Abstract
In recent years there has been a flood of interest in the relationship between brain tumors and stem cells. Some investigators have focused on the sensitivity of normal stem cells to transformation, others have described phenotypic or functional similarities between tumor cells and stem cells, and still others have suggested that tumors contain a subpopulation of “cancer stem cells” that is crucial for tumor maintenance or propagation. While all these concepts are interesting and provide insight into the origins and properties of brain tumors, the use of similar terms to describe them has led to confusion. The goal of this review is to sort out some of that confusion and highlight what we know and what we have yet to learn.
Introduction
Tumors of the central nervous system are among the most lethal types of cancer. These tumors can present with glial or neuronal phenotypes, or can contain a mixture of cell types. Tumors consisting largely of cells resembling glia are referred to as gliomas (often subdivided into astrocytomas and oligodendrogliomas), whereas those in which neuronal cells predominate are called neuroblastomas or primitive neuroectodermal tumors.
Although some progress has been made in the treatment of brain tumors, overall survival and quality of life remain poor, especially for patients with the most aggressive forms of these diseases. For example, glioblastoma multiforme, the most common malignant brain tumor in adults, has a median survival time of 1 year.1 Conventional therapies can prolong survival by a few months, but these are often associated with significant toxicity, and most patients eventually relapse and die from their disease. Medulloblastoma, a primitive neuroectodermal tumor of the cerebellum, is the most common malignant brain tumor in children.2 With a combination of surgery, radiation, and chemotherapy, the majority of medulloblastoma patients now survive for > 5 years. However, young children and those who present with disseminated disease have much lower survival rates. Moreover, patients treated for medulloblastoma often develop cognitive and endocrine deficits and have an increased risk of secondary tumors later in life. Thus, more effective approaches to treating brain tumors are desperately needed.
Such approaches are likely to come from a deeper understanding of the origins of these diseases, and in particular, from identification of the cells that mediate tumor initiation and propagation. Identifying the cell type from which a tumor arises (the cell of origin) allows previous studies of that cell type – for example, a neural stem cell or a neuronal or glial progenitor – to be used as a basis for understanding the biology of the tumor. In addition, the cell of origin provides an important baseline for genomic and proteomic analyses of tumor cells, which are frequently used to define targets for diagnosis and therapy. In short, understanding what is abnormal about a tumor cell depends on knowing which normal cell to compare it to.
Identification of the cells that maintain the growth of an established tumor is equally important. Such cells – often referred to as cancer stem cells, tumor-initiating cells, or tumor propagating cells – may represent a small proportion of the tumor mass, but because they are capable of extensive self-renewal they can sustain the long-term growth of a tumor. Moreover, recent studies have suggested that these cells may promote angiogenesis and may be resistant to radiation and chemotherapy.3–5 If so, conventional therapies may destroy the bulk of the tumor but leave the cancer stem cells behind to regenerate it. If cancer stem cells are critical mediators of tumor resistance and recurrence, identifying these cells and understanding their vulnerabilities is essential for development of effective brain tumor therapies.
While there is broad consensus regarding the importance of these cell types, the use of similar terms to describe them has led to some confusion. For example, the term “tumor-initiating cell” may refer to a normal cell that gives rise to a tumor following oncogenic transformation (ie, the cell of origin), or it may refer to a tumor cell that can recreate a tumor after transplantation into an immunocompromised mouse (ie, a tumor-propagating cell). Likewise, the term “cancer stem cell” may be applied to a tumor cell that expresses stem cell markers and exhibits multipotency (ie, a stemlike cancer cell), or it may be defined (as described above) as a cell that has the ability to regenerate a tumor after transplantation. The phenotypic similarity between cancer stem cells and normal stem cells has added to the confusion, prompting some investigators to speculate that cancer stem cells arise from transformation of normal stem cells. While there may be an important relationship between normal stem cells, stemlike cancer cells, and cancer stem cells, in most cases this relationship has not been addressed experimentally. To make progress in understanding and targeting brain tumors, it is critical that we use a common terminology, and that the terms we use clearly describe what we know about the cells we are studying. With this in mind, we provide a brief review of stem cells, cells of origin, and cancer stem cells in tumors of the central nervous system.
Stem Cells and Progenitors
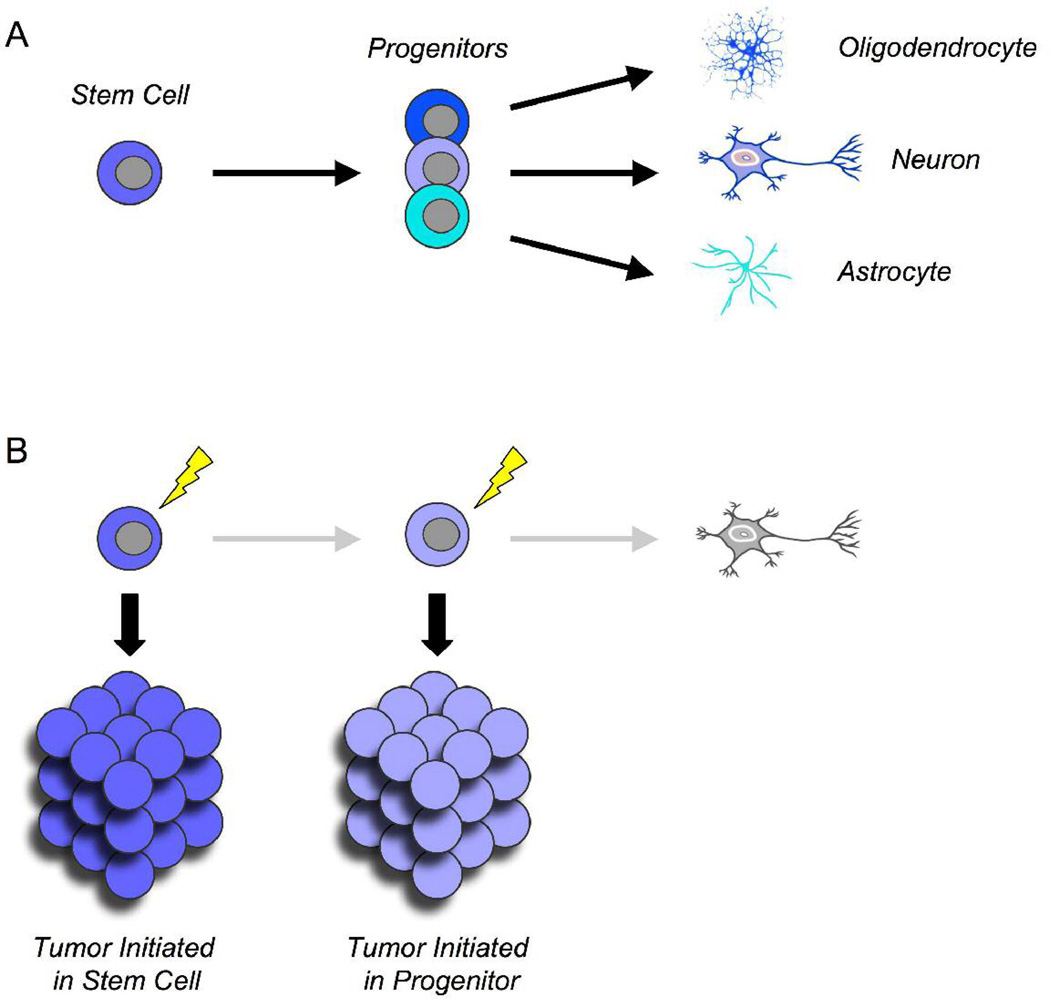
Stem cells can be defined by their ability to self-renew as well as differentiate into all the cell types of a given tissue (Figure 1A).6,7 In the nervous system, stem cells are believed to give rise to neurons, astrocytes, and oligodendrocytes. During embryogenesis, such cells can be found throughout the brain and spinal cord, most commonly in the ventricular zone. After birth, multipotent stem cells can still be found in some regions, including the cerebellum and hippocampus.8,9 In the adult brain, stem cells have been most widely studied in the subventricular zone lining the lateral ventricles and in the dentate gyrus of the hippocampus.3
Figure 1.
Normal cells that give rise to brain tumors. (A) Stem cells and progenitors. Stem cells are normal cells that can self-renew and give rise to all the cell types within a tissue. Progenitors, which arise from stem cells, can also proliferate but can only give rise to restricted cell types. In the brain, lineage-restricted progenitors give rise to neurons, astrocytes, and oligodendrocytes. (B) Cells of origin. The cell of origin for a tumor is the normal cell type that can give rise to the tumor following a particular oncogenic mutation. Both stem cells and progenitors can serve as cells of origin for brain tumors.
In contrast to stem cells, progenitors are more restricted in potential, although they may still possess significant proliferative capacity (Figure 1A). Lineage-restricted progenitors have been identified for neurons, astrocytes, and oligodendrocytes.11–14 An example of a glial progenitor is the oligodendrocyte/type 2 astrocyte progenitor. Although this cell was originally named based on its ability to generate oligodendrocytes and astrocytes in vitro, transplantation studies suggest that in vivo it may only give rise to oligodendrocytes.15 Oligodendrocyte/type 2 astrocyte progenitor cells express the markers A2B5, NG2, and platelet-derived growth factor receptor-alpha , proliferate in response to platelet-derived growth factor, and differentiate in response to thyroid hormone and retinoic acid.4 An example of a neuronal progenitor is the granule neuron precursor of the cerebellum. Granule neuron precursors originate from multipotent stem cells in the cerebellar ventricular zone, but before they commit to the granule lineage, they move away from the ventricular zone and form a distinct germinal zone on the outside of the cerebellum (the external granule layer).12 Granule neuron precursors in the external granule layer proliferate in response to Sonic hedgehog,5 and then exit the cell cycle and migrate away from the surface of the cerebellum to form mature granule neurons. The external granule layer is a transient structure: by 2 weeks to 3 weeks after birth in mice6 (6 months to 8 months after birth in humans7), all granule neuron precursors have differentiated into granule neurons. Thus, unlike stem cells, oligodendrocyte/type 2 astrocyte progenitor cells and granule neuron precursors only proliferate for a limited time, and only give rise to one cell type in vivo.
The distinction between stem cells and progenitors is often made based on expression of markers. Perhaps the most commonly used marker for neural stem cells is the intermediate filament protein Nestin, which is expressed in both embryonic ventricular zone and adult subventricular zone.19,20 More recently described stem cell markers include the transcription factor Sox28 and the cell surface glycoproteins CD133/Prominin122,23 and CD15/Lewis X.9 Although these markers are found on many stem cell populations, they can also be expressed by some lineage-restricted progenitors. For example, Nestin is expressed by Bergmann glia as well as granule neuron precursors in the cerebellum,12,25 CD133 has been observed in ependymal cells as well as cells expressing markers of mature astrocytes, oligodendrocytes, and neurons,8,26 and Sox2 has been shown to be expressed by restricted neuronal progenitors.8 Likewise, markers that are associated with specific neuronal and glial lineages can also be found on stem cells. For example, glial fibrillary acidic protein was for many years considered a canonical marker of the astrocytic lineage, but it is now clear that a subset of glial fibrillary acidic protein+ cells represent multipotent stem cells.27,28 Thus, marker expression alone may be an imperfect indicator of stem cell status.
Another approach that has been used to identify stem cells is the neurosphere assay.10 In this assay, cells are grown at clonal density on uncoated plastic (so that they cannot adhere) in the presence of epidermal growth factor and basic fibroblast growth factor. Under these conditions, individual stem cells are capable of proliferating and generating large clusters of cells, termed neurospheres. However, there are several caveats to using the neurosphere assay to identify stem cells. First, the ability to form neurospheres is not entirely stem cell specific, as several populations of progenitors are capable of forming such spheres as well.10 Second, the majority of the cells within a neurosphere are differentiated progeny, making identification of the stem cell population within a sphere troublesome.29,30 Finally, culturing cells in basic fibroblast growth factor has been shown to confer multipotency on cells that, in vivo, are not necessarily multipotent.31 Thus, cells capable of forming neurospheres in vitro may not necessarily represent true stem cells in vivo. Ultimately, the multipotency of a cell population can only be determined reliably by marking this population (using transgenic or retroviral approaches) and showing that its progeny include both neurons and glia.32,33
Although lineage-restricted progenitors and stem cells differ in their capacity for multilineage differentiation, these cells share many important characteristics. For example, while progenitors may not have the ability to self-renew indefinitely, they often possess some degree of self-renewal ability.13,34 Furthermore, genes and signaling pathways that are associated with self-renewal in stem cells can also regulate the growth and differentiation of progenitors.16,32,35,36 In light of these similarities, it is perhaps not surprising that both progenitors and stem cells can be targets of transformation.
Cell of Origin for Cancer
The cell of origin for cancer can be defined as the normal cell from which a tumor arises (Figure 1B). In principle, this can be either a stem cell or a more restricted progenitor. Historically, the cell of origin for human tumors has been inferred based on expression of markers that are associated with particular cell types. For example, tumors that express neurofilament or synaptophysin have been suggested to arise from neuronal progenitors, those that express glial fibrillary acidic protein have been postulated to arise from astrocytic precursors, and those that express Nestin have been suggested to arise from multipotent stem cells.37–39 However, this approach is flawed for several reasons. First, as mentioned above, many commonly used markers can be expressed on stem cells as well as progenitors. Second, expression of markers may change during transformation, causing tumor cells to resemble cells that are distinct from their cells of origin. Lastly, cells within a given tumor may be heterogeneous with respect to expression of lineage markers. In such cases, inferring the cell of origin becomes nearly impossible.
In light of these limitations, a number of investigators have turned to animal models as an alternative way to examine the cell of origin for brain tumors. These models provide several advantages over human studies. First, whereas human tumors vary significantly from patient to patient, animal models usually represent a single subtype of brain tumor and are genetically and phenotypically consistent from animal to animal. This allows robust conclusions to be drawn about the origins of these tumors (although such conclusions may not be generalizable to other tumor subtypes). Second, unlike tumor cells from patients, cells from animal models can be analyzed alongside normal stem cells or progenitors, allowing for direct comparisons between tumor cells and their putative cells of origin. Perhaps the most significant advantage of animal models is the ability to introduce an oncogenic mutation into a particular cell type and then test whether this results in a tumor. Using standard transgenic or knockout technology or focal injection of retroviruses, oncogenes can be overexpressed (or tumor suppressor genes knocked out) in almost any cell lineage at any stage of development. The strength of this approach is that it allows investigators to test the cell of origin prospectively and with appropriate experimental controls.
This strategy has been used to examine the cell of origin for various types of brain tumors. For example, deletion of the neurofibromatosis 1 gene in cells expressing glial fibrillary acidic protein results in malignant astrocytomas; the fact that early lesions in these animals reside within the subventricular zone and express stem cell markers suggests that these tumors arise from multipotent stem cells.11 Likewise, infection of Nestin+ or glial fibrillary acidic protein+ cells in the neonatal forebrain with retroviruses encoding platelet-derived growth factor leads to development of oligodendrogliomas or mixed oligoastrocytomas,41 suggesting that stem cells and progenitors can give rise to these tumors. Injection of platelet-derived growth factorencoding retroviruses into the adult white matter also leads to development of malignant glioma; since this approach predominantly targets NG2+/ glial fibrillary acidic protein-negative glial progenitors, the authors conclude that these tumors arise from oligodendrocyte progenitors.42 Lastly, our own studies indicate that activation of the hedgehog pathway in granule neuron precursor or stem cells can lead to medulloblastoma, implying that each of these cell types can serve as a cell of origin for this tumor (Yang et al, unpublished observations).
Although retroviral or transgenic studies can provide insight into the cell of origin for brain tumors, there is an important caveat to these approaches: tumors may result from introduction of an oncogenic lesion into a particular cell, but this cell may undergo differentiation or dedifferentiation into a phenotypically distinct cell type before becoming fully transformed. For example, transduction of multipotent hematopoietic stem cells with leukemogenic retroviruses results in leukemia, but only after cells have committed to the myeloid lineage.12 Conversely, infection of astrocytes with platelet-derived growth factor-encoding or epidermal growth factorencoding retroviruses has been shown to result in glioma, but only after these cells dedifferentiate into cells resembling glial progenitors or stem cells.41,44 Indeed, the ability of certain oncogenes and tumor suppressors to alter the differentiation state of a cell may be a crucial element of their ability to promote tumor formation.
Given the difficulties involved in identifying the cell of origin, it is worth reiterating the importance of such studies. Simply put, if we can determine which cell type gives rise to a particular tumor, we can apply what we know about the biology of that cell type to understand the behavior of the tumor. Additionally, identification of the cell of origin allows direct comparisons to be made between tumor cells and their normal counterparts, using genomic or proteomic approaches. Unlike comparisons between brain tumors and normal adult brain tissue (which consists largely of nondividing neurons and glia), comparisons between tumor cells and their respective cells of origin can provide insight into the genes and proteins that are involved in tumor initiation and progression.13 Thus, studies directed at identifying the cell of origin for brain tumors can lead to identification of novel markers for early detection and critical targets for therapy.
Cancer Stem Cells
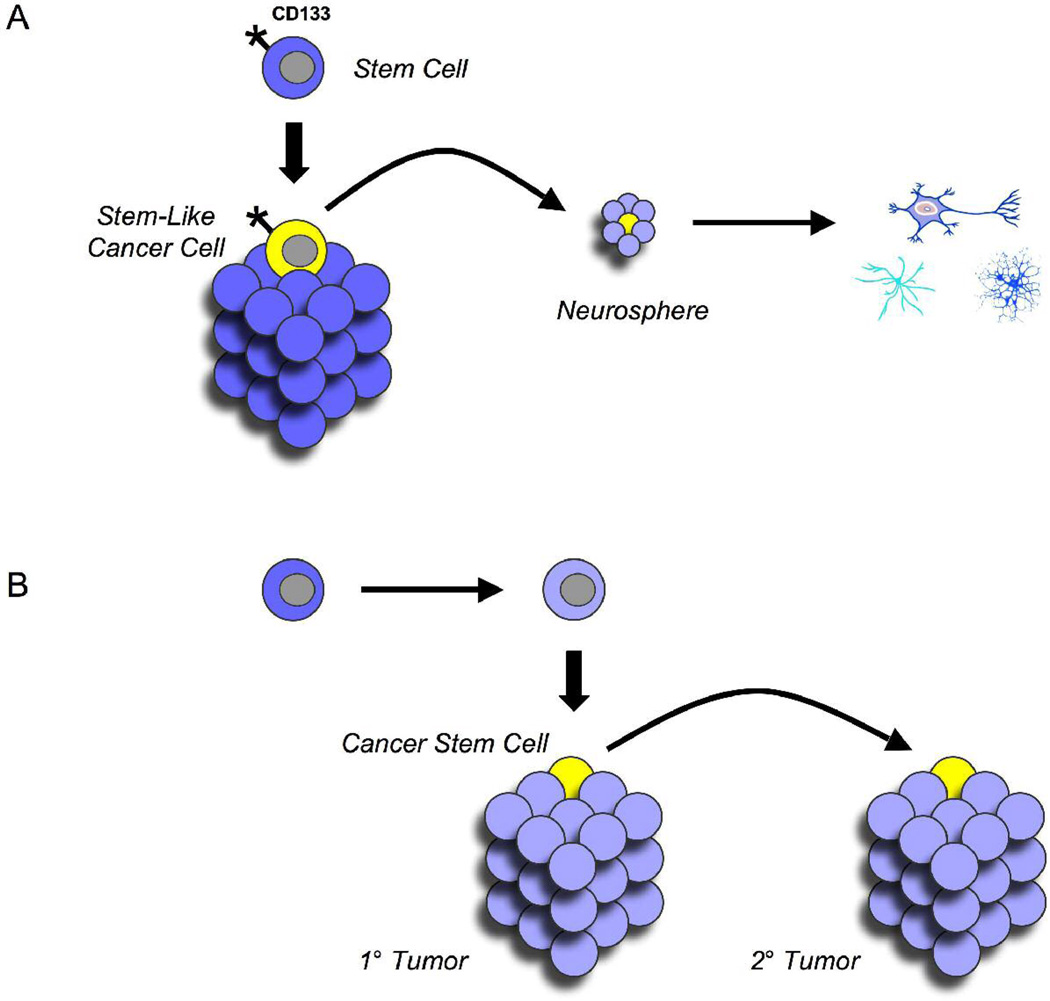
During the past 5 years, the term cancer stem cell has appeared in more than 500 papers, with at least 50 of these focusing on brain tumors. However, the sense in which this term has been used varies significantly among these publications. Although it is sometimes used to refer to tumor cells that phenotypically resemble stem cells (Figure 2A), it is more commonly used to describe cells within a tumor that are capable of maintaining the growth of the tumor in vivo or after transplantation6,46,47 (Figure 2B). The concept of a cancer stem cell was first given credence by investigators studying leukemia, who showed that a subpopulation of cells from human acute myeloid leukemia was uniquely capable of generating tumors after transplantation into immunodeficient mice.47,48 Since then, similar populations of cells have been identified in a variety of solid tumors, including cancers of the breast, brain, colon, prostate, skin, and pancreas.46,49–53
Figure 2.
Tumor cells with unique characteristics. (A) Stemlike cancer cells. Some cells within brain tumors resemble normal neural stem cells in terms of markers (such as CD133) or in terms of functional properties (such as the ability to form neurospheres and differentiate into neurons and glia). These stemlike cells may or may not be critical for the long-term growth of the tumors from which they arise. (B) Cancer stem cells. A subpopulation of cells within a tumor may have an extensive capacity for self-renewal and may be critical for tumor propagation. This capacity is usually demonstrated by testing the ability of these cells to give rise to secondary tumors after transplantation. Cancer stem cells need not be derived from, or resemble, normal tissue stem cells.
Some cancer stem cells share phenotypic characteristics with stem cells from corresponding tissues. This is true of leukemia-initiating cells, which express the hematopoietic stem cell marker CD34 and are also capable of tumor propagation after transplantation.47,48 Similarly, cancer stem cells described in human medulloblastoma and glioma express CD133 and can form neurospheres that differentiate into neurons, astrocytes, and oligodendrocytes in culture. Transplantation of CD133+ cells (but not CD133-negative cells) results in tumors that resemble the tumor from which they were derived, suggesting that these represent true cancer stem cells.46,54
However, the similarity between cancer stem cells and tissue stem cells is not universal. For example, leukemias can be propagated by cells that resemble committed granulocyte macrophage progenitors,55 and cancer stem cells in multiple myeloma have been found to resemble postgerminal center B cells.56 In the context of brain tumors, recent studies have suggested that not all gliomas are propagated by stemlike CD133+ cells, and that some may be propagated by CD133 negative cells.57,58 Thus, showing that tumors contain cells that express stem cell markers does not necessarily prove that these represent cancer stem cells. Such stemlike cells may have interesting or important properties, but they are not necessarily critical for tumor maintenance or propagation.
It is also worth mentioning that some investigators have identified “cancer stem cell” populations in cell lines that have been grown in culture for many years.59–61 These cells share some important characteristics with cancer stem cells isolated from primary tumors: in many cases they express stem cell markers and have the ability to form neurospheres, and have even been shown to selectively mediate tumor initiation after transplantation. While studies of these cells may shed light on the biology of cancer stem cells, it is important to keep in mind that these cells have undergone extensive selection in culture, and that the genetic and epigenetic adaptations that have allowed these cells to maintain themselves in culture may not be the same as the changes that allow primary tumor cells to do so in an animal or in a patient. Thus, studies of cancer stem cells from cell lines must be interpreted with caution until they have been validated using similar cells derived from primary tumors.
An important element of the cancer stem cell hypothesis is the notion that cancer stem cells are resistant to conventional chemotherapy and radiation. This would provide an explanation for tumor resistance and recurrence: if conventional therapies do not eradicate cancer stem cells, these cells can simply self-renew and regenerate the tumor once therapy has been withdrawn. In support of this notion, a number of studies have demonstrated that CD133+ cells from human gliomas are resistant to conventional radiation and chemotherapy.3,5 Moreover, research into therapies that can specifically target cancer stem cells has yielded some promising candidates, including bone morphogenetic proteins,14 inhibitors of Notch signaling,63 and inhibitors of angiogenesis (which may target cancer stem cells by disrupting their “niche”).15 But just as not all cancer stem cells phenotypically resemble stem cells, it is possible that not all cancer stem cells (as defined by their ability to propagate tumors) are resistant to radiation or chemotherapy. Examining this property in various types of brain tumors will be crucial to determining the importance of targeting cancer stem cells for therapy.
Analogies Between Stem Cells and Cancer
In the end, the most interesting relationships between brain tumors and stem cells may be functional ones. Cancer cells need to accomplish many of the same tasks as stem cells: just as stem cells need to self-renew as well as generate all the cell types in a tissue, cancer stem cells need to self-renew and generate all the cell types in a tumor. In doing so, they often use similar strategies and similar signals. Signaling pathways such as the Shh and Wnt pathways promote self-renewal and proliferation in normal neural precursors during development.16 It is therefore not surprising that these pathways have been implicated in medulloblastoma and glioma.61,66–68 Likewise, the Polycomb family transcriptional repressor BMI1 has been shown to regulate self-renewal of normal stem cells and progenitors69 and has been implicated in the pathogenesis of medulloblastoma and glioma.35,70 Lastly, the lipid phosphatase PTEN has been shown to regulate growth of normal neural stem cells and progenitors and is among the most common deletions in glioma.17 By studying the signals that control growth and differentiation of normal stem cells and progenitors, we can gain insight into how these processes become corrupted in cancer, and use this information to develop novel ways to target tumor cells.
Acknowledgment
This work was supported by funds from the Pediatric Brain Tumor Foundation, Golfers Against Cancer of the Triad, the American Cancer Society, the National Institute of Mental Health (Grant# MH067916) and the National Institute of Neurological Disorders and Stroke (Grant# NS052323).
Footnotes
This article was presented at the Neurobiology of Disease in Children: Symposium on Central Nervous System Tumors, in conjunction with the 36th annual meeting of the Child Neurology Society, Quebec City, Quebec, October 10, 2007.
References
- 1.Behin A, Hoang-Xuan K, Carpentier AF, Delattre JY. Primary brain tumours in adults. Lancet. 2003;361:323–331. doi: 10.1016/S0140-6736(03)12328-8. [DOI] [PubMed] [Google Scholar]
- 2.Crawford JR, MacDonald TJ, Packer RJ. Medulloblastoma in childhood: new biological advances. Lancet Neurol. 2007;6:1073–1085. doi: 10.1016/S1474-4422(07)70289-2. [DOI] [PubMed] [Google Scholar]
- 3.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 4.Bao S, Wu Q, Sathornsumetee S, et al. Stem Cell-like Glioma Cells Promote Tumor Angiogenesis through Vascular Endothelial Growth Factor. Cancer Res. 2006;66:7843–7848. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 5.Liu G, Yuan X, Zeng Z, et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 7.Temple S. The development of neural stem cells. Nature. 2001;414:112–117. doi: 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- 8.Lee A, Kessler JD, Read TA, et al. Isolation of neural stem cells from the postnatal cerebellum. Nat Neurosci. 2005;8:723–729. doi: 10.1038/nn1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shetty AK, Turner DA. In vitro survival and differentiation of neurons derived from epidermal growth factor-responsive postnatal hippocampal stem cells: inducing effects of brain-derived neurotrophic factor. J Neurobiol. 1998;35:395–425. doi: 10.1002/(sici)1097-4695(19980615)35:4<395::aid-neu7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 10.Emsley JG, Mitchell BD, Kempermann G, Macklis JD. Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog Neurobiol. 2005;75:321–341. doi: 10.1016/j.pneurobio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Rao MS. Glial progenitors in the CNS and possible lineage relationships among them. Biol Cell. 2004;96:279–290. doi: 10.1016/j.biolcel.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Alder J, Cho NK, Hatten ME. Embryonic precursor cells from the rhombic lip are specified to a cerebellar granule neuron identity. Neuron. 1996;17:389–399. doi: 10.1016/s0896-6273(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 13.Mayer-Proschel M, Kalyani AJ, Mujtaba T, Rao MS. Isolation of lineage-restricted neuronal precursors from multipotent neuroepithelial stem cells. Neuron. 1997;19:773–785. doi: 10.1016/s0896-6273(00)80960-5. [DOI] [PubMed] [Google Scholar]
- 14.Gregori N, Proschel C, Noble M, Mayer-Proschel M. The tripotential glial-restricted precursor (GRP) cell and glial development in the spinal cord: generation of bipotential oligodendrocyte-type-2 astrocyte progenitor cells and dorsal-ventral differences in GRP cell function. J Neurosci. 2002;22:248–256. doi: 10.1523/JNEUROSCI.22-01-00248.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Espinosa de los Monteros A, Zhang M, De Vellis J. O-2A progenitor cells transplanted into the neonatal rat brain develop into oligodendrocytes but not astrocytes. Proc Natl Acad Sci U S A. 1993;90:50–54. doi: 10.1073/pnas.90.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog [see comments] Neuron. 1999;22:103–114. doi: 10.1016/s0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- 17.Altman J, Bayer SA. Development of the Cerebellar System: In Relation to Its Evolution, Structure and Functions. Boca Raton, FL: CRC Press; 1997. [Google Scholar]
- 18.Abraham H, Tornoczky T, Kosztolanyi G, Seress L. Cell formation in the cortical layers of the developing human cerebellum. Int J Dev Neurosci. 2001;19:53–62. doi: 10.1016/s0736-5748(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 19.Tohyama T, Lee VM, Rorke LB, et al. Nestin expression in embryonic human neuroepithelium and in human neuroepithelial tumor cells. Lab Invest. 1992;66:303–313. [PubMed] [Google Scholar]
- 20.Dahlstrand J, Lardelli M, Lendahl U. Nestin mRNA expression correlates with the central nervous system progenitor cell state in many, but not all, regions of developing central nervous system. Brain Res Dev Brain Res. 1995;84:109–129. doi: 10.1016/0165-3806(94)00162-s. [DOI] [PubMed] [Google Scholar]
- 21.Graham V, Khudyakov J, Ellis P, Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39:749–765. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- 22.Uchida N, Buck DW, He D, et al. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci U S A. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corti S, Nizzardo M, Nardini M, et al. Isolation and characterization of murine neural stem/progenitor cells based on Prominin-1 expression. Exp Neurol. 2007;205:547–562. doi: 10.1016/j.expneurol.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 24.Capela A, Temple S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35:865–875. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 25.Sotelo C, Alvarado-Mallart RM, Frain M, Vernet M. Molecular plasticity of adult Bergmann fibers is associated with radial migration of grafted Purkinje cells. J Neurosci. 1994;14:124–133. doi: 10.1523/JNEUROSCI.14-01-00124.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfenninger CV, Roschupkina T, Hertwig F, et al. CD133 is not present on neurogenic astrocytes in the adult subventricular zone, but on embryonic neural stem cells, ependymal cells, and glioblastoma cells. Cancer Res. 2007;67:5727–5736. doi: 10.1158/0008-5472.CAN-07-0183. [DOI] [PubMed] [Google Scholar]
- 27.Doetsch F, Caille I, Lim DA, et al. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 28.Imura T, Kornblum HI, Sofroniew MV. The predominant neural stem cell isolated from postnatal and adult forebrain but not early embryonic forebrain expresses GFAP. J Neurosci. 2003;23:2824–2832. doi: 10.1523/JNEUROSCI.23-07-02824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reynolds BA, Rietze RL. Neural stem cells and neurospheres--re-evaluating the relationship. Nat Methods. 2005;2:333–336. doi: 10.1038/nmeth758. [DOI] [PubMed] [Google Scholar]
- 30.Singec I, Knoth R, Meyer RP, et al. Defining the actual sensitivity and specificity of the neurosphere assay in stem cell biology. Nat Methods. 2006;3:801–806. doi: 10.1038/nmeth926. [DOI] [PubMed] [Google Scholar]
- 31.Gabay L, Lowell S, Rubin LL, Anderson DJ. Deregulation of dorsoventral patterning by FGF confers trilineage differentiation capacity on CNS stem cells in vitro. Neuron. 2003;40:485–499. doi: 10.1016/s0896-6273(03)00637-8. [DOI] [PubMed] [Google Scholar]
- 32.Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–897. doi: 10.1038/nature03994. [DOI] [PubMed] [Google Scholar]
- 33.McCarthy M, Turnbull DH, Walsh CA, Fishell G. Telencephalic neural progenitors appear to be restricted to regional and glial fates before the onset of neurogenesis. J Neurosci. 2001;21:6772–6781. doi: 10.1523/JNEUROSCI.21-17-06772.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bogler O, Wren D, Barnett SC, et al. Cooperation between two growth factors promotes extended self-renewal and inhibits differentiation of oligodendrocyte-type-2 astrocyte (O-2A) progenitor cells. Proc Natl Acad Sci U S A. 1990;87:6368–6372. doi: 10.1073/pnas.87.16.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leung C, Lingbeek M, Shakhova O, et al. Bmi1 is essential for cerebellar development and is overexpressed in human medulloblastomas. Nature. 2004;428:337–341. doi: 10.1038/nature02385. [DOI] [PubMed] [Google Scholar]
- 36.Molofsky AV, Pardal R, Iwashita T, et al. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–967. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roessmann U, Velasco ME, Gambetti P, Autilio-Gambetti L. Neuronal and astrocytic differentiation in human neuroepithelial neoplasms. An immunohistochemical study. J Neuropathol Exp Neurol. 1983;42:113–121. doi: 10.1097/00005072-198303000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Yang HY, Lieska N, Shao D, et al. Proteins of the intermediate filament cytoskeleton as markers for astrocytes and human astrocytomas. Mol Chem Neuropathol. 1994;21:155–176. doi: 10.1007/BF02815349. [DOI] [PubMed] [Google Scholar]
- 39.Valtz NL, Hayes TE, Norregaard T, et al. An embryonic origin for medulloblastoma. New Biol. 1991;3:364–371. [PubMed] [Google Scholar]
- 40.Zhu Y, Guignard F, Zhao D, et al. Early inactivation of p53 tumor suppressor gene cooperating with NF1 loss induces malignant astrocytoma. Cancer Cell. 2005;8:119–130. doi: 10.1016/j.ccr.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dai C, Celestino JC, Okada Y, et al. PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes Dev. 2001;15:1913–1925. doi: 10.1101/gad.903001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Assanah M, Lochhead R, Ogden A, et al. Glial progenitors in adult white matter are driven to form malignant gliomas by platelet-derived growth factor-expressing retroviruses. J Neurosci. 2006;26:6781–6790. doi: 10.1523/JNEUROSCI.0514-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cozzio A, Passegue E, Ayton PM, et al. Similar MLL-associated leukemias arising from self-renewing stem cells and short-lived myeloid progenitors. Genes Dev. 2003;17:3029–3035. doi: 10.1101/gad.1143403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bachoo RM, Maher EA, Ligon KL, et al. Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell. 2002;1:269–277. doi: 10.1016/s1535-6108(02)00046-6. [DOI] [PubMed] [Google Scholar]
- 45.Oliver TG, Read TA, Kessler JD, et al. Loss of patched and disruption of granule cell development in a pre-neoplastic stage of medulloblastoma. Development. 2005;132:2425–2439. doi: 10.1242/dev.01793. [DOI] [PubMed] [Google Scholar]
- 46.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 47.Wang JC, Dick JE. Cancer stem cells: lessons from leukemia. Trends Cell Biol. 2005;15:494–501. doi: 10.1016/j.tcb.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 48.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 49.Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xin L, Lawson DA, Witte ON. The Sca-1 cell surface marker enriches for a prostatere-generating cell subpopulation that can initiate prostate tumorigenesis. Proc Natl Acad Sci U S A. 2005;102:6942–6947. doi: 10.1073/pnas.0502320102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fang D, Nguyen TK, Leishear K, et al. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65:9328–9337. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- 52.O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 53.Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 54.Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 55.Krivtsov AV, Twomey D, Feng Z, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature. 2006;442:818–822. doi: 10.1038/nature04980. [DOI] [PubMed] [Google Scholar]
- 56.Matsui W, Huff CA, Wang Q, et al. Characterization of clonogenic multiple myeloma cells. Blood. 2004;103:2332–2336. doi: 10.1182/blood-2003-09-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang J, Sakariassen PO, Tsinkalovsky O, et al. CD133 negative glioma cells form tumors in nude rats and give rise to CD133 positive cells. Int J Cancer. 2008;122:761–768. doi: 10.1002/ijc.23130. [DOI] [PubMed] [Google Scholar]
- 58.Beier D, Hau P, Proescholdt M, et al. CD133(+) and CD133(−) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67:4010–4015. doi: 10.1158/0008-5472.CAN-06-4180. [DOI] [PubMed] [Google Scholar]
- 59.Kondo T, Setoguchi T, Taga T. Persistence of a small subpopulation of cancer stem-like cells in the C6 glioma cell line. Proc Natl Acad Sci U S A. 2004;101:781–786. doi: 10.1073/pnas.0307618100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ghods AJ, Irvin D, Liu G, et al. Spheres isolated from 9L gliosarcoma rat cell line possess chemoresistant and aggressive cancer stem-like cells. Stem Cells. 2007;25:1645–1653. doi: 10.1634/stemcells.2006-0624. [DOI] [PubMed] [Google Scholar]
- 61.Bar EE, Chaudhry A, Lin A, et al. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 2007;25:2524–2533. doi: 10.1634/stemcells.2007-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Piccirillo SG, Reynolds BA, Zanetti N, et al. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444:761–765. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- 63.Fan X, Matsui W, Khaki L, et al. Notch pathway inhibition depletes stem-like cells and blocks engraftment in embryonal brain tumors. Cancer Res. 2006;66:7445–7452. doi: 10.1158/0008-5472.CAN-06-0858. [DOI] [PubMed] [Google Scholar]
- 64.Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 65.Fogarty MP, Kessler JD, Wechsler-Reya RJ. Morphing into cancer: the role of developmental signaling pathways in brain tumor formation. J Neurobiol. 2005;64:458–475. doi: 10.1002/neu.20166. [DOI] [PubMed] [Google Scholar]
- 66.Zurawel RH, Chiappa SA, Allen C, Raffel C. Sporadic medulloblastomas contain oncogenic beta-catenin mutations. Cancer Res. 1998;58:896–899. [PubMed] [Google Scholar]
- 67.Pietsch T, Waha A, Koch A, et al. Medulloblastomas of the desmoplastic variant carry mutations of the human homologue of Drosophila patched. Cancer Res. 1997;57:2085–2088. [PubMed] [Google Scholar]
- 68.Taylor MD, Liu L, Raffel C, et al. Mutations in SUFU predispose to medulloblastoma. Nat Genet. 2002;31:306–310. doi: 10.1038/ng916. [DOI] [PubMed] [Google Scholar]
- 69.Molofsky AV, He S, Bydon M, et al. Bmi-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16Ink4a and p19Arf senescence pathways. Genes Dev. 2005;19:1432–1437. doi: 10.1101/gad.1299505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bruggeman SW, Hulsman D, Tanger E, et al. Bmi1 controls tumor development in an Ink4a/Arf-independent manner in a mouse model for glioma. Cancer Cell. 2007;12:328–341. doi: 10.1016/j.ccr.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 71.Chow LM, Baker SJ. PTEN function in normal and neoplastic growth. Cancer Lett. 2006;241:184–196. doi: 10.1016/j.canlet.2005.11.042. [DOI] [PubMed] [Google Scholar]