Abstract
Preterm infants exposed to inflammation are at increased risk of white matter injury and/or cerebral palsy. To investigate the effect of chronic inflammation on developing white matter, we administered low-dose lipopolysaccharide once a day from postnatal day 3 to 11 examined white matter changes at postnatal day 12, and monitored serum levels of insulin-like growth factor 1 (IGF-1) and insulin-like factor binding protein-3 (IGFBP-3). A single injection of lipopolysaccharide decreased the serum IGF-1 level but not the IGFBP-3 level. At postnatal day 12, quantification of immunohistochemical staining for axonal, myelin, and oligodendrocyte markers revealed impaired myelination in subcortical white matter. In addition, brain grey matter volume decreased and spleen and liver weight increased at postnatal day 12. These data suggest chronic subclinical inflammation hampers development of white and grey matter in early life, which may be associated with IGF-1 deficiency.
Keywords: white matter damage, inflammation, neonatal brain injury, IGF-1
Introduction
Experimental evidence suggests that intrauterine inflammation induced by the gram negative bacterial cell wall component lipopolysaccharide is closely related to the development of perinatal brain injury, including white matter injury,1–4 similar to that seen in preterm human infants.4–7 Recently, similar white matter damage was also observed in both midgestation and near-term fetal sheep,8 suggesting the injurious effects of lipopolysaccharide may contribute to damage throughout pregnancy and that white matter injury may not only be limited to vulnerability of immature (O4+/O1−) oligodendroglia.9
Insulin-like growth factor 1 (IGF-1) plays an important role in childhood growth and continues to have anabolic effects in adults. It is also a neurotrophic factor promoting cell proliferation and differentiation during normal brain development and maturation, and it exerts biological effects on many cells, including oligodendrocytes in the central nervous system.10 In addition, poor growth of head circumference has been associated with low serum IGF-1 levels in preterm infants.11 After hypoxia-ischemia in infant rats, IGF-1 expression increases in the brain;12 IGF-1 administration protects the immature white matter from injury after cerebral ischemia in near-term fetal sheep.10 Lipopolysaccharide administration in rats decreases circulating IGF-1 and its gene expression in the liver.13, 14 However, the effect of lipopolysaccharide on IGF-1 in neonatal mice is not known. We investigated the effects of lipopolysaccharide on IGF-1 and IGF-1 binding protein 3 (IGFBP-3) serum expression in neonatal mice. Furthermore, using a model of repeated low-dose lipopolysaccharide administration, mimicking a clinic situation of subclinical chronic inflammation, we also examined the effect on white and grey matter development.
Materials and Methods
Animals
Time-mated pregnant C57BL/6 mice were purchased from Charles River Laboratories (Sulzfeld, Germany) and gave birth in the animal facility (Experimental Biomedicin, University of Gothenburg). Day of birth was counted as postnatal day 0. Mice were housed with a 12-hour light/dark cycle. Free access to a standard laboratory chow diet (B&K, Solna, Sweden) and drinking water was provided. All animal experimentation was approved by the Ethical Committee of Gothenburg (No. 314/05 or 375/08).
Lipopolysaccharide Administration
Dose response study: At postnatal day 6, mice offspring of both sexes were injected intraperitoneally with lipopolysaccharide (Escherichia coli 055:B5; Sigma, Stockholm, Sweden) 0.1mg/kg, 0.3mg/kg, and 0.5mg/kg, or saline. Mice were sacrificed at 6 hours after injection by decapitation. Blood was collected and serum levels of interleukin 6 (IL-6), IGF-1, and IGFBP-3 were analyzed. For the repeated LPS experiments, the dose of 0.3mg/kg LPS was chosen based on the IL-6 results above and our previous studies that show low mortality with this dose.15 Mice were injected intraperitoneally once a day from postnatal day 3 to postnatal day 11 and pups were sacrificed at postnatal day 12 and brain, spleen, and liver were collected and weighed.
Analysis of IL-6, IGF-1, and IGFBP-3
Serum IL-6 levels were measured using a mouse IL-6 ELISA (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. Dilution factor used for lipopolysaccharide-treated mice was 1:100. Samples from control animals were under detection limit when diluted 1:5. According to the manufacturer, the intra-assay coefficients of variation for the IL-6 assay were 7.0%, 3.9%, and 3.5% at concentrations of 30 pg/mL, 87 pg/mL, and 232 pg/mL, respectively, and the inter-assay coefficients of variation were 6.1%, 8.9%, and 7.6% at concentrations of 29 pg/mL, 8 pg/mL, and 238 pg/mL, respectively.
Serum IGF-1 levels were measured using an IGFBP3-blocked radioimmunoassay with a large excess of IGF-II for determination of IGF-1 (Mediagnost GmbH, Tübingen, Germany) according to a method described previously with some modification.16, 17 The intra assay CVs for the IGF-I assay were 11.1%, 7.2%, and 7.4% at concentrations of 36 µg/L, 204 µg/L, and 545 µg/L, respectively, and the inter-assay CVs at these concentrations were 13.5%, 8.8%, and 9.9%.
Serum IGFBP-3 levels were measured using a mouse IGFBP-3 ELISA (R&D Systems, Minneapolis, Minnesota) according to the manufacturer’ instructions. The samples were diluted 1:500. The recovery of a spiked control was 90% in spiked serum sample and 94% in spiked control, diluted 1:500 to 1:4000. The linearity was within the range of 80% to 120% for dilutions 1:500 to 1:4000.
Immunohistochemical Staining
Pups at postnatal day 12 were deeply anesthetized and perfused intracardially with saline and 5% buffered formaldehyde (Histofix; Histolab, Göteborg, Sweden). Brains were removed and fixed in 5% buffered formaldehyde for 24 hours. After dehydration with graded ethanol and xylene, brains were paraffin-embedded and cut into 9 µm frontal sections. Parallel sections were used for various stains, as previously described.18 Briefly, nonspecific binding was blocked for 30 minutes with 4% horse serum in phosphate buffered saline. The following primary antibodies were used: microtubule associated protein-2 (MAP-2, clone HM-2, Sigma, Stockholm, Sweden), rabbit anti-myelin basic protein (MBP, Sternberger Monoclonal Incorporated, SMI 94, Lutherville, MA, USA), anti-2’,3’-cyclic nucleotide 3’ phosphodiesterase (CNPase, MS-349-P; Lab Vision, Fremont, California), anti-phosphorylated neurofilament (NF, SMI 312; Sternberger Monoclonals), and oligodendrocyte lineage transcription factor 2 (Olig 2, AB15328, CHEMICON International, Inc, Temecula, CA, USA). After incubating the primary antibodies for 60 minutes at room temperature, the appropriate biotinylated secondary antibodies (all from Vector, Burlingame, California) were applied for 60 minutes at room temperature. Visualization was performed using Vectastain ABC Elite with 0.5 mg/mL 3′-diaminobenzidine enhanced with 15 mg/mL ammonium nickel sulfate, 2 mg/mL beta-D-glucose, 0.4 mg/mL ammonium chloride, and 0.01 mg/mL beta-glucose oxidase (all from Sigma).
Grey Matter Volume and Subcortical White Matter Area Measurement
Grey matter area was determined by measuring the MAP-2 immunoreactive area and myelination was examined by measuring the subcortical white matter area stained for MBP, CNPase, and NF using Micro Image, version 4.0 (Micro-Macro AB, Gothenburg, Sweden) as previously described.1, 8, 19 The volume of MAP-2-positive tissue was calculated according to the method described previously20 using the following formula: V=SA · p · T, where V is the total volume, SA is the sum of the areas measured, p is the inverse of the section sampling fraction, and T is the section thickness. Olig2-positive cell counting was performed using stereological principles (Stereo investigator 7, MicroBrightField System Inc.). Cells were counted at 8 levels in each brain with a counting frame of 75x75 µm per section and the average number of positive cells/mm2 was calculated. Results are presented as mean ± standard error of the mean.
Statistics
Statistical programs StatView and SPSS were used. Analysis of variance followed by Fisher’s protected least significant difference post hoc test was used for comparing the IL-6 results with different doses of lipopolysaccharide. For all other analysis, Student unpaired t test was used to compare results between lipopolysaccharide and saline groups.
Results
IL-6, IGF-1, and IGFBP-3 Changes after LPS Administration
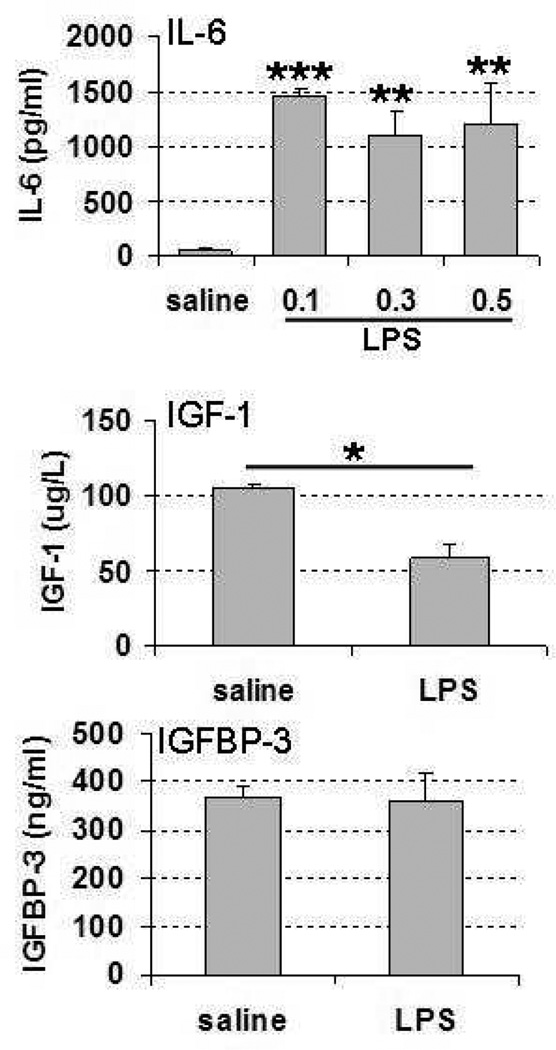
There was no mortality at any of the lipopolysaccharide doses used. To confirm that lipopolysaccharide induced an appropriate inflammatory response, we measured the pro-inflammatory cytokine IL-6 at 3 different lipopolysaccharide doses: 0.1 mg/kg, 0.3 mg/kg, and 0.5 mg/kg. IL-6 increased at 6 hours after lipopolysaccharide administration with all 3 doses (Figure 1) and there were no significant differences between the different lipopolysaccharide doses. Therefore, on the basis of these results and our previous experiments,15 a dosage of 0.3 mg/kg was chosen for all subsequent experiments.
Figure 1.
Serum IL-6, IGF-1, and IGFBP-3 levels at 6 hours after lipopolysaccharide administration on postnatal day 6. Lipopolysaccharide dose in IL-6 analysis: 0.1 mg/kg, 0.3 mg/kg, and 0.5 mg/kg. Lipopolysaccharide dose in IGF-1 and IGFBP-3 analysis: 0.3mg/kg, *P < .05, **P < .01, ***P < .001. Abbreviations: IGFBP-3, insulin-like growth factor binding protein; IGF-1, insulin-like growth factor 1; IL-6, interleukin-6.
To monitor changes in the IGF-1 system, serum levels of IGF-1 and IGFBP-3 were measured at 6 hours after administration of 0.3 mg/kg lipopolysaccharide to postnatal day 6 mouse pups. The lipopolysaccharide injection caused a significant decrease in IGF-1 serum levels at 6 hours after the injection (45% reduction) (Figure 1). IGFBP-3 serum levels were not altered at 6 hours after lipopolysaccharide in mice injected with 0.3 mg/kg lipopolysaccharide at postnatal day 6 (Figure 1).
Brain, Liver, and Spleen Weight Changes after LPS Administration
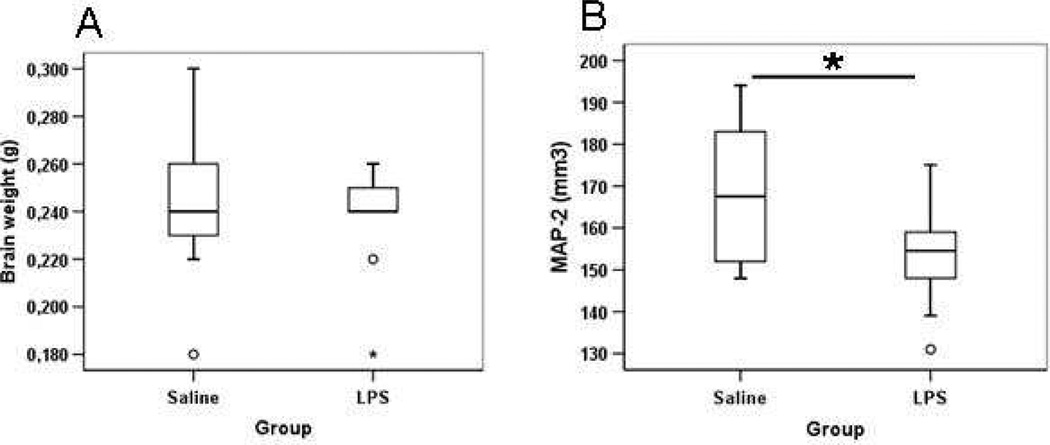
After administration of repeated lipopolysaccharide doses (0.3 mg/kg) once a day from postnatal day 3 to postnatal day 11, absolute brain weight (Figure 2, Panel A) and the relative brain weight to body weight (data not shown) were not different in lipopolysaccharide-exposed animals compared with saline-treated animals at postnatal day 12 and we found no infarctions, dilatation of the cerebral ventricles, or morphological signs of cell death after lipopolysaccharide. To examine the grey matter changes after repeated lipopolysaccharide treatment, brain grey matter volume was measured using immunohistochemical staining for the neuronal marker MAP-2. A significantly decreased grey matter volume was found in the lipopolysaccharide-treated mouse brain compared with saline-treated pup brains at postnatal day 12 (Figure 2, Panel B).
Figure 2.
Brain weight (A) and brain grey matter volume (B) at postnatal day 12 after repeated lipopolysaccharide administration from postnatal day 3 to postnatal day 11, *P < .05.
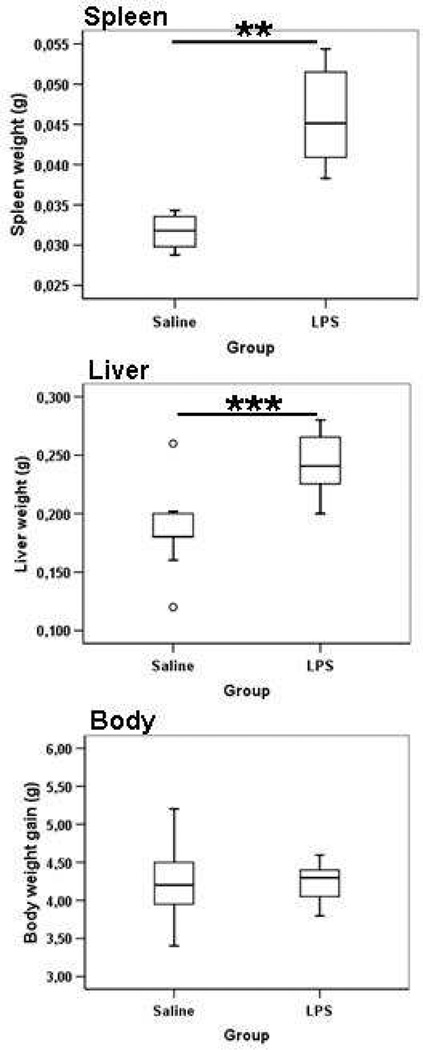
Moreover, there was a significant increase in both the absolute spleen and liver weights (Figure 3) as well as the relative spleen-and-liver weight-to-body-weight ratio (data not show) at postnatal day 12 in animals repeatedly treated with lipopolysaccharide compared with those treated with saline; the difference was largest for the liver weight (Figure 3).The whole body weight gain was not different between groups at postnatal day 12 (Figure 3).
Figure 3.
Liver weight, spleen weight, and body weight at postnatal day 12 after repeated lipopolysaccharide administration from postnatal day 3 to postnatal day 11, **P < .01, ***P <. 001.
White Matter Changes After Lipopolysaccharide Administration
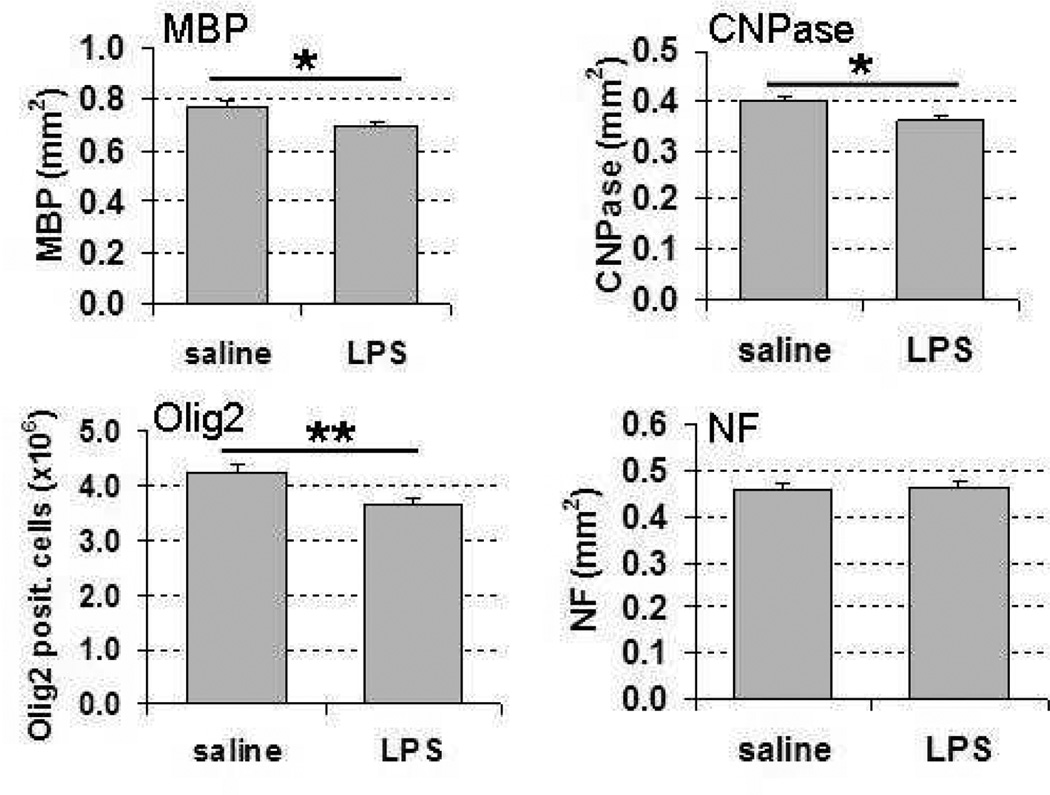
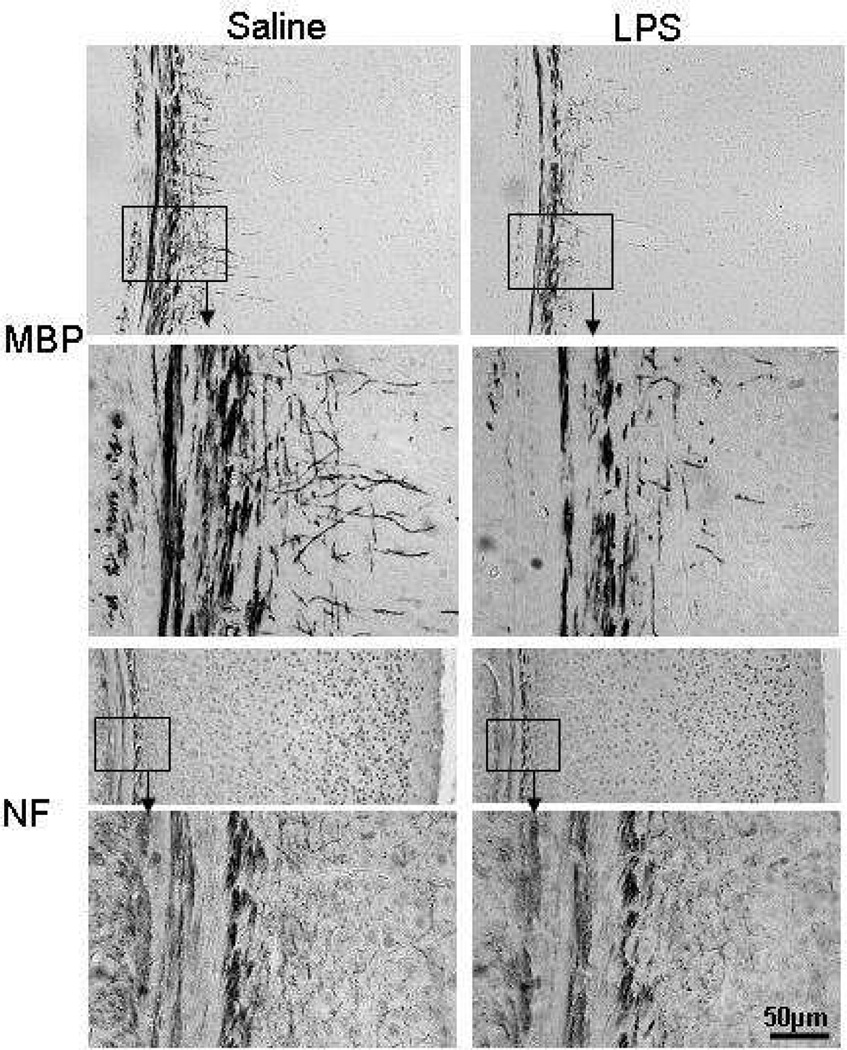
To investigate the impact of repeated lipopolysaccharide administration on white matter, parallel sections from postnatal day 12 mouse brains were immunohistochemically stained with oligodendrocyte markers CNPase and Olig2, myelin marker MBP, and axonal marker NF. Quantification of the immunohistochemical staining for these markers demonstrated significantly decreased myelination, as indicated by reduced area of MBP and CNPase staining in the subcortical white matter in lipopolysaccharide-treated animals compared with saline-treated animals (Figure 4 and Figure 5). Similarly, the number of cells positive for Olig 2, a marker expressed both in early progenitors and at later stages in the oligodendrocyte lineage,21 was significantly decreased in the lipopolysaccharide-treated animals compared with the saline-treated ones (Figure 4). Axons were visualized with NF, one of the major cytoskeletal proteins of the axon. There were no significant differences in NF immunohistochemical staining between the lipopolysaccharide- and saline-treated animals at postnatal day 12 (Figure 4 and Figure 5).
Figure 4.
Subcortical white matter area quantification of myelin basic protein (MBP), 2’,3’-cyclic nucleotide 3’ phosphodiesterase (CNPase), neurofilament (NF) immunoreactivity, and oligodendrocyte lineage transcription factor 2 (Olig 2)-positive cell counts at postnatal day 12 after repeated lipopolysaccharide administration from postnatal day 3 to postnatal day 11, *P <.05, **P < .01. Abbreviations: CNPase, 2’,3’-cyclic nucleotide 3’ phosphodiesterase; MBP, myelin basic protein; NF, neurofilament.
Figure 5.
Representative photomicrograph images of myelin basic protein (MBP) and neurofilament (NF) immunoreactivity in subcortical white matter areas at postnatal day 12 after repeated lipopolysaccharide administration from postnatal day 3 to postnatal day 11. Scale bar = 50µL. Abbreviations: MBP, myelin basic protein; NF, neurofilament.
Discussion
We found that repeated administration of a low dose of lipopolysaccharide from postnatal day 3 to postnatal day 11 impaired brain development (ie, induced a decrease in the number of oligodendrocytes and reduced myelination of the white matter, as well as reduction in grey matter volume. Lipopolysaccharide was also found to decrease serum IGF-1 levels, which may suggest a role for this growth factor in the neuropathology observed.
In the rodent, there is a major growth spurt of the brain in the first postnatal week22 and the dominant cell type in the white matter is the pre-oligodendrocyte.23, 24 This time period equates to the second-third trimester in human pregnancy, a window when white matter damage or deficiency of white matter growth is presumed to occur in the human.9 This study used a repeated lipopolysaccharide exposure model from postnatal day 3 up to postnatal day 11, therefore covering the period of rapid brain growth in rodents.
Lipopolysaccharide administered during pregnancy, either to the mother, directly to the fetus, or into the brain of newborn animals, caused white matter injury, indicated by either reduced number of oligodendrocytes and/or hypomyelination.1–4, 25–29 The mechanisms behind this remain unclear and multiple factors have been suggested, such as hypoglycemia,30,31 hypoxemia,3 selectively reduced white matter blood flow,31 reduced cerebral oxygen delivery to the brain,32 inflammation,4, 33, 34 specific vulnerability of precursor oligodendrocytes,35 excitotoxicity, and oxidative stress.27, 36,27 In addition, recently published work suggests impaired development/deficiency of growth of both white and grey matter is common in preterm infants and actually more common than overt lesions.37,38,39,40 Similarly, in the present study, we observed hypomyelination in the subcortical white matter along with a decreased number of Olig2-positive cells, a marker for both precursor oligodendrocytes and mature oligodendrocytes,21 suggesting impairment of white matter development either as a result of reduced proliferation/differentiation of oligodendrocytes or cellular loss. In contrast, axonal development, as shown by the quantification of NF immunoreactivity, was not affected by repeated lipopolysaccharide treatment, agreeing with a previous study when lipopolysaccharide was administrated prenatally.1 Taken together, these results suggest that inflammation affects oligodendroglia and myelin formation to a greater extent than the axons.
IGF-1 signaling plays an important role in myelination, and a number of studies have shown that exogenous IGF-1 administration is beneficial. IGF-1 was shown to prevent post-ischemic oligodendrocyte cell loss and associated demyelination and to stimulate proliferation, differentiation, and myelin production by oligodendrocytes in vitro.41 In the neonate, IGF-1 provides neuroprotection to oligodendrocyte progenitor cells after cerebral hypoxia-ischemia.42 In the present study, we found that a single intraperitoneal injection of low-dose lipopolysaccharide was sufficient to decrease IGF-1 serum levels. Similarly, lipopolysaccharide administration in adult rats induced a significant decrease in serum concentrations of IGF-1 and IGFBP-3 and body weight.13,43, 44 Given that IGF-1 has such a central role in the growth and development of the central nervous system,45 it would be interesting to further investigate how diminished IGF-1 levels may contribute to lipopolysaccharide-induced impairment of grey and white matter development.
The brain weight and whole body weight did not differ between the lipopolysaccharide group and saline controls. This agrees with a previous study in neonatal mice that shows that if lipopolysaccharide was given once at postnatal day 2, body weight and brain weight was not affected at postnatal days 5, 7, 14, and 2846 but not with a study in rats.47 The different effects on weight among various studies might reflect the different lipopolysaccharide dose used and the administration period. The liver and spleen play a central role in the systemic lipopolysaccharide response because the liver clears lipopolysaccharide from the circulation, and both liver and spleen release cytokines and reactive oxygen intermediates after lipopolysaccharide, which may reflect the liver and spleen weight increase observed in the present study.
In conclusion, chronic subclinical inflammation in early life inhibited white and grey matter development in neonatal mice. These cerebral changes were associated with reduced IGF-1 levels. Future studies will explore whether systemic inflammation impairs brain development through attenuation of IGF-1 tropic support.
Acknowledgment
Supported by grants from the National Institutes of Health (5R13NS040925-09), the Cerebral Palsy International Research Foundation, the Kennedy Krieger Institute, and the Child Neurology Society.
Footnotes
Presented at the Neurobiology of Disease in Children Conference: Symposium on Injury to the Preterm Brain and Cerebral Palsy, in conjunction with the 37th Annual Meeting of the Child Neurology Society, Santa Clara, California, November 5, 2008.
References
- 1.Wang X, Hagberg H, Nie C, et al. Dual role of intrauterine immune challenge on neonatal and adult brain vulnerability to hypoxia-ischemia. J Neuropathol Exp Neurol. 2007;66:552–561. doi: 10.1097/01.jnen.0000263870.91811.6f. [DOI] [PubMed] [Google Scholar]
- 2.Peebles DM, Miller S, Newman JP, et al. The effect of systemic administration of lipopolysaccharide on cerebral haemodynamics and oxygenation in the 0.65 gestation ovine fetus in utero. BJOG. 2003;110:735–743. [PubMed] [Google Scholar]
- 3.Duncan JR, Cock ML, Scheerlinck JP, et al. White matter injury after repeated endotoxin exposure in the preterm ovine fetus. Pediatr Res. 2002;52:941–949. doi: 10.1203/00006450-200212000-00021. [DOI] [PubMed] [Google Scholar]
- 4.Mallard C, Welin AK, Peebles D, et al. White matter injury following systemic endotoxemia or asphyxia in the fetal sheep. Neurochem Res. 2003;28:215–223. doi: 10.1023/a:1022368915400. [DOI] [PubMed] [Google Scholar]
- 5.Dammann O, Leviton A. Inflammatory brain damage in preterm newborns--dry numbers, wet, lab, causal inferences. Early Hum Dev. 2004;79:1–15. doi: 10.1016/j.earlhumdev.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Leviton A, Dammann O. Coagulation, inflammation, and the risk of neonatal white matter damage. Pediatr Res. 2004;55:541–545. doi: 10.1203/01.PDR.0000121197.24154.82. [DOI] [PubMed] [Google Scholar]
- 7.Garnier Y, Berger R, Alm S, et al. Systemic endotoxin administration results in increased S100B protein blood levels and periventricular brain white matter injury in the preterm fetal sheep. Eur J Obstet Gynecol Reprod Biol. 2006;124:15–22. doi: 10.1016/j.ejogrb.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Svedin P, Kjellmer I, Welin A, et al. Maturational effects of lipopolysaccharide on white-matter injury in fetal sheep. J Child Neurol. 2005;20:960–964. doi: 10.1177/08830738050200120501. [DOI] [PubMed] [Google Scholar]
- 9.Back SA, Han BH, Luo NL, et al. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci. 2002;22:455–463. doi: 10.1523/JNEUROSCI.22-02-00455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan J, Bennet L, George S, et al. Insulin-like growth factor-1 reduces postischemic white matter injury in fetal sheep. J Cereb Blood Flow Metab. 2001;21:493–502. doi: 10.1097/00004647-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Lofqvist C, Engstrom E, Sigurdsson J, et al. Postnatal head growth deficit among premature infants parallels retinopathy of prematurity and insulin-like growth factor-1 deficit. Pediatrics. 2006;117:1930–1938. doi: 10.1542/peds.2005-1926. [DOI] [PubMed] [Google Scholar]
- 12.Gluckman P, Klempt N, Guan J, et al. A role for IGF-1 in the rescue of CNS neurons following hypoxic-ischemic injury. Biochem Biophys Res Commun. 1992;182:593–599. doi: 10.1016/0006-291x(92)91774-k. [DOI] [PubMed] [Google Scholar]
- 13.Fan J, Molina PE, Gelato MC, Lang CH. Differential tissue regulation of insulinlike growth factor-I content and binding proteins after endotoxin. Endocrinology. 1994;134:1685–1692. doi: 10.1210/endo.134.4.7511091. [DOI] [PubMed] [Google Scholar]
- 14.Soto L, Martin AI, Millan S, et al. Effects of endotoxin lipopolysaccharide administration on the somatotropic axis. J Endocrinol. 1998;159:239–246. doi: 10.1677/joe.0.1590239. [DOI] [PubMed] [Google Scholar]
- 15.Eklind S, Mallard C, Leverin AL, et al. Bacterial endotoxin sensitizes the immature brain to hypoxic--ischaemic injury. Eur J Neurosci. 2001;13:1101–1106. doi: 10.1046/j.0953-816x.2001.01474.x. [DOI] [PubMed] [Google Scholar]
- 16.Blum WF, Breier BH. Radioimmunoassays for IGFs and IGFBPs. Growth Regul. 1994;4(Suppl 1):11–19. [PubMed] [Google Scholar]
- 17.Upton Z, Webb H, Tomas FM, et al. Characterization of serum-derived and recombinant rat IGF-I and their use for measuring true concentrations of IGF-I in rat plasma. J Endocrinol. 1996;149:379–387. doi: 10.1677/joe.0.1490379. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Zhu C, Qiu L, et al. Activation of ERK1/2 after neonatal rat cerebral hypoxia-ischaemia. J Neurochem. 2003;86:351–362. doi: 10.1046/j.1471-4159.2003.01838.x. [DOI] [PubMed] [Google Scholar]
- 19.Hedtjarn M, Mallard C, Arvidsson P, Hagberg H. White matter injury in the immature brain: role of interleukin-18. Neurosci Lett. 2005;373:16–20. doi: 10.1016/j.neulet.2004.09.062. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Svedin P, Nie C, et al. N-acetylcysteine reduces lipopolysaccharide-sensitized hypoxic-ischemic brain injury. Ann Neurol. 2007;61:263–271. doi: 10.1002/ana.21066. [DOI] [PubMed] [Google Scholar]
- 21.Back SA, Riddle A, McClure MM. Maturation-dependent vulnerability of perinatal white matter in premature birth. Stroke. 2007;38:724–730. doi: 10.1161/01.STR.0000254729.27386.05. [DOI] [PubMed] [Google Scholar]
- 22.Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- 23.Craig A, Ling Luo N, Beardsley DJ, et al. Quantitative analysis of perinatal rodent oligodendrocyte lineage progression and its correlation with human. Exp Neurol. 2003;181:231–240. doi: 10.1016/s0014-4886(03)00032-3. [DOI] [PubMed] [Google Scholar]
- 24.Pang Y, Cai Z, Rhodes PG. Disturbance of oligodendrocyte development, hypomyelination and white matter injury in the neonatal rat brain after intracerebral injection of lipopolysaccharide. Brain Res Dev Brain Res. 2003;140:205–214. doi: 10.1016/s0165-3806(02)00606-5. [DOI] [PubMed] [Google Scholar]
- 25.Bell MJ, Hallenbeck JM. Effects of intrauterine inflammation on developing rat brain. J Neurosci Res. 2002;70:570–579. doi: 10.1002/jnr.10423. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Hagberg H, Zhu C, et al. Effects of intrauterine inflammation on the developing mouse brain. Brain Res. 2007;1144:180–185. doi: 10.1016/j.brainres.2007.01.083. [DOI] [PubMed] [Google Scholar]
- 27.Fan LW, Mitchell HJ, Tien LT, et al. alpha-Phenyl-n-tert-butyl-nitrone reduces lipopolysaccharide-induced white matter injury in the neonatal rat brain. Dev Neurobiol. 2008;68:365–378. doi: 10.1002/dneu.20591. [DOI] [PubMed] [Google Scholar]
- 28.Pang Y, Cai Z, Rhodes PG. Effects of lipopolysaccharide on oligodendrocyte progenitor cells are mediated by astrocytes and microglia. J Neurosci Res. 2000;62:510–520. doi: 10.1002/1097-4547(20001115)62:4<510::AID-JNR5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 29.Cai Z, Pan ZL, Pang Y, et al. Cytokine induction in fetal rat brains and brain injury in neonatal rats after maternal lipopolysaccharide administration. Pediatr Res. 2000;47:64–72. doi: 10.1203/00006450-200001000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Eklind S, Arvidsson P, Hagberg H, Mallard C. The role of glucose in brain injury following the combination of lipopolysaccharide or lipoteichoic acid and hypoxiaischemia in neonatal rats. Dev Neurosci. 2004;26:61–67. doi: 10.1159/000080713. [DOI] [PubMed] [Google Scholar]
- 31.Young RS, Hernandez MJ, Yagel SK. Selective reduction of blood flow to white matter during hypotension in newborn dogs: a possible mechanism of periventricular leukomalacia. Ann Neurol. 1982;12:445–448. doi: 10.1002/ana.410120506. [DOI] [PubMed] [Google Scholar]
- 32.Dalitz P, Harding R, Rees SM, Cock ML. Prolonged reductions in placental blood flow and cerebral oxygen delivery in preterm fetal sheep exposed to endotoxin: possible factors in white matter injury after acute infection. J Soc Gynecol Investig. 2003;10:283–290. doi: 10.1016/s1071-5576(03)00090-x. [DOI] [PubMed] [Google Scholar]
- 33.Yesilirmak DC, Kumral A, Baskin H, et al. Activated protein C reduces endotoxin-induced white matter injury in the developing rat brain. Brain Res. 2007;1164:14–23. doi: 10.1016/j.brainres.2007.04.083. [DOI] [PubMed] [Google Scholar]
- 34.Kumral A, Baskin H, Yesilirmak DC, et al. Erythropoietin attenuates lipopolysaccharide-induced white matter injury in the neonatal rat brain. Neonatology. 2007;92:269–278. doi: 10.1159/000105493. [DOI] [PubMed] [Google Scholar]
- 35.Lehnardt S, Lachance C, Patrizi S, et al. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci. 2002;22:2478–2486. doi: 10.1523/JNEUROSCI.22-07-02478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Volpe JJ. Cerebral white matter injury of the premature infant-more common than you think. Pediatrics. 2003;112:176–180. doi: 10.1542/peds.112.1.176. [DOI] [PubMed] [Google Scholar]
- 37.Counsell SJ, Edwards AD, Chew AT, et al. Specific relations between neurodevelopmental abilities and white matter microstructure in children born preterm. Brain. 2008;131:3201–3208. doi: 10.1093/brain/awn268. [DOI] [PubMed] [Google Scholar]
- 38.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ment LR, Kesler S, Vohr B, et al. Longitudinal brain volume changes in preterm and term control subjects during late childhood and adolescence. Pediatrics. 2009;123:503–511. doi: 10.1542/peds.2008-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inder TE, Anderson NJ, Spencer C, et al. White matter injury in the premature infant: a comparison between serial cranial sonographic and MR findings at term. AJNR Am J Neuroradiol. 2003;24:805–809. [PMC free article] [PubMed] [Google Scholar]
- 41.D'Ercole AJ, Ye P, Calikoglu AS, Gutierrez-Ospina G. The role of the insulin-like growth factors in the central nervous system. Mol Neurobiol. 1996;13:227–255. doi: 10.1007/BF02740625. [DOI] [PubMed] [Google Scholar]
- 42.Lin S, Fan LW, Pang Y, et al. IGF-1 protects oligodendrocyte progenitor cells and improves neurological functions following cerebral hypoxia-ischemia in the neonatal rat. Brain Res. 2005;1063:15–26. doi: 10.1016/j.brainres.2005.09.042. [DOI] [PubMed] [Google Scholar]
- 43.Priego T, Ibanez de Caceres I, Martin AI, et al. Endotoxin decreases serum IGFBP-3 and liver IGFBP-3 mRNA: comparison between Lewis and Wistar rats. Mol Cell Endocrinol. 2003;199:23–28. doi: 10.1016/s0303-7207(02)00356-8. [DOI] [PubMed] [Google Scholar]
- 44.Granado M, Martin AI, Priego T, et al. Inactivation of Kupffer cells by gadolinium administration prevents lipopolysaccharide-induced decrease in liver insulin-like growth factor-I and IGF-binding protein-3 gene expression. J Endocrinol. 2006;188:503–511. doi: 10.1677/joe.1.06585. [DOI] [PubMed] [Google Scholar]
- 45.D'Ercole AJ, Ye P, O'Kusky JR. Mutant mouse models of insulin-like growth factor actions in the central nervous system. Neuropeptides. 2002;36:209–220. doi: 10.1054/npep.2002.0893. [DOI] [PubMed] [Google Scholar]
- 46.Thomas DJ, Winchurch RA. Altered organ growth and zinc and copper distribution in endotoxin-treated neonatal mice. Pediatr Res. 1991;30:141–145. doi: 10.1203/00006450-199108000-00003. [DOI] [PubMed] [Google Scholar]
- 47.Fan LW, Pang Y, Lin S, et al. Minocycline attenuates lipopolysaccharide-induced white matter injury in the neonatal rat brain. Neuroscience. 2005;133:159–168. doi: 10.1016/j.neuroscience.2005.02.016. [DOI] [PubMed] [Google Scholar]