Abstract
Background
From January 2011 onward, the Swiss newborn screening (NBS) program has included a test for cystic fibrosis (CF). In this study, we evaluate the first year of implementation of the CF-NBS program.
Methods
The CF-NBS program consists of testing in two steps: a heel prick sample is drawn (= Guthrie test) for measurement of immunoreactive trypsinogen (IRT) and for DNA screening. All children with a positive screening test are referred to a CF center for further diagnostic testing (sweat test and genetic analysis). After assessment in the CF center, the parents are given a questionnaire. All the results of the screening process and the parent questionnaires were centrally collected and evaluated.
Results
In 2011, 83 198 neonates were screened, 84 of whom (0.1%) had a positive screening result and were referred to a CF center. 30 of these 84 infants were finally diagnosed with CF (positive predictive value: 35.7%). There was an additional infant with CF and meconium ileus whose IRT value was normal. The 31 diagnosed children with CF correspond to an incidence of 1 : 2683. The average time from birth to genetically confirmed diagnosis was 34 days (range: 13–135). 91% of the parents were satisfied that their child had undergone screening. All infants receiving a diagnosis of CF went on to receive further professional care in a CF center.
Conclusion
The suggested procedure for CF-NBS has been found effective in practice; there were no major problems with its implementation. It reached high acceptance among physicians and parents.
Since 1979, it has been possible to measure immunoreactive trypsinogen (IRT) as a simple and reliable screening test for cystic fibrosis (CF) in neonates (1, 2). The first newborn screening (NBS) programs for CF were introduced in New Zealand and Australia in 1981 (3). The timely diagnosis and treatment of CF improves the affected infants’ food intake and digestion, growth, brain development, and lung function and lowers the frequency of CF exacerbations and hospitalizations (4, 5). There are now CF-NBS programs in the United States, England, Ireland, Scotland, France, Austria, Poland, the Netherlands, and regions of Italy and Spain (6, 7). American and European guidelines for CF screening have been developed (8, 9). In Germany, the inclusion of CF in the existing newborn screening program is currently under discussion (10).
There has been a NBS program in Switzerland for more than 40 years; at present, a Guthrie test is routinely performed to screen for six congenital metabolic disorders. For that purpose, a heel prick sample is taken from every neonate on the fourth day of life and dried on filter paper (= Guthrie card). Since 2006, the NBS program has been centrally performed in the national NBS laboratory of the University Children’s Hospital in Zurich.
In November 2010, the Swiss Federal Office of Public Health (FOPH) approved the implementation of testing for CF as the seventh disease to be included in the NBS program; a two-year pilot phase of the CF-NBS program began on 1 January 2011 (11). A retrospective study showed that the planned two-step procedure (IRT and DNA screening) would have captured 98% of the children who were given a clinical diagnosis of CF in the years 2006 to 2009 (12).
In the pilot phase of the CF-NBS program, its feasibility, efficacy, and acceptance were supposed to be evaluated (13). In this study, we evaluate the first year of the pilot phase—specifically, the following aspects:
the feasibility of the screening procedure,
the temporal course of the various steps of screening, and
the parents’ attitude toward the screening.
Methods
Procedure of the CF-NBS program
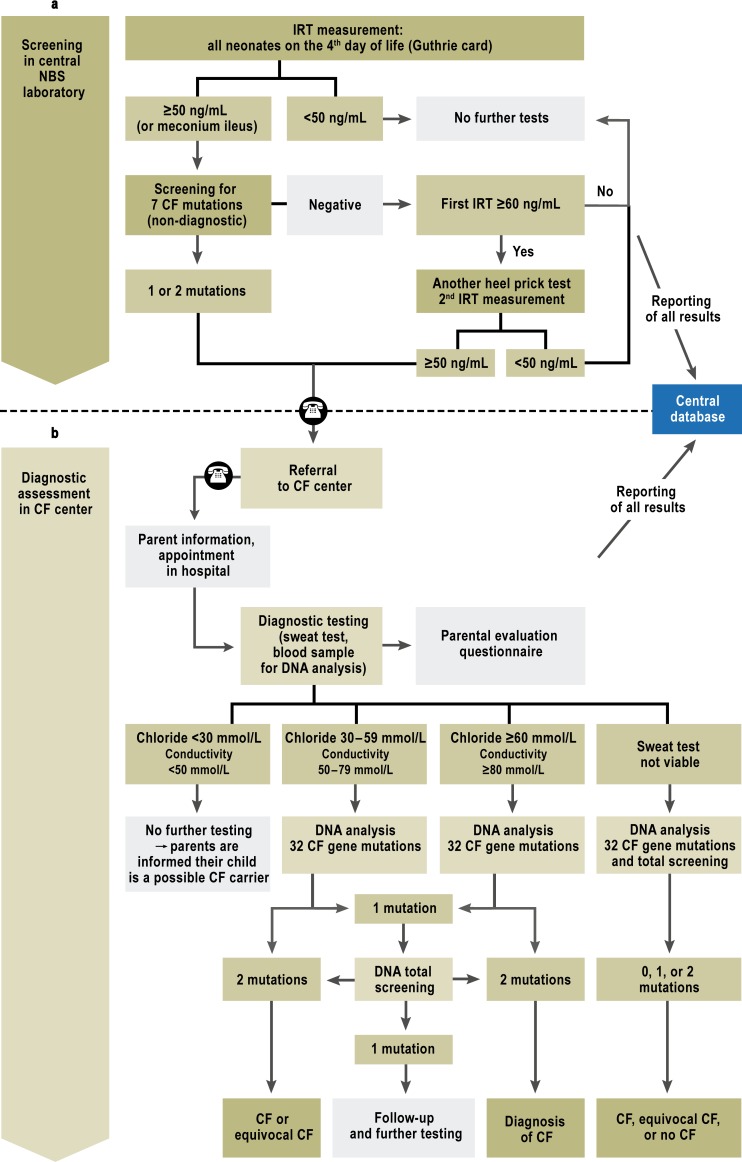
The NBS program consists of two parts (Figure 1): a) the screening per se in the NBS laboratory, and, where indicated, b) the further diagnostic assessment in the CF centers.
Figure 1.
Swiss CF newborn screening protocol after adjustments in the first pilot year. This protocol has been in use since 1 January 2012. The following four adjustments to the protocol were made in the first pilot year:
The IRT cutoff value was raised from 45 to 50 ng/mL;
A second heel prick test was performed only if the initial IRT was 60 ng/mL or higher, without any CF gene mutation on screening.
If the sweat test did not yield a valid result, genetic analysis was performed immediately and the sweat test was repeated later.
Children with meconium ileus had genetic screening at the same time as IRT measurement.
CF, cystic fibrosis; IRT, immunoreactive trypsinogen;
NBS, newborn screening
a) The screening process in the NBS laboratory: The national NBS laboratory screens all children born in Switzerland, unless their parents voice an objection to the screening. To screen for CF, IRT is measured in a heel prick sample (Figure 1). When the measured IRT is above the 99th percentile (45 ng/mL) (11), the same blood is subjected to DNA screening for the seven commonest CF gene mutations in Switzerland (F508del, 3905insT, G542X, R553X, W1282X, 1717–1G>A, N1303K) (12). If one or more mutations are found, the positive finding is reported to the CF center in whose catchment area the child was born; the center is not told which genetic mutation was detected. If DNA screening is negative (after an initially elevated IRT), IRT is measured again in a new heel prick sample (second Guthrie card), which is requested from the hospital where the child was born or from the treating pediatrician.
The screening procedure was slightly modified after an interim analysis of four months (Figure 1): The IRT cutoff value was raised to 50 ng/mL, and a second heel prick test was conducted only if the initial IRT was 60 ng/mL or higher, and DNA screening revealed no CF gene mutation (see Results, a), below). If the sweat test was not viable, a genetic analysis was conducted immediately and the sweat test was repeated later (see Results, d), below).
b) Diagnostic assessment and patient care in CF centers: There are eight pediatric CF centers in Switzerland (Aarau, Basel, Bern, Geneva, Lausanne, Lucerne, St. Gallen, and Zurich) and one regional CF care unit for the Canton of Ticino. The heads of the centers were personally informed of all positive screening results in infants belonging to their catchment population. They then telephoned the parents immediately to offer further diagnostic tests (14). The first diagnostic test used was the macroduct method measuring the chloride concentration in sweat (mmol/L) (15). A nanoduct sweat test was also performed to determine conductivity (which is related to the ion concentration; mmol/L). The latter was done at the request of the FOPH because it requires less sweat than the macroduct test and more frequently yields a result (16, 17). Both sweat tests were performed according to international guidelines (15, 18). As conductivity measurement is currently accepted only as a screening test (8), the result of the nanoduct sweat test was taken into account to establish the diagnosis only when the macroduct method failed to yield a valid result.
When the macroduct test yielded a positive result, a CFTR gene analysis was performed at the molecular level, the parents were informed of the diagnosis of CF, and the child was given further care according to the relevant guidelines (19– 21). When the macroduct test yielded a borderline finding, or when it failed to yield a valid finding and the nanoduct test was either positive or borderline, molecular-genetic analysis was performed and the macroduct test was later repeated to establish the diagnosis definitively. When the sweat test yielded a negative finding, the parents were informed that the child might be a carrier of CF; this information was generally delivered by a pediatric pulmonologist or, if desired, by a genetic counseling service. The treating pediatrician was informed in all cases.
Evaluation of the CF-NBS program
The CF-NBS program was evaluated at the Institute of Social and Preventive Medicine (ISPM) of the University of Bern. The results were documented at the NBS laboratory in Zurich (for part a), screening) and at the ISPM (for part b), diagnostic assessment). The results of diagnostic tests were reported by the clinicians of the CF centers to the ISPM on a standardized form.
The parents were given a questionnaire at the CF center (eSupplement), in which they were asked
how understandable the information on the screening was,
what feelings were aroused by the screening, and
whether they were satisfied with the screening overall.
The questionnaires were anonymous and were sent directly to the ISPM.
Statistical evaluation
The following parameters were calculated and analyzed:
Screening procedure:
optimization of the IRT cutoff value
number of parents that refused to participate in the NBS program
recall rate (the percentage of children who needed further measures after a positive screening test) (22)
referral rate
number of diagnoses
positive predictive value (PPV)
further care
sensitivity
specificity
incidence
Temporal course of the screening: To assess the efficiency of the screening procedure, the dates of each screening step were recorded. The goal was to establish the diagnosis of CF as rapidly as possible, in order to provide the affected neonates with adequate treatment at the earliest possible time.
Parent satisfaction: from the questionnaires.
Results
Screening procedure
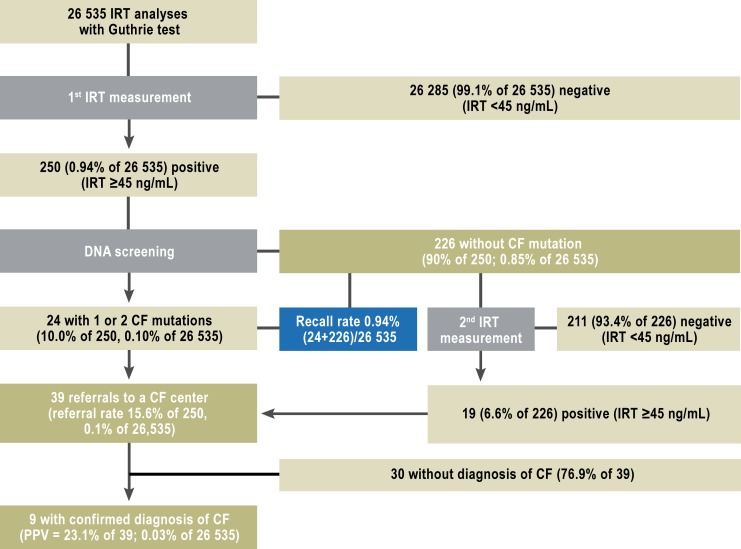
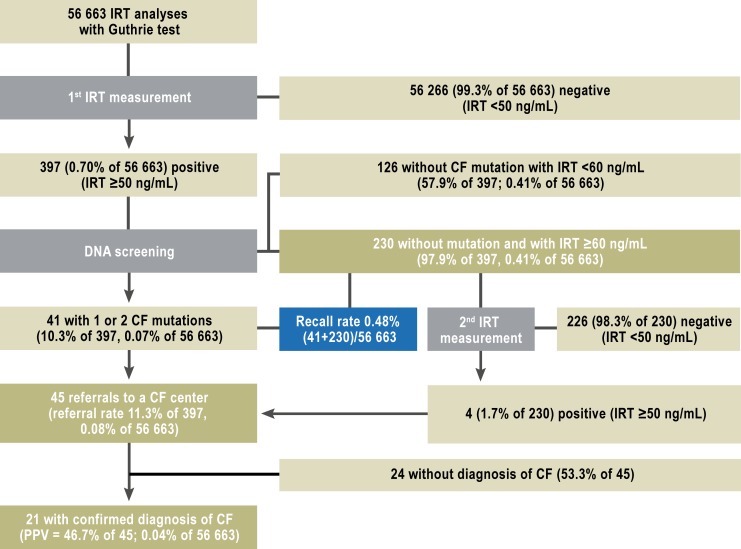
a) Optimization of the IRT cutoff value: After an interim assessment at 4 months, the IRT cutoff value was raised to 50 ng/mL (percentile 99.2) (Figure 1) and a second heel prick test was no longer performed on children whose initial IRT value was under 60 ng/mL and who did not have any of the seven CFTR mutations. This lowered the rate of a second heel prick test from 0.85% to 0.41% (a relative reduction of 52%). For this reason, results a–c were additionally stratified for the two temporal periods before and after these changes (eFigures 1 and 2).
eFigure 1.
Results of the screening from January to April 2011: the number of children in each step of the screening protocol in the first four months of 2011, and the calculated quality variables.
CF, cysric fibrosis; IRT, immunoreactive trypsinogen; PPV, positive predictive value
eFigure 2.
Results of the screening from May to December 2011: the number of children in each step of the screening protocol from May to December 2011, and the calculated quality variables.
CF, cysric fibrosis; IRT, immunoreactive trypsinogen; PPV, positive predictive value
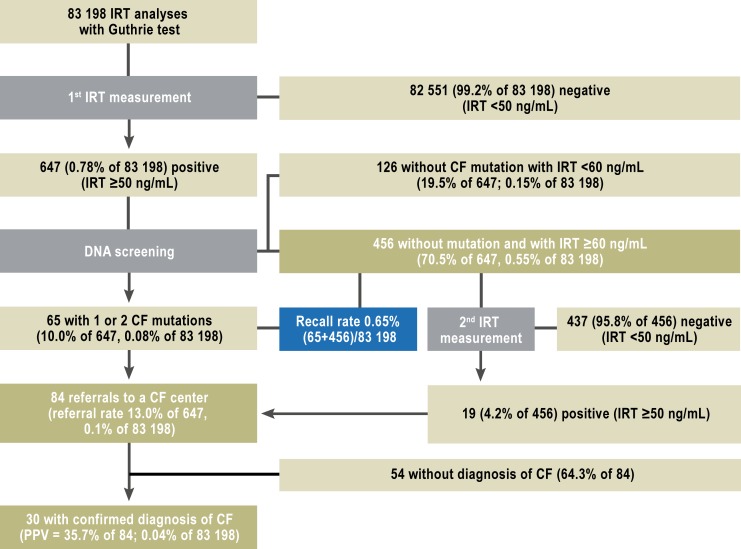
b) Number of test refusers and recall rate: In the year 2011, 83 198 infants were tested in the NBS program (Figure 2). The NBS laboratory was notified of 5 infants whose parents refused screening. There were 647 infants (0.80%) with an elevated IRT value who underwent DNA screening. 65 of them were found to have CF mutations, and the heel prick test was repeated in 456 (recall rate = 0.63%).
Figure 2.
Results of screening in the year 2011: the number of children in each step of the screening protocol in 2011, and the calculated quality variables. CF, cysric fibrosis; IRT, immunoreactive trypsinogen; PPV, positive predictive value
c) Referral rates, children diagnosed, PPV, and further care: A total of 84 infants were referred to the CF centers (referral rate 13.0%; Figure 2). The diagnosis of CF was confirmed in 30 of them (PPV = 35.7%). In the second period (May–December), the PPV was 46.7 % (eFigure 2). All 30 infants received further care in a CF center. Three of them had rare CF mutations of uncertain clinical relevance (“equivocal CF”) (23). Of the 27 infants with classic CF, 26 were directly referred because of a finding of at least one mutation in the screening, and one was referred after two elevated IRT values were measured. Of the 3 infants with equivocal CF, two were referred because of a finding of at least one mutation, and the third one after two elevated IRT values were measured.
A sweat test was attempted in 80 infants and yielded a valid result on the first attempt in 66. Four infants underwent genetic analysis immediately because of severe clinical disease. Because of the long waiting time until the sweat test could be repeated (until the newborn is heavy enough and produces enough sweat), it was decided 4 months into the pilot phase to perform the genetic analysis at once for any infants whose results on an initial sweat test were unclear. In the year 2011, a total of 35 diagnostic genetic analyses were performed:
18 because of a positive sweat test,
5 because of a borderline sweat test,
8 because of unavailable sweat test results due to technical failure (inadequate amount of sweat, defective electrodes),
4 because of severe clinical disease, as mentioned above (direct genetic analysis).
In cases without valid sweat-test results, the sweat test was repeated in the CF centers for definitive diagnosis.
d) Preliminary sensitivity and specificity: The clinical diagnosis of meconium ileus was made in 8 infants before NBS, and these children were specifically studied for CF. The disease was diagnosed in seven, six of whom had a positive screening test (included in the group of 84 referred infants). One child had a normal IRT value (39 ng/mL) and thus a negative screening test. CF has not been diagnosed to date in any other child born in 2011. Thus, the false-negative rate of the IRT test was 3.2% (1/31), and the false-positive rate was 0.06% (54/83 167). One year into the pilot phase, the preliminary sensitivity was 96.8% (30/31) and the specificity 99.9 % (83 113/83 167).
e) Incidence: The incidence of CF in 2011 (31 diagnoses in 83 198 neonates screened) was 1 : 2683, or 37 per 100 000 person-years. Not counting the 3 children with equivocal CF, the incidence was 1 : 2971.
Temporal course
The mean age at genetic confirmation of the diagnosis was 34 days (range, 13–135) (Table 1). On average, the screening result was reported to the responsible CF center before the infant was 20 days old. The parents were contacted after an average of 4 days, and the child began evaluation in a CF center an average of 2 days later. The diagnosis was then genetically confirmed or CF ruled out 14 days after that.
Table 1. Time variables over the course of the screening.
| Variable (days) | Median | Range | Median | Range | Median | Range | ||
| a) Birth | 3 | 3–19 | a) to e)birth to sweat test in CF center | 26 | 4–314* | a) to g)birth to genetically confirmed diagnosis | 34 | 13–135 |
| – drawing of heel prick sample | ||||||||
| b) Drawing of heel prick sample | 2 | 1–5 | ||||||
| – screening for CF | ||||||||
| c) screening for CF | 13 | 0–37 | ||||||
| – reporting of screening result to CF center | ||||||||
| d) Reporting of screening result to CF center | 4 | 0–38 | ||||||
| – telephone call to parents | ||||||||
| e) Telephone call to parents | 2 | 0–16 | ||||||
| – sweat test in CF center | ||||||||
| f) Sweat test in CF center | 7 | 0–63 | f) to g)sweat test in CF center to genetically confirmed diagnosis | 14 | 1–80 | |||
| – arrival of blood in genetics laboratory | ||||||||
| g) Arrival of blood in genetics laboratory | 6 | 1–22 | ||||||
| – genetically confirmed diagnosis |
*Outlier of 314 days to sweat test in CF center: this family emigrated to a foreign country immediately after the birth of their child, returned nearly a year later and was then offered a sweat test. The finding in this case was negative for CF. CF, cystic fibrosis
Parental satisfaction
81 of 84 parents received a questionnaire in a CF center; 47 (58%) responded, after an average of two weeks. The response rate was similar among parents of children that did and did not receive a diagnosis of CF (64% versus 56%, p = 0.460; Table 2).
Table 2. Results from parent questionnaire: general and stratified by CF diagnosis (CF, cystic fibrosis).
| All families(n = 47) | Families with CF diagnosis (n = 18) | Families without CF diagnosis (n = 29) | p value | |
| Response rate | 58% (47/81) | 64% (18/28) | 56% (29/52) | 0.460 |
| Time from receipt of questionnaire to response (median/range) | 14 days (3–455) | 16 days (7–158) | 13.5 days (3–455) | 0.818 |
| Overall satisfaction with screening | 43 (91%) | 18 (100%) | 25 (86%) | 0.257 |
| Worried after phone call from CF center | 35 (76%) | 13 (76%) | 22 (76%) | 0.963 |
| Worried after visit to CF center | 16 (34%) | 11 (61%) | 5 (17%) | 0.002 |
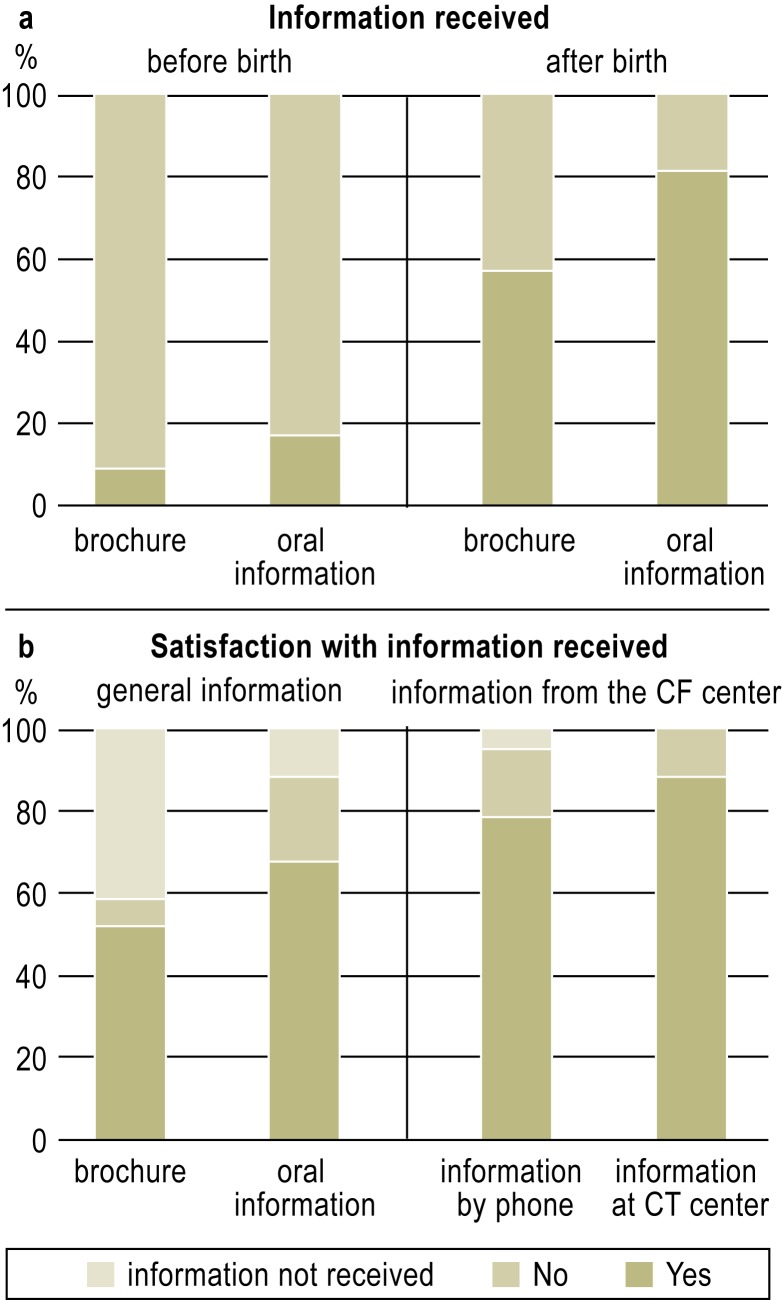
a) Information received about the screening: 200 of 203 maternity hospitals ordered the NBS brochure, but only 8 of 47 parents (8.5%) remembered having received or read it before their child’s birth; 27 (57.5%) did so afterward (Figure 3a). 8 of 47 parents recalled having received oral information before birth (17.0%), compared to 38 (80.9%) afterward.
Figure 3.
Parent information on CF neonatal screening:
The Figure shows the results of the parent questionnaire: how many of the parents recalled having been informed about the CF-NBS program before or after the birth of their child, and how satisfied they were with the information they received at each stage of the screening. CF-NBS, newborn screening for cystic fibrosis
The parents’ satisfaction with the information they received increased after each successive step of the screening (Figure 3b): 52% of those who had read the brochure were satisfied with it, 68% were satisfied with the oral information they received before or after the birth of their child, 79% (37 parents) with the information they received when they were first contacted over the telephone by the CF center, and 90% (42 sets of parents) with the ensuing discussion in the CF center.
b) Emotional response to the screening: After the telephone call from the CF center, 76% (35 sets of parents) of the families were worried and 24% (11 sets of parents) were optimistic (Table 2). After the visit to the CF center, only 34% (16 sets of parents) were still worried (61% of the families with a diagnosis of CF, and 17% of the families without a diagnosis of CF [p = 0.002]).
c) Overall attitude toward the screening: Overall, 91% of the parents (43 sets of parents) were satisfied that their child had been screened (Table 2): 18, or 100%, of those whose child had CF, and 25, or 86%, of those whose child did not have CF (p = 0.257).
Discussion
CF screening was successfully implemented as a component of the nationwide Swiss newborn screening program. Of the 83 198 infants screened in 2011, 84 were referred to a CF center and 30 received a diagnosis of CF. All infants with CF were treated in a CF center from the 26th day of life onward. Switzerland is thereby acting in accordance with the recommendations of the European Cystic Fibrosis Society (8). The genetic confirmation of the diagnosis was available, on average, on the 34th day of life. Screening met with high acceptance among parents and physicians; the number of refusals of NBS was comparable to that observed in previous years, before CF screening was added (5 versus 6.4 refusals per year in 2006 to 2010; range, 4–11). None of the refusers mentioned the new CF screening as a reason for refusal. The incidence of CF in the first year of the screening was 1 per 2683 infants. The incidence of CF has been previously estimated to be 1 in 2500 in Switzerland, 1 in 4150 in France, and 1 in 3500 in Austria (6).
Strengths and weaknesses
The success of CF-NBS program was attributable to the already well-established nationwide newborn screening program in Switzerland, which was centralized at the University Childrens’ Hospital in Zurich. The well-established procedure in which parents are expressly given the right of refusal certainly accounted in large measure for the high acceptance of the screening among parents. Moreover, further testing (where needed) was confined to 8 specialized centers, in analogous fashion to the diagnostic tests that are performed in the same children’s hospitals for the other six metabolic diseases included in the screening program.
The CF specialists across the country collaborated well throughout the pilot phase of the CF-NBS program, and the head of the NBS laboratory communicated personally with the heads of the CF centers. Interim analyses enabled optimization of the procedure with immediate addressing of any problems that came up, followed by the implementation of appropriate changes. For example, the IRT cutoff value was optimized to lower the recall rate. This is important, so that parents can be spared unnecessary worry. High sensitivity and specificity were achieved with an acceptable amount of extra work for the physicians involved, ensuring their compliance. A further important factor for success was the good collaboration with the FOPH, which supported the screening and contributed part of the costs of the evaluation. There was a relatively high response rate to the parent questionnaire, even though no reminders were sent in order not to compromise anonymity.
The potential problems of any CF screening program include false-positive findings and the identification of children with rare CF mutations whose further clinical course cannot be clearly predicted. The latter may account for the higher number of diagnoses in the screening year 2011 compared to previous years. Furthermore, the protocol change by which genetic testing is performed immediately whenever a sweat test fails to yield a valid result might lead to the detection of mild CFTR variants that would not cause any overt disease manifestations until the patient reaches adulthood (19).
It is known that infants with meconium ileus may have a normal IRT value even if they do, in fact, have CF. Therefore, for all infants with meconium ileus, genetic analysis is performed in the NBS laboratory at the same time as IRT measurement, so that such cases will not be missed. A limitation of the present study is that the interpretation of the parents’ answers about their satisfaction with the screening is based on a relatively small number (47) of returned questionnaires.
Interpretation of the findings
IRT screening failed to detect only one infant with CF, who received the diagnosis anyway because meconium ileus was present. The apparently excellent sensitivity of screening (30/31) should be interpreted cautiously, however, because CF in unscreened children may not become clinically evident until years later if its manifestations are relatively mild. For this reason, the evaluation of the CF newborn screening program will continue. All of the clinicians who make the diagnosis of CF in the Swiss CF centers are also participants in the NBS program and members of the Swiss Working Group for CF, which meets regularly every six months. Data on diagnoses that are made outside the screening program are exchanged and entered into the central database. This enables long-term monitoring of the specificity and sensitivity of the CF-NBS program. Thus, even in the future when screening has become universal, it will still be important to think of CF whenever children present with its characteristic manifestations (including recurrent cough, failure to thrive, fatty stools, and chronic abdominal pain).
The changes made in the screening protocol after four months markedly lowered the number of false-positive screenings. This was important to ensure acceptance of the screening among parents and physicians and to spare the parents unnecessary worry. Most parents had a positive attitude toward screening, regardless of the final diagnosis: 86% were satisfied that their child had been screened, even when they were asked to come to the hospital because of an, in the end, false-positive screening result. The responses to the parent questionnaires confirm the finding of a French study that the worry aroused by a false-positive screening test is rapidly relieved by a subsequent, normal sweat test (24).
The age at diagnosis was markedly lowered (mean age at genetic confirmation of the diagnosis: 34 days), which is important to enable an early start of treatment. An analysis of the available data from the genetic laboratory performing most CFTR analyses in Switzerland revealed that the diagnosis was made at a mean age of 198 days (range, 13–1307 days) before screening was introduced. The analysis included all 113 children with classic CF who received their diagnosis in this laboratory from 2000 to 2010 and who were under 4 years old at the time of diagnosis.
The parents’ responses to the questionnaires revealed the importance of avoiding delays between the telephone call to the parents and the subsequent evaluation in a CF center. To this end, the parents were only telephoned when an appointment in the CF center could be scheduled within 2 days. Thus, they were never called before the weekend. This accounts for the longer time between the report to the CF center and the telephone call to the parents (4 days) than between the telephone call and the sweat test (2 days).
Conclusion
The introduction of a CF-NBS program in Switzerland encountered no major problems, and further optimization was possible in the first year of the pilot phase. The implementation of the program met with high acceptance from physicians and parents. On average, the diagnosis of CF was genetically confirmed on the 34th day of life, and all affected infants received further treatment in a CF center.
Supplementary Material
Key Messages.
The implementation of newborn screening for cystic fibrosis (CF) in Switzerland met with high acceptance from physicians and parents. The number of parents refusing to have their children screened was no higher than in previous years.
One year after its introduction, the two-step screening process involving IRT measurement and DNA screening was found to have a (preliminary) sensitivity of 96.8% and a specificity of 99.9%.
All infants with CF received further treatment in a specialized center, starting from the 26th day of life (on average).
In comparison to the years before the introduction of CF screening, CF was diagnosed more frequently and at younger ages. 30 cases were diagnosed, compared to an average of 16 diagnoses per year before screening, at a mean age of 34 days, compared to 198 days before screening.
91% of the parents responding to a questionnaire were satisfied their child had been screened.
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
The two-year pilot project of neonatal screening for cystic fibrosis is supported by the Swiss Cystic Fibrosis Society (Schweizerische Gesellschaft für Cystische Fibrose, CFCH), The Lung Leagues of the Cantons of St. Gallen, Bern, Solothurn, Vaud, Zurich, and Ticino, the Telethon Foundation, the Dr. Pierluigi Crivelli Foundation, the Swiss Federal Office of Public Health, and the Novartis, Abbott, Glaxo, AstraZeneca, Vifor, and Solvay companies. The evaluation of the pilot project was financed by the Swiss Cystic Fibrosis Society and the Swiss Federal Office of Public Health.
We thank all the members of the Swiss CF Screening Task Force for their helpful collaboration: PD Dr. med. Jürg Barben, St. Gallen (president); PD Dr. med. Alex Möller, Zurich; Dr. med. Anne Mornand, Genevaf; Dr. med. Carmen Casaulta, Bern; Prof. Dr. med. Claudia Kuehni, Bern; Dr. med. Gaudenz Hafen, Lausanne; Prof. Dr. med. Martin Schöni, Bern; Prof. Dr. med. Mathias Baumgartner, Zurich; Prof. Dr. med. Nicolas Regamey, Bern; PD Dr. Ralph Fingerhut, Zurich; Prof. Dr. Sabina Gallati, Bern; Dr. Toni Torresani, Zurich; Prof. Dr. med. Constance Barazzone, Geneva; Dr. med. Peter Eng, Aarau; Prof. Dr. med. Jürg Hammer, Basel; Dr. med. Dominik Müller, Aarau; Dr. med. Isabelle Rochat, Lausanne; Dr. med. Renate Spinas, Zurich;
Dr. med. Johannes Spalinger, Luzern; PD Dr. med. Daniel Trachsel, Basel;
Dr. med. Maura Zanolari, Lugano.
Footnotes
Conflict of interest statement
The authors received support for this study from the above parties.
Prof. Baumgartner, Dr. Torresani, and PD Dr. Barben received partial reimbursement of conference participation fees and travel expenses from the Abbott company.
Prof. Gallati states that no conflict of interest exists.
PD Dr. Barben, Dr. Torresani, Prof. Baumgartner, and Prof. Gallati participated in the development and implementation of neonatal screening for CF. Dr. Rueegg and Prof. Kuehni participate in the performance and evaluation of screening.
References
- 1.Crossley JR, Elliot RB, Smith PA. Dried blood spot screening for cystic fibrosis in the newborn. Lancet. 1979;313:472–474. doi: 10.1016/s0140-6736(79)90825-0. [DOI] [PubMed] [Google Scholar]
- 2.Crossley JR, Smith PA, Edgar BW, Gluckman PD, Elliott RB. Neonatal screening for cystic fibrosis, using immunoreactive trypsin assay in dried blood spots. Clin Chim Acta. 1981;113:111–121. doi: 10.1016/0009-8981(81)90145-5. [DOI] [PubMed] [Google Scholar]
- 3.Massie J, Clements B. Diagnosis of cystic fibrosis after newborn screening: The Australasian experience—twenty years and five million babies later: a consensus statement from the Australasian Paediatric Respiratory Group. Pediatr Pulmonol. 2005;39:440–446. doi: 10.1002/ppul.20191. [DOI] [PubMed] [Google Scholar]
- 4.Balfour-Lynn IM. Newborn screening for cystic fibrosis: evidence for benefit. Arch Dis Child. 2008;93:7–10. doi: 10.1136/adc.2007.115832. [DOI] [PubMed] [Google Scholar]
- 5.Brice P, Jarrett J, Mugford M. Genetic screening for cystic fibrosis: An overview of the science and the economics. J Cyst Fibros. 2007;6:255–261. doi: 10.1016/j.jcf.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Southern KW, Munck A, Pollitt R, et al. A survey of newborn screening for cystic fibrosis in Europe. J Cyst Fibros. 2007;6:57–65. doi: 10.1016/j.jcf.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Grosse SD, Boyle CA, Botkin JR, et al. Newborn screening for Cystic Fibrosis: Evaluation of Benefit and Risks and Recommendations for State Newborn Screening Programs. MMWR. 2004;53(RR-13):1–36. [PubMed] [Google Scholar]
- 8.Castellani C, Southern KW, Brownlee K, et al. European best practice guidelines for cystic fibrosis neonatal screening. J Cyst Fibros. 2009;8:153–173. doi: 10.1016/j.jcf.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Comeau AM, Accurso FJ, White TB, et al. Guidelines for Implementation of Cystic Fibrosis Newborn Screening Programs: Cystic Fibrosis Foundation Workshop Report. Pediatrics. 2007;119:e495–e518. doi: 10.1542/peds.2006-1993. [DOI] [PubMed] [Google Scholar]
- 10.Harms E, Olgemöller B. Neonatal screening for metabolic and endocrine disorders. Dtsch Arztebl Int. 2011;108(1-2):11–22. doi: 10.3238/arztebl.2011.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barben J, Torresani T, Schoeni M, Gallati S, Baumgartner M. Neugeborenen-Screening auf Cystische Fibrose - ab 1. Januar auch in der Schweiz. Paediatrica. 2010;21:38–39. [Google Scholar]
- 12.Barben J, Gallati S, Fingerhut R, Schoeni MH, Baumgartner MR, Torresani T. Retrospective analysis of stored dried blood spots from children with cystic fibrosis and matched controls to assess the performance of a proposed newborn screening protocol in Switzerland. J Cyst Fibros. 2012;11:332–336. doi: 10.1016/j.jcf.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Barben J, Baumgartner M, Torresani T. Neugeborenen-Screening auf Cystische Fibrose. Forum News. 2011;3:24–27. [Google Scholar]
- 14.Farrell PM, Rosenstein BJ, White TB, et al. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr. 2008;153:4–14. doi: 10.1016/j.jpeds.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LeGrys VA, Yankaskas JR, Quittell LM, Marshall BC, Mogayzel PJ., Jr Diagnostic sweat testing: the Cystic Fibrosis Foundation guidelines. J Pediatr. 2007;151:85–89. doi: 10.1016/j.jpeds.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Barben J, Ammann RA, Metlagel A, Schoeni MH. Conductivity determined by a new sweat analyzer compared with chloride concentrations for the diagnosis of cystic fibrosis. J Pediatr. 2005;146:183–188. doi: 10.1016/j.jpeds.2004.08.085. [DOI] [PubMed] [Google Scholar]
- 17.Desax MC, Ammann R, Hammer J, Schoeni M, Barben J. Nanoduct sweat testing for rapid diagnosis in newborns, infants and children with cystic fibrosis. Eur J Pediatr. 2008;167:299–304. doi: 10.1007/s00431-007-0485-0. [DOI] [PubMed] [Google Scholar]
- 18.Green A, Kirk J. Guidelines for the performance of the sweat test for the diagnosis of cystic fibrosis. Ann Clin Biochem. 2007;44:25–34. doi: 10.1258/000456307779596011. [DOI] [PubMed] [Google Scholar]
- 19.Borowitz D, Parad RB, Sharp JK, et al. Cystic Fibrosis Foundation practice guidelines for the management of infants with cystic fibrosis transmembrane conductance regulator-related metabolic syndrome during the first two years of life and beyond. J Pediatr. 2009;155(6 Supplement):106–116. doi: 10.1016/j.jpeds.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borowitz D, Robinson KA, Rosenfeld M, et al. Cystic Fibrosis Foundation evidence-based guidelines for management of infants with cystic fibrosis. J Pediatr. 2009;155(6 Supplement):73–93. doi: 10.1016/j.jpeds.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerem E, Conway S, Elborn S, Heijerman H. Standards of care for patients with cystic fibrosis: a European consensus. J Cyst Fibros. 2005;4:7–26. doi: 10.1016/j.jcf.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 22.International Society for Neonatal Screening (ISNS), Standing Committee on Quality Assurance. Lexicon of terms to be used in newborn screening. www.isns-neoscreening.org/htm/facts_sheets.htm. 2005. Last accessed April 2013.
- 23.Mayell SJ, Munck A, Craig JV, et al. A European consensus for the evaluation and management of infants with an equivocal diagnosis following newborn screening for cystic fibrosis. J Cyst Fibros. 2009;8:71–78. doi: 10.1016/j.jcf.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Beucher J, Leray E, Deneuville E, et al. Psychological effects of false-positive results in cystic fibrosis newborn screening: a two-year follow-up. J Pediatr. 2010;156:771–776. doi: 10.1016/j.jpeds.2009.12.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.