Abstract
Brain injury after hypoxic-ischemic encephalopathy often develops with delayed appearance, opening a therapeutic window. Clinical studies in newborns show that post-hypoxic-ischemic hypothermia improves outcome. This has generated renewed interest in the molecular mechanisms of hypoxic-ischemic brain injury. In this brief review, we propose that mitochondrial permeabilization is crucial for injury to advance beyond the point of no return. We suggest that excitatory amino acids, nitric oxide, inflammation, trophic factor withdrawal, and an increased pro- versus anti-apoptotic Bcl-2 protein ratio will trigger Bax-dependent mitochondrial outer membrane permeabilization (MOMP). MOMP, in turn, elicits mitochondrial release of cytochrome C, apoptosis-inducing factor, SMAC/Diablo, and HtrA2/Omi. Cytochrome C efflux activates caspase-9/-3, leading to DNA fragmentation. Apoptosis-inducing factor interacts with cyclophilin A and induces chromatinolysis. Blockage of MOMP holds promise as a strategy for perinatal brain protection.
Pathophysiology of Neonatal Brain Injury
Perinatal encephalopathy develops after peripartum hypoxia-ischemia in 1 to 3 out of every 1000 births and remains an important problem. It is associated with a high risk of death or major neurological and neurodevelopmental abnormalities resulting from injury in the basal ganglia/thalamus and cerebral cortex.1 The etiology behind these lesions is complex. Besides the intensity and duration of the primary/secondary insult, genetic background, gender,2,3 stage of brain development,4 and the activity of repair/adaptive processes5 during the recovery phase are important. Furthermore, the presence or absence of various antecedents (eg, infection, growth retardation) are likely to be of great significance.
The development of injury can be considerably delayed after ischemic episodes (eg, global ischemia in adult gerbils and rats results in complete initial recovery of function and structural integrity but is followed by delayed selective loss of pyramidal cells in the cornu ammonis 1 of hippocampus 3 days to 4 days after the primary insult).6,7 Experimental work in neonatal models has also demonstrated near-complete recovery after the primary insult, followed by secondary disruption of high-energy phosphates8 and loss of glucose-metabolizing brain tissue9 6 hours to 48 hours later. Infants with hypoxic-ischemic encephalopathy show characteristic abnormalities in cerebral energy metabolism, which is frequently normal soon after birth but shows a progressive decline in [PCr]/[Pi] and increase in lactate some hours later.10,11 Infants displaying this phenomenon develop severe neurodevelopmental impairment or die, and there is a relationship between the magnitude of the late decline in [PCr]/[Pi] and the severity of neurodevelopmental impairment.12,13 These findings suggest that brain injury develops with a certain delay after hypoxia-ischemia, which is also supported by the fact that various treatments given only after the insult in animals14 can reduce injury and that outcome in newborns with neonatal encephalopathy can be improved to some extent by post-hypoxic-ischemic hypothermia.15,16 Such proof of concept that neuroprotection is feasible not only experimentally but also in the clinical setting has created renewed interest in the pathophysiology of secondary brain injury after hypoxia-ischemia, with the hope of finding novel and more efficient treatments for the future.
The mechanisms leading to secondary brain injury are still partly unknown, but neurotransmitters, including excitatory amino acids, intracellular calcium, nitric oxide/reactive oxygen species, immuno-inflammatory activation, trophic factor withdrawal, and Bcl-2 family proteins all seem to be involved.17–19 We believe these upstream perpetrators converge on mitochondria, and at a certain level of stress mitochondrial outer membrane permeabilization (MOMP) occurs, which shifts reversible injury to irreversible cell death (Figure). In this review we summarize current evidence that supports the central role of mitochondria in neonatal brain injury.
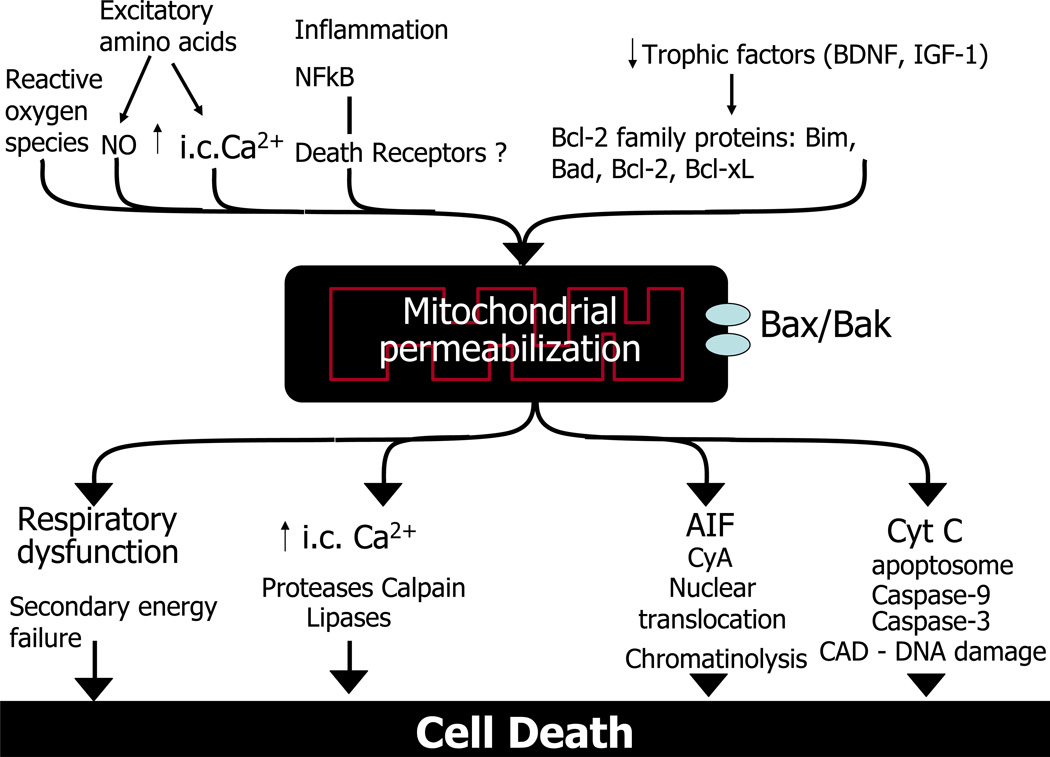
Figure.
Mitochondrial outer membrane permeabilization (MOMP). Many upstream events like excitatory amino acids, intracellular calcium (Ca2+ic), nitric oxide/reactive oxygen species, inflammation, nuclear factor kappa B, trophic factor withdrawal, and Bcl-2 family proteins contribute to mitochondrial stress after neonatal brain injury. These perpetrators converge on mitochondria and together trigger MOMP, leading to irreversible caspase- and non-caspase-dependent cell injury. Abbreviations: AIF, apoptosis-inducing factor; CAD, caspase-activated Dnase; cyA, cyclophilin A; cytC, cytochrome C; BDNF, brain-derived neurotrophic factor; i.c., intracellular; IGF-1, insulin-like growth factor 1; MOMP, mitochondrial outer membrane permeabilization; NF-kB, nuclear factor kappa B.
Mitochondrial Permeabilization After Neonatal Hypoxia-Ischemia?
Electron microscopy has shown swollen “giant” mitochondria with ruptured outer membranes with large amounts of calcium in the matrix often in neuronal cells early after neonatal hypoxia-ischemia.20 Using [14C]2-deoxyglucose entrapment in the mitochondrial matrix as an indicator of mitochondrial permeabilization,21 we found increased mitochondrial permeability 0 hours to 1.5 hours and 6.5 hours to 8 hours after hypoxia-ischemia, indicating, at least qualitatively, that the inner mitochondrial membrane was permeabilized to some degree after the insult. However, there is stronger support for a role of the outer mitochondrial membrane in hypoxia-ischemia.
Proteins are normally localized in the intermembrane space or on the outer surface of the inner mitochondrial membrane but can translocate to the cytosol or the nucleus. For example, cytochrome C, which takes part in electron transfer in the electron transport chain, detaches from cardiolipin in the inner mitochondrial membrane22 and translocates to the cytosol,23 which leads to the assembly of the apoptosome24 and activation of caspase-925–27 and caspase-3.28–30 Another mitochondrial protein, apoptosis-inducing factor, is normally attached to the outer surface of the inner mitochondrial membrane. In response to poly(ADP-ribose)polymerase-1 (PARP-1)-dependent apoptotic signaling, a peptidase is activated and apoptosis-inducing factor is liberated and can, if MOMP occurs, translocate to the cytosol and nucleus.31 Indeed, PARP-1 is activated after neonatal hypoxia-ischemia32 and stroke,33 and apoptosis-inducing factor translocates from the mitochondria to the nucleus in the cerebral cortex, thalamus, and hippocampus.34 Recently, it was demonstrated that apoptosis-inducing factor interacts with cyclophilin A in the cytosol and the complex translocates to the nucleus and forms a degradasome, and chromatinolysis is initiated.35 Also, Smac/Diablo and HtrA2/Omi localized in the mitochondrial intermembrane space translocate to the nucleus after hypoxia-ischemia.26
In summary, evidence is strong that MOMP occurs during reperfusion in brain regions affected by hypoxia-ischemia, but at present it is uncertain to what degree MOMP is accompanied by inner membrane depolarization and bioenergetic dysfunction after hypoxia-ischemia. Basic research indicates that induction of MOMP results in respiratory failure due to caspase-dependent degradation of critical components of complex I in the electron transport chain.36
Relationship Between MOMP and Brain Injury
The extent of apoptosis-inducing factor translocation to the nucleus correlates with the morphological distribution of neuronal injury after hypoxia-ischemia,34 and apoptosis-inducing factor deficiency confers considerable protection in mice subjected to neonatal hypoxia-ischemia.37 Gene deletion of both PARP-132 and cyclophilin A 35 decreases apoptosis-inducing factor translocation to the nucleus and reduces brain injury. Caspase inhibitors have also been shown to be protective in hypoxia-ischemia28 and the broad-spectrum caspase inhibitor Q-VD-OPh reduces injury also in apoptosis-inducing factor-deficient mice,37 suggesting that MOMP induces activation of caspase-dependent and caspase nondependent processes, and that both contribute to brain injury.
Mechanisms of Mitochondrial Permeabilization
Most researchers agree that at least 2 alternative routes lead to mitochondrial permeabilization, an event that marks the point of no return in multiple pathways leading to cell death.38–40 The first relies on the opening of the permeability transition pore in the inner mitochondrial membrane, and it is enhanced by cyclophilin D and desensitized by the cyclophilin D inhibitor cyclosporine A; the other requires a direct permeabilization of the outer membrane by Bax/Bak, and is considered to be cyclophilin D-independent and cyclosporine A-resistant.
In the adult brain, ischemia induces opening of the cyclophilin D-dependent mitochondrial permeability transition pore, leading to necrotic cell death.41,42 The cyclophilin-D inhibitor cyclosporine A reduces ischemic injury41 and cyclophilin-D gene deficiency confers marked protection in adult brain ischemia.43 In the immature brain, however, the situation appears to be quite different. Cyclosporine A had no effect on mitochondrial respiration, inner mitochondrial permeabilization (as measured with the deoxyglucose entrapment technique), or brain injury after hypoxia-ischemia.44 Mitochondria from 16- to 18-day-old rats exhibited greater calcium uptake capacity (in the absence of adenosine triphosphate) than adult rats and calcium-induced cytochrome C release was unaffected by cyclosporine A.45 In agreement, the calcium retention capacity was higher in mitochondria from postnatal day 9 mice compared with mitochondria from the adult brain, and the calcium retention capacity was less cyclophilin D-dependent in the immature setting.46 In vivo experiments demonstrated that cyclophilin D gene deficiency increased brain injury, cytochrome C translocation, and caspase activation after hypoxia-ischemia in postnatal day 9 mice, whereas injury was markedly decreased after hypoxia-ischemia at postnatal day 60.43,46
In the immature brain, Bax, rather than cyclophilin D, seems to be crucial for mitochondrial permeabilization. Bax (and Bak) is highly expressed in immature mitochondria47,48 and is particularly sensitive to Bax-BH3-peptide-induced cytochrome C release in vitro.46,47 Bax translocates from cytosol to mitochondria after neonatal hypoxia-ischemia, 23 and Bax knock-out mice are protected from hypoxic-ischemic brain injury.49 Recently, Bax-inhibitory peptide was shown to reduce injury (by 75%) and down-stream caspase activation after hypoxia-ischemia in the immature, but not in adult, brain.46
In summary, cyclophilin D-dependent mitochondrial permeability transition is critical for mitochondrial permeabilization and brain injury in the adult, whereas Bax-dependent mechanisms prevail in the immature brain. The role of cyclophilin D in hypoxia-ischemia shifts from an anti-apoptotic protein in the immature brain to a pro-necrotic mediator in the adult brain.46
Upstream Factors Involved in Triggering MOMP Bcl-2 Family Proteins
The above data suggest that Bax-dependent MOMP is a critical event in neonatal brain injury, and it is therefore of interest to understand which factors regulate MOMP. Although it has been long recognized that pro- and anti-apoptotic Bcl-2 family proteins regulate Bax-dependent MOMP and are critical regulators of apoptotic cell death,22,50 their role in neonatal hypoxia-ischemia has only partly been clarified. Most pro-apoptotic multidomain (Bax, Bak, bok), BH3 only (Bad, Bim, Bid, Puma) proteins as well as anti-apoptotic Bcl-2 and Bcl-xL are highly expressed postnatally, followed by downregulation (except for Bcl-xL) with brain maturation.47,51 Transgenic Bcl-xL overexpressing52 as well as Bad or Bim knock-out53 mice are all resistant to postnatal hypoxia-ischemia. Phosphorylation of Bcl-2 at serine-24 in the BH4 region (leading to inactivation of its anti-apoptotic effect) coincides with cytochrome C release after neonatal hypoxia-ischemia and precedes caspase- 3 activation. In addition, the trophic factors IGF-1, BDNF, and hexarellin, which at least partly act through increasing anti- vs. pro-apoptotic Bcl-2 family protein balance, all decrease downstream caspase activation and injury in the postnatal brain.54–57 In summary, these studies suggest that several Bcl-2 family proteins have an important role in neonatal brain injury, probably through regulation of Bax-dependent MOMP.
Excitatory Amino Acid Receptors, Nitric Oxide, and MOMP
Excitatory amino acids are released during and after neonatal hypoxia-ischemia, activating AMPA and NMDA receptors that in turn trigger production of both reactive oxygen species and nitric oxide.17 Furthermore, NMDA/nitric oxide activates poly(ADP-ribose) polymerase, which depletes mitochondrial NAD(H) levels and triggers mitochondrial apoptosis-inducing factor release (above). NMDA receptor antagonists improve mitochondrial respiration, attenuate caspase-3 activation, and decrease brain injury after neonatal hypoxia-ischemia.58–60 Furthermore, the brain protection provided by the nitric oxide synthase inhibitor 2-iminobiotin was also accompanied by near-complete inhibition of caspase-3 and reduction of apoptotic cell death.61 Taken together, these data suggest that excessive NMDA receptor activation and nitric oxide production impair mitochondrial function and contribute somehow to MOMP and subsequent activation of executional caspases and cell death.
Inflammation, Nuclear Factor Kappa B (NF-kB), and MOMP
Inflammation has previously been shown to be involved in neonatal brain injury.14 Hence, interleukin-18,62 caspase-1,63 or complement C1q gene deficiency64 or treatment with interleukin-1 receptor antagonist,65 platelet activating factor antagonist,66 and inducible nitric oxide synthase inhibitors67 all reduce neonatal brain injury. Many of the proinflammatory mediators are regulated by the transcription factor nuclear factor kappa B (NF-kB). Recently; it was shown that post-hypoxia-ischemia treatment with a highly selective NF-kB inhibitor peptide (TAT-NBD) reduced brain injury by 80%.68 Interestingly, the protection was accompanied by marked attenuation of mitochondrial accumulation of p53, mitochondrial cytochrome C release, and activation of caspase-3. The data suggest there may be a link between NF-kB-mediated inflammation and MOMP (Figure) or, alternatively, that NF-kB is directly regulating apoptosis.
In summary, we propose that many upstream events (eg, excitatory amino acids, nitric oxide, inflammation, NF-kB, trophic factor withdrawal, and Bcl-2 family proteins) previously shown to be important in neonatal brain injury all contribute to mitochondrial stress during the post-hypoxia-ischemia phase. At a certain stress threshold, MOMP will occur, leading to irreversible caspase- and non-caspase-dependent cell death (Figure).
Acknowledgments
This work was supported by grants from the Swedish Medical Research Council (VR 2006-3396 to HH) and Swedish governmental grants to researchers in the Public Health Service (ALFGBG2863 to HH), MRC strategic award (UK, P19381 to HH), and National Natural Science Foundation of China (30571972 to XW). Presented at the Neurobiology of Disease in Children Conference: Symposium on Injury to the Preterm Brain and Cerebral Palsy, in conjunction with the 37th Annual Meeting of the Child Neurology Society, Santa Clara, California, November 5, 2008. Supported by grants from the National Institutes of Health (5R13NS040925-09), the Cerebral Palsy International Research Foundation, the Kennedy Krieger Institute, and the Child Neurology Society.
References
- 1.Jouvet P, Cowan FM, Cox P, et al. Reproducibility and accuracy of MR imaging of the brain after severe birth asphyxia. AJNR Am J Neuroradiol. 1999;20:1343–1348. [PMC free article] [PubMed] [Google Scholar]
- 2.Johnston MV, Hagberg H. Sex and the pathogenesis of cerebral palsy. Dev Med Child Neurol. 2007;49:74–78. doi: 10.1017/s0012162207000199.x. [DOI] [PubMed] [Google Scholar]
- 3.Zhu C, Xu F, Wang X, et al. Different apoptotic mechanisms are activated in male and female brains after neonatal hypoxia-ischaemia. J Neurochem. 2006;96:1016–1027. doi: 10.1111/j.1471-4159.2005.03639.x. [DOI] [PubMed] [Google Scholar]
- 4.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Z, Covey MV, Bitel CL, et al. Sustained neocortical neurogenesis after neonatal hypoxic/ischemic injury. Ann Neurol. 2007;61:199–208. doi: 10.1002/ana.21068. [DOI] [PubMed] [Google Scholar]
- 6.Ito U, Spatz M, Walker JT, Jr, Klatzo I. Experimental cerebral ischemia in mongolian gerbils. I. Light microscopic observations. Acta Neuropathol. 1975;32:209–223. doi: 10.1007/BF00696570. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki R, Yamaguchi T, Kirino T, et al. The effects of 5-minute ischemia in Mongolian gerbils: I. Blood-brain barrier, cerebral blood flow, and local cerebral glucose utilization changes. Acta Neuropathol. 1983;60:207–216. doi: 10.1007/BF00691868. [DOI] [PubMed] [Google Scholar]
- 8.Penrice J, Lorek A, Cady EB, et al. Proton magnetic resonance spectroscopy of the brain during acute hypoxia-ischemia and delayed cerebral energy failure in the newborn piglet. Pediatr Res. 1997;41:795–802. doi: 10.1203/00006450-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Gilland E, Bona E, Hagberg H. Temporal changes of regional glucose use blood flow, and microtubule-associated protein 2 immunostaining after hypoxia-ischemia in the immature rat brain. J Cereb Blood Flow Metab. 1998;18:222–228. doi: 10.1097/00004647-199802000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Azzopardi D, Wyatt JS, Cady EB, et al. Prognosis of newborn infants with hypoxic-ischemic brain injury assessed by phosphorus magnetic resonance spectroscopy. Pediatr Res. 1989;25:445–451. doi: 10.1203/00006450-198905000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Hanrahan D, Cox IJ, Edwards AD. Persistent increases in cerebral lactate concentration after birth asphyxia. Pediatr Res. 1998;44:304–311. doi: 10.1203/00006450-199809000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Hanrahan D, Cox IJ, Azzopardi D, et al. Relation between proton magnetic resonance spectroscopy within 18 hours of birth asphyxia and neurodevelopment at one year of age. Dev Med Child Neurol. 1999;41:76–82. doi: 10.1017/s0012162299000171. [DOI] [PubMed] [Google Scholar]
- 13.Roth SC, Baudin J, Cady E, et al. Relation of deranged neonatal cerebral oxidative metabolism with neurodevelopmental outcome and head circumference at 4 years. Dev Med Child Neurol. 1997;39:718–725. doi: 10.1111/j.1469-8749.1997.tb07372.x. [DOI] [PubMed] [Google Scholar]
- 14.Hagberg H, Rousset CI, Wang X, Mallard C. Mechanisms of perinatal brain damage and protective possibilities. Drug Discovery Today: Disease Mechanisms. 2006;3:397–407. [Google Scholar]
- 15.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 16.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. National Institute of Child Health and Human Development Neonatal Research Network. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 17.Johnston MV. Excitotoxicity in perinatal brain injury. Brain Pathol. 2005;15:234–240. doi: 10.1111/j.1750-3639.2005.tb00526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferriero DM. Neonatal brain injury. N Engl J Med. 2004;351:1985–1995. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- 19.Blomgren K, Hagberg H. Free radicals, mitochondria, and hypoxia-ischemia in the developing brain. Free Radic Biol Med. 2006;40:388–397. doi: 10.1016/j.freeradbiomed.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 20.Puka-Sundvall M, Gajkowska B, Cholewinski M, et al. Subcellular distribution of calcium and ultrastructural changes after cerebral hypoxia-ischemia in immature rats. Dev Brain Res. 2000;125:31–41. doi: 10.1016/s0165-3806(00)00110-3. [DOI] [PubMed] [Google Scholar]
- 21.Griffiths EJ, Halestrap AP. Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem J. 1995;307:93–98. doi: 10.1042/bj3070093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 23.Northington FJ, Ferriero DM, Flock DL, Martin LJ. Delayed neurodegeneration in neonatal rat thalamus after hypoxia-ischemia is apoptosis. J Neurosci. 2001;21:1931–1938. doi: 10.1523/JNEUROSCI.21-06-01931.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gill R, Soriano M, Blomgren K, et al. Role of Caspase-3 activation in cerebral ischemia-induced neurodegeneration in adult and neonatal brain brain. J Cereb Blood Flow Metab. 2002;22:420–430. doi: 10.1097/00004647-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Benjelloun N, Joly LM, Palmier B, et al. Apoptotic mitochondrial pathway in neurones and astrocytes after neonatal hypoxia-ischaemia in the rat brain. Neuropathol Appl Neurobiol. 2003;29:350–360. doi: 10.1046/j.1365-2990.2003.00467.x. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Zhu C, Hagberg H, et al. X-linked inhibitor of apoptosis (XIAP) protein protects against caspase activation and tissue loss after neonatal hypoxia-ischemia. Neurobiol Dis. 2004;16:179–189. doi: 10.1016/j.nbd.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 27.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91(4):479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 28.Cheng Y, Deshmukh M, D'Costa A, et al. Caspase inhibitor affords neuroprotection with delayed administration in a rat model of neonatal hypoxic-ischemic brain injury. J Clin Invest. 1998;101:1992–1999. doi: 10.1172/JCI2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu BR, Liu CL, Ouyang Y, et al. Involvement of caspase-3 in cell death after hypoxia-ischemia declines during brain maturation. J Cereb Blood Flow Metab. 2002;22:420–430. doi: 10.1097/00004647-200009000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Zhu C, Wang X, Hagberg H, Blomgren K. Correlation between caspase-3 activation and three different markers of DNA damage in neonatal cerebral hypoxia-ischemia. J Neurochem. 2000;75:819–829. doi: 10.1046/j.1471-4159.2000.0750819.x. [DOI] [PubMed] [Google Scholar]
- 31.Modjtahedi N, Giordanetto F, Madeo F, Kroemer G. Apoptosis-inducing factor: vital and lethal. Trends Cell Biol. 2006;16:264–272. doi: 10.1016/j.tcb.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 32.Hagberg H, Wilson MA, Matsushita H. PARP-1 gene disruption in mice preferentially protects males from perinatal brain injury. J Neurochem. 2004;90:1068–1075. doi: 10.1111/j.1471-4159.2004.02547.x. [DOI] [PubMed] [Google Scholar]
- 33.Joly LM, Benjelloun N, Plotkine M, Charriaut-Marlangue C. Distribution of Poly(ADP-ribosyl) ation and cell death after cerebral ischemia in the neonatal rat. Pediatr Res. 2003;53:776–782. doi: 10.1203/01.PDR.0000059751.00465.F6. [DOI] [PubMed] [Google Scholar]
- 34.Zhu C, Qiu L, Wang X, et al. Involvement of apoptosis-inducing factor in neuronal death after hypoxia-ischemia in the neonatal rat brain. J Neurochem. 2003;86:306–317. doi: 10.1046/j.1471-4159.2003.01832.x. [DOI] [PubMed] [Google Scholar]
- 35.Zhu C, Wang X, Deinum J, et al. Cyclophilin A participates in the nuclear translocation of apoptosis inducing factor in neurons after cerebral hypoxia-ischemia. J Exp Med. 2007;204:1741–1748. doi: 10.1084/jem.20070193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ricci JE, Munoz-Pinedo C, Fitzgerald P, et al. Disruption of mitochondrial function during apoptosis is mediated by caspase cleavage of the p75 subunit of complex I of the electron transport chain. Cell. 2004;117:773–786. doi: 10.1016/j.cell.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 37.Zhu C, Wang X, Huang Z, et al. Apoptosis-inducing factor is a major contributor to neuronal loss induced by neonatal cerebral hypoxia-ischemia. Cell Death Differ. 2007;14:775–784. doi: 10.1038/sj.cdd.4402053. [DOI] [PubMed] [Google Scholar]
- 38.Scorrano L, Nicolli A, Basso E, et al. Two modes of activation of the permeability transition pore: the role of mitochondrial cyclophilin. Mol Cell Biochem. 1997;174:181–184. [PubMed] [Google Scholar]
- 39.Zamzami N, Larochette N, Kroemer G. Mitochondrial permeability transition in apoptosis and necrosis. Cell Death Differ. 2005;12(Suppl2):1478–1480. doi: 10.1038/sj.cdd.4401682. [DOI] [PubMed] [Google Scholar]
- 40.Zoratti M, Szabo I, De Marchi U. Mitochondrial permeability transitions: how many doors to the house? Biochim Biophys Acta. 2005;1706:40–52. doi: 10.1016/j.bbabio.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 41.Khaspekov L, Friberg H, Halestrap A, et al. Cyclosporin A and its nonimmunosuppressive analogue N-Me-Val-4-cyclosporin A mitigate glucose/oxygen deprivation-induced damage to rat cultured hippocampal neurons. Eur J Neurosci. 1999;11:3194–3198. doi: 10.1046/j.1460-9568.1999.00743.x. [DOI] [PubMed] [Google Scholar]
- 42.Basso E, Fante L, Fowlkes J, et al. Properties of the permeability transition pore in mitochondria devoid of Cyclophilin D. J Biol Chem. 2005;280:18558–18561. doi: 10.1074/jbc.C500089200. [DOI] [PubMed] [Google Scholar]
- 43.Schinzel AC, Takeuchi O, Huang Z, et al. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci USA. 2005;102:12005–12010. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Puka-Sundvall M, Gilland E, Hagberg H. Cerebral hypoxia-ischemia in immature rats: involvement of mitochondrial permeability transition? Dev Neurosci. 2001;23:192–197. doi: 10.1159/000046142. [DOI] [PubMed] [Google Scholar]
- 45.Robertson CL, Bucci CJ, Fiskum G. Mitochondrial response to calcium in the developing brain. Dev Brain Res. 2004;151:141–148. doi: 10.1016/j.devbrainres.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 46.Wang X, Carlsson Y, Basso E, et al. Developmental shift of cyclophilin D contribution to hypoxic-ischemic brain injury. J Neurosci. 2009;29:2588–2596. doi: 10.1523/JNEUROSCI.5832-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Polster BM, Robertson CL, Bucci CJ, et al. Postnatal brain development and neural cell differentiation modulate mitochondrial bax and BH3 peptide-induced cytochrome c release. Cell Death Differ. 2003;10:365–370. doi: 10.1038/sj.cdd.4401158. [DOI] [PubMed] [Google Scholar]
- 48.Soane L, Siegel ZT, Shuh RA, Fiskum G. Postnatal developmental regulation of Bcl-2 family proteins in brain mitochondria. J Neurosci Res. 2008;86:1267–1276. doi: 10.1002/jnr.21584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gibson ME, Han HH, Choi, et al. Bax contributes to apoptotic-like death following neonatal hypoxia-ischemia: evidence for distinct apoptosis pathways. Mol Med. 2001;7:644–655. [PMC free article] [PubMed] [Google Scholar]
- 50.Youle RJ, Strasser A. The Bcl-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 51.Shimohama S, Fujimoto S, Sumida Y, Tanino H. Differential expression of rat brain bcl-2 family proteins in development and aging. Biochem Biophys Res Commun. 1998;252:92–96. doi: 10.1006/bbrc.1998.9577. [DOI] [PubMed] [Google Scholar]
- 52.Parsadanian AS, Cheng Y, Keller-Peck CR, et al. Bcl-xL is an antiapoptotic regulator for postnatal CNS neurons. J Neurosci. 1998;18:1009–1019. doi: 10.1523/JNEUROSCI.18-03-01009.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ness JM, Harvey CA, Strasser A, et al. Selective involvement of BH3-only Bcl-2 family members Bim and Bad in neonatal hypoxia–ischemia. Brain Res. 2006;1099:150–159. doi: 10.1016/j.brainres.2006.04.132. [DOI] [PubMed] [Google Scholar]
- 54.Cheng Y, Gidday JM, Yan Q, et al. Marked age-dependent neuroprotection by brain-derived neurotrophic factor against neonatal hypoxic-ischemic brain injury. Ann Neurol. 1997;41:521–529. doi: 10.1002/ana.410410416. [DOI] [PubMed] [Google Scholar]
- 55.Han BH, D'Costa A, Back SA, et al. BDNF blocks caspase-3 activation in neonatal hypoxia-ischemia. Neurobiol Dis. 2000;7:38–53. doi: 10.1006/nbdi.1999.0275. [DOI] [PubMed] [Google Scholar]
- 56.Brywe KG, Mallard C, Gustavsson M, et al. IGF-I neuroprotection in the immature brain after hypoxia-ischemia, involvement of Akt and GSK3beta? Eur J Neurosci. 2005;21:1489–1502. doi: 10.1111/j.1460-9568.2005.03982.x. [DOI] [PubMed] [Google Scholar]
- 57.Brywe KG, Leverin AL, Gustavsson M, et al. Growth hormone-releasing peptide hexarelin reduces neonatal brain injury and alters Akt/glycogen synthase kinase-3beta phosphorylation. Endocrinology. 2005;146:4665–4672. doi: 10.1210/en.2005-0389. [DOI] [PubMed] [Google Scholar]
- 58.Hagberg H, Gilland E, Diemer NH, Andiné P. Hypoxia-ischemia in the neonatal rat brain: histopathology after post-treatment with NMDA and non-NMDA receptor antagonists. Biol Neonate. 1994;66:205–213. doi: 10.1159/000244109. [DOI] [PubMed] [Google Scholar]
- 59.Gilland E, Puka-Sundvall M, Hillered L, Hagberg H. Mitochondrial function and energy metabolism after hypoxia-ischemia in the immature rat brain: involvement of NMDA-receptors. J Cereb Blood Flow Metab. 1998;18:297–304. doi: 10.1097/00004647-199803000-00008. [DOI] [PubMed] [Google Scholar]
- 60.Puka-Sundvall M, Wallin C, Gilland E, et al. Impairment of mitochondrial respiration after cerebral hypoxia-ischemia in immature rats: relationship to activation of caspase-3 and neuronal injury. Dev Brain Res. 2000;125:43–50. doi: 10.1016/s0165-3806(00)00111-5. [DOI] [PubMed] [Google Scholar]
- 61.Peeters-Scholte C, Koster J, Veldhuis W, et al. Neuroprotection by selective nitric oxide synthase inhibition at 24 hours after perinatal hypoxia-ischemia. Stroke. 2002;33:2304–2310. doi: 10.1161/01.str.0000028343.25901.09. [DOI] [PubMed] [Google Scholar]
- 62.Hedtjärn M, Leverin AL, Eriksson K, et al. Interleukin-18 involvement in hypoxic-ischemic brain injury. J Neurosci. 2002;22:5910–5919. doi: 10.1523/JNEUROSCI.22-14-05910.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu XH, Kwon D, Schielke GP, et al. Mice deficient in interleukin-1 converting enzyme are resistant to neonatal hypoxic-ischemic brain damage. J Cereb Blood Flow Metab. 1999;19:1099–1108. doi: 10.1097/00004647-199910000-00006. [DOI] [PubMed] [Google Scholar]
- 64.Ten VS, Sosunov SA, Mazer SP, et al. C1q-deficiency is neuroprotective against hypoxicischemic brain injury in neonatal mice. Stroke. 2005;36:2244–2250. doi: 10.1161/01.STR.0000182237.20807.d0. [DOI] [PubMed] [Google Scholar]
- 65.Martin D, Chinookoswong N, Miller G. The interleukin-1 receptor antagonist (rhIL-1ra) protects against cerebral infarction in a rat model of hypoxia-ischemia. Exp Neurol. 1994;130:362–367. doi: 10.1006/exnr.1994.1215. [DOI] [PubMed] [Google Scholar]
- 66.Liu XH, Eun BL, Barks JD. Platelet-activating factor antagonist BN 50730 attenuates hypoxic-ischemic brain injury in neonatal rats. Pediatr Res. 2001;49:804–811. doi: 10.1203/00006450-200106000-00016. [DOI] [PubMed] [Google Scholar]
- 67.Tsuji M, Higuchi Y, Shiraishi K, et al. Protective effect of aminoguanidine on hypoxic-ischemic brain damage and temporal profile of brain nitric oxide in neonatal rat. Pediatr Res. 2000;47:79–83. doi: 10.1203/00006450-200001000-00015. [DOI] [PubMed] [Google Scholar]
- 68.Nijboer CH, Heijnen CJ, Groenendaal F, et al. Strong neuroprotection by inhibition of NF-kappaB after neonatal hypoxia-ischemia involves apoptotic mechanisms but is independent of cytokines. Stroke. 2008;39:2129–2137. doi: 10.1161/STROKEAHA.107.504175. [DOI] [PubMed] [Google Scholar]