to parents that embryos and children have an increased risk of aneuploidy, not only of sex chromosomes but also of chromosomes 18 and 21. Invasive genetic prenatal testing is therefore recommended.
Overall, treatment of infertility in patients with Klinefelter syndrome remains only partly successful, and counseling provided for couples should not raise their hopes too high.
Key Messages.
Klinefelter syndrome is one of the commonest forms of chromosome aneuploidy and the commonest form of hypogonadism and genetically-determined infertility.
Although diagnosis is easy (small testicular volume), Klinefelter syndrome still often remains overlooked or untreated.
Children and adolescents with Klinefelter syndrome can suffer from impaired verbal development, learning difficulties, and problems in the development of social skills.
Klinefelter syndrome is associated with a high comorbidity rate and increased mortality.
Testosterone substitution and prevention of concomitant disorders are the most important elements of treatment. Nowadays some patients can become fathers via TESE/ICSI.
Acknowledgments
Translated from the original German by Caroline Devitt, M.A.
Footnotes
Conflict of interest statement
Prof. Nieschlag has received fees for a Springer textbook on andrology and a textbook on testosterone from Cambridge University Press. He has also received reimbursement of conference participation fees and travel and accommodation expenses from the Association of German Internal Medicine Specialists (BDI, Berufsverband der Deutschen Internisten).
References
- 1.Klinefelter HF, Reifenstein EC, Albright F. Syndrome characterized by gynecomastia, aspermatogenesis without A-Leydigism, and increased excretion of follicle-stimulating hormone. J Clin Endocrinol. 1942;II:615–627. [Google Scholar]
- 2.Lanfranco F, Kamischke A, Zitzmann M, Nieschlag E. Klinefelter’s syndrome. Lancet. 2004;364:273–283. doi: 10.1016/S0140-6736(04)16678-6. [DOI] [PubMed] [Google Scholar]
- 3.Nieschlag E, Behre HM, Wieacker P, Meschede D, Kamischke A, Kliesch S. Störungen im Bereich der Testes. Andrologie. In: Nieschlag E, Behre HM, Nieschlag S, editors. Grundlagen und Klinik der reproduktiven Gesundheit des Mannes. 3rd edition. Heidelberg: Springer; 2009. pp. 199–244. [Google Scholar]
- 4.Swerdlow AJ, Higgins CD, Schoemaker MJ, Wright AF, Jacobs PA. Mortality in patients with Klinefelter syndrome in Britain: a cohort study. J Clin Endocrinol Metab. 2005;90:6516–6522. doi: 10.1210/jc.2005-1077. [DOI] [PubMed] [Google Scholar]
- 5.Bojesen A, Juul S, Birkebaek NH, Gravholt CH. Morbidity in Klinefelter syndrome: a Danish register study based on hospital discharge diagnoses. J Clin Endocrinol Metab. 2006;91:1254–1260. doi: 10.1210/jc.2005-0697. [DOI] [PubMed] [Google Scholar]
- 6.Bojesen A, Stochholm K, Juul S, Gravholt CH. Socioeconomic trajectories affect mortality in Klinefelter syndrome. J Clin Endocrinol Metab. 2011;96:2098–2104. doi: 10.1210/jc.2011-0367. [DOI] [PubMed] [Google Scholar]
- 7.Bade K. Das Klinefelter-Syndrom: Berücksichtigung in der ärztlichen Praxis und Literatur. Inauguraldissertation, Medizinische Fakultät der Universität Münster. 2006 [Google Scholar]
- 8.Groth KA, Skakkebaek A, Host C, Gravholt CH, Bojesen A. Klinefelter-Syndrome—A clinical update. J Clin Endocrinol Metab. 2013;98:20–30. doi: 10.1210/jc.2012-2382. [DOI] [PubMed] [Google Scholar]
- 9.Kamischke A, Baumgardt A, Horst J, Nieschlag E. Clinical and diagnostic features of patients with suspected Klinefelter syndrome. J Androl. 2003;24:41–48. [PubMed] [Google Scholar]
- 10.Tüttelmann F, Gromoll J. Novel genetic aspects of Klinefelter’s syndrome. Mol Hum Reprod. 2010;16:386–395. doi: 10.1093/molehr/gaq019. [DOI] [PubMed] [Google Scholar]
- 11.Maiburg M, Repping S, Giltay J. The genetic origin of Klinefelter syndrome and its effect on spermatogenesis. 2012;98:253–260. doi: 10.1016/j.fertnstert.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 12.Wikström AM, Hoei-Hansen CE, Dunkel L, Rajpert-De Meyts E. Immunoexpression of androgen receptor and nine markers of maturation in the testes of adolescent boys with Klinefelter syndrome: evidence for degeneration of germ cells at the onset of meiosis. J Clin Endocrinol Metab. 2007;92:714–719. doi: 10.1210/jc.2006-1892. [DOI] [PubMed] [Google Scholar]
- 13.Zitzmann M, Depenbusch M, Gromoll J, Nieschlag E. X-chromosome inactivation patterns and androgen receptor functionality influence phenotype and social characteristics as well as pharmacogenetics of testosterone therapy in Klinefelter patients. J Clin Endocrinol Metab. 2004;89:6208–6217. doi: 10.1210/jc.2004-1424. [DOI] [PubMed] [Google Scholar]
- 14.Gravholt CH, Jensen AS, Høst C, Bojesen A. Body composition, metabolic syndrome and type 2 diabetes in Klinefelter syndrome. Acta Paediatr. 2011;100:871–877. doi: 10.1111/j.1651-2227.2011.02233.x. [DOI] [PubMed] [Google Scholar]
- 15.Nieschlag E, Nieschlag S, Behre HM. Lifespan and testosterone. Nature. 1993;366 doi: 10.1038/366215a0. [DOI] [PubMed] [Google Scholar]
- 16.Foresta C, Caretta N, Palego P, et al. Reduced artery diameters in Klinefelter syndrome. Int J Androl. 2012;35:720–725. doi: 10.1111/j.1365-2605.2012.01269.x. [DOI] [PubMed] [Google Scholar]
- 17.Corona G, Petrone L, Paggi F, et al. Sexual dysfunction in subjects with Klinefelter’s syndrome. Int J Androl. 2010;33:574–580. doi: 10.1111/j.1365-2605.2009.00986.x. [DOI] [PubMed] [Google Scholar]
- 18.Bojesen A, Birkebæk N, Kristensen K, et al. Bone mineral density in Klinefelter syndrome is reduced and primarily determined by muscle strength and resorptive markers, but not directly by testosterone. Osteoporos Int. 2011;22:1441–1450. doi: 10.1007/s00198-010-1354-7. [DOI] [PubMed] [Google Scholar]
- 19.Ratcliffe S. Long-term outcome in children of sex chromosome abnormalities. Arch Dis Child. 1999;80:192–195. doi: 10.1136/adc.80.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ross JL, Roeltgen DP, Stefanatos G, et al. Cognitive and motor development during childhood in boys with Klinefelter syndrome. Am J Med Genet A. 2008;146A:708–719. doi: 10.1002/ajmg.a.32232. [DOI] [PubMed] [Google Scholar]
- 21.Verri A, Cremante A, Clerici F, Destefani V, Radicioni A. Klinefelter’s syndrome and psychoneurologic function. Mol Hum Reprod. 2010;16:425–433. doi: 10.1093/molehr/gaq018. [DOI] [PubMed] [Google Scholar]
- 22.Lewejohann L, Damm OS, Luetjens CM, et al. Impaired recognition memory in male mice with a supernumerary X chromosome. Physiol Behav. 2009;96:23–29. doi: 10.1016/j.physbeh.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Nieschlag E, Schlatt S, Behre HM, et al. WHO. Deutsche Übersetzung von. 5th edition. Heidelberg: Springer; 2012. Laborhandbuch zur Untersuchung des menschlichen Ejakulates und der Spermien-Zervikalschleim-Interaktion. [Google Scholar]
- 24.Shores MM, Smith NL, Forsberg CW, Anawalt BD, Matsumoto AM. Testosterone treatment and mortality in men with low testosterone levels. J Clin Endocrinol Metab. 2012;97:2050–2058. doi: 10.1210/jc.2011-2591. [DOI] [PubMed] [Google Scholar]
- 25.Swerdloff RS, Wang CCL. Review of guidelines on diagnosis and treatment of testosterone deficiency. In: Nieschlag E, Behre HM, editors. Testosterone: action, deficiency, substitution. 4th edition. Cambridge: Cambridge University Press; 2012. pp. 408–420. [Google Scholar]
- 26.Behre HM, Nieschlag E. Testosterone preparations for clinical use in males. In: Nieschlag E, Behre HM, editors. Testosterone: action, deficiency, substitution. 4th edition. Cambridge: Cambridge University Press; 2012. pp. 309–335. [Google Scholar]
- 27.Zitzmann M, Nieschlag E. Androgen receptor gene CAG repeat length and body mass index modulate the safety of long-term intramuscular testosterone undecanoate therapy in hypogonadal men. J Clin Endocrinol Metab. 2007;92:3844–3853. doi: 10.1210/jc.2007-0620. [DOI] [PubMed] [Google Scholar]
- 28.Kühnert B, Byrne M, Simoni M, et al. Testosterone substitution with a new transdermal, hydroalcoholic gel applied to scrotal or non-scrotal skin: a multicentre trial. Eur J Endocrinol. 2005;153:317–326. doi: 10.1530/eje.1.01964. [DOI] [PubMed] [Google Scholar]
- 29.Behre HM, Tammela TL, Arver S, et al. A randomized, double-blind, placebo-controlled trial of testosterone gel on body composition and health-related quality-of-life in men with hypogonadal to low-normal levels of serum testosterone and symptoms of androgen deficiency over 6 months with 12 months open-label follow-up. Aging Male. 2012;15:198–207. doi: 10.3109/13685538.2012.699562. [DOI] [PubMed] [Google Scholar]
- 30.Zitzmann M, Faber S, Nieschlag E. Association of specific symptoms and metabolic risks with serum testosterone in older men. J Clin Endocrinol Metab. 2006;91:4335–4343. doi: 10.1210/jc.2006-0401. [DOI] [PubMed] [Google Scholar]
- 31.Bhasin S, Pencina M, Jasuja GK, et al. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart Study and applied to three geographically distinct cohorts. J Clin Endocrinol Metab. 2011;96:2430–2439. doi: 10.1210/jc.2010-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelleher S, Conway AJ, Handelsman DJ. Blood testosterone threshold for androgen deficiency symptoms. J Clin Endocrinol Metab. 2004;89:3813–3817. doi: 10.1210/jc.2004-0143. [DOI] [PubMed] [Google Scholar]
- 33.Nieschlag E, Behre HM. Therapie mit Testosteron: Andrologie. In: Nieschlag E, Behre HM, Nieschlag S, editors. Grundlagen und Klinik der reproduktiven Gesundheit des Mannes. 3nd edition. Heidelberg: Springer; 2009. pp. 445–464. [Google Scholar]
- 34.Wang C, Nieschlag E, Swerdloff R, et al. Untersuchung, Behandlung und Überwachung des Altershypogonadismus (Late-onset hypogonadism) des Mannes: ISA, ISSAM, EAU, EAA und ASA Empfehlungen. J Repromed Endokrinol. 2009;7:60–66. [Google Scholar]
- 35.Simm PJ, Zacharin MR. The psychosocial impact of Klinefelter syndrome—a 10 year review. J Ped Endrocrinol Metab. 2006;19:499–505. [PubMed] [Google Scholar]
- 36.Rogol AD, Tartaglia N. Considerations for androgen therapy in children and adolescents with Klinefelter syndrome (47, XXY) Pediatr Endocrinol Rev. 2010;8(Suppl 1):145–150. [PubMed] [Google Scholar]
- 37.Braunisch S. Deliktprävention durch ambulante kriminaltherapeutische Behandlung des Forensik-Ambulatoriums des Forensisch-Psychiatrischen Dienstes der Universität Bern. Schweizerische Zeitschrift für Kriminologie. 2011;2:4–8. [Google Scholar]
- 38.Mehta A, Paduch DA. Klinefelter syndrome: an argument for early aggressive hormonal and fertility management. Fertil Steril. 2012;98:274–283. doi: 10.1016/j.fertnstert.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Van Saen D, Gies I, De Schepper J, Tournaye H, Goossens E. Can pubertal boys with Klinefelter syndrome benefit from spermatogonial stem cell banking? Hum Reprod. 2012;27:323–330. doi: 10.1093/humrep/der425. [DOI] [PubMed] [Google Scholar]
- 40.Kliesch S, Zitzmann M, Behre HM. Fertilität beim Klinefelter-Syndrom (47,XXY) Urologe A. 2011;50:26–32. doi: 10.1007/s00120-010-2443-0. [DOI] [PubMed] [Google Scholar]
- e1.Schiff JD, Palermo GD, Veeck LL, Goldstein M, Rosenwaks Z, Schlegel PN. Success of testicular sperm extraction [corrected] and intracytoplasmic sperm injection in men with Klinefelter syndrome. J Clin Endocrinol Metab. 2005;90:6263–6267. doi: 10.1210/jc.2004-2322. Erratum in: J Clin Endocrinol Metab 2006; 91: 4027. [DOI] [PubMed] [Google Scholar]
- e2.Kamischke A, Niesclag E. Analysis of medical treatment of male infertility. Hum Reprod (Suppl) 1999;14:1–23. doi: 10.1093/humrep/14.suppl_1.1. [DOI] [PubMed] [Google Scholar]
- e3.Okada H, Goda K, Yamamoto Y, et al. Age as a limiting factor for successful sperm retrieval in patients with nonmosaic syndrome. Fertil Steril. 2005;84:1662–1664. doi: 10.1016/j.fertnstert.2005.05.053. [DOI] [PubMed] [Google Scholar]
- e4.Bakircioglu ME, Erden HF, Kaplancan T, Ciray N, Bener F, Bahceci M. Aging may adversely affect testicular sperm recovery in patients with Klinefelter syndrome. Urology. 2006;68:1082–1086. doi: 10.1016/j.urology.2006.05.028. [DOI] [PubMed] [Google Scholar]
- e5.Gies I, De Schepper J, Goossens E, van Saen D, Pennings G, Tournaye H. Spermatogonial stem cell preservation in boys with Klinefelter syndrome: to bank or not to bank, that’s the question. Fertil Steril. 98 doi: 10.1016/j.fertnstert.2012.04.023. [DOI] [PubMed] [Google Scholar]
- e6.Sciurano RB, Luna Hisano CV, Rahn MI, et al. Focal spermatogenesis originates in euploid germ cells in classical Klinefelter patients. Hum Reprod. 2009;24:2353–2360. doi: 10.1093/humrep/dep180. [DOI] [PubMed] [Google Scholar]
- e7.Swerdlow AJ, Schoemaker MJ, Higgins CD, Wright AF, Jacobs PA UK Clinical Cytogenetics Group. Cancer incidence and mortality in men with Klinefelter syndrome: a cohort study. J Natl Cancer Inst. 2005;97:1204–1210. doi: 10.1093/jnci/dji240. [DOI] [PubMed] [Google Scholar]