Abstract
Background
Failure of adaptive plasticity with increasing pathology is suggested to contribute to progression of disability in multiple sclerosis (MS). However, functional impairments can be reduced with practice, suggesting that brain plasticity is preserved even in patients with substantial damage.
Objective
Here, functional magnetic resonance imaging (fMRI) was used to probe systems-level mechanisms of brain plasticity associated with improvements in visuomotor performance in MS patients and related to measures of microstructural damage.
Methods
23 MS patients and 12 healthy controls underwent brain fMRI during the first practice session of a visuomotor task (short-term practice) and after 2 weeks of daily practice with the same task (longer-term practice). Participants also underwent a structural brain MRI scan.
Results
Patients performed more poorly than controls at baseline. Nonetheless, with practice, patients showed performance improvements similar to controls and independent of the extent of MRI measures of brain pathology. Different relationships between performance improvements and activations were found between groups: greater short-term improvements were associated with lower activation in the sensorimotor, posterior cingulate, and parahippocampal cortices for patients, whereas greater long-term improvements correlated with smaller activation reductions in the visual cortex of controls.
Conclusions
Brain plasticity for visuomotor practice is preserved in MS patients despite a high burden of cerebral pathology. Cognitive systems different from those acting in controls contribute to this plasticity in patients. These findings challenge the notion that increasing pathology is accompanied by an outright failure of adaptive plasticity, supporting a neuroscientific rationale for recovery-oriented strategies even in chronically disabled patients.
Introduction
The neuropathology of multiple sclerosis (MS) is characterized by multifocal inflammatory demyelination and neuroaxonal injury.1,2 Whereas remyelination is an important mechanism of repair after acute inflammatory demyelination,3 clinical recovery is facilitated by adaptive functional reorganization.4,5,6-7 Although the occurrence and amount of lesional damage may trigger functional reorganization,6,8 the integrity of the extralesional brain tissue explains part of the potential for network plasticity underlying clinical recovery.9,10,11 An increasing burden of pathology limiting the availability of such tissue has been proposed to lead to the progression of disability in MS through a failure of brain plasticity.12,13
Our previous work, however, suggests that adaptive brain plasticity may be substantially preserved across levels of disability and damage in MS.14 We showed that practice-related improvements in visuomotor performance of MS patients were present even in patients with higher MRI lesion volumes and greater disabilities and could not be distinguished from those of healthy controls.14 This and related evidence challenge the hypothesis that increasing MS pathology leads to disability progression through a general failure of adaptive brain plasticity.14,15
Here, we investigate the relationship between microstructural damage and brain plasticity in patients with MS. We probe brain plasticity using functional magnetic resonance imaging (fMRI) in patients undergoing short-(minutes) and longer-term (days) practice of a visuomotor task. We first test the relationship between microstructural pathology, as reflected in brain MRI, and improvements in visuomotor performance with practice. We then characterize brain functional plasticity underlying these visuomotor improvements. We contrast patients with healthy controls to test the hypothesis that the capacity for performance improvement with practice is preserved in patients14 despite high levels of microstructural damage. However, we expect that functional patterns characterizing the systems-level mechanisms of brain plasticity underlying this preserved capacity in patients differ from those of healthy controls.16
Because motor practice induces functional changes of cognitive systems similar to those occurring during recovery after brain injury,17 our investigation provides insights into mechanisms supporting motor recovery in MS. As visuomotor integration constitutes the basis for reaching, grasping, and locomotion,18 our findings also inform about important functional substrates of rehabilitative interventions.
Methods
Participants and Study Design
A total of 35 right-handed individuals (23 MS patients and 12 healthy controls) participated in this study approved by the Oxfordshire Ethics Committee. Only patients who had been free of relapses for the 6 months prior to screening for the study were included. Patients were asked to keep their medications constant during the study.
Two phases of motor practice were assessed in this study: short-term practice was assessed over 12 minutes of the visuomotor task during the first functional MRI session (fMRI1); longer-term practice was tested over days of daily home practice with the same task. Participants were asked to train for at least 15 days, although shorter practice periods were not an exclusion criterion. Compliance was checked at the end of the study using the output files automatically saved on the practice laptop. At the end of the home training, participants underwent a second scanning session (fMRI2). In the separate scanning session, participants underwent a structural brain MRI scan.
Data Acquisition
Clinical measures
At baseline, patients and controls underwent a behavioral assessment of limb and cognitive functions using the 9-hole peg test (9-HP), the 25-foot walk, and the Paced Auditory Serial Addition Test of the Multiple Sclerosis Functional Composite.19 In patients, clinical disability was quantified by the Expanded Disability Status Scale score by a trained assessor (VT).20
Visuomotor performance
Visuomotor performance was evaluated using an isometric visuomotor tracking task.14,21 Participants were asked to track the vertical movements of a computer-controlled bar (the target bar) by altering the height of an adjacent bar controlled through varying the grip pressure applied to a handheld plastic rod. The target bar moved in a smoothly oscillatory fashion with the amplitude changing in a repetitive fashion to form the “sequence.” Continuous feedback on performance was provided by the color of the participant’s bar changing from red to blue when the difference between the heights of the 2 bars fell below 10 mm. Participants alternated 38-s sequence blocks with 38-s rest blocks, during which they made no response while watching a random sequence of movements generated to simulate their performance. A total of 7 blocks of each condition were performed during the fMRI sessions. Participants undertook a practice session before the start of the experiment. After fMRI1, they underwent a 13-minute daily training session at home with 10 blocks of sequence alternated with 10 blocks of rest.
Functional MRI
Data acquisition was performed on 3-T Varian Inova MRI system (Oxford, UK) using multislice gradient-echo EPI sequence [repetition time (TR) = 3000 ms; echo time (TE) = 30 ms; 43 axial slices (3.5-mm thick) providing whole-brain coverage; field of view (FOV), 256 × 192 mm2; matrix 64 × 64].
Structural MRI
The structural scans were performed on a 1.5-T Siemens Sonata scanner. T1-weighted images (T1-WIs) were acquired for brain volume calculation (TR = 12 ms, TE = 5.65 ms, flip angle = 19°, with elliptical sampling of k-space, giving a voxel size of 1 × 1 × 1 mm). Diffusion-WIs (DWIs) were acquired using echo planar imaging [60 axial slices (2.5-mm thick), matrix size 128 × 104, FOV = 320×260 mm, giving a voxel size of 2.5 × 2.5 × 2.5 mm]. Diffusion weighting was isotropically distributed along 60 directions using a b value of 1000 s/mm2.
Data Analysis
Measures of visuomotor performance
The distance between the target bar and the pressure-sensing bar constituted the tracking error. The 90th percentile of the tracking error (p90) for sequence represented the summary measure of error across each block for each participant. The mean of p90 tracking errors across all blocks in each scanning session represented the overall tracking error for sequence. Short-term practice was quantified by a reduction of the p90 over 7 blocks of sequence during fMRI1. We used the slope of a linear fit to the individual tracking errors over 7 blocks of sequence as a measure of the rate of short-term practice-related improvements. The mean daily tracking error of the home sequence practice was used to quantify the longer-term practice-related improvements, the slope over days being the rate of longer-term practice-related improvements. All behavioral measures are reported in arbitrary units as mean ± standard error (SE).
Changes in mean tracking error were tested using repeated-measures analysis of variance (ANOVA) with group (controls and patients) as a between-subject factor and either block (1 to 7, for short-term data) or day (1 to 15, for longer-term data) as a within-subject factor.
Structural MRI analysis
Analysis was carried out using tools from the FMRIB Software Library (FSL) (www.fmrib.ox.ac.uk/fsl) to characterize the amount and distribution of damage in patients.
Details on lesion volume calculation are reported in the supplementary material (available at http://nnr.sagepub.com/supplemental). Brain parenchyma volumes were measured on T1-WIs using SIENAX,22,23 which measures normalized brain volume (NBV).24,25
All DWIs were corrected for head motion and eddy currents. Mean fractional anisotropy (FA) images were created using DTIFit.26 With Tract-Based Spatial Statistics,27 individual FA maps were nonlinearly aligned to a common FA template. The across-subject mean FA image was calculated and used to generate a “skeleton” of white matter (WM) tracts, which was thresholded at FA > 0.2. Individual-participant maximum FA values nearest to the mean FA skeleton were perpendicularly projected onto this skeleton for statistical analysis. Mean FA values were calculated for the participants within the skeleton. To test for voxelwise differences in FA values, we carried out permutation-based nonparametric testing.28 Results were considered significant for P < .05 after applying threshold-free cluster enhancement29 as a correction for multiple comparisons. To characterize WM properties, similar analysis was performed on mean diffusivity as well as on axial (L1) and radial (L2L3) diffusivity maps. Axial diffusivity was obtained by averaging the second and third eigenvalues.
T1-WIs were analyzed with FSL-VBM30,31 incorporating nonlinear registration and correction for local expansion or contraction. To test for local differences between gray-matter (GM) volumes in patients versus controls, a voxelwise general linear model (GLM) was applied using permutation-based nonparametric testing, forming clusters with threshold-free cluster enhancement and testing clusters for significance at P < .05, corrected for multiple comparisons.
Analysis of the behavioral-structural relationships
Spearman correlation tested the relationship between the 9-HP test and overall tracking error as well as the relationship between measures of performance and structural MRI measures. The relationship between performance changes with short- and longer-term practice and structural MRI measures was tested in a partial correlation, which included initial performance as a covariate. Two-tailed unpaired t tests or the Mann-Whitney test were used to test for significant between-group differences in the mean age, disability, structural MRI measures of damage, overall tracking error, rates of improvements, and days of practice. Levene test for equality of error variances was performed on slopes of short- and longer-term tracking error changes across groups.
For all the statistical tests, differences were considered significant at the P < .05 level, 2-tailed. Values are quoted as mean ± SE, unless stated otherwise.
fMRI of motor practice
Analysis was carried out using tools from FSL. First-level (step 1) data preprocessing included motion correction, brain extraction, spatial smoothing (Gaussian kernel of 8-mm full width at half maximum), high-pass temporal (available at http://nnr.sagepub.com/supplemental) (150-s cutoff), and correction for field inhomogeneities through fieldmap-based EPI unwarping using FEAT of FSL.26 Nonlinear registration from high-resolution T1 structural to MNI standard space was carried out. The time series was analyzed using a GLM approach with local autocorrelation correction. The canonical gamma variate hemodynamic response function was used. Two principal explanatory variables (along with their temporal derivatives) specified (1) the onset and duration of sequence task periods to identify the mean effect associated with the task and (2) a linear trend over the course of the scanning session in signal change associated with the sequence task, which was orthogonalized with respect to the mean effect of the task. Head motion parameters were added to the model as confound regressors.
Higher-level (group) analyses were conducted32 with automatic outlier deweighting.33 GM partial volume information based on the individual structural images was added to the model as a voxel-dependent covariate. Group Z statistical images were restricted to gray matter and then thresholded using clusters determined by Z > 2.3 and a cluster-extent corrected significance threshold of P = .05.34,35-36
A mean within-session group effect associated with the sequence task as well as with sequence linear change was identified (step 2). Next, a mean difference between sessions was tested to identify changes associated with home practice. This was achieved by using a fixed-effects, between-session, within-subject analysis (step 3), followed by a mixed-effects, between-session, group-level analysis (step 4).
The relationship between variation in behavioral measures and variation in BOLD signal change was tested in correlation analyses. To identify short-term BOLD behavioral relationships, individual-participant outputs from analysis of the first fMRI session in step 1 were tested for correlation with de-meaned individual measures of (1) p90 tracking error during the first block of sequence, (2) slopes of tracking error over 7 blocks of sequence, and, in a separate design, (3) baseline overall motor performance, that is, mean of p90 tracking error, across 7 blocks of fMRI1. To identify longer-term BOLD-behavioral relationships, individual-participant outputs quantifying BOLD change between sessions from step 3 were tested for correlation with de-meaned individual measures of (1) mean performance during the first day of home practice and (2) individual slopes of tracking error changes over the home practice period. Number of days of practice was included as a confound regressor.
Brain functional activations were labeled using the Harvard-Oxford Structural Atlas, the Juelich Histological Atlas, and the Oxford Thalamic Connectivity Probability Atlas (http://www.fmrib.ox.ac.uk/fsl/data/atlas-descriptions.html).
fMRI of control tasks
To assess any nonspecific brain BOLD signal changes over time, participants performed control tasks. During the visual checkerboard task, they were asked to fixate on a cross in the middle of the screen. No motor response was required. During the motor task, they were asked to tap their right index finger at a frequency of 1 Hz. The visual checkerboard was used to cue the motor response. Both tasks used a 30-s block design (5 blocks of “on” alternated with 5 blocks of “off”). Analysis was performed with the same general schema as for the visuomotor task. However, high-pass temporal filtering with a 60-s cutoff was used to remove low-frequency drifts.
Results
Baseline Characteristics
The MS patients (19 relapsing-remitting and 4 secondary progressive) had lower manual dexterity but were well matched to the healthy controls for demographic and cognitive measures (Table 1). The Expanded Disability Status Scale functional systems had the following median (range) scores: pyramidal = 3 (0-4); sensory = 2 (0-4); cerebellar = 2 (0-4); brainstem = 1 (0-4); bladder/bowel = 1 (0-3); visual = 1 (0-4); mental = 1 (0-2).
Table I.
Demographic,Clinical, and Structural Characteristics of Controls and Patientsa
| Controls (n = 12) | MS Patients (n = 23) | PValue | |
|---|---|---|---|
| Age, y | 43 ± 2.7 | 45 ± 8.5 | .53 |
| Sex (F/M) | 9/3 | 18/5 | .83b |
| Disease duration, y | — | 12 ± 1.5 | — |
| EDSS, median, range | — | 4.0, 0-7.0 | — |
| Number of relapses, median, range | — | 4, 1-12 | — |
| Right-hand grip test | 33.0 ± 2.7 | 26.6 ± 2.0 | .02c |
| Left-hand grip test | 31.4 ± 2.8 | 25.7 ± 2.1 | .04c |
| Right-hand 9-HPTd | 18.2 ± 0.5 | 25.3 ± 3.1 | .06c |
| Left-hand 9-HPTd | 19.5 ± 0.4 | 31.7 ± 6.9 | .01c |
| 25-FWd | 6.2 ± 0.2 | 17.0 ± 7.5 | <.0001c |
| PASAT 3 s | 42.08 ± 3.7 | 40.61 ± 3.5 | .92c |
| PASAT 2 s | 33.7 ± 3.6 | 33.3 ± 3.0 | .93c |
| T2-LV (cm3) | — | 18.2 ± 2.9 | — |
| TI-LV (cm3) | — | 14.4 ± 2.5 | — |
| NBV (cm3) | 1563.5 ± 20.4 | 1498.6 ± 20.0 | .03 |
| FA | 0.43 ± 0.003 | 0.39 ± 0.007 | <.0001 |
| MD | 8.2 × 10−4 ± 7 × 10−6 | 8.8 × 10−4 ± 11 × 10−6 | .0001 |
| LI | 12.4 × 10−4 ± 8 × 10−6 | 12.7 × 10−4 ± 7 × 10−6 | .008 |
| L2L3 | 6.1 × 10−4 ± 7 × 10−6 | 6.8 × 10−4 ± 13 × 10−6 | <.0001 |
| GM volume | 0.47 ± 0.008 | 0.37 ±0.01 | <.0001 |
Abbreviations: MS, multiple sclerosis; EDSS, Expanded Disability Status Scale; 9-HPT, 9-hole peg test; 25-FW, 25-foot walk; PASAT, Paced Auditory Serial Addition Test; T2-LV,T2-hyperintense lesion volume;TI-LV,TI-hypointense lesion volume; NBV, normalized brain volume; FA, fractional anisotropy; MD, mean diffusivity; LI, axial diffusivity; L2L3, radial diffusivity; GM, gray matter.
Values are reported as mean ± standard error and the significance was determined using an unpaired t test, unless stated otherwise.
Pearson χ2.
Mann-Whitney U test.
Mean of 2 consecutive trials.
Supplementary Figure S1 shows the distribution of WM lesions in the patient group. Patients had smaller NBVs than controls, and there was widespread microstructural damage with reduced GM volume and WM FA (Table 1, Figure 1).
Visuomotor Performance and Its Changes With Practice
At baseline, patients had higher overall tracking error for the visuomotor task (177.1 ± 11.1 units) than did the controls (134.6 ± 8.2 units; P = .004; Figure 2A). The overall tracking error correlated significantly with performance of the right hand 9-HP test for the patients (ρ = 0.58; P = .004), but not for the controls (ρ = −0.20; P = .54).
Figure 2.
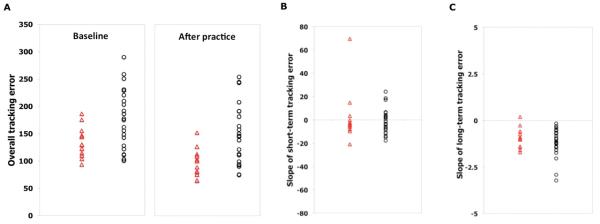
(A), Individual measures of overall tracking error at baseline (fMRI1) and after longer-term practice (fMRI2); (B), individual slope of short-term tracking error during the first scanning session (fMRI1); and (C) individual slopes of longer-term tracking performance error with home practice in controls (triangle) and in patients (circle).
Both groups improved their performance during the first practice session (F = 2.5; df = 3.4; P = .06), with no significant between-group differences (Block × Group interaction: F = 0.47; df = 3.4; P = .73; Figure 2B). The mean rate of short-term improvements was lower, although not significantly so, for patients (−1.1 ± 2.2 unit/block) than for controls (−3.8 ± 2.4 unit/block); P = .46). There was no significant difference in variance associated with rates of short-term improvements between patients and controls (F = 1.5; P = .23).
The duration of practice was shorter than 15 days in 2 patients and in 2 controls. The length of home practice was similar for patients (median, 17 days; range, 9-24 days; only 1 patient with <2 weeks of practice) and controls (median, 17 days; range, 13-21 days; only 1 control with <2 weeks of practice) as were rates of performance improvement over the practice period (controls, −1.1 ± 0.2 units/d; patients, −1.3 ± 0.2 units/d; P = .35; Figure 2C). The variance in the rates of longer-term performance improvement for controls also was not significantly different from that observed for patients (F = 1.3; P = .27). The overall tracking error at the end of the training period remained greater for the patients (controls, 96.7 ± 7.5 units; patients, 151.13 ± 11.4 units; P < .0001; Figure 2A).
Visuomotor Performance and MRI Measures of Brain Pathology
At baseline, the mean tracking error during block 1 of sequence correlated with the NBV and mean FA within the major WM tracts in patients (NBV: ρ = −0.60, P = .003; mean FA: ρ = −0.77, P < .0001) but not in controls (NBV: ρ = −0.18, P = .57; mean FA: ρ = −0.10; P = .76). When controlling for initial performance in block 1, there was no significant relationship between rates of short-term improvements and NBV or mean FA for the patients (NBV: ρ = 0.03, P = .90; mean FA: ρ = 0.19, P = .41) or for the controls (NBV: ρ = 0.23, P = .50; mean FA: ρ = 0.12, P = .72). Similarly, after accounting for differences in performance on the first day of practice, there were no significant correlations between rates of performance improvement and brain structural MRI measures for either the patients (NBV: ρ = 0.22, P = .33; mean FA: ρ = −0.10, P = .66) or the controls (NBV: ρ = −0.10, P = .78; mean FA: ρ = −0.08, P = .82).
Visuomotor Performance and fMRI Measures of Brain Plasticity
Relating initial performance to brain activity at baseline
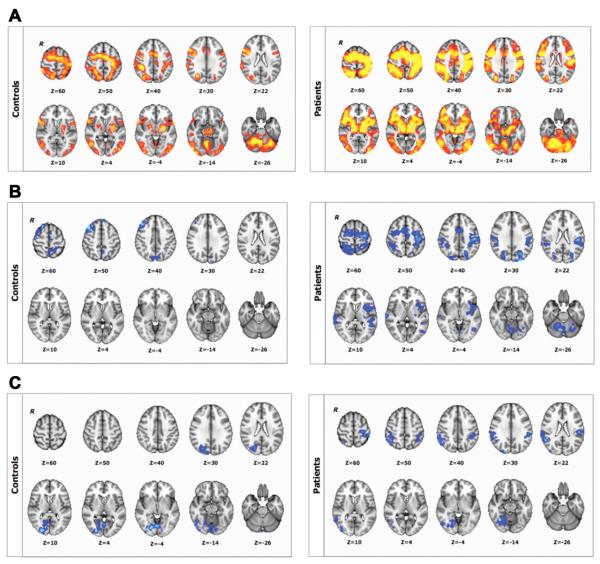
Execution of the visuomotor task and the control visual and motor tasks were associated with activation of bilateral cortical, subcortical, and cerebellar regions both in controls and in patients (Figure 3A). There was no significant between-group difference in either the visuomotor or the control task.
Figure 3.
BOLD signal changes associated with (A) baseline sequence-related activation (sequence vs rest contrast); (B), performance improvements for short-term (during fMRI1); and (C) long-term practice (contrasting fMRI1 > fMRI2) for controls (upper panel) and for patients (lower panel); Z > 2.3, P < .05, corrected. R identifies the right hemisphere.
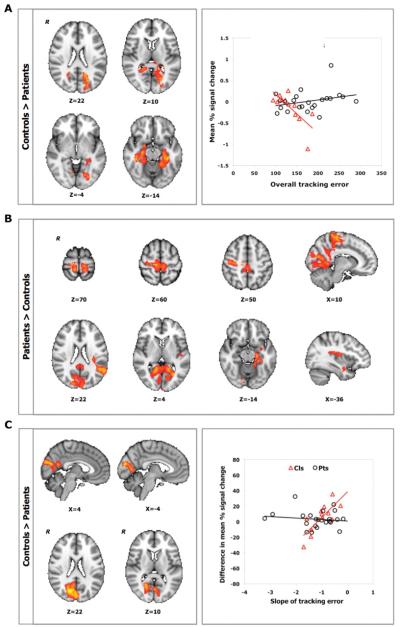
Within the control group, higher mean tracking error correlated with lower task-related activation in posterior parietal, occipital, and parahippocampal cortices during the first practice session (fMRI1; Table 2). No significant correlation was identified for the patient group, although a positive correlation between overall motor performance and BOLD signal in the left hippocampus, right superior parietal lobule, and right primary motor cortex was suggested using a voxelwise threshold of Z > 3.1 (uncorrected P < .001). Contrasting the 2 groups directly, we found significant differences in the relationships between performance and brain activation during fMRI1: a negative correlation between overall mean tracking error during fMRI1 and mean percentage signal change in the posterior parietal, occipital, parahippocampal, cingulate, and temporal cortices and in the hippocampus was observed for the controls but not for the patients (Figure 4A).
Table 2.
Correlations Between Task-Related Brain Activation (Sequence vs Rest) and (1) Baseline Overall Motor Performance, (2) Short-term Practice-Related Improvements, and (3) Longer-term Practice-Related Improvements in Visuomotor Tracking Errora
| Controls |
Controls < Patients |
|||||||
|---|---|---|---|---|---|---|---|---|
| MNI Coordinates |
MNI Coordinates |
|||||||
| Region of Interest | Z | x | y | z | Z | x | y | z |
| Baseline overall motor performance | ||||||||
| L cingulate gyrus, posterior division | 3.0 | 12 | −40 | 30 | ||||
| L SPL | 2.9 | −22 | −56 | 40 | ||||
| L precuneus | 3.8 | −20 | −66 | 24 | ||||
| R precuneus | 3.50 | 16 | −48 | 14 | ||||
| R lingual gyrus (V2) | 2.5 | 8 | −64 | −4 | ||||
| L intracalcarine cortex (VI) | 3.4 | −22 | −68 | 10 | ||||
| L occipital fusiform cortex (V4) | 3.1 | −26 | −76 | −4 | ||||
| R temporal fusiform cortex | 3.7 | 40 | −34 | −14 | ||||
| L hippocampus | 3.1 | −18 | −12 | 20 | ||||
| R hippocampus | 3.6 | 30 | −14 | −22 | ||||
| L parahippocampal gyrus | 3.9 | −34 | −32 | −16 | ||||
| R parahippocampal gyrus | 3.2 | 22 | −36 | −18 | ||||
| Short-term practice-related improvement | ||||||||
| R precentral gyrus, M1 | 3.5 | 12 | −26 | 60 | 4.1 | 10 | −26 | 66 |
| L precentral gyrus, M1 | 3.3 | −12 | −28 | 68 | ||||
| R precentral gyrus, PMd | 3.8 | 12 | −22 | 66 | ||||
| L cingulate gyrus, anterior division | 2.5 | −6 | −10 | 38 | 2.9 | −4 | −8 | 38 |
| L cingulate gyrus, posterior division | 3.0 | −6 | −46 | 18 | 2.9 | −6 | −46 | 20 |
| R cingulate gyrus, anterior division | 3.4 | 12 | −12 | 40 | ||||
| R cingulate gyrus, posterior division | 3.5 | 8 | −48 | 16 | 2.8 | 6 | −46 | 20 |
| R postcentral gyrus, S1 | 4.6 | 6 | −38 | 72 | 3.7 | 6 | −38 | 72 |
| L insular cortex | 3.3 | −36 | −14 | 12 | ||||
| R parietal opercular cortex | 3.3 | 34 | −22 | 22 | ||||
| L parietal opercular cortex | 4.4 | −32 | −30 | 18 | 3.6 | −32 | −28 | 18 |
| L central opercular cortex | 3.0 | −36 | −10 | 16 | ||||
| L SPL | 3.1 | −16 | −40 | 70 | 3.9 | −8 | −38 | 56 |
| L angular gyrus (IPL-Pga) | 3.7 | −50 | −52 | 24 | ||||
| R precuneus | 3.0 | 4 | −58 | 40 | ||||
| R lingual gyrus (V2) | 3.4 | 12 | −46 | 0 | 3.5 | 14 | −50 | 2 |
| L lingual gyrus (V2) | 2.8 | −16 | −52 | 0 | 3.7 | −12 | −70 | −2 |
| L intracalcarine cortex (V1) | 3.7 | −24 | −66 | 8 | ||||
| R intracalcarine cortex (V1) | 3.1 | 22 | −58 | 8 | 3.1 | 16 | −70 | 12 |
| L middle temporal gyrus | 3.0 | −62 | −20 | −14 | ||||
| R middle temporal gyrus, posterior division |
3.4 | 58 | −22 | −12 | ||||
| R Heschls gyrus (primary auditory cortex) |
3.7 | 42 | −26 | 6 | ||||
| L planum temporale (primary auditory cortex) |
4.4 | −32 | −34 | 18 | ||||
| R hippocampus | 3.3 | 26 | −20 | −16 | ||||
| L hippocampus | 4.3 | −20 | −16 | −18 | 3.8 | −26 | −22 | −20 |
| L parahippocampal gyrus | 4.4 | −32 | −6 | −26 | 3.2 | −18 | −38 | −16 |
| L thalamus (connected to posterior parietal cortex) |
2.4 | −18 | −24 | 2 | ||||
| R thalamus (connected to posterior parietal cortex) |
3.3 | 12 | −26 | 0 | ||||
| Longer-term practice-related improvement | ||||||||
| L precuneus | 3.5 | 6 | −56 | 18 | ||||
| L cuneal cortex (V2) | 4.2 | −4 | −86 | 18 | 4.2 | −4 | −86 | 18 |
| R supracalcarine cortex (V2) | 5.1 | 22 | −60 | 14 | 5.0 | 22 | −60 | 14 |
Abbreviations: SPL, superior parietal lobule; IPL, incraparietal lobule; PMd, lateral premotor cortex dorsal; S1, primary somatosensory cortex; M1, primary motor cortex;V, visual cortex; R, right; L. left.
Localization of clusters is in Montreal Neurological Institute (MNI) Standard Brain Space. Z score of the peak voxel is reported for each cluster showing a significant correlation between baseline overall motor performance and activation during the sequence versus rest or a significant practice-related reduction of BOLD signal for the sequence versus rest contrast (random effects, Z > 2.3, P < .05, corrected). The brain functional activation clusters are labeled using anatomical definitions in the Harvard-Oxford Structural Atlas and, where relevant, the Juelich Histological Atlas (in brackets) or the Oxford Thalamic Connectivity Probability Atlas.
Figure 4.
A. Relationship between individual variation in overall tracking error at baseline and BOLD percentage signal change during fMRI1 (Z > 2.3, P < .05, corrected) for controls (triangle) and patients (circle). B. Relationship between individual variation in the rate of short-term practice-related improvements and brain activation for sequence versus rest during fMRI1 (Z > 2.3, P < .05, corrected). C. Relationship between individual variation in longer-term practice-related improvements and difference between sessions (fMRI1 vs fMRI2) in percentage signal change for the sequence versus rest contrast (Z > 2.3, P < .05, corrected) for controls (triangle) and patients (circle). Results are controlled for initial performance (day 1). R identifies the right hemisphere.
Changes in brain activity with visuomotor practice
Reductions in BOLD signal with practice were observed in the prefrontal, premotor, motor, and parietal cortices in controls over the course of fMRI1. Additional reductions in activation were found in the temporal and occipital cortices and in the cerebellum in the patients (Figure 3B). Increases in BOLD signal change were not observed in the first practice session for either group. After longer-term practice (fMRI1 vs fMRI2), there were significant decreases in visuomotor task–related activation in the occipital cortex for controls and in both the occipital and parietal cortices for patients (Figure 3C). We did not find increases in BOLD signal change between sessions for either group. There were no significant between-session changes in BOLD signal for the control visual or motor tasks for either group.
Relating performance improvements to changes in brain activity
Brain regions relevant for visuomotor improvements differed between patients and controls. Greater performance improvements with short-term practice (fMRI1) were associated with smaller task-related mean BOLD signal in sensorimotor, premotor, cingulate, temporal, and parahippocampal cortices for the patient group (Table 2). In many of these same regions, the mean BOLD signal was more strongly correlated with performance improvements in patients than in controls (Table 2, Figure 4B). We found no correlation between the mean BOLD signal and performance improvements for controls using fully corrected statistics. However, a voxelwise threshold of Z > 3.1 (uncorrected P < .001) suggested a greater BOLD signal in the left angular gyrus for controls who showed greater performance improvements with practice.
Greater improvements in performance after longer-term practice (fMRI1 vs fMRI2) were associated with smaller changes in BOLD signal in the occipital cortex for the control group (Table 2). This relationship was not found for patients, resulting in a significant between-group difference (Table 2, Figure 4C). However, a post hoc analysis using a voxelwise threshold of Z > 3.1 (uncorrected P < .001) suggested a trend for a relationship between greater longer-term performance improvements and smaller changes in BOLD signal over time in the left superior parietal lobule and in the right lateral occipital cortex in the patient group.
Discussion
Our results demonstrate that performance improvements in MS patients can occur despite considerable brain damage and disability. Baseline patterns of activation for the visuomotor and control tasks suggest adaptive plasticity in patients. The comparison of activation changes with visuomotor task practice for the 2 study groups provides direct evidence for functional plasticity that is both qualitatively and quantitatively different between patients and controls, extending previous neurophysiological observations.15,16
Relating Brain Damage to Brain Plasticity in MS
Along with previous behavioral evidence for a substantial preservation of brain plasticity with increasing burden of disability,14 our results challenge the hypothesis that accumulation of greater burdens of microstructural damage leads to clinical progression in MS through an exhaustion of plastic reserve. They suggest that the concept of the progression of disability in MS should be extended from the simple focus on outright and general failure of adaptive plasticity to the consequences of more specific functional impairments arising from pathology in critical pathways6 combined with those of chronic limb disuse.37 Although systems-level adaptive plasticity may compensate for damage,8 this compensatory potential can be exceeded with progression of neuroaxonal loss affecting functional “bottlenecks” (eg, the major afferent optic nerves for vision, the efferent corticospinal tracts for movement). Substantial evidence has highlighted that the integrity of long pathways correlates functionally with measures of impairment38,39-40 and behaviorally with measures of disability.6,41 Therefore, although we show that brain plasticity is largely preserved even at higher burdens of microstructural damage, the ultimate behavioral impact of systems-level adaptive plasticity may be limited by the extent of chronic damage to functionally relevant long pathways. Learned disuse of the limb42 with disease progression may also contribute to progression of disability in MS. We found a significant correlation of WM integrity or NBV with performance at baseline but not with performance improvements in the patients. Simple relationships between lesion size and prognosis for recovery with neurorehabilitation have also been weak for patients after stroke.43 Learned disuse has provided a compelling interpretation of these results and motivated successful neurorehabilitation approaches based on forced use of the affected limb.44,45 Our observations suggest that learned disuse, rather than pathology alone, could contribute to disability in MS through maladaptive mechanisms,37 highlighting a specific new focus for neurorehabilitation interventions.44,46
Characterizing Adaptive Brain Plasticity in MS
Patients showed different functional patterns underlying performance improvements with practice compared with controls. Short-term practice-related improvements were associated with signal changes in regions for sensorimotor control and integrative aspects of task encoding47: lower levels of activity in these regions were found in patients who improved their performance more with practice. Evidence that plasticity in the primary sensorimotor cortex mediates the establishment of practice-related and task-specific motor memories comes from a broad range of studies in both animal models48,49 and healthy humans.50 Previous studies in MS have suggested that recruitment of sensorimotor areas ipsilateral to the hand moved may either limit the behavioral impact of brain pathology8,16,51 or may represent a functional marker of more severe microstructural damage that is reflected in impaired performance.52,53,54-55 The localized relationship between greater visuomotor improvements and lower levels of activity in sensorimotor and premotor cortices, which also show reduced activity with practice, provides direct evidence for an adaptive role of the ipsilateral sensorimotor areas, whose activity is no longer required when performance has improved with practice. These results support the rationale for therapeutic modulation of the sensorimotor activity ipsilateral to the hand moved as part of neurorehabilitation strategies.56,57-58
Brain activation changes after home practice could be consistently related to performance improvements only for the controls, for whom a sustained activation in the primary visual cortex correlated with larger improvements in performance. This relationship may reflect an aspect of visual memory related to directed attention for the visuomotor task.59,60-61 Although an exploratory analysis suggests that longer-term performance changes may be associated with changes in activity in the parietal and lateral occipital cortices, the failure to identify consistent correlations in the patients is striking. In the control experiment, the visual response to the checkerboard was indistinguishable between patients and controls, demonstrating that the potential for activation of the visual cortex was not impaired in patients. These results could reflect smaller adaptive functional changes, previously proposed as a disease trait associated with loss of functional connectivity.12 Alternatively, there may be a greater heterogeneity of patterns between the patients arising from differences in individual pathologies and the functional systems that are predominantly impaired as well as from differences in the individual potential for improvement revealed or enhanced by practice. Differences in patterns of activation with a simple motor task across different stages of MS suggested the latter hypothesis.62 In our study, indirect evidence supporting this notion comes from the identification of practice-related signal reductions in a wider range of areas in patients than in controls.
There were no significant between-session changes in BOLD signal for the control visual or motor tasks for either group. This suggests that practice of the visuomotor task over this short period does not influence low-level visual or motor responses. This observation and the localization of functional changes observed with practice of the task together emphasize that the major modulatory effects of practice of this task are expressed in higher-order visuomotor control regions rather than in primary sensory and motor regions.
Potential Limitations of the Study
When comparing participants over a broad range of disabilities, the choice of an optimal task to probe visuomotor learning is problematic. We developed an isometric grip task to allow acquisition of a motor action sequence without need for fine finger control. We previously tested this task behaviorally in healthy controls21 as well as in a separate cohort of neurological patients.14,63 We have previously demonstrated a relationship between task performance and increasing MS disability and lesional damage,14 suggesting that the task reflects clinically relevant aspects of MS pathology. The impact of differences in impairment of hand function in the patients was limited by patient selection and study design: All patients were able to grip well and perform the 9-HP test, and each practice session had an initial calibration phase.
The home practice design potentially introduces inconsistencies in the way the participants practice. To limit this, each participant was trained in the use of the equipment before the home practice sessions started. We confirmed good compliance with the practice schedule for all participants by examination of the electronic records of each practice session.
There are also potential analysis confounds intrinsic to imaging-based comparisons of pathological and normal brains, which vary nonlinearly in size and shape. To limit type I error caused by GM volume loss in patients, our analysis approach included GM as a covariate, allowing us to control for differences in local anatomy, which can contribute to differences in BOLD signal generation. We also controlled the quality of our imaging preprocessing by ensuring corrections of distortions through field mapping before nonlinear registration of serial images. We took into account the potential confound of motion by including it as a regressor in the GLM analysis.
Finally, there is the potential for differential variability of fMRI responses between patients and controls. However, our previous studies of intersession variability have confirmed good reproducibility for fMRI measures both in controls and in patients.64 Along with the findings from the control visual and motor tasks, this supports our interpretation of the fMRI changes as reflecting brain plasticity specific to the task.
Conclusions
The results of this study refute the hypothesis of a general failure of adaptive brain plasticity in disabled MS patients with diffuse microstructural damage. They suggest that the concept of the progression of disability in MS should be extended from the simple focus on general failure of adaptive plasticity to the combined consequences of functional impairments arising from pathology in critical pathways and from chronic limb disuse. In this view, our study not only provides a novel framework for neurorehabilitation strategies in MS,44,45 but it also encourages efforts to develop repair strategies65 and neuroprosthetic approaches66 for functionally critical pathways in disabled MS patients.
Figure 1.
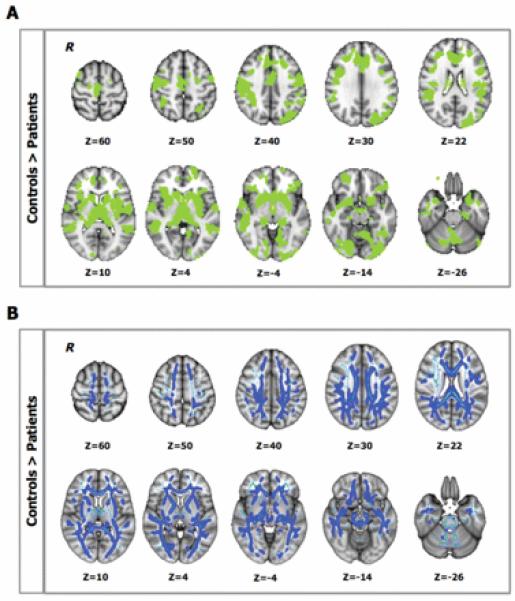
A. Between-group differences in gray-matter (GM) volume (P < .05, corrected): patients showed lower GM volume than controls in the highlighted (green) regions. B. Between-group differences in white matter (WM) fractional anisotropy (FA; P < .05, corrected): light blue defines the WM “skeleton” in which the group-based statistical contrast was carried out and superimposed; dark blue indicates regions where patients show lower FA than controls. R identifies the right hemisphere.
Acknowledgments
The authors wish to thank Dr Zsigmond T. Kincses and Mr Iain Wilson for their technical support with the experimental equipment. We are also grateful to Dr Mark Jenkinson for his support with correction of field distortions on functional data. We thank the people with MS who participated in the study.
Funding The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a grant of the Multiple Sclerosis Society UK (Grant ref. 829/05), a Training Fellowship of the Multiple Sclerosis Society Italy (VT), and the Oxford Biomedical Research Centre funded by the UK National Institute for Health Research (VT). The Wellcome Trust funds HJ-B. RGW is grateful to the UK Medical Research Council.
Footnotes
Declaration of Conflicting Interests The authors have no conflicts of interest to declare. PMM is a full-time employee of GlaxoSmithKline, which is engaged in the development of new drugs for multiple sclerosis.
References
- 1.Frischer JM, Bramow S, Dal-Bianco A, et al. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain. 2009;132(pt 5):1175–1189. doi: 10.1093/brain/awp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evangelou N, Konz D, Esiri MM, Smith S, Palace J, Matthews PM. Regional axonal loss in the corpus callosum correlates with cerebral white matter lesion volume and distribution in multiple sclerosis. Brain. 2000;123(pt 9):1845–1849. doi: 10.1093/brain/123.9.1845. [DOI] [PubMed] [Google Scholar]
- 3.Crawford DK, Mangiardi M, Xia X, Lopez-Valdes HE, Tiwari-Woodruff SK. Functional recovery of callosal axons following demyelination: a critical window. Neuroscience. 2009;164:1407–1421. doi: 10.1016/j.neuroscience.2009.09.069. [DOI] [PubMed] [Google Scholar]
- 4.Mezzapesa DM, Rocca MA, Rodegher M, Comi G, Filippi M. Functional cortical changes of the sensorimotor network are associated with clinical recovery in multiple sclerosis. Hum Brain Mapp. 2008;29:562–573. doi: 10.1002/hbm.20418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pantano P, Mainero C, Lenzi D, et al. A longitudinal fMRI study on motor activity in patients with multiple sclerosis. Brain. 2005;128(pt 9):2146–2153. doi: 10.1093/brain/awh549. [DOI] [PubMed] [Google Scholar]
- 6.Reddy H, Narayanan S, Matthews PM, et al. Relating axonal injury to functional recovery in MS. Neurology. 2000;54:236–239. doi: 10.1212/wnl.54.1.236. [DOI] [PubMed] [Google Scholar]
- 7.Wegner C, Filippi M, Korteweg T, et al. Relating functional changes during hand movement to clinical parameters in patients with multiple sclerosis in a multi-centre fMRI study. Eur J Neurol. 2008;15:113–122. doi: 10.1111/j.1468-1331.2007.02027.x. [DOI] [PubMed] [Google Scholar]
- 8.Lee M, Reddy H, Johansen-Berg H, et al. The motor cortex shows adaptive functional changes to brain injury from multiple sclerosis. Ann Neurol. 2000;47:606–613. [PubMed] [Google Scholar]
- 9.He Y, Dagher A, Chen Z, et al. Impaired small-world efficiency in structural cortical networks in multiple sclerosis associated with white matter lesion load. Brain. 2009;132(pt 12):3366–3379. doi: 10.1093/brain/awp089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giorgio A, Portaccio E, Stromillo ML, et al. Cortical functional reorganization and its relationship with brain structural damage in patients with benign multiple sclerosis. Mult Scler. 2010;16:1326–1334. doi: 10.1177/1352458510377333. [DOI] [PubMed] [Google Scholar]
- 11.Sumowski JF, Wylie GR, Deluca J, Chiaravalloti N. Intellectual enrichment is linked to cerebral efficiency in multiple sclerosis: functional magnetic resonance imaging evidence for cognitive reserve. Brain. 2010;133(pt 2):362–374. doi: 10.1093/brain/awp307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cifelli A, Matthews PM. Cerebral plasticity in multiple sclerosis: insights from fMRI. Mult Scler. 2002;8:193–199. doi: 10.1191/1352458502ms820oa. [DOI] [PubMed] [Google Scholar]
- 13.Schoonheim MM, Geurts JJ, Barkhof F. The limits of functional reorganization in multiple sclerosis. Neurology. 2010;74:1246–1247. doi: 10.1212/WNL.0b013e3181db9957. [DOI] [PubMed] [Google Scholar]
- 14.Tomassini V, Johansen-Berg H, Leonardi L, et al. Preservation of motor skill learning in patients with multiple sclerosis. Mult Scler. 2010;17:103–115. doi: 10.1177/1352458510381257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeller D, aufm Kampe K, Biller A, et al. Rapid-onset central motor plasticity in multiple sclerosis. Neurology. 2010;74:728–735. doi: 10.1212/WNL.0b013e3181d31dcf. [DOI] [PubMed] [Google Scholar]
- 16.Morgen K, Kadom N, Sawaki L, et al. Training-dependent plasticity in patients with multiple sclerosis. Brain. 2004;127(pt 11):2506–2517. doi: 10.1093/brain/awh266. [DOI] [PubMed] [Google Scholar]
- 17.Matthews PM, Johansen-Berg H, Reddy H. Non-invasive mapping of brain functions and brain recovery: applying lessons from cognitive neuroscience to neurorehabilitation. Restor Neurol Neurosci. 2004;22:245–260. [PubMed] [Google Scholar]
- 18.Georgopoulos AP, Grillner S. Visuomotor coordination in reaching and locomotion. Science. 1989;245:1209–1210. doi: 10.1126/science.2675307. [DOI] [PubMed] [Google Scholar]
- 19.Cutter GR, Baier ML, Rudick RA, et al. Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain. 1999;122(pt 5):871–882. doi: 10.1093/brain/122.5.871. [DOI] [PubMed] [Google Scholar]
- 20.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 21.Tomassini V, Jbabdi S, Kincses ZT, et al. Structural and functional bases for individual differences in motor learning. Hum Brain Mapp. 2011;32:494–508. doi: 10.1002/hbm.21037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith SM, Zhang Y, Jenkinson M, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17:479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- 23.Battaglini M, Smith SM, Brogi S, De Stefano N. Enhanced brain extraction improves the accuracy of brain atrophy estimation. Neuroimage. 2008;40:583–589. doi: 10.1016/j.neuroimage.2007.10.067. [DOI] [PubMed] [Google Scholar]
- 24.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 25.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 26.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 27.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 28.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 30.Ashburner J, Friston KJ. Voxel-based morphometry: the methods. Neuroimage. 2000;11(6, pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 31.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1, pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 32.Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- 33.Woolrich M. Robust group analysis using outlier inference. Neuroimage. 2008;41:286–301. doi: 10.1016/j.neuroimage.2008.02.042. [DOI] [PubMed] [Google Scholar]
- 34.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 35.Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- 36.Friston KJ, Worsley KJ, Frackowiak RS, Maziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp. 1994;1:214–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- 37.Reddy H, Narayanan S, Woolrich M, et al. Functional brain reorganization for hand movement in patients with multiple sclerosis: defining distinct effects of injury and disability. Brain. 2002;125(pt 12):2646–2657. doi: 10.1093/brain/awf283. [DOI] [PubMed] [Google Scholar]
- 38.Trip SA, Schlottmann PG, Jones SJ, et al. Optic nerve magnetization transfer imaging and measures of axonal loss and demyelination in optic neuritis. Mult Scler. 2007;13:875–879. doi: 10.1177/1352458507076952. [DOI] [PubMed] [Google Scholar]
- 39.Conte A, Lenzi D, Frasca V, et al. Intracortical excitability in patients with relapsing-remitting and secondary progressive multiple sclerosis. J Neurol. 2009;256:933–938. doi: 10.1007/s00415-009-5047-0. [DOI] [PubMed] [Google Scholar]
- 40.Narayanan S, Fu L, Pioro E, et al. Imaging of axonal damage in multiple sclerosis: spatial distribution of magnetic resonance imaging lesions. Ann Neurol. 1997;41:385–391. doi: 10.1002/ana.410410314. [DOI] [PubMed] [Google Scholar]
- 41.De Stefano N, Narayanan S, Francis GS, et al. Evidence of axonal damage in the early stages of multiple sclerosis and its relevance to disability. Arch Neurol. 2001;58:65–70. doi: 10.1001/archneur.58.1.65. [DOI] [PubMed] [Google Scholar]
- 42.Taub E, Crago JE, Burgio LD, et al. An operant approach to rehabilitation medicine: overcoming learned nonuse by shaping. J Exp Anal Behav. 1994;61:281–293. doi: 10.1901/jeab.1994.61-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sterr A, Shen S, Szameitat AJ, Herron KA. The role of corticospinal tract damage in chronic motor recovery and neurorehabilitation: a pilot study. Neurorehabil Neural Repair. 2010;24:413–419. doi: 10.1177/1545968309348310. [DOI] [PubMed] [Google Scholar]
- 44.Mark VW, Taub E, Bashir K, et al. Constraint-induced movement therapy can improve hemiparetic progressive multiple sclerosis: preliminary findings. Mult Scler. 2008;14:992–994. doi: 10.1177/1352458508090223. [DOI] [PubMed] [Google Scholar]
- 45.Taub E, Miller NE, Novack TA, et al. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil. 1993;74:347–354. [PubMed] [Google Scholar]
- 46.Nudo RJ. Plasticity. NeuroRx. 2006;3:420–427. doi: 10.1016/j.nurx.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doyon J, Benali H. Reorganization and plasticity in the adult brain during learning of motor skills. Curr Opin Neurobiol. 2005;15:161–167. doi: 10.1016/j.conb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 48.Kleim JA, Barbay S, Nudo RJ. Functional reorganization of the rat motor cortex following motor skill learning. J Neurophysiol. 1998;80:3321–3325. doi: 10.1152/jn.1998.80.6.3321. [DOI] [PubMed] [Google Scholar]
- 49.Martin SJ, Morris RG. Cortical plasticity: it’s all the range. Curr Biol. 2001;11:R57–R59. doi: 10.1016/s0960-9822(01)00015-x. [DOI] [PubMed] [Google Scholar]
- 50.Floyer-Lea A, Matthews PM. Distinguishable brain activation networks for short- and long-term motor skill learning. J Neurophysiol. 2005;94:512–518. doi: 10.1152/jn.00717.2004. [DOI] [PubMed] [Google Scholar]
- 51.Reddy H, Narayanan S, Arnoutelis R, et al. Evidence for adaptive functional changes in the cerebral cortex with axonal injury from multiple sclerosis. Brain. 2000;123(pt 11):2314–2320. doi: 10.1093/brain/123.11.2314. [DOI] [PubMed] [Google Scholar]
- 52.Manson SC, Palace J, Frank JA, Matthews PM. Loss of interhemispheric inhibition in patients with multiple sclerosis is related to corpus callosum atrophy. Exp Brain Res. 2006;174:728–733. doi: 10.1007/s00221-006-0517-4. [DOI] [PubMed] [Google Scholar]
- 53.Johansen-Berg H, Rushworth MF, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci U S A. 2002;99:14518–14523. doi: 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pelled G, Bergstrom DA, Tierney PL, et al. Ipsilateral cortical fMRI responses after peripheral nerve damage in rats reflect increased interneuron activity. Proc Natl Acad Sci U S A. 2009;106:14114–14119. doi: 10.1073/pnas.0903153106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cerasa A, Fera F, Gioia MC, et al. Adaptive cortical changes and the functional correlates of visuo-motor integration in relapsing-remitting multiple sclerosis. Brain Res Bull. 2006;69:597–605. doi: 10.1016/j.brainresbull.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 56.Fregni F, Boggio PS, Mansur CG, et al. Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport. 2005;16:1551–1555. doi: 10.1097/01.wnr.0000177010.44602.5e. [DOI] [PubMed] [Google Scholar]
- 57.Ward NS, Cohen LG. Mechanisms underlying recovery of motor function after stroke. Arch Neurol. 2004;61:1844–1848. doi: 10.1001/archneur.61.12.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lindenberg R, Renga V, Zhu LL, Nair D, Schlaug G. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology. 2010;75:2176–2184. doi: 10.1212/WNL.0b013e318202013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cooke SF, Bear MF. Visual experience induces long-term potentiation in the primary visual cortex. J Neurosci. 2010;30:16304–16313. doi: 10.1523/JNEUROSCI.4333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frenkel MY, Sawtell NB, Diogo AC, Yoon B, Neve RL, Bear MF. Instructive effect of visual experience in mouse visual cortex. Neuron. 2006;51:339–349. doi: 10.1016/j.neuron.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 61.Kourtzi Z, DiCarlo JJ. Learning and neural plasticity in visual object recognition. Curr Opin Neurobiol. 2006;16:152–158. doi: 10.1016/j.conb.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 62.Rocca MA, Colombo B, Falini A, et al. Cortical adaptation in patients with MS: a cross-sectional functional MRI study of disease phenotypes. Lancet Neurol. 2005;4:618–626. doi: 10.1016/S1474-4422(05)70171-X. [DOI] [PubMed] [Google Scholar]
- 63.Bosnell R, Kincses TZ, Stagg C, et al. Motor practice promotes increased activity in brain regions structurally disconnected after subcortical stroke. Neurorehabil Neural Repair. 2011;25:607–616. doi: 10.1177/1545968311405675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bosnell R, Wegner C, Kincses ZT, et al. Reproducibility of fMRI in the clinical setting: implications for trial designs. Neuroimage. 2008;42:603–610. doi: 10.1016/j.neuroimage.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 65.Martino G, Franklin RJ, Van Evercooren AB, Kerr DA. Stem cell transplantation in multiple sclerosis: current status and future prospects. Nat Rev Neurol. 2010;6:247–255. doi: 10.1038/nrneurol.2010.35. [DOI] [PubMed] [Google Scholar]
- 66.Nicolelis MA, Lebedev MA. Principles of neural ensemble physiology underlying the operation of brain-machine interfaces. Nat Rev Neurosci. 2009;10:530–540. doi: 10.1038/nrn2653. [DOI] [PubMed] [Google Scholar]