Abstract
Graft versus host disease (GVHD) remains a significant complication of allogeneic transplantation. We previously reported that the adenosine A2A receptor (A2AR) specific agonist, ATL146e, decreases the incidence and severity of GVHD in a mouse transplant model. There is increasing interest in treatments that increase CD4+CD25highFoxp3+ regulatory T cells (Tregs) to suppress GVHD. Our current study found in vitro that A2AR selective agonists enhanced TGF-β induced generation of mouse Tregs 2.3–3 fold. We demonstrated in vivo suppression of GVHD with specific A2AR agonists in two different murine GVHD transplant models associated with profound increases in both circulating and target tissue Tregs of donor origin. Three different A2AR agonists of differing potency, ATL146e, ATL370 and ATL1223 all significantly inhibited GVHD-associated weight loss and mortality. At the same time, Tregs shown to be of donor origin increased 5.1–7.4 fold in spleen, 2.7–4.6 fold in peripheral blood, 2.3–4.7 fold in colon and 3.8–4.6 fold in skin. We conclude that specific activation of A2AR inhibits acute GVHD through an increase of donor-derived Tregs. Furthermore, the increased presence of Tregs in target tissues (colon and skin) of A2AR specific agonist treated mice is likely the mechanistic basis for the anti-inflammatory effect preventing acute GVHD.
Introduction
Graft versus host disease (GVHD) continues to be a significant cause of morbidity and mortality after allogeneic hematopoietic stem cell transplantation [1]. Targeted methods to prevent or treat Graft versus host disease are in high demand. T-regulatory cells (Tregs) are a subset of CD4+CD25high T cells that express the forkhead transcription factor P3 (FoxP3) and have been shown to suppress the proliferation of T conventional cells and help promote tolerance [2,3]. There is increasing recognition that Tregs are important in preventing the development, reducing the severity, and/or mediating the resolution of GVHD [4,5]. It has been reported that donor Tregs infusions will suppress the development of GVHD in a mouse model [6–8], and that the number of Tregs in the peripheral blood and affected tissues is decreased during the development of acute GVHD in humans [9]. It is likely that Tregs act to modulate immune responses at anatomic sites of GVHD inflammation, but may also act to modulate immunity in central and peripheral lymphoid organs as well as in peripheral blood.
Cyclic AMP-elevating (c-AMP) agents are known to induce alloreactive T cell tolerance and prevent GVHD lethality in murine models [10]. Because the activation of the Gs-coupled adenosine A2A receptor (A2AR) appears to terminate inflammation through the regulation of cells that mediate both innate and adaptive immunity, it is a promising pharmacological target for the treatment of GVHD [11]. The selective activation of the A2AR has been shown to potently limit inflammation and injury in various inflammatory disease models, and the data suggests a central role for this receptor involves a feedback mechanism that inhibits the inflammation associated with activation of either innate or acquired immunity. A2AR agonists have significant protective effects in multiple models of ischemia-reperfusion injury, and inhibit the progression of inflammatory bowel disease and reduce joint destruction due to septic arthrosis [12–16]. Furthermore, it has been shown that the anti-inflammatory effects of methotrexate are mediated in part via the induction of adenine nucleotide release from injured tissue and the subsequent activation of A2ARs on local immune cells [17, 18]. Notably, T cell tolerance by T cell anergy can also be induced by selective A2AR agonist treatment [19].
We have previously shown that treatment with the specific A2AR agonist, ATL146e, decreases the incidence and severity of GVHD as well as improves survival of mice in a GVHD transplant model [20]. However, the mechanism of action of ATL146e mediating this reduction of GVHD mortality was not clearly determined. There had been a few reports exploring the relationship between Tregs and A2AR [21–24], but there was no prior evidence to show that activation of A2AR could actually induce immunosuppressive Tregs in the setting of GVHD. In our current study we found that selective A2AR agonists potently enhanced the TGF-β induced generation of mouse Tregs in vitro. In vivo in two GVHD mouse transplant models, treatment with selective A2AR agonists greatly increased the number of CD4+CD25high FoxP3+ Treg cells in peripheral blood and lymphatic tissue such as the spleen, as well as locally in tissues (skin and colon) that are the target of GVHD in these models. Our findings in vitro and in vivo strongly link the action of selective A2AR agonists to the induction of Tregs that act to reduce the development of GVHD in target tissues.
Material and Methods
Mice
For these studies, five mouse strains (with relevant H2 major histocompatibility type indicated) were purchased from Jackson Laboratory (Bar Harbor, ME): C57BL/6J (H2-Kb); B6.PL-Thy1a/CyJ (congenic to C57BL/6J carrying the Thy 1.1 allele [H2-Kb]); Balb/c (H2-Kd); B6D2F1/J (F1 hybrid cross between C57BL/6J female x DBA/2J male [H2-Kb/d]); B6.Cg-Foxp3sf/J (FoxP3 deficient scurfy on C57BL/6J background [H2-Kb]). The National Institute of Allergy and Infectious Disease Animal Care and Use Committee (Bethesda, MD, USA) approved all animal studies (ACUC approved protocol LHD-3E).
Specific A2AR agonists
The highly specific A2AR agonists ATL146e, ATL370 and ATL1223 were gifts from Forest Laboratories, Inc, New York, NY (or their fully owned subsidiaries) under material transfer agreements. ATL146e (apadenoson; Stedivaze™), the most potent of the three agonists used in our murine studies is in clinical development by Forest Laboratories, Inc as a coronary vasodilator for use in nuclear Single Photon Emission Computed Topography myocardial perfusion imaging.
Murine transplant models used for the study of GVHD induction
Exclusively female mice, 8–12 weeks old, were used both as transplant recipients and as sources of donor hematopoietic stem cells in the form of CD3+ T cell-depleted bone marrow (TCD-BM) and donor spleen-derived purified CD3+ T lymphocytes. Bone marrow cells were collected from the femurs and tibia of donors and depleted of T cells as previous described [25] to yield T cell depleted bone marrow (TCD-BM). Purified T cells were obtained from the spleens of donors using mouse CD3+ T cell enrichment columns (R&D Systems, Minneapolis, MN, USA), resulting in a T cell product of 93–97% purity.
Two transplant recipient models of murine GVHD were studied: C57BL/6J (B6) or B6.PL-Thy1a/CyJ (B6-Thy1.1) donor cells transplanted into B6D2F1/J (B6-D2) recipients (H2-Kb into H2-Kb/d model); or B6 donor cells transplanted into Balb/C recipients (H2-Kb into H2-Kd model). For some experiments B6.Cg-Foxp3sf/J (B6-FoxP3 deficient) donor cells were transplanted into B6-D2 recipients (Fox P3 deficient H2-Kb into H2-Kb/d).
Transplants were performed by conditioning recipients with 8.5 cGy for the B6-D2 transplant recipients or with 6.5 cGy for the Balb/C transplant recipients. Twenty-four hours later, mice were transplanted by lateral tail vein infusion with donor TCD-BM (10×106 cells if B6-D2 was the recipient and 5×106 cells if Balb/C was the recipient). Induction of GVHD was achieved by also infusing purified T cells (10×106 cells for B6-D2 recipients and 5×106 cells for Balb/C recipients). Transplanted B6-D2 controls that received donor TCD-BM but not purified T cells served as one type of non-GVHD control. A second control used B6-D2 mice transplanted with B6-D2 T replete marrow (referred to as BM only).
Unless alternatively specified in the results, primed Alzet osmotic minipumps (Durect Corp., Cupertino, CA, USA) were implanted subcutaneously 24 h before irradiation for a constant rate delivery of 10 ng/kg/min ATL146e, 50ng/kg/min ATL370, 200 ng/kg/min ATL1223, or vehicle for 14 days. These specified doses of the different A2AR specific agonists were determined from preliminary dose finding experiments used to delineate doses with maximal benefit and minimal side effects. Weights were monitored daily and animals sacrificed when total weight loss exceeded 25% of their starting body weight.
Flow cytometry for T regulatory cells
Peripheral blood was collected from the tail vein or when euthanized, via cardiac puncture. Spleens were also collected from euthanized mice. Finely minced spleen cell suspensions were passed through a 40-mm nylon cell strainer (BD Biosciences, San Jose, CA, USA), and collected in phosphate buffered saline (PBS). RBCs were removed with ACK lysis buffer (Quality Biological Inc., Gaithersburg, MD, USA). Cells were washed and resuspended at 5×106 cells/ml in PBS with 0.2% bovine serum albumin (Sigma-Aldrich, St. Louis, MO, USA). Aliquots (0.1 ml) were then placed at 4 °C and labeled for 30 min in the dark with fluorochrome-labeled anti-mouse CD4 (RM4-5) and anti-mouse CD25 (PC61.5)(eBioscience, San Diego, CA, USA). FoxP3 was stained by intracellular analysis after fixation and permeabilization using a PE-conjugated anti-mouse FoxP3 (FJK-16s) antibody (eBioscience, SanDiego, USA). Control samples were labeled with isotype-matched control antibodies. The samples were run on a benchtop flow cytometer (FACSort, Becton Dickinson, San Diego, CA, USA) with a minimum of 10,000 events collected. Analysis was performed with Cell Quest (Tree Star, Inc., Ashland, OR, USA).
Measurement of serum cytokines
Cytokine concentrations in serum samples were measured using mouse cytokine/chemokine multiplex immunoassay kits (Linco, St. Charles, MO, USA) with the BioPlex system (BioRad, Hercules, CA, USA) using Luminex technology. Serum levels of TGF-β were measured by ELISA (enzyme-linked immunoabsorbent assay, R&D System Inc., Minneapolis, MN, USA).
Histopathological analysis and immunohistochemistry
Skin and colon were harvested from euthanized mice and fixed in 4% paraformaldehyde in PBS, pH 7.4, and embedded in paraffin. Sections (4 μm) were subjected to standard Heamatoxylin and Eosin (H&E) staining and immunochemical staining. Each section was then scored separately by a board certified toxicologic pathologist, blinded to animal status, according to the published criteria [26–28]. Briefly, liver was scored according to bile duct damage, presence of apoptotic bodies, damaged biliary epithelium with lymphocytic infiltrate and cell debris in both hepatocytes and Kupffer cells. Skin was scored by changes in the subcutaneous fat, an influx of neutrophil and mononuclear cells, and increased skin thickening with hyperkeratosis and epidermal hyperplasia. Colon was scored by presence of apoptotic bodies in the crypt epithelium, damage and reactive epithelial changes as well as neutrophils in the lumen of the central crypt. Immunostaining for FoxP3+ T regulatory cells was performed using an anti-FoxP3 (150D/D4) primary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Cell counts were performed over five areas chosen randomly in at least 3 different tissue sections each from a minimum of 3 mice and analyzed in a blinded manner.
Preparation of lymphocytes from colonic lamina propria or skin
To isolate infiltrating lymphocytes in the skin, 100 mg of skin was pooled, minced, and then digested with 2.5 mg/ml type I collagenase (Sigma-Aldrich, St. Louis. MO) and 0.25 mg/ml hyaluronidase (Sigma-Aldrich, St. Louis. MO) for 2 h at 37 °C [29]. Colonic lamina propria lymphocytes were isolated as described previously [30]. Briefly, 100 mg of colonic tissue was washed in medium (RPMI 1640 supplemented with penicillin and streptomycin), then minced. The specimen was then digested in RPMI 1640 medium containing 2% fetal bovine serum (FBS) and 1 mg/ml type IV collagenase (Sigma-Aldrich, St. Louis. MO) for 2 h at 37°C with 5% CO2. A single-cell suspension was obtained by passing the digested tissue through a cell strainer. Absolute cell number was determined as described previously [30].
RNA extraction and real-time reverse transcription–PCR for FoxP3
Total RNA from colon and skin was isolated using TRIzol (Invitrogen, Grand Island, NY). cDNA was synthesized from 500 ng total RNA using Maxima First strand cDNA synthesis kit (Fermentas Inc, Glen Burnie, MD) according to the manufacturer’s recommendation. For FoxP3 and glyceraldehyde phosphate dehydrogenase (GAPDH) transcripts, real-time polymerase chain reaction (PCR) was performed with a 7500 Real Time PCR system (Applied Biosystems, Foster City, CA) based on specific primers and general fluorescence detection with SYBR Green. The following primer combinations were used: mouse FoxP3, forward 5′-AGGAGCCGCAAGCTAAAAGC-3′ and reverse 5′-TGCCTTCGTGCCCACTGT-3′; mouse GAPDH, forward 5′-CTCATGACCACAGTCCATGC-3′ and reverse, 5′-CACATTGGGGGTAGGAACAC-3′. All PCRs were performed using Maxima SYBR Green/ROX qPCR Master Mix (Fermentas Inc, Glen Burnie, MD). Relative gene expressions of FoxP3 in skin or colonic tissues were normalized to GAPDH.
Generation of Tregs in vitro by culture of CD4+CD25− T cells with TGF-β
CD4+CD25− T cells were isolated from single cell suspensions of spleens from naive B6 mice using components of the Miltenyi CD4+CD25+ Regulatory T Cell Isolation Kit (Miltenyi BioTec Inc, Auburn, CA) to first positively select for CD4+ T cells and then negatively select the purified CD4+ T cells to isolate the CD25 negative subset following manufacturer’s instructions. The purified CD4+CD25− T cells were cultured in a 24-well plate (3×105 cells/well), stimulated with anti-CD3 and anti-CD28 antibody Dynabeads (Invitrogen, Carlsbad, CA, USA) for 3 days in the presence of TGF-β (2 ng/ml) (Peprotech, Rocky Hill, NJ), without or with the addition of rapamycin (10 nM) (LC Laboratories, Woburn, MA), Cyclosporine A (CsA, 10 nM) (LC Laboratories, Woburn, MA), the A2AR antagonist ZM241385 at 1 μM (Sigma Aldrich) or one of the selective A2AR agonists (ATL370 or ATL1223, 10 nM). The experiment was repeated independently three times.
Statistics
Prism software (GraphPad Software, Inc. La Jolla, CA) was used for all statistical analyses. Student’s t tests or one-way analysis of variance (ANOVA) with post-hoc Dunnett’s multiple comparison were used to compare experimental groups with a control group. Histopathological semi quantitative scoring was analyzed using nonparametric Kruskal-Wallis test and Dunn’s Multiple Comparison Test.
Results
Selective A2AR agonists augment in vitro TGF-β stimulated differentiation of FoxP3+ T cells from naïve CD4+CD25− T cells
CD4+CD25− T cells purified from B6 spleens were stimulated in culture with anti-CD3 and anti-CD28 antibody Dynabeads together with TGF-β, in the absence or presence of the selective A2AR agonists ATL370 or ATL1223 for 3 days. Initially, less than 0.1% of the CD4+CD25− T cells were FoxP3 positive. Controls substituted rapamycin or CsA for the selective A2AR agonists. Other controls omitted TGF-β from the culture. At 3 days with TGF-β alone, the proportion of CD4+FoxP3+ T cells increased to 4.9 ± 0.3% compared to 0.47 ± 0.7% for the control cultures without TGF-β (Fig. 1A upper group [representative experiment] and Fig. 1C [average of 3 independent experiments]). The addition of rapamycin (known to enhance generation of Tregs, [31]) enhanced the TGF-β stimulated generation of CD4+FoxP3+ T cells to 9.7 ± 2.5%. In contrast, addition of CsA (known to interfere with generation of Tregs, [31]) inhibited the TGF-β stimulated generation of CD4+FoxP3+ T cells to 0.3 ± 0.2% (Fig. 1C).
Figure 1. The effects of selective A2AR agonists on TGFβ-mediated differentiation of FoxP3+ cells from naïve CD4 cells.
(A) CD4 cells were isolated from normal B6 mouse spleen by MACS. The cells were stimulated with Dynabeads-mouse T cell activator (anti-CD3/anti-CD28 antibody) in the presence of no TGF-β, TGF-β (2 ng/ml), TGF-β plus rapamycin (rapa, 10 nM) or TGF-β plus Cyclosporine A (CsA, 10 nM), TGF-β plus ATL370 (10 nM), or TGF-β plus ATL1223 (10 nM), and analyzed for FoxP3 expression. (B) Addition of A2AR antagonist ZM241385 (ZM, 1 μM) 24hr pretreatment on panel (A) and experiment (C). The data shown are representative of 3 independent experiments and gives the mean percentage of positive cells ± SEM. P values were calculated, comparing TGF-β only versus TGF-β plus ATL370 or TGF-β plus ATL1223 and comparing TGF-β plus ATL370 versus TGF-β plus ATL370 plus ZM241385 or TGF-β plus ATL1223 versus TGF-β plus ATL1223 plus ZM241385 by student’s t tests.
The addition of the selective A2AR agonists ATL370 or ATL1223 enhanced the TGF-β stimulated generation of CD4+FoxP3+ T cells to 14.5 ± 1.0% or 11.5 ± 1.3%, respectively (Fig. 1A lower group and Fig. 1C block bar). Thus, when used at equimolar concentrations (10 nM), the two selective A2AR agonists, ATL370 or ATL1223 were more effective than rapamycin at enhancing TGF-β stimulated generation of CD4+FoxP3+ T cells in vitro, increasing generation by 2.3 to 3 fold over TGF-β alone (p = 0.0009 and p = 0.0257 comparing TGF-β alone vs. with ATL370 or ATL1223 respectively, over 3 experiments). This observation in vitro suggested that selective A2AR agonists could act in vivo to directly increase Treg generation as a mechanism to reduce development of GVHD.
Additionally, use of the A2AR antagonist (ZM 241385) 24hrs before the addition of the selective A2AR agonists was used to further confirm that the increase of the T regulatory population was due to the effect of the A2A receptor agonist. ZM 241385 treatment combined with the A2AR agonist decreased the generation of CD4+FoxP3+ T cells in vitro by 2.9 to 3.5 fold compared to ATL370 or ATL1223 alone (p = 0.0008 and p = 0.0131 respectively comparing ATL370 or ATL1223 alone vs. with the antagonist over 3 experiments, Fig. 1B). In fact the number of CD4+FoxP3+ T cells with both ZM 241385 and A2AR agonist treatment (4.1 ± 1.5% with ATL370 or 3.9 ± 1.2% with ATL1223, Fig. 1C) was similar to treatment with TGF-β alone (4.9 ± 1.3%, Fig. 1C). Interestingly, ZM 241385 did not affect CD4+FoxP3+ T cell generation in cells treated with either TGF-β or rapamycin (Fig. 1C).
Selective A2AR agonists ameliorates weight loss and mortality due to acute GVHD
We previously reported that the A2AR specific agonist, ATL146e, can decrease the incidence and severity of GVHD induced weight loss as well as improve survival of mice in a B6 into B6-D2 model of transplant-related GVHD [20], but did not delineate a mechanism for this effect. In the current study we also studied the effects of additional A2AR specific agonists, ATL370 and ATL1223 as well as used a second model with a greater degree of mismatch, that is the B6 into Balb/C model.
In Figures 2A and 2B we confirmed our previous observation that continuous administration of ATL146e by subcutaneous osmotic pump for 14 days, beginning 2 days before transplant of B6 TCD-BM plus T cells into B6-D2 mice reduces both GVHD associated weight loss and mortality compared to mice treated with vehicle control. In addition the protective effect of ATL146e administered for 12 days post transplant is observable up to 40 days post-transplant shown in this experiment. In Figures 2C and 2D both ATL370 and ATL1223 similarly delivered for 14 days by osmotic pump beginning 2 days before transplant of B6 TCD-BM plus T cells into B6-D2 mice reduces both GVHD associated weight loss and mortality compared to mice treated with vehicle control. Furthermore, the effect is again observable out to 30 days post-transplant as shown in this experiment. Finally, using the more severe GVHD model of B6 into Balb/C, we examined the ability of ATL370 or ATL1223 again delivered for 14 days by osmotic pump beginning 2 days before transplant to reduce the development of severe GVHD associated weight loss and accelerated mortality compared to mice treated with vehicle control (Figs. 2E and 2F).
Figure 2. A2AR agonists ameliorate GVHD after allogeneic HST.
Weight loss and survival are shown in mice receiving TCD-BM plus Donor T cells after treatment with either ATL146e (A and B) or ATL370 or ATL1223 (C and D) in the B6 into B6-D2 model, with ATL370 or ATL1223 (E and F) in the B6 into Balb/C model. Data shown are from three independent experiments (N = 15); error bars indicate SEM. Mortality was defined as mice that were found dead or had reached a moribund state due to excessive body weight loss, necessitating euthanasia.
Mice in all groups that received donor TCD-BM only (without additional donor T cells) did not develop acute GVHD associated weight loss nor did they have significant mortality over the indicated observation periods (data not shown). All of the A2AR agonist treated mice had reduced GVHD-associated weight loss and mortality as compared to vehicle only-treated mice. Further, when using ATL370 or ATL1223 (Figs. 2D and 2F) more than 50 % of the A2AR agonist-treated animals survived beyond 30 days post transplant as compared to 0% survival of vehicle-treated controls in either transplant model. This effect was dose dependent as osmotic pump administration of ATL370 at 10 ng/kg/min and ATL1223 at 50 ng/kg/min did not at all inhibit GVHD associated weight loss in either model (data not shown), but the use of 50 ng/kg/min of ATL370 and 200 ng/kg/min of ATL1223 as shown in Figures 2C–F significantly reduced weight loss and mortality.
Inhibitory effect of selective A2AR agonists on proinflammatory cytokine and chemokine production in the acute GVHD mouse model
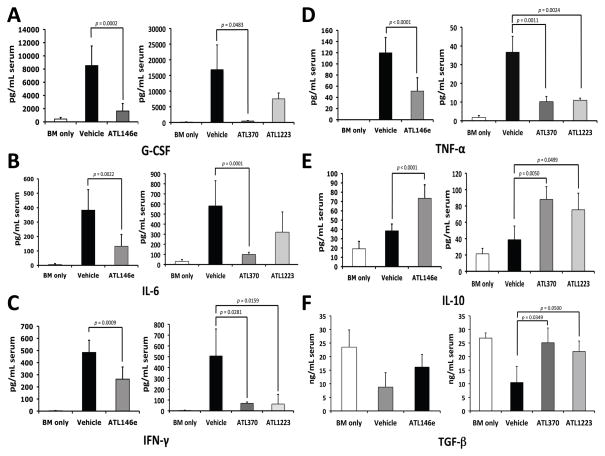
In the setting of GVHD, G-CSF, IL-6, IFN-γ and TNF-α appear to be proinflammatory cytokines that correlate with disease severity, whereas increased levels of IL-10 and TGF-β may be protective, or may correlate with control or resolution of GVHD [32–34]. Serum levels of G-CSF, IL-6, IFN-γ, TNF-α, IL-10 and TGF-β were thus analyzed as an indicator of inflammatory activity of acute GVHD at 16 to 20 days in the B6 into B6-D2 model of TCD-BM plus T cell transplant-related GVHD. We examined the effect of vehicle, ATL146e (10 ng/kg/min), ATL370 (50 ng/kg/min), or ATL1223 (200 ng/kg/min) delivered for 14 days by osmotic pump beginning 2 days prior to transplant. For these studies, we used the levels from B6-D2 mice transplanted with cells from B6-D2 donors (BM only) as our baseline control.
The serum levels of TNF-α, IFN-γ, IL-6 and G-CSF were significantly lower in mice treated with the A2AR agonists compared to the vehicle-treated controls (Fig 3A–D). Furthermore, when we measured serum levels of the anti-inflammatory, tolerance-inducing cytokines IL-10 and TGF-β (Fig 3E and 3F), these were significantly increased by 1.5 or 1.5–2.5 fold, respectively, when compared to vehicle only. It should be noted that in the BM only control, baseline levels of pro-inflammatory cytokines were very low while the levels of the anti-inflammatory cytokines were much higher compared to any of the experimental values. Treatment of the experimental mice with A2AR agonists resulted in all cases in reversion of the cytokine levels toward the values seen in the BM only controls. These data indicate that activation of the A2Areceptor can reduce systemic levels of proinflammatory cytokines and increase immunotolerogenic cytokines in the setting of transplant-related GVHD.
Figure 3. GVHD-induced elevation of proinflammatory cytokine and chemokine levels is reduced by A2AR agonists administration in the B6 into B6-D2 model.
Blood was collected 16 to 20 days after transplant and serum concentrations of G-CSF (A), IL-6 (B), IFN-γ (C), TNF-α (D), IL-10 (E) and TGF-β (F) were measured using multiplex immunoassay kits with the BioPlex system and ELISA assay. Data shown are from three independent experiments (N = 9); error bars indicate SEM. P values were calculated comparing agonist to vehicle control by student’s t tests.
Selective A2AR agonist induces CD4+ CD25high FoxP3+ T regulatory cells in acute GVHD mouse model
Here we showed in vitro that A2AR activation significantly augments TGF-β induced generation of mouse CD4+FoxP3+ T cells. Furthermore, our in vivo studies in the transplant-related GVHD mouse model demonstrated that the A2AR activation-associated amelioration of GVHD is associated with significant reduction in levels of serum proinflammatory cytokines and significant increase in serum levels of IL-10 and TGF-β, which are known to be factors in the generation of Tregs. In addition there is further evidence in vivo from a mouse model of autoimmunity that A2AR induced amelioration of autoimmunity is associated with generation of Tregs [24].
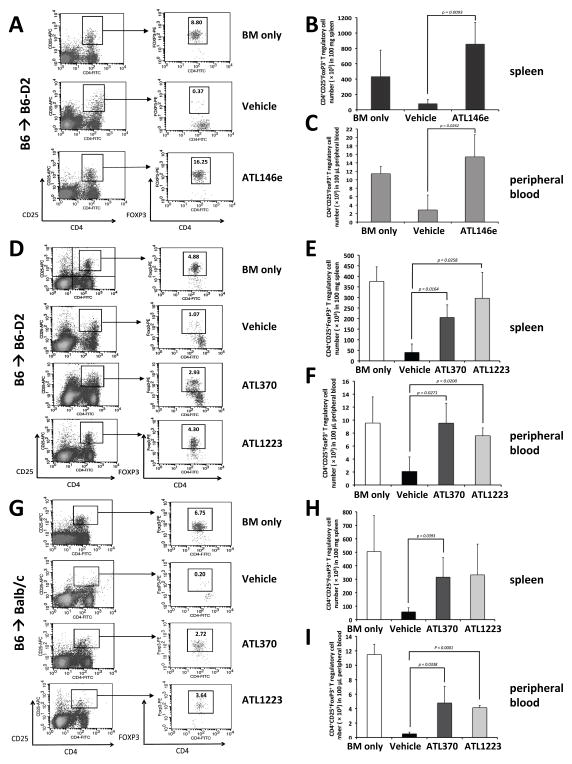
We therefore next sought to determine whether specific A2AR agonist treatment in the mouse transplant-related GVHD models was associated with changes in Tregs in lymphoid organs, peripheral blood, and at anatomic target sites of GVHD. In the analysis shown in Figure 4 we used our mouse GVHD models (B6 into B6-D2 shown in Figs. 4A–F; B6 into Balb/c shown in Figs. 4G–I) to examine the absolute number of CD4+CD25highFoxP3+ Tregs appearing in the spleen and peripheral blood 16–20 days post transplant. We compared treatment with vehicle versus specific A2AR agonists delivered by route, dose, and time noted in the previous results and as describe in the Methods section. Again, “BM only” refers to the B6-D2 into B6-D2 control transplanted with unmanipulated marrow and no additional T cells.
Figure 4. A2AR agonists increase T regulatory cells in acute GVHD mouse models.
The percentages or number of CD4, CD25, FoxP3 positive cells in various tissues post transplant are shown. Panel (A): data from a representative experiment using the B6-D2 into B6 model. Panels (B) and (C): cumulative number of CD4, CD25, FoxP3 positive cells data from 3 experiments analyzing 100 mg of spleen and 100 μl of peripheral blood respectively. Panel (D): data from a representative experiment using the same model but treated with either ATL370 or ATL1223. Panels (E) and (F): cumulative data from 3 independent experiments for both agonists. Data from the B6 into Balb/C recipients is shown in Panels G, H and I where G is the data from one representative experiment and Panels H and I, the cumulative data for spleen and peripheral blood from 3 separate experiments. For all experiments, P values were calculated comparing vehicle control to drug using student’s t test.
As expected, splenic CD4+CD25highFoxP3+ Tregs were significantly decreased in both of the vehicle treated acute GVHD mouse models compared to “BM only” controls. Conversely, the number of Tregs in the “BM only” groups was similar to that measured in the spleens of untransplanted/untreated mice (data not shown). Treatment of mice from both GVHD models with any of the three specific A2AR agonists resulted in a profound increase in CD4+CD25highFoxP3+ Tregs in the spleen compared to the vehicle treated GVHD mice (Fig. 4A, 4D and 4G). With the B6 into B6-D2 model this increase in number compared to vehicle treatment averaged almost 10 fold with ATL146e (p = 0.0093) treatment, and 5–7 fold with ATL370 (p = 0.0164) or ATL1223 (p = 0.0258) treatment (Fig. 4B and 4E). In the greater mismatched B6 into Balb/C model the increase in splenic Tregs averaged almost 5–6 fold with ATL370 (p = 0.0393) or ATL1223 (p = 0.0616) treatment (Fig. 4H).
In addition to the spleen, we also measured the absolute number of CD4+CD25highFoxP3+ Tregs in the peripheral blood finding a 5.4 fold increase with ATL146e (p = 0.0262) treatment and 3.6–4.6 fold increase with ATL370 (p = 0.0271) or ATL1223 (p = 0.0200) treatment in the B6 into B6-D2 model (Fig. 4C and 4F). In the B6 into Balb/C model, peripheral blood Tregs were increased 8.1–9.4 fold with ATL370 (p = 0.0338, Fig. 4I) or ATL1223 (p < 0.0001, Fig. 4I). These results suggest that specific activation of A2AR inhibits acute GVHD through decreased proinflammatory cytokine production, induction of IL-10 and TGF-β and an increase in CD4+ CD25high FoxP3+ immunosuppressive T regulatory cells in spleen and peripheral blood.
T regulatory cells increased by the selective A2AR agonist in the GVHD mouse model were of donor origin
We determined the origin of A2AR induced Tregs by substituting donor cells from B6-Thy1.1 instead of B6 for transplant into B6-D2 recipients to allow detection of Thy1.1 on Tregs appearing in the transplanted mice. This B6-Thy1.1 into B6-D2 model also replicated the acute GVHD symptoms similar to the B6 into B6-D2 model, and also responded to treatment with ATL146e, resulting in less weight loss and improved survival (data not shown). As shown in Figure 5A–B, measuring Tregs in spleen and peripheral blood, we confirmed again the severe depletion of Tregs in the vehicle treated GVHD model (2.91 ± 2.52 % of CD4+ cells in spleen, 0.88 ± 0.51 % of CD4+ cells in peripheral blood) compared to “BM only” control (12.06 ± 3.43 % of CD4+ cells in spleen, 15.64 ± 6.55 % of CD4+ cells in peripheral blood), but significant restoration of the level of Tregs almost to the level of the “BM only” control in the B6-Thy1.1 into B6-D2 GVHD model treated with ATL146e (10.66 ± 5.18 % of CD4+ cells in spleen, 12.10 ± 4.28 % of CD4+ cells in peripheral blood, Figure 5C–D). Furthermore, gating on Tregs and assessing expression of the Thy1.1 donor marker versus Thy1.2 recipient marker (Fig. 5A and 5B right hand panels) demonstrated that in the “BM only” control all Tregs expressed only Thy1.2, as expected, while no Thy1.2 Tregs are detected in the vehicle or ATL146e treated B6-Thy1.1 into B6-D2 GVHD model transplant groups. Virtually all of the Tregs arising in the GVHD model transplant mice were Thy1.1 donor origin. This was seen in both the vehicle treated GVHD model, where there is a relative paucity of Tregs compared to the “BM only” control, and in the ATL146e treated GVHD model where relative numbers of Tregs are restored closer to levels seen in the “BM only” control. We also observed the same results when we used either ATL370 or ATL1223 (data not shown).
Figure 5. T regulatory cells that are increased by A2AR agonists are donor derived in acute GVHD.
Shown are the percentages of CD4, CD25, FoxP3 positive cells found in B6-D2 recipients using B6-Thy1.1 donors and treated with either vehicle or ATL146e or receiving T replete marrow from B6-D2 donors (BM only) found in the spleen (A) or peripheral blood (B) with the data from 3 separate experiments shown in (C) and (D). P values were calculated comparing treatment to vehicle control by student’s t test (N = 9). Panels (E) and (F): weight loss and mortality of B6-D2 recipients despite using either ATL 370 or 1223 when Fox P3 deficient mice are used as transplant donors. Panels (G–L): A2AR agonist administration does not effect GVHD-induced elevation of proinflammatory cytokine and chemokine levels in the B6-FoxP3 deficient mice into B6-D2 model. Blood was collected 16 to 20 days after transplant and serum concentrations of G-CSF (G), IFN-γ (H), IL-6 (I), TNF-α (J), IL-10 (K) and TGF-β (L) were measured using multiplex immunoassay kits with the BioPlex system and ELISA assay. Data is from two independent experiments (N = 10); error bars indicate SEM.
Since the Tregs arising in the GVHD model of transplant are all of donor origin, we next performed experiments to determine whether functional FoxP3 in donor cells was required for A2AR agonist efficacy. B6-FoxP3 deficient mice, (scurfy mouse on B6 background) have been shown to be incapable of generating functional Tregs and suffer from a variety of autoimmune problems. When B6-FoxP3 deficient donors were transplanted into B6 recipients, the two groups of A2AR agonist treatment mice (ATL370 and ATL1223) experienced the same accelerated body weight loss and mortality as the Vehicle treated mice (Fig. 5E and 5F; compare to Fig. 2C and 2D). We measured serum levels of G-CSF, IL-6, IFN-γ, TNF-α, IL-10 and TGF-β at 16 to 20 days in the B6-FoxP3 deficient mice into B6-D2 model. Proinflammatory cytokines (G-CSF, IL-6, IFN-γ and TNF-α) were not significantly decreased in two groups of A2AR agonist treatment mice (ATL370 and ATL1223; Fig 5G–J). This result can be compared to the serum levels of proinflammatory cytokine in the B6 into B6-D2 model (Fig. 3A–D). Moreover, serum levels of IL-10 and TGF-β were also no different between the vehicle control group and A2AR agonist treatment groups (ATL370 and ATL1223, Fig. 5K and 5L; compared to Fig. 3E and 3F). This then confirms the absolute requirement for the donor graft to develop functional Tregs in order for A2AR agonist treatment to provide protection against increased mortality from GVHD in this model.
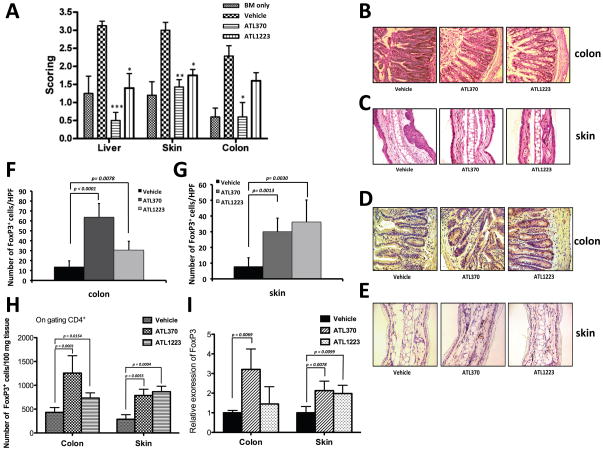
Mice treated with the A2AR have increased numbers of FoxP3+ cells in both skin and colon
As the skin and gastrointestinal tract are target organs of GVHD, we performed histopathological analysis by Heamatoxylin and Eosin (H&E) staining and immunohistochemical staining on liver, ear and colon biopsies obtained at day 16 to 20 in our B6 into B6-D2 GVHD mouse model recipients after treatment with either of the two A2AR agonists (ATL370 or ATL1223) or Vehicle only. Pathological scoring for evaluation of GVHD in the target tissues (liver, skin and colon) was performed by a pathologist blinded to the treatment status of the mouse (Table 1). Animals treated with either agonists had significantly lower scores than vehicle treated mice and in some cases was similar to that of bone marrow only transplanted mice (Fig. 6A). Vehicle treated mice group demonstrated severe inflammation, necrosis, edema, and amyloid-like material in the lamina propria of the colon (Fig. 6B), and inflammation with thickness, ulceration and pustule formation in the skin (Fig. 6C). However, ATL370 or ATL1223 mice had minimal focal ulceration, a less-pronounced submucosal edema in the colon (Fig. 6B) and mostly normal-appearing skin (Fig. 6D).
Table 1. Semiquantitative histopathological analysis.
The pathologist was blinded to the samples and graded for GVHD in multiple samples from animals treated with bone marrow transplant only (a non GVHD control), Vehicle, or either A2A agonist. The mean score (± SEM) is given for each tissue type.
| BM only | Vehicle | ATL370 | ATL1223 | |
|---|---|---|---|---|
| Liver | 1.250 ±0.957 | 3.125 ±0.354 | 0.500 ±0.548 | 1.400 ±0.894 |
| Skin | 1.200 ±0.837 | 3.000 ±0.577 | 1.429 ±0.535 | 1.750 ±0.463 |
| Colon | 0.600 ±0.548 | 2.286 ±0.756 | 0.600 ±0.894 | 1.600 ±0.548 |
Figure 6. ATL370 and ATL1223 increase T regulatory cells in colon and skin of acute GVHD mouse model.
(A) The cumulative results of the histopathological scoring are represented in bar graph form. (*: P < 0.05, **: P<0.01, *** P<0.001 comparison with Vehicle). Colon (B and D) or Skin (C and E) was obtained, placed in 4% paraformaldehyde 16 days to 20days after transplantation and paraffin embedded and Heamatoxylin and Eosin staining (B and C) and immunohistochemistry (IHC) by mouse FoxP3 antibody (D and E) was performed. Panels (F) and (G), the numbers of FoxP3+ cells (dark brown stained cells) were counted per high power field (HFP) for colon or skin respectively with magnification of 40X. Data shown are the cells counted from a random area (N = 5); error bars indicate SEM. Panel (H), FACS analysis of infiltrating T regulatory cells numbers per 100 mg of skin or colon tissues in B6-D2 recipients. Skin or colon tissues were digested as described in Material and Methods and stained with antibodies for CD4, CD25 and FoxP3. Data are shown mean (± SEM) from 5 independent skin or colon samples from each group. Panel (I), Relative gene expression of FoxP3 in skin or colon tissues in B6-D2 recipients. Total RNA was extracted from skin or colon tissues and analyzed by real-time RT-PCR. Levels were normalized using GAPDH mRNA. Data represent the mean (± SEM) from 4 independent skin or colon samples from each group. P value vs. vehicle control as assessed by student’s t test.
As shown in Fig. 6D (colon) or Fig. 6E (skin) immunohistochemical staining for FoxP3 showed that transplanted mice receiving the A2AR agonists appeared to have more FoxP3+ cells than those treated with Vehicle. We quantified this observation by having a blinded observer count the number of FoxP3 positive cells in a random sampling of high power fields as noted in the Methods. The results of this analysis for colon and for skin are shown in Figs. 6F and 6G, respectively. In mice treated with vehicle control and with active GVHD the number of FoxP3+ cells detectable in colon was 14 ± 6 per high power field. However, in the ATL370 treated mice, there were 64 ± 14 FoxP3+ cells per high power field in colon, a difference that was statistically significant compared to the Vehicle control group (p < 0.0001, Fig. 6F middle bar). The trend was similar in the colon of ATL1223 treated mice, though the increase in FoxP3+ cells also reached statistical significance (p = 0.0078, Fig. 6F right bar). On the other hand, the number of FoxP3+ T cells per high power field in skin was only 8 ± 6 in the vehicle control, but increased to a highly significant 30 ± 7 in the ATL370 treated mice (p = 0.0013, Fig. 6G middle bar) and to 36 ± 14 in the ATL1223 treated mice (p = 0.0030, Fig. 6G right bar). We also determined the number of infiltrating T regulatory cells in colon and skin by flow cytometry analysis after extraction of lymphocytes from the tissues. In mice treated with vehicle control and with active GVHD, the number of CD4+FoxP3+ cells in 100 mg of colon was 432 ± 101. However, we measured 1256 ± 365 of CD4+FoxP3+ cells in the ATL370 treated mice and 732 ± 110 of CD4+FoxP3+ cells in ATL1223 treated mice which is statistically significant higher than the Vehicle control group (p = 0.0001, p = 0.0154, Fig. 6H). As well, the number of T regulatory cells in the skin was 287 ± 97 in the vehicle control mice, but increased to 786 ± 132 in the ATL370 treated mice (p = 0.0055) and to 865 ± 115 in the ATL1223 treated mice (p = 0.0004). Therefore the number of infiltrating T regulatory cells in skin or colonic tissues were increased 1.7 to 3 fold after treatment with an A2AR agonist for acute GVHD. Furthermore, we observed FoxP3 gene expression increased 3.21 fold (p = 0.0069, Fig. 6I) in the colonic tissues of ATL370 treated mice as measured by increased mRNA levels, although we did not see a difference when using ATL1223. In the skin, FoxP3 gene expression also increased 2 fold (p = 0.0078 for ATL370, p = 0.0099 for ATL 1223 compare to vehicle control, Fig. 6I) for each of the A2AR agonists treated groups.
Discussion
Allogeneic transplantation is being increasingly used to cure a wide variety of cancers and monogenetic disorders of blood and other organs. However, acute and chronic GVHD remain as some of the most significant adverse effects of this potentially lifesaving procedure. Current treatment for GVHD involves the use of high dose immunosuppressants including corticosteroids, various inhibitors of T cell function and monoclonal antibodies directed at T and B cell antigens or pro-inflammatory signaling pathways, which in themselves impair innate and acquired immunity, may impair engraftment or impede anti-tumor effects and lead to a significantly increased risk of infection. There is a need for new agents with alternate mechanisms of action to prevent GVHD, particularly those that act to enhance tolerance rather than merely suppress T cell function. More targeted prevention and treatment of GVHD is therefore a goal of many transplanters. Our study demonstrates that specific activation of A2AR mediates reduction of acute GVHD pathology, symptoms, and improves survival rate in mouse mismatch transplant models in large part by enhancing development of Tregs that act to increase graft tolerance to host antigens.
Additionally, A2AR blocks the function of effector T lymphocytes by suppressing proinflammatory cytokine production, and decreasing expression of activation markers and proliferation [35–37]. It has been reported that activated conventional T cells in the presence of an A2AR agonist in vitro are anergic [24], and it has been shown that activation of the A2AR can prevent T cell proliferation or production of IL-2 or IFN-γ upon restimulation. Our study suggests that in vivo these effects of A2AR agonist treatment are likely augmented or mediated by an increased production of Tregs. In this study, we examined the selective A2AR agonists of differing potency (ATL146e, ATL370 and ATL1223) in the reduction of GVHD pathology in two models of transplant related GVHD in mice. We showed a profound protection against weight loss and mortality, which was associated with a decrease in proinflammatory cytokines, increase in protective anti-inflammatory cytokines, and enhanced production of protective Tregs in spleen, peripheral blood and target tissues (colon and skin). We demonstrated that these A2AR agonist induced protective Tregs were of donor origin, and that failure to produce Tregs (using FoxP3 deficient donor cells) resulted in failure to provide protection against GVHD mediated mortality. Tregs unequivocally have been shown to play an important role in development of donor tolerance to host, with protection against development of GVHD [38–42]. In a recent clinical study it was shown that infusion of Tregs derived in vitro from umbilical cord blood can safely reduce the incidence of GVHD [43,44]. These reports provided an important basis for initiating our assessment of the effect of A2AR activation on generation of Tregs in our acute GVHD mouse models. We showed that CD4+CD25highFoxP3+ Tregs are increased by all three of the A2AR agonists that we studied, though in general the most potent and specific agonist, ATL146e, had the most profound effect. We also documented significant reduction of peripheral blood proinflammatory cytokine levels, including IL-6, INF-γ, IL-2 and TNF-α and increased levels of the immunosuppressive cytokine, IL-10 and TGF-β from administration of A2AR agonist relative to vehicle controls.
There have also been some reports suggesting that adenosine activation, via the A2AR, not only can enhance generation of Tregs, but that the effectiveness of the tolerogenic action of those Tregs can be increased by engaging the A2AR on the target effector T cells [21]. In fact some have suggested that generation of adenosine by Tregs suppresses effector T cells via the A2AR. In an immune mediated colitis model, it was found that CD45RBlow or CD25+ Tregs from A2AR−/− mice failed to suppress CD45RBhigh cells and prevent disease induced by pathogenic CD45RBhigh T cells, and that CD45RBhigh effector T cells from A2AR−/− mice are not suppressed by suppressor Treg cells derived from wild type mice [23]. Furthermore, the Powell laboratory group has reported that extracellular adenosine stimulates the A2AR to promote T cell anergy and the generation of FoxP3+ and LAG3+ Tregs by reducing the production of IL-6 and enhancing the production of TGF-β after antigen stimulation [24] It has also been shown that mice lacking the CD73/ecto-5′-nucleotidase appear to have increased GVHD due to the inability to convert AMP to adenosine [45]. Further, autoimmunity in ADA-SCID patients has been linked to a dysregulation of the CD39/73 adenosinergic pathways resulting in aberrant T regulatory function [46]. Finally, methotrexate, a commonly used drug in the management and treatment of GVHD as well as autoimmune disorders such as rheumatoid arthritis has been shown to increase levels of AMP and extracellular adenosine and administration of adenosine A2A receptor agonists reversed its anti-inflammatory effects in a mouse model of inflammation [47, 48]. However binding of adenosine to other receptors can also trigger proinflammatory effects perhaps explaining some of the failures seen with methotrexate use [49].
In our current study we not only observed that A2AR specific agonists improved survival rate and inhibited weight loss, but we also observed a profound reduction in clinical manifestations of skin sores and diarrhea in the treated mice. While these manifestations of clinical improvement in the treated mice were difficult to quantify compared to measures of weight loss and survival, the amelioration of skin sores and diarrhea was clearly evident to the investigators and animal care team.
A number of studies have documented that rapamycin mediates GVHD prevention in part through the induction of Tregs, and that these cells act within the affected tissues [50, 51]. Our current studies demonstrated a similar effect from A2AR agonists. Rapamycin and A2AR agonists have different proximal mechanisms of action, though downstream effects clearly converge in their ability to enhance generation of Tregs. We have conducted preliminary experiments in vitro to determine if perhaps A2AR agonists and rapamycin effects would be synergistic in enhancing generation of Tregs, but to date these preliminary experiments indicate only an additive and not synergistic effect. However, even additive effects might be clinically useful if dose limiting toxicities from combined rapamycin plus A2AR agonist could be reduced relative to the Treg generating activities of these agents. Such combination treatment studies in our GVHD mouse models in vivo are ongoing.
Finally we observed that A2AR agonists, but not cyclosporine A, promoted TGF-β mediated differentiation of Tregs in vitro (Fig. 1). Treg development and homeostasis are controlled by T cell receptor (TCR) engagement, cytokines such as IL-2, IL-10, and TGF-β and costimulatory receptors such as CD28 and CTLA-4 [52]. It has also been reported that TCR signaling through phosphatidyl inositol 3-kinase (PI3K), protein kinase B (Akt), and mammalian target of rapamycin (mTOR) regulates FoxP3 expression in activated CD4 lineage thymoctyes and peripheral T cells. Conversely, constitutive activation of the PI3K/AKT/mTOR signaling pathway in choromosome 10 (PTEN, major negative regulator of PI3K/Akt signaling)-deficient T cells reduces FoxP3 induction, which can be restored by PI3K inhibitor [53]. The fact that we do not see a synergistic effect in combination with rapamycin suggests that there is some overlap through the m-TOR pathway as a mechanism for T regulatory cell development, however this needs to explored further. There is also data suggesting that A2AR stimulation enhances Foxp3 transcription by decreasing IL-6 expression and enhancing TGF-β expression [24]. As seen in the scurfy model, the level of proinflammatory cytokines remained high and there was no impact by the agonists in the severity of GVHD, insinuating that the generation of T regulatory cells may be dampened by the high cytokine levels and lack of IL-6 production. However, we still have questions as to whether activation of the A2AR is directly involved in TGF-β induced FoxP3 expression on GVHD mouse model or if this is an indirect effect. Additional studies are needed to further elucidate the mechanism by which A2AR specific agonist increases Tregs.
In summary, we have demonstrated that specific activation of A2AR enhances development of Tregs in vitro, and that treatment with specific agonist of A2AR inhibits the development of acute GVHD in two types of HLA mismatched mouse transplant models through the increase of donor-derived Tregs in spleen, blood and target tissues of GVHD (colon and skin). Our pre-clinical studies in these mouse models along with clinical studies using A2A agonists in normal volunteers documenting their low toxicity profile, provide strong support for bringing highly specific agonists of A2AR to the clinic for the prevention and treatment of GVHD.
Acknowledgments
This work was supported by the intramural NIAID.
The authors acknowledge Dr. So Yoon Kim for help with Immunohistochemistry and CMB animal care facility technicians.
Footnotes
Authorship
K. L. H, S. V. T., S. M. K., S. H. and C. M. C. performed experiments; K. L. H., S. H. and S. V. T. collected and analyzed data; K. L. H, H. L. M. and E. M. K designed the research; and K. L.H., E. M. K., and H. L. M. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References
- 1.Reddy P. Pathophysiology of acute graft-versus-host disease. Hematol Oncol. 2003;21:149–161. doi: 10.1002/hon.716. [DOI] [PubMed] [Google Scholar]
- 2.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 3.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 4.Semple K, Yu Y, Wang D, Anasetti C, Yu XZ. Efficient and selective prevention of GVHD by antigen-specific induced Tregs via linked-suppression in mice. Biol Blood Marrow Transplant. 2011;17:309–318. doi: 10.1016/j.bbmt.2010.12.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng G, Chan T, Wood KJ, Bushell A. Donor reactive regulatory T cells. Curr Opin Organ Transplant. 2009;14:432–438. doi: 10.1097/MOT.0b013e32832c58f1. [DOI] [PubMed] [Google Scholar]
- 6.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4+CD25+ regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196:389–399. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wysocki CA, Jiang Q, Panoskaltsis-Mortari A, Taylor PA, McKinnon KP, Su L, Blazar BR, Serody JS. Critical role for CCR5 in the function of donor CD4+CD25+ regulatory T cells during acute graft-versus-host disease. Blood. 2005;106:3300–3307. doi: 10.1182/blood-2005-04-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tawara I, Shlomchik WD, Jones A, Zou W, Nieves E, Liu C, Toubai T, Duran-Struuck R, Sun Y, Clouthier SG, Evers R, Lowler KP, Levy RB, Reddy P. A crucial role for host APCs in the induction of donor CD4+CD25+ regulatory T cell-mediated suppression of experimental graft-versus-host disease. J Immunol. 2010;185:3866–3872. doi: 10.4049/jimmunol.1001625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolf D, Wolf AM, Fong D, Rumpold H, Strasak A, Clausen J, Nachbaur D. Regulatory T-cells in the graft and the risk of acute graft-versus-host disease after allogeneic stem cell transplantation. Transplantation. 2007;83:1107–1113. doi: 10.1097/01.tp.0000260140.04815.77. [DOI] [PubMed] [Google Scholar]
- 10.O’Shaughnessy MJ, Chen ZM, Gramaglia I, Taylor PA, Panoskaltsis-Mortari A, Vogtenhuber C, Palmer E, Grader-Beck T, Boussiotis VA, Blazar BR. Elevation of intracellular cyclic AMP in alloreactive CD4+ T Cells induces alloantigen-specific tolerance that can prevent GVHD lethality in vivo. Biol Blood Marrow Transplant. 2007;13:530–542. doi: 10.1016/j.bbmt.2007.01.071. [DOI] [PubMed] [Google Scholar]
- 11.Lappas CM, Sullivan GW, Linden J. Adenosine A2A agonists in development for the treatment of inflammation. Expert Opin Investig Drugs. 2005;14:797–806. doi: 10.1517/13543784.14.7.797. [DOI] [PubMed] [Google Scholar]
- 12.Awad AS, Huang L, Ye H, Duong ET, Bolton WK, Linden J, Okusa MD. Adenosine A2A receptor activation attenuates inflammation and injury in diabetic nephropathy. Am J Physiol Renal Physiol. 2006;290:F828–837. doi: 10.1152/ajprenal.00310.2005. [DOI] [PubMed] [Google Scholar]
- 13.Lappas CM, Day YJ, Marshall MA, Engelhard VH, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med. 2006;203:2639–2648. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linden J. Adenosine in tissue protection and tissue regeneration. Mol Pharmacol. 2005;67:1385–1387. doi: 10.1124/mol.105.011783. [DOI] [PubMed] [Google Scholar]
- 15.Naganuma M, Wiznerowicz EB, Lappas CM, Linden J, Worthington MT, Ernst PB. Cutting edge: Critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis. J Immunol. 2006;177:2765–2769. doi: 10.4049/jimmunol.177.5.2765. [DOI] [PubMed] [Google Scholar]
- 16.Odashima M, Bamias G, Rivera-Nieves J, Linden J, Nast CC, Moskaluk CA, Marini M, Sugawara K, Kozaiwa K, Otaka M, Watanabe S, Cominelli F. Activation of A2A adenosine receptor attenuates intestinal inflammation in animal models of inflammatory bowel disease. Gastroenterology. 2005;129:26–33. doi: 10.1053/j.gastro.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 17.Montesinos MC, Desai A, Cronstein BN. Suppression of inflammation by low-dose methotrexate is mediated by adenosine A2A receptor but not A3 receptor activation in thioglycollate-induced peritonitis. Arthritis Res Ther. 2006;8:R53. doi: 10.1186/ar1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montesinos MC, Yap JS, Desai A, Posadas I, McCrary CT, Cronstein BN. Reversal of the antiinflammatory effects of methotrexate by the nonselective adenosine receptor antagonists theophylline and caffeine: evidence that the antiinflammatory effects of methotrexate are mediated via multiple adenosine receptors in rat adjuvant arthritis. Arthritis Rheum. 2000;43:656–663. doi: 10.1002/1529-0131(200003)43:3<656::AID-ANR23>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 19.Palmer TM, Trevethick MA. Suppression of inflammatory and immune responses by the A2A adenosine receptor: an introduction. Br J Pharmacol. 2008;153(Suppl 1):S27–34. doi: 10.1038/sj.bjp.0707524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lappas CM, Liu PC, Linden J, Kang EM, Malech HL. Adenosine A2A receptor activation limits graft-versus-host disease after allogenic hematopoietic stem cell transplantation. J Leukoc Biol. 2010;87:345–354. doi: 10.1189/jlb.0609388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sitkovsky M, Lukashev D, Deaglio S, Dwyer K, Robson SC, Ohta A. Adenosine A2A receptor antagonists: blockade of adenosinergic effects and T regulatory cells. Br J Pharmacol. 2008;153(Suppl 1):S457–464. doi: 10.1038/bjp.2008.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naganuma M, Wiznerowicz EB, Lappas CM, Linden J, Worthington MT, Ernst PB. Cutting edge: Critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis. J Immunol. 2006;177:2765–2769. doi: 10.4049/jimmunol.177.5.2765. [DOI] [PubMed] [Google Scholar]
- 24.Zarek PE, Huang CT, Lutz ER, Kowalski J, Horton MR, Linden J, Drake CG, Powell JD. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood. 2008;111:251–259. doi: 10.1182/blood-2007-03-081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zakrzewski JL, Kochman AA, Lu SX, Terwey TH, Kim TD, Hubbard VM, Muriglan SJ, Suh D, Smith OM, Grubin J, Patel N, Chow A, Cabrera-Perez J, Radhakrishnan R, Diab A, Perales MA, Rizzuto G, Menet E, Pamer EG, Heller G, Zuniga-Pflucker JC, Alpdogan O, van den Brink MR. Adoptive transfer of T-cell precursors enhances T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Nat Med. 2006;12:1039–1047. doi: 10.1038/nm1463. [DOI] [PubMed] [Google Scholar]
- 26.Choi SW, Islam S, Greenson JK, Levine J, Hutchinson R, Yanik G, Teitelbaum DH, Ferrara JL, Cooke KR. The use of laparoscopic liver biopsies in pediatric patients with hepatic dysfunction following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2005;36:891–896. doi: 10.1038/sj.bmt.1705158. [DOI] [PubMed] [Google Scholar]
- 27.Zhou L, Askew D, Wu C, Gilliam AC. Cutaneous gene expression by DNA microarray in murine sclerodermatous graft-versus-host disease, a model for human scleroderma. J Invest Dermatol. 2007;127:281–292. doi: 10.1038/sj.jid.5700517. [DOI] [PubMed] [Google Scholar]
- 28.Akpek G, Chinratanalab W, Lee LA, Torbenson M, Hallick JP, Anders V, Vogelsang GB. Gastrointestinal involvement in chronic graft-versus-host disease: a clinicopathologic study. Biol Blood Marrow Transplant. 2003;9:46–51. doi: 10.1053/bbmt.2003.49999. [DOI] [PubMed] [Google Scholar]
- 29.Vokaer B, Van Rompaey N, Lemaitre PH, Lhomme F, Kubjak C, Benghiat FS, Iwakura Y, Petein M, Field KA, Goldman M, Le Moine A, Charbonnier LM. Critical role of regulatory T cells in Th17-mediated minor antigen-disparate rejection. J Immunol. 2010;185:3417–3425. doi: 10.4049/jimmunol.0903961. [DOI] [PubMed] [Google Scholar]
- 30.Chaput N, Louafi S, Bardier A, Charlotte F, Vaillant JC, Menegaux F, Rosenzwajg M, Lemoine F, Klatzmann D, Taieb J. Identification of CD8+CD25+Foxp3+ suppressive T cells in colorectal cancer tissue. Gut. 2009;58:520–529. doi: 10.1136/gut.2008.158824. [DOI] [PubMed] [Google Scholar]
- 31.Zeiser R, V, Nguyen H, Beilhack A, Buess M, Schulz S, Baker J, Contag CH, Negrin RS. Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood. 2006;108:390–399. doi: 10.1182/blood-2006-01-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lappas CM, Rieger JM, Linden J. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J Immunol. 2005;174:1073–1080. doi: 10.4049/jimmunol.174.2.1073. [DOI] [PubMed] [Google Scholar]
- 33.Koreth J, Antin JH. Current and future approaches for control of graft-versus-host disease. Expert Rev Hematol. 2008;1:111. doi: 10.1586/17474086.1.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holler E. Cytokines, viruses, and graft-versus-host disease. Curr Opin Hematol. 2002;9:479–484. doi: 10.1097/00062752-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Kim SS. Treatment options in steroid-refractory acute graft-versus-host disease following hematopoietic stem cell transplantation. Ann Pharmacother. 2007;41:1436–1444. doi: 10.1345/aph.1K179. [DOI] [PubMed] [Google Scholar]
- 36.Sullivan GW. Adenosine A2A receptor agonists as anti-inflammatory agents. Curr Opin Investig Drugs. 2003;4:1313–1319. [PubMed] [Google Scholar]
- 37.Butler JJ, Mader JS, Watson CL, Zhang H, Blay J, Hoskin DW. Adenosine inhibits activation-induced T cell expression of CD2 and CD28 co-stimulatory molecules: role of interleukin-2 and cyclic AMP signaling pathways. J Cell Biochem. 2003;89:975–991. doi: 10.1002/jcb.10562. [DOI] [PubMed] [Google Scholar]
- 38.Coenen JJ, Koenen HJ, van Rijssen E, Kasran A, Boon L, Hilbrands LB, Joosten I. Rapamycin, not cyclosporine, permits thymic generation and peripheral preservation of CD4+ CD25+ FoxP3+ T cells. Bone Marrow Transplant. 2007;39:537–545. doi: 10.1038/sj.bmt.1705628. [DOI] [PubMed] [Google Scholar]
- 39.Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S, Negrin RS. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9:1144–1150. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 40.Cohen JL, Trenado A, Vasey D, Klatzmann D, Salomon BL. CD4+CD25+ immunoregulatory T Cells: new therapeutics for graft-versus-host disease. J Exp Med. 2002;196:401–406. doi: 10.1084/jem.20020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gaidot A, Landau DA, Martin GH, Bonduelle O, Grinberg-Bleyer Y, Matheoud D, Gregoire S, Baillou C, Combadiere B, Piaggio E, Cohen JL. Immune reconstitution is preserved in hematopoietic stem cell transplantation coadministered with regulatory T cells for GVHD prevention. Blood. 2011;117:2975–2983. doi: 10.1182/blood-2010-08-299974. [DOI] [PubMed] [Google Scholar]
- 42.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4+CD25+ immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99:3493–3499. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 43.Komanduri KV, Champlin RE. Can Treg therapy prevent GVHD? Blood. 2011;117:751–752. doi: 10.1182/blood-2010-11-317305. [DOI] [PubMed] [Google Scholar]
- 44.Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J, Defor T, Levine BL, June CH, Rubinstein P, McGlave PB, Blazar BR, Wagner JE. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117:1061–1070. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsukamoto H, Chernogorova P, Ayata K, Gerlach UV, Rughani A, Ritchey JW, Ganesan J, Follo M, Zeiser R, Thompson LF, Idzko M. Deficiency of CD73/ecto-5′-nucleotidase in mice enhances acute graft-versus-host disease. Blood. 2012;119:4554–4564. doi: 10.1182/blood-2011-09-375899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sauer AV, Brigida I, Carriglio N, Hernandez RJ, Scaramuzza S, Clavenna D, Sanvito F, Poliani PL, Gagliani N, Carlucci F, Tabucchi A, Roncarolo MG, Traggiai E, Villa A, Aiuti A. Alterations in the adenosine metabolism and CD39/CD73 adenosinergic machinery cause loss of Treg cell function and autoimmunity in ADA-deficient SCID. Blood. 2012;119:1428–1439. doi: 10.1182/blood-2011-07-366781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morabito L, Montesinos MC, Schreibman DM, Balter L, Thompson LF, Resta R, Carlin G, Huie MA, Cronstein BN. Methotrexate and sulfasalazine promote adenosine release by a mechanism that requires ecto-5′-nucleotidase-mediated conversion of adenine nucleotides. J Clin Invest. 1998;101:295–300. doi: 10.1172/JCI1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cronstein BN, Naime D, Firestein G. The antiinflammatory effects of an adenosine kinase inhibitor are mediated by adenosine. Arthritis Rheum. 1995;38:1040–1045. doi: 10.1002/art.1780380804. [DOI] [PubMed] [Google Scholar]
- 49.Blackburn MR, Vance CO, Morschl E, Wilson CN. Adenosine receptors and inflammation. Handb Exp Pharmacol. 2009;193:215–269. doi: 10.1007/978-3-540-89615-9_8. [DOI] [PubMed] [Google Scholar]
- 50.Chen BJ, Morris RE, Chao NJ. Graft-versus-host disease prevention by rapamycin: cellular mechanisms. Biol Blood Marrow Transplant. 2000;6:529–536. doi: 10.1016/s1083-8791(00)70062-0. [DOI] [PubMed] [Google Scholar]
- 51.Palmer JM, Chen BJ, DeOliveira D, Le ND, Chao NJ. Novel mechanism of rapamycin in GVHD: increase in interstitial regulatory T cells. Bone Marrow Transplant. 2010;45:379–384. doi: 10.1038/bmt.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Demirkiran A, Hendrikx TK, Baan CC, van der Laan LJ. Impact of immunosuppressive drugs on CD4+CD25+FOXP3+ regulatory T cells: does in vitro evidence translate to the clinical setting? Transplantation. 2008;85:783–789. doi: 10.1097/TP.0b013e318166910b. [DOI] [PubMed] [Google Scholar]
- 53.Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, Knight ZA, Cobb BS, Cantrell D, O’Connor E, Shokat KM, Fisher AG, Merkenschlager M. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci U S A. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]