Abstract
The corpus luteum (CL) is a transient endocrine organ that is essential for maintenance of pregnancy in both ruminants and primates. The cellular and endocrine mechanisms that regulate the CL in these species have commonalities and some distinct and intriguing differences. Both species have similar cellular content with large luteal cells derived from the granulosa cells of the follicle, small luteal cells from follicular thecal cells, and large numbers of capillary endothelial cells that form the vasculature that has an essential role in optimal CL function. Intriguingly, the large luteal cells in ruminants grow larger than in primates and acquire a capacity for high constitutive progesterone (P4) production that is independent of stimulation from LH. In contrast, the primate CL and the granulosa lutein cells from primates continue to require stimulation by LH/CG throughout the luteal phase. Although the preovulatory follicle of women and cows had similar size and steroidogenic output (10 to 20 mg/h), the bovine CL had about ten-fold greater P4 output compared to the human CL (17.4 vs. 1.4 mg/h), possibly due to the development of high constitutive P4 output by the bovine large luteal cells. The continued dependence of the primate CL on LH/CG/cAMP also seems to underlie luteolysis, as there seems to be a requirement for greater luteotropic support in the older primate CL than is provided by the endogenous LH pulses. Conversely, regression of the ruminant CL is initiated by PGF from the nonpregnant uterus. Consequently, the short luteal phase in ruminants is primarily due to premature secretion of PGF by the nonpregnant uterus and early CL regression, whereas CL insufficiency in primates is related to inadequate luteotropic support and premature CL regression. Thus, the key functions of the CL, pregnancy maintenance and CL regression in the absence of pregnancy, are produced by common cellular and enzymatic pathways regulated by very distinct luteotropic and luteolytic mechanisms in the CL of primates and ruminants.
Keywords: corpus luteum, primate, progesterone, ruminant
Introduction
The corpus luteum (CL) is a transient endocrine gland that is essential for pregnancy in mammalian species, including humans and cattle. In 1672, deGraaf described the CL, calling them "globules", with the number of globules matching the number of pregnancies in rabbits (Jocelyn, 1972). Although Malpighi first introduced the term, "corpus luteum", in 1685 in order to describe the color of the structure (alternatively translated as a reddish-orange or yellow color; Malpighi, 1685), there remained until the early 1900s no scientifically-tested physiological role for the CL. In the late 1800s, Gustav Born discussed with his students the indirect evidence that the CL is a gland of internal secretion. In 1901, two papers were published by students of Gustav Born, one by Ludwig Fraenkel in Germany and the other by Vilhelm Magnus in Norway that independently demonstrated that mated rabbits did not maintain their pregnancies after bilateral ovariectomy or galvano-cautery of all CL (Fraenkel and Cohn, 1901; Magnus, 1901). Later research demonstrated that the active compound could be extracted from the CL using alcohol but not saline (Corner, 1929). In 1934, isolation of pure crystalline progesterone (P4) from the CL was reported by four different laboratories (Allen, 1974). Many studies have now demonstrated the essential role for P4 in maintaining pregnancy, as well as a role for P4 in many other aspects of reproductive physiology (Morris and Diskin, 2008; Wiltbank et al., 2012). The primate CL, unlike the bovine CL, also secretes substantial amounts of estradiol-17β (E2), androgens, and inhibin. Secretion of these products, which are normally considered follicular products, by the CL underlies some of the unique features of the primate menstrual cycle (Devoto et al., 2009).
Although P4 production is the most recognized and critical feature of the CL, some of the most interesting biology is related to the transient nature of this structure. Ovulation of the follicle by the LH surge produces substantial growth in cellular mass and clear differentiation of the follicular cell types into a structure that is uniquely specialized for the distinct endocrine and transient properties of the CL (see section “Cellular characteristics of the ruminant and primate CL” below). Development of the CL into a fully operational P4 factory involves growth and development of the steroidogenic cells, as well as development of an extraordinary capillary network to efficiently deliver nutrients and remove luteal P4. The primary luteal cell types are discussed in section “Cellular characteristics of the ruminant and primate CL”. The role of luteotropins in development of the CL as well as a role for luteolysins in the subsequent regression of the CL have been actively researched and discussed by CL researchers during much of the last century. Nevertheless, there are still surprising gaps in our understanding of the nature of the luteotropins and luteolysins in different species and indeed, the requirement for exogenous hormonal regulation of CL development and regression, as compared to endogenous luteal regulatory mechanisms (sections “Role of LH in the primate and ruminant CL” and “Mechanisms of luteal regression”). Finally infertility problems in both cattle and women can result from inadequate function of the CL, termed a short estrous cycle in cattle and a luteal phase defect in women. The physiology of these two luteal deficiencies is intriguingly quite distinct (section “Mechanisms of luteal regression”). Thus, this review will focus on these key aspects of differences between the human and bovine CL and will not attempt to be comprehensive on the many other structural, molecular, and physiological characteristics of CL from various species that have been reviewed in previous manuscripts (Keyes and Wiltbank, 1988; Wiltbank et al., 1991; Pate, 1994, 1995; Niswender et al., 2000; Anderson et al., 2001; Pate and Keyes, 2001).
Cellular characteristics of the ruminant and primate CL
There are three major cell-types that inhabit the CL: large steroidogenic luteal cells, small steroidogenic luteal cells, and vascular endothelial cells. In addition, there are many other important cell types in the CL including fibroblasts, other vascular cell types, and immune cells (Pate and Keyes, 2001). These other cell types generally represent only a minor part of the total luteal volume, however, these cells, particularly immune cells, appear to have a critical role in CL function, particularly in the demise of the CL (Pate, 1994, 1995; Pate and Keyes, 2001). In ruminants, there has been clear evidence for two distinct populations of steroidogenic luteal cells, small and large (Rodgers and Oshea, 1982; Rodgers et al., 1983, 1984; Hoyer and Niswender, 1985, 1986; Hoyer et al., 1988; Niswender et al., 1994). In primates, the excellent flow cytometry results by Hild-Petito et al. (1989) clearly demonstrate three steroidogenic cell populations, based on luteal diameter (Hild-Petito et al., 1991). The first population is smaller than small luteal cells from ruminants with a cell size around 12 μm. The second population contains medium luteal cells with diameters of 18–20 μm. The large luteal cells show a clear peak at 25 μm. All of these peaks were determined for cells that stained positively for 3β-hydroxysteroid-dehydrogenase (3β-HSD), a key enzyme in synthesis of P4 (Hild-Petito et al., 1991). The majority (97.4%) of the non-steroidogenic luteal cells (3β-HSD negative) were <15 μm in diameter. The presence of a third populations of steroidogenic luteal cells in primates and not in ruminants does not seem to be due to methodological differences between studies because similar flow cytometric studies of isolated cells from the sheep CL produced only two populations of cells with small luteal cells of 15–22 μm and large luteal cells with a broad peak at 30–40 μm (Brannian et al., 1993). Nevertheless, most studies on luteal cells from women or from other primates have generally evaluated two populations of steroidogenic cells. Thus, the discussion below will focus on studies that have evaluated these two steroidogenic populations in ruminants or primates, due to a lack of research on subpopulations at this time.
Large luteal cells
The histological section of bovine CL shown in Fig. 1 illustrates that large luteal cells take up a great deal of cellular volume in the CL, occupying about 40% of the total luteal volume. Nevertheless, as can be seen by the staining of nuclei in Masson’s trichrome stained section, the large luteal cells represent very few of the luteal cells, maybe as few at 3.5% of the total luteal cell numbers in the bovine (Oshea et al., 1989). For example, Parkinson et al. (1994) did an extensive morphometric analysis of the CL in pregnant and nonbred heifers (Friesian by Bos taurus beef). The average cell diameter was 13.94 μm, although the median cell size was 12.48 μm with a cellular distribution that they described as "leptokurtotic" (extended tail of data in the positive direction) until days 12 to 19 of pregnancy when the distribution became clearly bimodal, with up to 10% of cells in the large cell category and 90% in the smaller categories. Nevertheless, the volume of the CL occupied by the large luteal cells (>22.5 μm in diameter) is greater than 60% in fully functional CL (Parkinson et al., 1994). The distinctive cellular features of the bovine large luteal cell have been extensively described (Sawyer et al., 1979, 1990).
Figure 1.
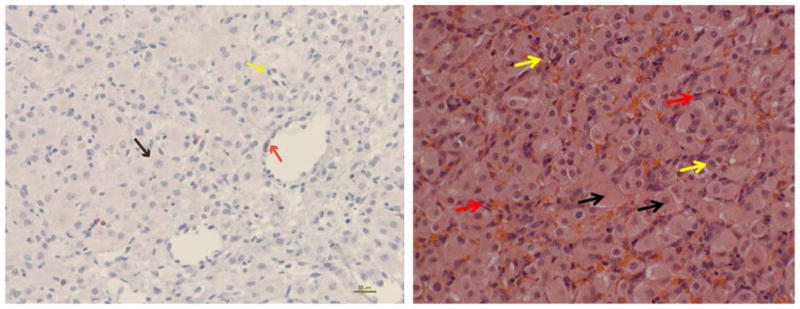
Histological sections of mid-cycle bovine corpus luteum. Left – Hematoxylin and eosin staining; Right – Masson’s trichrome staining (red arrows indicate endothelial cells, black arrow – Large luteal cells; yellow arrow – small luteal cells).
Micrographs of the primate CL, and particularly the human CL, demonstrate areas that are primarily granulosalutein cells and other areas that primarily contain smaller theca-lutein cells. Thus, during development of the primate CL there is not as complete of an integration process of the granulosa and thecal cells into a more homogenous tissue as found in ruminant CL or the CL of most other species.
The cellular origin of the large luteal cell has generally been ascribed to the granulosa cell in both primates and ruminants. This origin seems obvious in the primate based on even a superficial evaluation of luteal micrographs. In the ruminant CL this origin is based on sequential temporal evaluations of the CL after ovulation, luteinization, and development of the CL, however this origin has been questioned by various researchers. Nevertheless, luteinization of granulosa cells gives rise to cells with cellular features and mRNA expression that are similar to the large luteal cell (Meidan et al., 1990).
The differentiation process that gives rise to the large luteal cell is impressive. From a mass perspective, the granulosa cell is only 10 μm in diameter prior to the LH surge but increases to 38 μm in the fully-functional bovine large luteal cell (Oshea et al., 1989). This calculates to an increase in volume from about 500 μm3 in the granulosa cell to almost 30,000 μm3 in the large luteal cell, over a 50-fold increase in cellular volume (Oshea et al., 1989, Wiltbank, 1994). In the primate CL the granulosa cells increase from 10 μm to about 25 μm, a 16-fold increase in volume from ~500 μm3 to ~8000 μm3 (Hild- Petito et al., 1989). Thus, in both primates and ruminants the granulosa cells undergo an impressive hypertrophy during development of the CL, with about a 4-fold greater hypertrophy during development of large luteal cells in ruminants than primates. The number of large luteal cells is similar to the number of granulosa cells in the preovulatory follicle (Smith et al., 1994), again consistent with the idea that the granulosa cells differentiate into the large luteal cells without hyperplasia but with substantial hypertrophy.
One intriguing calculation is whether the calculated number and volume of large luteal cells would fit within the volume of the CL. O'Shea et al. (1989) evaluated the volume and number of large luteal cells in cows that had been induced to ovulate with two prostaglandin Fα (PGF) injections given 11 days apart. The CL were taken 14 days after the final PGF treatment, resulting in CL expected to be on day 12 of the cycle but in reality could be between days 8 and 12. Using morphometric techniques, it was determined that 1 g of luteal tissue would be expected to contain about 13.8 million large luteal cells of about 30,000 μm3 volume calculated to represent about 40% of the luteal volume (Oshea et al., 1989). Parkinson et al. (1994), also found that the CL on days 8 to 12 of the estrous cycle had about 40% of the luteal volume occupied by large luteal cells. However, these authors, found that more than 60% of luteal volume was occupied by large luteal cells on day 16 of pregnancy, increasing to almost 70% by day 19 of pregnancy. Calculation of the maximal volume of a sphere (i.e. the mature CL) that can be occupied by smaller spheres (i.e. large luteal cells) is determined by the Kepler conjecture and is found to maximize at about 0.74 (Torquato and Stillinger, 2010). In other words, the theoretical maximal volume of the CL that could be occupied by large luteal cells is about 74%, if the placement and orientation of all spheres are optimized. Thus, the CL volume in the cow seems to be almost optimized based on "sphere within a sphere" calculations. Obviously, other cells, such as the luteal vasculature, can easily fit in the rest of the luteal tissue volume. Nevertheless, this illustrates the essential nature of the large luteal cell in determining and occupying CL volume. We were unable to find similar calculations for the CL of humans or other primates.
Steroidogenesis in the granulosa cell also dramatically increases after the LH surge and luteinization. In the cow, P4 concentrations continue to increase until day 14 of the estrous cycle, even though volume of the CL does not significantly increase after day 7 (Fig. 2; Sartori et al., 2004). The increase in volume and circulating P4 during the first seven days is likely due to dramatic increases in P4 production and hypertrophy in the large luteal cells. There is also clear hyperplasia happening during this time period, most likely due to proliferation of capillary endothelial cells developing the vasculature that underlies the extremely high rate of blood flow that is characteristic of the CL. As clearly shown in Fig. 2, there is almost as much increase in circulating P4 during the second week of CL development as during the first week, with no detectable increase in luteal volume. It is also clear that the increased circulating P4 without increased CL volume occurs in both heifers and lactating cows with similar dynamics. The mechanisms that underlie this disconnection between increasing circulating P4 with static CL have not been experimentally evaluated but an obvious explanation would be continued hyperplasia of large luteal cells, as the proportion of CL volume occupied by these cells increases as well as increasing steroidogenic capacity in these cells (Parkinson et al., 1994). A second paradox shown in Fig. 2 is that CL volume is much greater in lactating cows than heifers, however circulating P4 is substantially lower in lactating cows than heifers. The greater CL volume in lactating cows appears to be accounted for by increased size of the ovulatory follicle (Sartori et al., 2002, 2004). In addition, the reduced circulating P4 seems almost completely accounted for by the much greater metabolic clearance rate for P4 in lactating dairy cows than in heifers (Sangsritavong et al., 2002; Wiltbank et al., 2006). A third paradox, that is not obvious from Fig. 2, is that P4 continues to increase, while LH pulses are decreasing due to the rising negative feedback from the increasing P4 concentrations. This is one of many evidences (discussed below) that luteal P4 production is largely independent of the "luteotropic" actions of LH in ruminant CL. Calculations by Niswender et al. (1994) indicated that over 80% of the circulating P4 in ewes is derived from the large luteal cells. Large luteal cells in ruminants have very high constitutive P4 production, apparently independent of LH or cAMP action.
Figure 2.
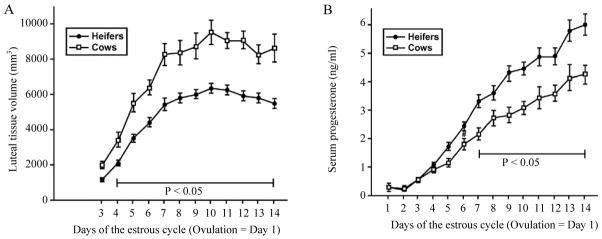
Volume of CL tissue (A) and concentrations of circulating P4 (B) in lactating dairy cows and heifers during the estrous cycle (Sartori et al., 2004).
Similar to ruminants, primates also display a dramatic increase in circulating P4 and CL volume following the LH surge and development of the CL. Nevertheless, the pattern of increase in P4 is distinct in primates compared to ruminants. There is increasing CL volume and increasing P4 only during the first 5–6 days after ovulation with a subsequent plateau in circulating P4 for about 4–7 days, followed by a decline in circulating P4 with a P4 nadir and menses occurring, on average, at 13 days after ovulation (Baerwald et al., 2005). This is obviously a shorter CL lifespan than observed in cattle with a much shorter time of increasing P4 concentrations. In addition, unlike the CL of ruminants, the CL of primates has the capacity for substantial production of estrogens. Thus, the LH surge in ruminants dramatically decreases the expression of aromatase in the granulosa cells, even before ovulation of the preovulatory follicle (Tsai et al., 1996). Although mRNA for aromatase also seems to decline after the LH surge in primate granulosa cells, the capacity for aromatization of androgens continues, granted at a somewhat diminished capacity (Sanders et al., 1996; Xu et al., 2005). Aromatase mRNA is detected in granulosa-lutein cells of the midcycle primate CL and circulating E2 peaks above 100 pg/ml, a substantial concentration, although somewhat below the peak E2 concentration that is above 200 pg/ml during the periovulatory period (Sanders et al., 1996; Baerwald et al., 2005). Nevertheless, the requirement for two different cell types for production of E2, as extensively described in the follicle, seems to continue in the primate CL. There has been some variability in reports, however it appears that large luteal cells have little, if any, capacity for production of androgen. However, androgens produced by the theca-lutein cells can be readily converted to estrogens by the granulosa-lutein/large luteal cells due to the high aromatase activity in these cells (Sanders and Stouffer, 1997).
The indispensable role for granulosa cells/large luteal cells in the function of the CL has been most convincingly demonstrated by aspiration of granulosa cells from the preovulatory follicle. In an elegant study (Milvae et al., 1991), Holstein heifers had granulosa cells removed (n = 9), removed and then replaced (n = 7), or heifers were left untreated (n = 6). The heifers with removal of granulosa cells had much lower circulating concentrations of P4 (<2 ng/ml), compared to the other two groups (>5 ng/ml) on days 7–17 of the estrous cycle; however length of the estrous cycle was not altered. Recently, another study (O'Hara et al., 2012) evaluated the effect of granulosa cell aspiration on CL function in single-ovulating or superovulated Bos taurus beef heifers. Similar to the previous study, circulating P4 concentrations were lower from days 7 to 14 (when heifers were slaughtered) in aspirated compared to non-aspirated control heifers. Size of the CL was also evaluated in this study, based on transrectal ultrasound and, as expected, CL diameter was substantially smaller. The weight of the CL on day 14 was reduced from 8.42 g to 4.16 g in heifers that had follicle aspiration. Nevertheless, the concentrations of mRNA for CYP11A1, HSD3B1, and STAR, the three key proteins involved in production of P4, were not altered by follicle aspiration, although LHCGR mRNA was reduced after follicle aspiration. Similarly, ovulation of smaller follicles (likely to have fewer granulosa cells) results in smaller CL and lower circulating P4 concentrations (Vasconcelos et al., 2001; Peters and Pursley, 2003; Mussard et al., 2007). Thus, it seems clear that, in the cow, removal of granulosa cells from the preovulatory follicle results in a smaller CL with lower circulating P4. In primates, aspiration of granulosa cells from the dominant follicle after the LH surge also results in lower circulating P4 concentrations in studies in humans (Garcia et al., 1981; Vargyas et al., 1986) and rhesus monkeys (Kreitmann and Hodgen, 1980; Kreitmann et al., 1981), but has not been observed in all studies (Kerin et al., 1981). Almost all of these studies are consistent with the idea that ovulation of a follicle with reduced numbers of granulosa cells, either due to follicular aspiration or smaller follicle size, is likely to result in a CL with fewer large luteal cells and therefore reduced volume of steroidogenic tissue and reduced P4 production. Thus, it seems clear that numbers of granulosa cells in the ovulatory follicle and subsequent numbers of large luteal cells in the CL is critical for determining the size and steroidogenic capacity of the CL, in either primates or ruminants.
Small luteal cells
The small luteal cells develop from the steroidogenic thecal cells of the ovulated follicle. In cattle, small luteal cells have about one-tenth the volume of large luteal cells (3,000 vs. 30,000 μm3). However, there are about 100 million small luteal cells/g of tissue, compared to 13.8 million large luteal cells (Oshea et al., 1989). Thus, small luteal cells occupied a volume of 27.7% of the CL on day 8 to 12 of the estrous cycle (Oshea et al., 1989). In sheep (Farin et al., 1986) the volume of CL occupied by small luteal cells was 18% (day 8) to 23% (day 12). There are also morphological distinctions between these two steroidogenic cell types such as spherical nuclei in large cells but irregular nuclei in small cells and presence of rough endoplasmic reticulum and many secretory granules in large but not small luteal cells (see Sawyer et al., 1979; Farin et al., 1986; Oshea et al., 1990; Wiltbank, 1994). There are also differences in receptors for LH (generally greater in small cells), E2 (much greater in large cells), and PGF2α (much greater in large cells; see Fitz et al., 1982; Wiltbank, 1994).
In ruminants, production of P4 and cAMP are regulated by distinct mechanisms in large and small luteal cells. First, basal cAMP is undetectable in large or small luteal cells. After LH treatment there is a substantial increase in cAMP in small cells and a minor increase in large cells. Forskolin, a pharmacological activator of adenylate cyclase, stimulates a large increase in cAMP in both small and large luteal cells. Optimal production of P4 by luteal cells requires cholesterol substrate delivered by lipoproteins. In ruminants, HDL is the most abundant circulating lipoprotein (Grummer and Carroll, 1988) and the most stimulatory to luteal P4 production (Wiltbank et al., 1990; Carroll et al., 1992a, b). The data in Table 1 show the P4 production by large and small luteal cells with all cells in the presence of HDL (Wiltbank et al., 1993). Under basal conditions, the large cells had almost 100-fold greater P4 production than the small luteal cells. Treatment with forskolin reduced this difference to less than 10-fold due to the dramatic (15-fold) increase in P4 production by small luteal cells with only a minor increase (less than 50%) in large luteal cells. Thus, P4 production is under stimulatory control through the cAMP/PKA pathway in small cells but is at elevated levels in large cells without any external hormonal stimulation.
Table 1.
Response of plated ovine large (LLC) and small (SLC) luteal cells to various treatments (all incubations in the presence of 100 μg/ml HDL). Forskolin (For; 50 μM) treatment stimulated cAMP production by either large or small luteal cells. Forskolin stimulated P4 production by SLC in the absence (Control) but not in the presence of the hydroxylated cholesterol derivatives (25-OH, 20-OH, 22-OH chol) or pregnenolone (pregnen). There was a small increase in LLC in response to forskolin under control conditions but not in the presence of hydroxylated cholesterols or pregnenolone (Wiltbank et al., 1993). The P4 production by LLC was much greater than SLC under all of the conditions (significance not shown in Table).
| Progesterone [fg/(cell x min)]
| ||||||
|---|---|---|---|---|---|---|
| cAMP [fg/(cell* min)] | Control-P4 | +25-OH chol-P4 | +20-OH chol-P4 | +22-OH chol-P4 | +pregnen-P4 | |
| SLC - Cont | Undetectable | 0.29 ± .08 | 8.0 ± 1.6 | 42 ± 10 | 41 ± 9 | 124 ± 34 |
| SLC + For | 18 ± 3* | 4.54 ± 1.24* | 8.7 ± 1.9 | 38 ± 10 | 43 ± 9 | 126 ± 35 |
| LLC - Cont | Undetectable | 27.5 ± 3.6 | 42.3 ± 6.7 | 207 ± 35 | 259 ± 47 | 850 ± 129 |
| LLC + For | 19 ± 1* | 41.2 ± 7.2* | 36.1 ± 4.9 | 206 ± 36 | 254 ± 43 | 895 ± 135 |
Indicates differences (P < 0.05) within one column and for one cell type (SLC or LLC).
Table 1 also demonstrates the key pathways involved in luteal P4 production in the two steroidogenic cell types: regulated cellular movement of cholesterol and the enzymes P450scc and 3βHSD. Cholesterol is primarily a hydrophobic molecule and this makes it difficult for cholesterol to freely diffuse through hydrophilic environments such as the cytoplasm. In addition, cholesterol has a hydroxyl group at the 3-position that produces a discrete hydrophilic region making it difficult for "flip-flop" of cholesterol between membrane surfaces within the lipid bilayer of cellular membranes. Hydroxylated cholesterol derivatives (25-OH, 20-OH, or 22-OH cholesterol; as shown in Table 1) were used to measure P450scc activity because these compounds cross cellular membranes without the need for transport proteins such as StAR. It is clear that forskolin stimulated P4 production by small luteal cells but this did not alter metabolism of hydroxylated cholesterol derivatives (25-OH, 20-OH, or 22-OH cholesterol). These data indicate that P450scc activity is similar with or without forskolin treatment. Treatment with pregnenolone, as a measure of 3βHSD activity, caused very high P4 production that did not change after forskolin treatment. In large luteal cells, P4 production is much greater than in small luteal cells under any of the conditions. Incubation of large luteal cells with hydroxylated cholesterol or pregnenolone dramatically increased P4 production indicating that cholesterol movement may be still somewhat limiting even in this cell type. Nevertheless, it is clear that P450scc and 3βHSD activity are present in excess amounts in small and large luteal cells and do not appear to be the rate-limiting step regulated by stimulatory or inhibitory pathways (data not shown) in luteal cells. The key rate-limiting step in luteal P4 production appears to be movement of cholesterol from the outer to the inner mitochondrial membrane. Obviously, the large luteal cell more effectively utilizes steroidogenic enzyme activity under basal conditions.
In contrast to ruminants, large and small luteal cells in primates seem to be under important stimulatory control through pathways regulated by LH/hCG and cAMP. Under basal conditions, P4 production on a per cell basis is 12-fold (early luteal phase) to 22-fold (mid-luteal phase) greater in large luteal cells than small luteal cells (Hild-Petito et al., 1989). In luteal cells from the early luteal phase, treatment with hCG increased P4 production by small (2.3-fold) and large luteal (3.3-fold) cells. Treatment with a cAMP analogue also increased P4 in small (3.3-fold) and large (3.6-fold) luteal cells from the early luteal phase. However, treatment of luteal cells from the mid-luteal phase with hCG or cAMP had less of an effect on P4 production by luteal cells. In some studies only the large luteal cells showed stimulated P4 production (Hild-Petito et al., 1989), whereas in other studies, hCG only increased P4 production in small luteal cells (Ohara et al., 1987; Retamales et al., 1994). Unfortunately, the experiments with separated cell populations from primates did not add lipoprotein substrate and therefore P4 production is likely to not be optimized. In ruminants, luteal cells without provision of lipoprotein do not demonstrate optimal P4 production and do not have physiological responses to hormonal treatments (Wiltbank et al., 1989, 1993).
Capillary endothelial cells
The follicle has a distinct interstitial environment because blood vessels generally do not cross the follicular basement membrane and directly deliver blood components to the granulosa cells and oocyte that are contained in the follicular compartment. However, after degradation of the follicular basement membrane following the LH surge, there is intense blood vessel growth into this previously avascular compartment. Indeed, over 100 yr ago Andres Prenant hypothesized an endocrine function for the CL because of its abundant vascularity, a sign by which the histologist characterizes a gland of internal secretion pouring its products into the internal environment of the organism via the blood (Prenant, 1898). In the mature CL about 60% of the steroidogenic luteal cells are directly adjacent to a capillary (Dharmarajan et al., 1985; Farin et al., 1986; Niswender et al., 1994). The CL has one of the highest blood flows per mass of tissue in the body. The CL has higher blood flow per tissue mass than other tissues (Wiltbank et al., 1988, 1989) with blood flow of 30 ml/min/g of tissue compared to blood flow to the uterus (0.5 ml/min/g), adrenal gland (3 ml/min/g), or rest of the ovary without the CL (3 ml/min/g).
The high rate of blood flow to the CL could be essential not only for removal of P4 from the CL into the systemic circulation but also for efficient delivery of hormones, nutrients, and substrates to the CL (Meidan et al., 2005; Shirasuna et al., 2010). The exchange between the luteal cells and the bloodstream is also facilitated by the highly fenestrated nature of the luteal capillaries, which provides substantial permeability to large proteins (Ellinwood et al., 1978). Therefore, the high rate of luteal blood flow, the exaggerated plasma membrane surface area on the vascular side of luteal cells, and the highly permeable nature of the luteal capillaries allow for facile exchange of proteins and hormones between the luteal cells and blood stream. In the primate CL, the majority (97.4%) of the non-steroidogenic luteal cells (3β-HSD negative) were found to be <15 μm in diameter (Hild-Petito et al., 1989). In contrast in the sheep CL, there were two clear peaks of non-steroidogenic luteal cells, one peaking at about 9–10 μm and the second at 19–20 μm (Brannian et al., 1993). The identity of these non-steroidogenic luteal cell populations remains to be determined.
The most definitive research on the essential role for angiogenesis on the function of the CL were provided by the studies from the laboratory of Hamish Fraser (Fraser and Duncan, 2009). In one study, a VEGF receptor antagonist (VEGF trapA40) was given to marmoset monkeys either from the day of ovulation until luteal day 3 or on luteal day 3 for 1 day (Wulff et al., 2001). Inhibition of VEGF activity dramatically reduced luteal endothelial cell proliferation (proliferation index of >5 to <0.5) and endothelial cell area (75% reduction after 3 days; 44% reduction after 1 day). There was also a marked reduction in circulating P4 concentrations after VEGF antagonist treatment (P4 remained near 20 nM throughout the 3 days compared to increases to more than 80 nM in control monkeys). Even 1 day of VEGF antagonist treatment on day 3 reduced P4 by 50%. In other studies (Dickson et al., 2001; Fraser et al., 2005; Duncan et al., 2008), stump-tailed macaques were treated with different doses of VEGF antagonist in the early luteal phase (4, 1, or 0.25 mg/kg) or 1 mg/kg during the mid-luteal phase. Treatment with 4 or 1 mg/kg blocked the normal luteal phase elevation in P4. The 0.25 mg/kg dose had a more subtle effect on P4 production. Even at the mid-luteal phase, treatment with a single dose of 1 mg/kg caused a dramatic suppression in circulating P4 concentrations. Thus, VEGF is essential for the development of the CL, as would be expected, but continued action of VEGF is essential for maintenance of normal P4 production by the CL of primates. Similar experiments have not been performed in ruminants.
The inverse of this experiment was performed to evaluate the effects of excessive luteal vascularization. Marmosets were treated with an antibody to Delta-like ligand 4 (DLL4) in the early or mid-luteal phase (Fraser et al., 2012). This Notch ligand, DLL4, inhibits VEGF-mediated vessel sprouting and branching. Treatment with DLL4 antibodies in the early luteal phase increased luteal angiogenesis and increased vascular density on day 3 of the cycle. However, circulating P4 was significantly decreased. By day 10, the CL were smaller with involution of luteal cells and decreased circulating P4. Treatment with DLL4 in the mid-luteal phase produced a smaller but still significant depression in circulating P4 during the remainder of the luteal phase. Thus, it appears that either suppression of luteal angiogenesis or excessive angiogenesis may be detrimental to optimal function of the CL.
Role of LH in the primate and ruminant CL
The term "luteotropic hormone" may have first been used by Astwood (1941) to describe a partially purified pituitary factor that could maintain function of the rat CL. The luteotropic role in ruminants and primates has generally been ascribed to LH (Niswender et al., 2000). Nevertheless, P4 production by the CL of most species is generally not under acute stimulatory control in the same way as other endocrine glands. In his classic article on regulation of the CL, Dr. Irv Rothchild (Rothchild, 1981) expresses this concept eloquently: "The CL differs from other pituitary-dependent endocrine glands. The general level of activity of the adrenal and thyroid, for example, is determined mainly by a negative feedback control system. Such a control system is not typical of the CL . . . [there is relative] autonomy of P4 secretion, which appears to a variable extent among the mammalian species . . . [also] in spite of the presence of the gonadotropic hormone the CL inevitably regresses and P4 secretion stops."
In ruminants, there has been substantial research on the role of LH in luteal function (Niswender et al., 1994, 2000; Wiltbank, 1994). For example, when sheep were hypophysectomized on day 5 after estrus and CL recovered on day 12, serum P4 concentrations, luteal P4 concentrations, and weight of the CL were lower than would be expected for a day 12 CL but are usually similar or only slightly lower than expected for a day 5 CL (Farin et al., 1990; Juengel et al., 1995). For example, CL weighed about 300 mg on day 5 and about 600 mg on day 12, but were only about 300 mg on day 12 when ewes were hypophysectomized on day 5 (Juengel et al., 1995). Circulating P4 was about 1 ng/ml in ewes on day 5 or in hypophysectomized day 12 ewes as compared to about 2 ng/ml in intact ewes on day 12. Treatment of hypophysectomized ewes with LH, growth hormone, or a combination of LH and growth hormone restored circulating P4 to similar values as observed in day 12 intact ewes. Luteal weight was also increased by treatment with growth hormone and numerically but not statically increased by LH treatment. However, luteal weight was only restored to normal values by replacement of both growth hormone and LH. Hypophysectomy on day 12 appeared to decrease P4 in the presence or absence of the uterus (Kaltenbach et al., 1968; Denamur et al., 1973; Denamur, 1974). Purified LH was generally found to maintain the CL in the absence of the pituitary (Kaltenbach et al., 1968); although, partially purified prolactin and not LH has also been reported to maintain the CL in hypophysectomized ewes (Denamur et al., 1973). The early studies must be interpreted with caution due to the potential lack of purity of the hormonal preparations. Thus, pituitary hormones, particularly LH, may be important for maintenance of the CL but they appear to be particularly critical for luteal growth.
Pulsatile LH secretion can be eliminated by treatments with a GnRH antagonist. Treatment of ewes on day 12 of the estrous cycle with a GnRH antagonist eliminated pulsatile LH secretion but had no effect on circulating P4 concentrations (McNeilly et al., 1992). Peters et al. (1994) treated cattle with a potent GnRH antagonist during four different stages of luteal development. Treatment from days 2–7 (i.e. during early luteal development) caused the circulating P4 to reach about 50% of the normal concentrations but the CL still developed and regressed at the normal time. Treatment with GnRH antagonist from day 7–12 also reduced the maximal size of the CL and may have produced a slightly earlier time of luteal regression. Treatment with GnRH antagonist on day 12–17 did not alter circulating P4 concentrations although the time of luteolysis may have been slightly later in some cows. Any alterations in time of luteolysis may have been due to the reported changes in follicular growth after these treatments (Fike et al., 1997) because circulating E2 from follicles appears to be critical for timing of luteal regression (Araujo et al., 2009). However, we have completed a large study with treatment of Holstein cows with the GnRH antagonist Acyline (Wiltbank et al.; unpublished). Challenge with 100 μg of GnRH during the treatment period produced no increase in circulating LH, indicating the completeness of the GnRH receptor blockade. In addition, follicle growth stopped at about 8 mm, a time when the follicle becomes dependent on LH pulses. Nevertheless, we found no effect of GnRH antagonist treatment on circulating P4, no effect on size of the CL (as determined by transrectal ultrasound), and normal CL regression in response to PGF treatment on day 7. Thus, our study did not support a role for LH in development or maintenance of the ruminant CL, consistent with the study of McNeilly et al. (1992) in the ewe and most of the treatment groups in the study of Peters et al. (1994). It seems likely, based on the many studies with ruminant large luteal cells, that there is constitutive P4 production in the ruminant CL that is not acutely dependent on stimulation by LH. These results are consistent with the conclusions of Rothchild (1981) that a great deal of luteal P4 production is due to constitutive mechanisms.
Constitutive P4 production by the CL of ruminants is a reflection of the constitutive P4 production in the large luteal cell. We have worked with a very specific PKA inhibitor that almost completely blocks P4 production stimulated by LH or forskolin (Diaz et al., 2002). Intriguingly, treatment of unstimulated large luteal cells with this specific PKA inhibitor caused a dramatic reduction in P4 production, consistent with a constitutively active PKA in large luteal cells (Diaz et al., 2002). An elegant study by Bogan and Niswender (2007) showed that the PKA inhibitor, PKI, did not inhibit the cAMP concentrations in small or large luteal cells but again that it dramatically inhibited P4 production by large luteal cells. Further, they showed that StAR protein in unstimulated large luteal cells was primarily phosphorylated, or in an active state, and that StAR phosphorylation was dramatically inhibited by treatment with PKA inhibitor, both consistent with the idea that PKA was continually phosphorylating StAR even in the absence of increased cAMP (Bogan and Niswender, 2007). This contrasted with small luteal cells that had StAR protein that was primarily not phosphorylated under basal conditions but became phosphorylated by stimulation of cAMP production in small luteal cells. Thus, a constitutively active PKA appears to underlie the constitutive P4 production that is the hallmark of the ruminant large luteal cell.
In contrast to the CL of ruminants, the CL of primates is clearly dependent upon acute stimulation by LH pulses. Numerous studies during the 1970s, 1980s, and 1990s provide clear evidence that removal of LH pulses at any time during the luteal phase dramatically decreases circulating P4 concentrations and ultimately leads to loss of CL structure (Hutchison and Zeleznik, 1984; Moudgal, 1984; Hutchison et al., 1986; Benyo and Zeleznik, 1997; Zeleznik, 1998, 2001; Duffy et al., 1999; Fraser et al., 1999; Zeleznik and Somers, 1999, Stouffer, 2003; Xu et al., 2005; Jabbour et al., 2006; Bishop et al., 2009). For example, administration of GnRH antagonists during the mid-luteal phase led to a reduction in circulating P4 by 75% at 1 day, 88% by 2 days, and 96% by 3 days of treatment (Hall et al., 1991). In addition, treatment with GnRH antagonist after the end of the LH surge and during the first few days of the luteal phase prevented CL development, as shown by no increase in circulating P4, greatly reduced CL mass (Dubourdieu et al., 1991), and dramatically reduced luteal angiogenesis and luteal capillary endothelial cell numbers (Fraser and Lunn, 2001). Suppression of LH support for more than 72 h can cause irreversible loss of luteal P4 production and luteal structure (Fraser et al., 1987). Administration of LH or CG maintains normal circulating P4 concentrations and CL mass in monkeys treated with GnRH antagonist (Webley et al., 1991; Duffy et al., 1999). Thus, excellent ablation-replacement experiments have provided clear evidence that LH is the required and essential luteotropin in primates. In addition, most of the studies with primate luteal cells have found that LH is stimulatory to steroidogenesis in either the large or small luteal cells providing the underlying cellular basis for the LH dependence of the primate CL (Hild-Petito et al., 1989). The cAMP/PKA pathways are likely to underlie most, if not all, of the luteal response to LH stimulation of luteal cells, however, loss of CREB expression in granulosa-lutein cells eliminates this transcription factor pathway for transmission of the LH-cAMP-PKA signaling (Benyo and Zeleznik, 1997; Zeleznik and Somers, 1999; Zeleznik, 2001). In addition, there is strong evidence that maintenance of luteal function by LH is ultimately mediated by actions of intraluteal P4 (Stouffer, 2003), consistent with the Rothchild idea that intraluteal P4 is the ultimate luteotropin in many species (Rothchild, 1981).
Finally, it seems appropriate to consider the efficiency of P4 production by the CL of ruminants and primates. Unfortunately, we were not able to find valid determinations of the precise production rates of P4 by the CL of these species. Nevertheless, we used values from the literature to make comparisons of circulating hormonal concentrations, sizes of ovarian structures, metabolic clearance rate (MCR), and were able to calculate production rates for P4 and for E2 in humans and dairy cows (Table 2). Obviously, the weight of a cow is much greater than a woman and this leads to a greater MCR for E2 and P4 in cows (Sangsritavong et al., 2002) than women (Grow, 2002). The MCR is defined as the volume of blood cleared of a substance per unit of time and is expressed in liters of blood cleared per hour in Table 2. The MCR is greater than the weight difference for lactating cows than human probably due to the high rate of liver blood flow due to extremely high intake of feed (Sangsritavong et al., 2002, Vasconcelos et al., 2003, Wiltbank et al., 2006). In addition, the high sex hormone binding globulin concentrations in humans, will protect E2 and P4 from metabolism and reduce the MCR in humans (Grow, 2002). The diameter of the ovulatory follicle in women (Baerwald et al., 2005) and lactating cows (Sartori et al., 2004) is surprisingly similar. However, peak E2 concentrations are more than 20-fold greater in women (Baerwald et al., 2005) than cows (Sartori et al., 2004). This difference is expected if a follicle of similar size has a similar rate of E2 production in humans and cows, but a much lower rate of metabolism in the human than the cow. Indeed, our calculated values for production rates in mg/h are fairly similar in humans and cows (11.5 vs. 20.5) suggesting a similar efficiency for E2 production in the dominant follicle of either species. As might be expected, the volume or weight of CL is fairly similar in the two species since a similar size of follicle was ovulated to produce the CL. Again the MCR for P4 is much greater in cows than humans (32.3X). The extraordinary value that was discovered in these calculations was that the CL of the dairy cow had over 10-fold more production of P4 than the human CL. Even when the production of P4 was corrected for luteal tissue weight there was still a major advantage for the bovine CL. This was an unexpected finding and indicates that although the human and bovine follicles have similar E2 production efficiencies, there are extraordinary differences in P4 production efficiency in the bovine CL compared the human CL. Obviously, both species have an incredible increase in steroid output from the CL compared to the follicle. Indeed the human CL has 100 times more P4 production than E2 production by the human follicle. However, the bovine CL has about 1,000 times more P4 produced by the CL than E2 produced by the bovine follicle, further emphasizing the exceptional P4 production capacity of the bovine CL. This may be due to the extraordinary size of the bovine large luteal cell (3X-greater in bovine than human) and clear optimization of luteal cell organization within the CL (discussed above). There may also be greater P4 production efficiencies due to the high concentrations of mitochondria and steroidogenic enzyme activity that have been found in the large luteal cells of ruminants (Farin et al., 1986; Wiltbank et al., 1993; Diaz et al., 2002). It also seems very likely that constitutively active protein kinase A in the bovine large luteal cell produces increased efficiencies as compared to the LH-regulated activity of the human large luteal cell (Diaz et al., 2002; Bogan and Niswender, 2007). Thus, highly efficient mechanisms for P4 production based on constitutive P4 production by the large luteal cell may have evolved in ruminants and this strategy may be more efficient (in terms of mass of P4 produced per mass of tissue) than the LH-regulated P4 production strategy utilized by the primate large luteal cell.
Table 2.
Comparisons of human female and dairy cow for estradiol-17β (E2) and progesterone (P4) metabolic clearance rate (MCR in l/h; Grow, 2002, Sangsritavong et al., 2002), follicle diameter and peak E2 (Sartori et al., 2004; Baerwald et al., 2005), CL weight and peak P4, calculated from CL volume without luteal cavity (Sartori et al., 2004; Baerwald et al., 2005), and calculated values of follicular production of E2 and luteal production of P4. Follicular production of E2 (mg/h) = Peak E2 (mg/l) X MCR for E2 (l/h). Luteal production of P4 (mg/h) = Peak P4 (mg/l) X MCR for P4 (l/h).
| Woman | Dairy Cow | Species Difference (Cow/Human) | |
|---|---|---|---|
| Weight (kg) | 65 | 680 | 10.5 |
| MCR for E2 (l/h) | 56 | 2700 | 48.2 |
| MCR for P4 (l/h) | 96 | 3100 | 32.3 |
| Follicle diameter (mm) | 21.7 | 18.6 | 0.86 |
| Peak E2 (pg/ml) | 205 | 7.6 | 0.037 |
| Follicular production of E2 (mg/h) | 11.5 | 20.5 | 1.78 |
| CL Weight (g) | 5.3 | 7.6 | 1.43 |
| Peak P4 (ng/ml) | 14.2 | 5.6 | 0.39 |
| Luteal production of P4 (mg/h) | 1,363 | 17,360 | 12.7 |
| Luteal production of P4 (mg/h)/g CL tissue | 257 | 2284 | 8.89 |
| Luteal production/follicular production | 119X | 847X | 7X |
Mechanisms of luteal regression
CL regression in ruminants
In the absence of a viable embryo, functional and structural regression of the CL occurs. That the uterus was responsible for regression of the CL in ruminants was first demonstrated in elegant experiments in which the uterus was removed (or a sham operation), some of the CL were marked with India ink (Wiltbank and Casida, 1956), and ovaries evaluated up to 100 days after estrus in ewes or up to 154 days after estrus in cows. All control ewes had normal return to estrus, however, estrus was delayed in all ewes or cows with the uterus removed. In addition, it was found that removal of the uterus resulted in prolonged maintenance of the CL in both ewes and cows (Wiltbank and Casida, 1956). Subsequent well-designed studies showed that unilateral but not contralateral hysterectomy resulted in prolongation of the CL, showing that CL regression in ruminants was due to factors coming from the uterus on the same side as the ovary and not through the general circulation (Moor and Rowson, 1966a, b). A series of experiments using surgical anastomoses of ovarian veins and arteries in hemi-hysterectomized cows provided conclusive evidence that uterine luteolytic factor reached the CL by a local utero-ovarian pathway (Delcampo and Ginther, 1973; Ginther et al., 1973; Mapletoft et al., 1976). The uterine luteolytic factor was later identified as Prostaglandin F2α (PGF; Knickerbocker et al., 1988). The evidence that PGF is the main luteolysin in ruminants is substantial and convincing (reviewed in Knickerbocker et al., 1988). For example, 1) PGF increases near the time luteolysis, 2) inhibition of PG production delays luteolysis, 3) passive immunization to PGF blocks luteolysis, 4) PGF is efficiently transferred from the uterine artery to the ovarian vein (see Knickerbocker et al., 1988). Thus, regression of the CL is due to uterine PGF and specifically due to multiple pulses of PGF released by the nonpregnant uterus (McCracken et al., 1999; Niswender et al., 2000; Schams and Berisha, 2004). There is substantial variability in the frequency and amplitude of PGF pulses associated with ruminant luteolysis but typically there are 4–8 discrete pulses that occur at 6–14 h intervals (Kindahl et al., 1976; Silvia et al., 1991; Mann and Lamming, 2006). For example, in heifers evaluated at hourly intervals during the 7 days around luteolysis there was complete luteolysis, as defined by a decrease to basal P4, after 4 distinct PGF pulses that occurred during ~30 h (Kindahl et al., 1976). Recent studies have mimicked the normal pattern of PGF pulses by infusion of low doses of PGF into the uterus of heifers (Ginther et al., 2007, 2009). The PGF metabolite patterns were similar to the patterns observed during natural luteolysis. In addition, the changes in circulating P4 concentrations and luteal blood flow showed a similar pattern as occurred during natural luteolysis with an increase in circulating P4 and blood flow after the initial PGF pulse and a subsequent decrease in P4 and blood flow after the second, third, and fourth infusions of PGF (Ginther et al., 2007, 2009).
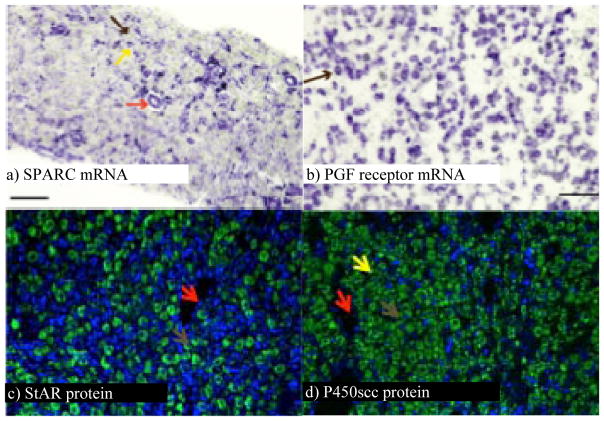
The primary location of PGF receptors is the large luteal cell in ruminants as can be clearly seen by in situ hybridization for PGFR in bovine CL (Fig. 3). There are numerous changes in gene expression during natural CL regression or during CL regression induced by exogenous PGF. A recent study demonstrated the changes in gene expression in response to a series of four low-amplitude pulses of uterine PGF (Atli et al., 2012). All pulses of PGF induce increases in early response genes such as FOS, JUN and EGR1 probably due to activation of the PGF receptor in response to each PGF pulse. Inhibition of mRNA for StAR, a key protein involved in luteal P4 production, begins after the second pulse of PGF, although decreases in mRNA for other key steroidogenic enzymes do not occur until after the third (NR5A1, LHCGR) or fourth (CYP11A1) PGF pulse. In addition to decreased luteal P4 production during luteolysis, there is also increased intraluteal production of PGF after treatment with PGF consistent with a key auto-amplification pathway for PGF in ruminant CL (Townson and Pate, 1994, Tsai and Wiltbank, 1997; Wiltbank and Ottobre, 2003). Induction of mRNA for enzymes involved in synthesis of PGF requires only one (PTGS2 also known as COX-2) or two (PTGFS also known as PGF synthase) low-amplitude pulses of PGF. However, a key enzyme involved in degradation of PGF, hydroxyprostaglandin dehydrogenase (HPGD), requires three pulses of PGF before its mRNA is inhibited. Thus, intraluteal PGF synthesis may be induced during the early stages of CL regression, however elimination of PGF degradation pathways may be required for complete luteolysis, as originally indicated by the studies of (Silva et al., 2000). The role of the immune cells in CL regression in ruminants has been extensively investigated (Pate, 1994; Pate and Keyes, 2001). As previously shown, mRNA for factors (FAS, FASLG, ILB1, IL8) that stimulate migration and activation of immune cells are increased by PGF and we observed an increase in all these factors after the second PGF pulse. Finally, inhibition of the factors involved in blood vessel growth and maintenance are decreased after the second (VEGFA) or fourth (FGF2) pulse of PGF. Thus, multiple molecular pathways are regulated by PGF pulses to induce the full luteolytic cascade during CL regression in ruminants.
Figure 3.
Micrographs of sections of bovine CL showing the distribution of specific mRNA and proteins. a) SPARC mRNA is localized to small luteal cells (yellow arrow) and endothelial cells (red arrow) but not to large luteal cells (brown arrow). b) PGF receptor mRNA is localized exclusively to large luteal cells (brown arrow). c) StAR protein is localized to large luteal cells (brown arrow). d) P450 cholesterol side chain cleavage protein is localized to large luteal cells (brown arrow), small luteal cells (yellow arrow), but not to endothelial cells of blood vessels (red arrow).
CL regression in primates
In contrast to the studies in ruminants, it was clear from early studies that complete removal of the uterus in primates did not prolong CL lifespan (Burford and Diddle, 1936). Thus, it is clear that uterine PGF is not the primary initiator of luteolysis in primates. Nevertheless, under some circumstance PGF treatment of primates can initiate the process of luteolysis (Wiltbank and Ottobre, 2003). For example, marmoset monkeys are sensitive to various analogs of PGF (Summers et al., 1985; Webley et al., 1991, 2010). However, it seems clear that the primary mechanism involved in initiation of luteolysis is lack of sufficient LH stimulation. Indeed, rescue of the primate CL during pregnancy is caused by an LH analog, CG, secreted by the developing chorion (Fraser et al., 1987; Auletta and Flint, 1988; Dubourdieu et al., 1991; Zeleznik, 1998). Nonetheless, CL regression is not due to an actual lack of LH pulses but a requirement for increasing LH stimulation during the later primate luteal phase (Duffy et al., 1999). In the GnRH antagonist treated monkey, a constant dose of LH pulses or treatment with low doses of hLH or hCG can maintain the early and mid-cycle CL, however the later phase CL seems to require increasing amounts of LH (Zeleznik, 1998, Duffy et al., 1999). There may be a number of molecular pathways that underlie the increasing requirement for LH in the later luteal phase. At least during the early stages of luteal regression there seems to be normal concentrations of LH receptor mRNA and protein (Duncan et al., 1996). However, during induced luteolysis, either by treatment with a PGF analog or treatment with a GnRH antagonist, there was a rapid loss of LH receptors in the marmoset CL (Duncan et al., 1998). In addition, there are distinct changes in the expression of LH receptor splice variants with an increase in the most truncated form of LH receptor, termed LHrd, and a decrease in expression of the full-length LH receptor (Dickinson et al., 2009). Expression of these receptors in COS-7 cells indicated that treatment with LH did not increase cAMP in cells expressing LHrd. Coexpression of LHrd with the full-length LH receptor prevented cAMP production in response to LH treatment. It appears that coexpression of LHrd with the full-length LH receptor reduced cell surface expression of the LH receptor and this probably mediated the inhibitory effect of LHrd on LH-stimulated cAMP production (Dickinson et al., 2009).
Although the primate CL can clearly synthesize PGF, the evidence is still equivocal that intraluteal PGF production underlies luteolysis in primates, reviewed by (Wiltbank and Ottobre, 2003). It seems clear that primate luteal cells express PGF receptors and treatment of human granulosa cells, in vitro, with a stimulator of cAMP production, forskolin, causes a dramatic induction of PGF receptor expression (Tsai et al., 2001). Intriguingly, treatment of human granulosa-lutein cells after 2 days of forskolin treatment had no effect on expression of COX-2 or LH receptor mRNA, however after 8 days of forskolin treatment PGF treatment caused an increase in COX-2 mRNA and a decrease in LH receptor mRNA, as would be expected during induced luteolysis in primates (Tsai et al., 2001). Thus, human granulosa cells that are luteinized in vitro undergo changes that are typical for acquisition of luteolytic capacity, as have been observed in ruminant luteinized-granulosa cells. Similarly, during the early luteal phase PGF is not effective in inducing luteolysis in most species, including marmoset monkeys, however PGF became effective as a luteolytic agent about 7 days after ovulation (Webley et al., 2010). Thus, the late luteal phase rise in PGF production may be indicative of an increase in intraluteal PGF production during the process of luteolysis. We postulate that elevated cAMP/PKA activity in luteal cells may suppress enzymes for PGF synthesis (PTGS2/COX-2; PTGFS/PGF Synthase) and may maintain the enzyme for PGF degradation (HPGD). As cAMP/PKA activity becomes reduced during the late luteal phase this may lead to increases in PGF synthesis and reduced PGF degradation and thus increases in intraluteal PGF production. This intraluteal PGF production may underlie at least part of the mechanism for luteolysis in the primate. Obviously, this model is highly speculative and would need to be tested in future experiments.
Luteal insufficiency in ruminants and primates
In ruminants, short cycles have been recognized for many years. The short cycle is clearly due to a shortened luteal phase of only about 7 days, followed by continued growth of the follicle and estrus at about 10 days after the previous estrus. Obviously, pregnancies cannot occur in this shortened cycle because of this early CL regression. A number of different hypotheses have been put forward for the early CL regression in ruminants, including improper luteinization and development of the CL or alternatively early secretion of PGF by the uterus. It is clear now that the second hypothesis is most supported by the scientific evidence. Removal of the uterus, or inhibition of PGF secretion prevents short luteal phases in ruminants (Southee et al., 1988; Hunter, 1991; Garverick et al., 1992b). In addition, intrauterine infusion of interferon-tau, the protein that normally causes maintenance of the CL during pregnancy in ruminants, can prevent short luteal phases (Garverick et al., 1992a). At least part of the action of interferon-tau is a reduction in uterine secretion of PGF. Thus, all of this evidence clearly shows that CL that develops in cattle destined to have a short luteal phase has the capacity for normal function and lifespan. However early secretion of uterine PGF causes regression of the CL as soon as it develops the ability to regress in response to exogenous PGF (about day 7 of the cycle).
The luteal phase defect (LPD) in women has been recognized since 1949 however, the prevalence and etiology have been debated for many years (Jones, 1976; Davis et al., 1989; Ginsburg, 1992; Bukulmez and Arici, 2004). Indeed, (Bukulmez and Arici, 2004) challenge the notion that luteal phase defect is a major problem of infertility or recurrent abortions stating: "LPD is a reality in assisted reproduction cycles with GnRHagonist/antagonist suppression. Otherwise, there is no convincing evidence to define LPD as a distinct clinical entity that leads to reproductive problems. It is not justified to include costly and cumbersome tests to diagnose LPD in patients who have infertility or recurrent abortion." Although research studies have provided objective methods for defining luteal phase defect, a number of studies have found similar rates of luteal phase defect, by various definitions, in women with normal fertility as in women diagnosed with infertility or recurrent abortion (Lenton et al., 1984; Davis et al., 1989; Castelbaum and Lessey, 1995). Nevertheless, almost all women that undergo stimulated IVF cycles have defects in subsequent CL function (Bukulmez and Arici, 2004). An initial hypothesis was that aspiration of granulosa cells during the oocyte retrieval resulted in inadequate granulosa-lutein cells in the subsequent CL, leading to lower P4 production (O'Hara et al., 2012). However, aspiration of the oocyte from naturally ovulating women did not cause a luteal phase defect, making this explanation unlikely (Kerin et al., 1981). Currently, most evidence points to lack of sufficient LH pulses to support normal CL development and function. Two possible explanations have been advanced. First, GnRH agonists are often used to prevent a spontaneous LH surge, down-regulate pituitary GnRH receptors, and permit total exogenous regulation of circulating gonadotropins. However, the inhibitory effects of these agonists may persist during the first week or more of the developing CL. Thus, insufficient circulating LH leads to insufficient CL function. Alternatively, the extremely elevated steroid concentrations present after superovulation of women during IVF treatments could also inhibit GnRH/LH pulses again leading to insufficient LH support for the CL and early CL regression. It is clear that there is a remarkable depression in circulating LH and luteal blood flow in women that have undergone superstimulation with GnRH agonist treatment and subsequent luteal phase support (Takasaki et al., 2011). Thus, the current explanations for luteal phase defect in women focuses on the lack of adequate LH support during the development and/or maintenance of the CL. It should be noted that ruminants do not demonstrate luteal phase defect following superovulation.
Final conclusions
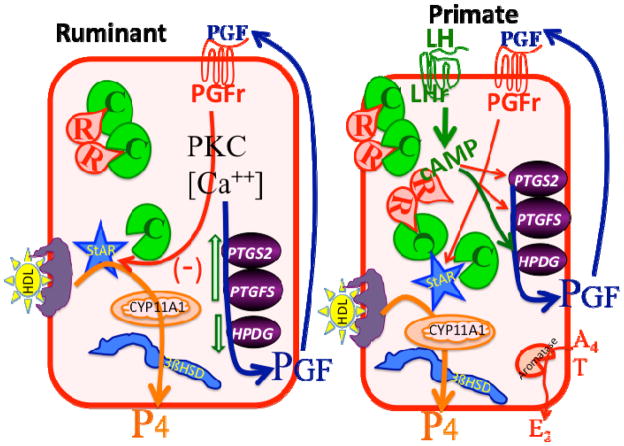
Figure 4 shows a simplified model of some of the concepts that have been discussed in this review. Obviously the key product of both the ruminant and primate luteal cells is P4. We provided some evidence that the ruminant CL can produce P4 at about 10-times the efficiency of P4 production by the primate CL. The cellular basis for high P4 production by the ruminant CL is the large luteal cell with development of high constitutive P4 production probably due to free catalytic subunit for PKA, even in the absence of cAMP. In contrast, the primate CL requires stimulation by LH to increase luteal P4 production probably through cAMP/PKA-mediated pathways. In addition to P4 production the primate CL also produces substantial quantities of E2 due to androgen production by the theca-lutein cells with aromatization in the granulosa-lutein cells. Luteolysis in the ruminant CL is initiated by PGF from the non-pregnant uterus through pathways that are becoming better characterized. In contrast, luteolysis in the primate is clearly related to insufficient stimulation by LH, due to an increasing requirement for LH-cAMP as the CL ages. We speculate that a reduction in the cAMP-stimulated pathways may lead to increased PGF stimulation in the late luteal phase, activation of the PGF receptors that are present on granulosa-lutein cells and ultimate regression of the primate CL.
Figure 4.
Postulated model for regulation of large luteal cells of ruminants (left) and primates (right). In the ruminant large luteal cell, there is excess catalytic subunit for PKA (C in green) and this can phosphorylate and activate StAR (steroidogenic acute regulatory protein) as well as other proteins. This produces very high constitutive progesterone (P4) production in the ruminant large luteal cell due to high amounts of cholesterol substrate, in the form of HDL, and high enzymatic activity for CYP11A1 (also known as P450 cholesterol side chain cleavage enzyme) and 3β-hydroxysteroid-dehydrogenase enzyme (3βHSD). Inhibitory pathways that are activated by Prostaglandin F2a (PGF) binding to its receptor (PGFr) are also present, leading to inhibition of StAR, increases in PGF synthesis enzymes (PTGS2 and PTGFS) and inhibition of the PGF degradation enzyme (HPGD). This produces an auto-amplification pathway for PGF with PGF production in the large luteal cell increasing due to PGF action. In the primate large luteal cell, similar pathways for high P4 production are present, however, these pathways are under direct control by LH. Activation of the LH receptor (LHr) by LH increases cAMP, which binds to the regulatory subunit of PKA (R in red), releasing catalytic subunit to phosphorylate StAR and other proteins. In addition, the large luteal cells of primates convert androgens (A4, T) to estrogens (E2). It is postulated that a reduction in LH stimulation would lead to changes in PGF synthesis and degradation enzymes that may increase intraluteal production of PGF and activation of inhibitory pathways by PGF.
Acknowledgments
Grant support: Wisconsin Experiment Station, NIH R01 HD050616, and FAPESP Brazil.
References
- Allen W. Recollections of my life with progesterone. Gynecol Invest. 1974;5:142–182. doi: 10.1159/000301649. [DOI] [PubMed] [Google Scholar]
- Anderson LE, Wu YL, Tsai SJ, Wiltbank MC. Prostaglandin F-2 alpha receptor in the corpus luteum: recent information on the gene, messenger ribonucleic acid, and protein. Biol Reprod. 2001;64:1041–1047. doi: 10.1095/biolreprod64.4.1041. [DOI] [PubMed] [Google Scholar]
- Araujo RR, Ginther OJ, Ferreira JC, Palhao MM, Beg MA, Wiltbank MC. Role of follicular estradiol-17beta in timing of luteolysis in heifers. Biol Reprod. 2009;81:426–437. doi: 10.1095/biolreprod.108.073825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astwood EB. The regulation of corpus luteum function by hypophyseal luteotrophin. Endocrinology. 1941;28:309–320. [Google Scholar]
- Atli MO, Bender RW, Mehta V, Bastos MR, Luo W, Vezina CM, Wiltbank MC. Patterns of gene expression in the bovine corpus luteum following repeated intrauterine infusions of low doses of prostaglandin F2alpha. Biol Reprod. 2012;86:130. doi: 10.1095/biolreprod.111.094870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auletta FJ, Flint AP. Mechanisms controlling corpus luteum function in sheep, cows, nonhuman primates, and women especially in relation to the time of luteolysis. Endocr Rev. 1988;9:88–105. doi: 10.1210/edrv-9-1-88. [DOI] [PubMed] [Google Scholar]
- Baerwald AR, Adams GP, Pierson RA. Form and function of the corpus luteum during the human menstrual cycle. Ultrasound Obstet Gynecol. 2005;25:498–507. doi: 10.1002/uog.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyo DF, Zeleznik AJ. Cyclic adenosine monophosphate signaling in the primate corpus luteum: Maintenance of protein kinase A activity throughout the luteal phase of the menstrual cycle. Endocrinology. 1997;138:3452–3458. doi: 10.1210/endo.138.8.5346. [DOI] [PubMed] [Google Scholar]
- Bishop CV, Hennebold JD, Stouffer RL. The effects of luteinizing hormone ablation/replacement versus steroid ablation/replacement on gene expression in the primate corpus luteum. Mol Hum Reprod. 2009;15:181–193. doi: 10.1093/molehr/gap005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogan RL, Niswender GD. Constitutive steroidogenesis in ovine large luteal cells may be mediated by tonically active protein kinase A. Biol Reprod. 2007;77:209–216. doi: 10.1095/biolreprod.106.059618. [DOI] [PubMed] [Google Scholar]
- Brannian JD, Stouffer RL, Shiigi SM, Hoyer PB. Isolation of ovine luteal cell subpopulations by flow-cytometry. Biol Reprod. 1993;48:495–502. doi: 10.1095/biolreprod48.3.495. [DOI] [PubMed] [Google Scholar]
- Bukulmez O, Arici A. Luteal phase defect: myth or reality. Obstet Gynecol Clin North Am. 2004;31:727–744. doi: 10.1016/j.ogc.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Burford TH, Diddle AW. Effect of total hysterectomy upon the ovary of the Macacus rhesus. Surg Gynecol Obstet. 1936;62:701–707. [Google Scholar]
- Carroll DJ, Grummer RR, Clayton MK. Stimulation of luteal cell progesterone production by lipoproteins from cows fed control or fat-supplemented diets. J Dairy Sci. 1992a;75:2205–2214. doi: 10.3168/jds.S0022-0302(92)77981-8. [DOI] [PubMed] [Google Scholar]
- Carroll DJ, Grummer RR, Mao FC. Progesterone production by cultured luteal cells in the presence of bovine low-density or high-density-lipoproteins purified by heparin affinity-chromatography. J Anim Sci. 1992b;70:2516–2526. doi: 10.2527/1992.7082516x. [DOI] [PubMed] [Google Scholar]
- Castelbaum AJ, Lessey BA. Corpus luteum defect - Alloyed gold standard. Fertil Steril. 1995;63:427–427. doi: 10.1016/s0015-0282(16)57384-1. [DOI] [PubMed] [Google Scholar]
- Corner GW, Allen WM. Physiology of the corpus luteum. II. Production of a special uterine reaction (progestational proliferation) by extracts of the corpus luteum. Am J Physiol. 1929;88:326–399. [Google Scholar]
- Davis OK, Cholst IN, Berkeley AS, Freedman KS, Naus GJ. The incidence of luteal phase defect in normal, fertile women, determined by serial endometrial biopsies. Fertil Steril. 1989;51:582–586. doi: 10.1016/s0015-0282(16)60603-9. [DOI] [PubMed] [Google Scholar]
- Delcampo CH, Ginther OJ. Vascular anatomy of uterus and ovaries and unilateral luteolytic effect of uterus - angioarchitecture in sheep. Am J Vet Res. 1973;34:1377–1385. [PubMed] [Google Scholar]
- Denamur R, Martinet J, Short RV. Pituitary control of ovine corpus luteum. J Reprod Fertil. 1973;32:207–220. doi: 10.1530/jrf.0.0320207. [DOI] [PubMed] [Google Scholar]
- Denamur R. Luteotropic factors in sheep. J Reprod Fertil. 1974;38:251–259. doi: 10.1530/jrf.0.0380251. [DOI] [PubMed] [Google Scholar]
- Devoto L, Fuentes A, Kohen P, Cespedes P, Palomino A, Pommer R, Munoz A, Strauss JF. The human corpus luteum: life cycle and function in natural cycles. Fertil Steril. 2009;92:1067–1079. doi: 10.1016/j.fertnstert.2008.07.1745. [DOI] [PubMed] [Google Scholar]
- Dharmarajan AM, Bruce NW, Meyer GT. Quantitative ultrastructural characteristics relating to transport between luteal cell cytoplasm and blood in the corpus luteum of the pregnant rat. Am J Anat. 1985;172:87–99. doi: 10.1002/aja.1001720107. [DOI] [PubMed] [Google Scholar]
- Diaz FJ, Anderson LE, Wu YL, Rabot A, Tsai SJ, Wiltbank MC. Regulation of progesterone and prostaglandin F-2 alpha production in the CL. Mol Cell Endocrinol. 2002;191:65–80. doi: 10.1016/s0303-7207(02)00056-4. [DOI] [PubMed] [Google Scholar]
- Dickinson RE, Stewart AJ, Myers M, Millar RP, Duncan WC. Differential expression and functional characterization of luteinizing hormone receptor splice variants in human luteal cells: implications for luteolysis. Endocrinology. 2009;150:2873–2881. doi: 10.1210/en.2008-1382. [DOI] [PubMed] [Google Scholar]
- Dickson SE, Bicknell R, Fraser HM. Mid-luteal angiogenesis and function in the primate is dependent on vascular endothelial growth factor. J Endocrinol. 2001;168:409–416. doi: 10.1677/joe.0.1680409. [DOI] [PubMed] [Google Scholar]
- Dubourdieu S, Charbonnel B, Massai MR, Marraoui J, Spitz I, Bouchard P. Suppression of corpus luteum function by the GnRH antagonis Nal-Glu: effect of the dose and timing of hCG administration. Fertil Steril. 1991;56:440–445. doi: 10.1016/s0015-0282(16)54537-3. [DOI] [PubMed] [Google Scholar]
- Duffy DM, Stewart DR, Stouffer RL. Titrating luteinizing hormone replacement to sustain the structure and function of the corpus luteum after gonadotropin-releasing hormone antagonist treatment in rhesus monkeys. J Clin Endocrinol Metab. 1999;84:342–349. doi: 10.1210/jcem.84.1.5362. [DOI] [PubMed] [Google Scholar]
- Duncan WC, McNeilly AS, Fraser HM, Illingworth PJ. Luteinizing hormone receptor in the human corpus luteum: lack of down-regulation during maternal recognition of pregnancy. Hum Reprod. 1996;11:2291–2297. doi: 10.1093/oxfordjournals.humrep.a019091. [DOI] [PubMed] [Google Scholar]
- Duncan WC, Illingworth PJ, Young FM, Fraser HM. Induced luteolysis in the primate: rapid loss of luteinizing hormone receptors. Hum Reprod. 1998;13:2532–2540. doi: 10.1093/humrep/13.9.2532. [DOI] [PubMed] [Google Scholar]
- Duncan WC, van den Driesche S, Fraser HM. Inhibition of vascular endothelial growth factor in the primate ovary up-regulates hypoxia-inducible factor-1 alpha in the follicle and corpus luteum. Endocrinology. 2008;149:3313–3320. doi: 10.1210/en.2007-1649. [DOI] [PubMed] [Google Scholar]
- Ellinwood WE, Nett TM, Niswender GD. Ovarian vasculature: structure and function. In: Jones RE, editor. The Vertebrate Ovary. New York: Plenum; 1978. pp. 583–614. [Google Scholar]
- Farin CE, Moeller CL, Sawyer HR, Gamboni F, Niswender GD. Morphometric analyses of cell-types in the ovine corpus luteum throughout the estrous cycle. Biol Reprod. 1986;35:1299–1308. doi: 10.1095/biolreprod35.5.1299. [DOI] [PubMed] [Google Scholar]
- Farin CE, Nett TM, Niswender GD. Effects of LH on luteal cell populations in hypophysectomized ewes. J Reprod Fertil. 1990;88:61–70. doi: 10.1530/jrf.0.0880061. [DOI] [PubMed] [Google Scholar]
- Fike KE, Bergfeld EG, Cupp AS, Kojima FN, Mariscal V, Sanchez T, Wehrman ME, Grotjan HE, Hamernik DL, Kittok RJ, Kinder JE. Gonadotropin secretion and development of ovarian follicles during oestrous cycles in heifers treated with luteinizing hormone releasing hormone antagonist. Anim Reprod Sci. 1997;49:83–100. doi: 10.1016/s0378-4320(97)00040-7. [DOI] [PubMed] [Google Scholar]
- Fitz TA, Mayan MH, Sawyer HR, Niswender GD. Characterization of 2 steroidogenic cell types in the ovine corpus luteum. Biol Reprod. 1982;27:703–711. doi: 10.1095/biolreprod27.3.703. [DOI] [PubMed] [Google Scholar]
- Fraenkel L, Cohn F. Experimentelle Untersuchungen des Corpus luteum auf die Insertion des Eies (Theorie von Born) Anat Anz. 1901;20:294–300. [Google Scholar]
- Fraser HM, Nestor JJ, Vickery BH. Suppression of luteal function by a LHRH antagonist during the early luteal phase in the stumptailed macaque monkey and the effects of subsequent administration of hCG. Endocrinology. 1987;121:612–618. doi: 10.1210/endo-121-2-612. [DOI] [PubMed] [Google Scholar]
- Fraser HM, Lunn SF, Harrison DJ, Kerr JB. Luteal regression in the primate: different forms of cell death during natural and gonadotropin-releasing hormone antagonist or prostaglandin analogue-induced luteolysis. Biol Reprod. 1999;61:1468–1479. doi: 10.1095/biolreprod61.6.1468. [DOI] [PubMed] [Google Scholar]
- Fraser HM, Lunn SF. Regulation and manipulation of angiogenesis in the primate corpus luteum. Reproduction. 2001;121:355–362. doi: 10.1530/rep.0.1210355. [DOI] [PubMed] [Google Scholar]
- Fraser HM, Wilson H, Morris KD, Swanston I, Wiegand SJ. Vascular endothelial growth factor trap suppresses ovarian function at all stages of the luteal phase in the macaque. J Clin Endocrinol Metab. 2005;90:5811–5818. doi: 10.1210/jc.2005-1199. [DOI] [PubMed] [Google Scholar]
- Fraser HM, Duncan WC. SRB reproduction, fertility and development award lecture 2008. Regulation and manipulation of angiogenesis in the ovary and endometrium. Reprod Fertil Dev. 2009;21:377–392. doi: 10.1071/rd08272. [DOI] [PubMed] [Google Scholar]
- Fraser HM, Hastings JM, Allan D, Morris KD, Rudge JS, Wiegand SJ. Inhibition of Delta-Like Ligand 4 induces luteal hypervascularization followed by functional and structural luteolysis in the primate ovary. Endocrinology. 2012;153:1972–1983. doi: 10.1210/en.2011-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J, Jones GS, Acosta AA, Wright GL. Corpus luteum function after follicle aspiration for oocyte retrieval. Fertil Steril. 1981;36:565–572. doi: 10.1016/s0015-0282(16)45852-8. [DOI] [PubMed] [Google Scholar]
- Garverick HA, Moser MT, Keisler DH, Hamilton SA, Roberts RM, Smith MF. Luteal function after intrauterine infusion of recombinant bovine interferon alpha-1 into postpartum beef cows expected to have short or normal luteal phases. J Reprod Fertil. 1992a;94:319–325. doi: 10.1530/jrf.0.0940319. [DOI] [PubMed] [Google Scholar]
- Garverick HA, Zollers WG, Smith MF. Mechanisms associated with corpus luteum life span in animals having normal or subnormal luteal function. Anim Reprod Sci. 1992b;28:111–124. [Google Scholar]
- Ginsburg KA. Luteal phase defect: etiology, diagnosis, and management. Endocrinol Metab Clin North Am. 1992;21:85–104. [PubMed] [Google Scholar]
- Ginther OJ, Delcampo CH, Rawlings CA. Local pathway between uterus and ovaries in ewes. J Anim Sci. 1973;37:312–313. [Google Scholar]
- Ginther OJ, Silva LA, Araujo RR, Beg MA. Temporal associations among pulses of 13,14-dihydro-15-keto-PGF2alpha, luteal blood flow, and luteolysis in cattle. Biol Reprod. 2007;76:506–513. doi: 10.1095/biolreprod.106.057653. [DOI] [PubMed] [Google Scholar]
- Ginther OJ, Araujo RR, Palhao MP, Rodrigues BL, Beg MA. Necessity of sequential pulses of prostaglandin F2alpha for complete physiologic luteolysis in cattle. Biol Reprod. 2009;80:641–648. doi: 10.1095/biolreprod.108.072769. [DOI] [PubMed] [Google Scholar]
- Grow DR. Metabolism of endogenous and exogenous reproductive hormones. Obstet Gynecol Clin North Am. 2002;29:425–436. doi: 10.1016/s0889-8545(02)00009-8. [DOI] [PubMed] [Google Scholar]
- Grummer RR, Carroll DJ. A review of lipoprotein cholesterol metabolism - Importance to ovarian function. J Anim Sci. 1988;66:3160–3173. doi: 10.2527/jas1988.66123160x. [DOI] [PubMed] [Google Scholar]
- Hall JE, Bhatta N, Adams JM, Rivier JE, Vale WW, Crowley WF. Variable tolerance of the developing follicle and corpus luteum to GnRH antagonis-induced gonadotropin withdrawal in the human. J Clin Endocrinol Metab. 1991;72:993–1000. doi: 10.1210/jcem-72-5-993. [DOI] [PubMed] [Google Scholar]
- Hild-Petito SA, Shiigi SM, Stouffer RL. Isolation and characterization of cell subpopulations from the monkey corpus luteum of the menstrual cycle. Biol Reprod. 1989;40:1075–1085. doi: 10.1095/biolreprod40.5.1075. [DOI] [PubMed] [Google Scholar]
- Hild-Petito SA, West NB, Brenner RM, Stouffer RL. Localization of androgen receptor in the follicle and corpus luteum of the primate ovary during the menstrual cycle. Biol Reprod. 1991;44:561–568. doi: 10.1095/biolreprod44.3.561. [DOI] [PubMed] [Google Scholar]
- Hoyer PB, Niswender GD. The regulation of steroidogenesis is different in the 2 types of ovine luteal cells. Can J Physiol Pharmacol. 1985;63:240–248. doi: 10.1139/y85-045. [DOI] [PubMed] [Google Scholar]
- Hoyer PB, Niswender GD. Adenosine 3',5' monophosphate binding capacity in small and large ovine luteal cells. Endocrinology. 1986;119:1822–1829. doi: 10.1210/endo-119-4-1822. [DOI] [PubMed] [Google Scholar]
- Hoyer PB, Kong W, Crichton EG, Bevan L, Krutzsch PH. Steroigogenic capacity and ultrastructural morphology of cultured oving luteal cells. Biol Reprod. 1988;38:909–920. doi: 10.1095/biolreprod38.4.909. [DOI] [PubMed] [Google Scholar]
- Hunter MG. Characteristics and causes of the inadequate corpus luteum. J Reprod Fertil Suppl. 1991;43:91–99. [PubMed] [Google Scholar]
- Hutchison JS, Zeleznik AJ. The rhesus monkey corpus luteum is dependent on pituitary gonadotropin secretion throughout the luteal phase of the menstrual cycle. Endocrinology. 1984;115:1780–1786. doi: 10.1210/endo-115-5-1780. [DOI] [PubMed] [Google Scholar]
- Hutchison JS, Nelson PB, Zeleznik AJ. Effects of different gonadotropin pulse frequencies on corpus luteum function during the menstrual cycle of rhesus monkeys. Endocrinology. 1986;119:1964–1971. doi: 10.1210/endo-119-5-1964. [DOI] [PubMed] [Google Scholar]
- Jabbour HN, Kelly RW, Fraser HM, Critchley HOD. Endocrine regulation of menstruation. Endocr Rev. 2006;27:17–46. doi: 10.1210/er.2004-0021. [DOI] [PubMed] [Google Scholar]
- Jocelyn H, Setchell BP. An annotated translation of Regnier deGraaf's new treatise concerning the generative organs of women (1672) J Reprod Fertil Suppl. 1972;17:77–206. [PubMed] [Google Scholar]
- Jones GS. Luteal phase defect. Fertil Steril. 1976;27:351–356. doi: 10.1016/s0015-0282(16)41769-3. [DOI] [PubMed] [Google Scholar]
- Juengel JL, Nett TM, Tandeski TR, Eckery DC, Sawyer HR, Niswender GD. Effects of LH and GH on luteal development in hypophysectomized ewes. Endocrine. 1995;3:323–326. doi: 10.1007/BF03021414. [DOI] [PubMed] [Google Scholar]
- Kaltenbach CC, Graber JW, Niswender GD, Nalbandov AV. Effect of hypophysectomy on formation and maintenance of corpora lutea in ewe. Endocrinology. 1968;82:753–759. doi: 10.1210/endo-82-4-753. [DOI] [PubMed] [Google Scholar]
- Kerin JF, Broom TJ, Ralph MM, Edmonds DK, Warnes GM, Jeffrey R, Crocker JM, Godfrey B, Cox LW, Seamark RF, Matthews CD. Human luteal phase function following oocyte aspiration from the immediately pre-ovular graafian follicle of spontaneous ovular cycles. Br J Obstet Gynaecol. 1981;88:1021–1028. doi: 10.1111/j.1471-0528.1981.tb01691.x. [DOI] [PubMed] [Google Scholar]
- Keyes PL, Wiltbank MC. Endocrine regulation of the corpus-luteum. Annu Rev Physiol. 1988;50:465–482. doi: 10.1146/annurev.ph.50.030188.002341. [DOI] [PubMed] [Google Scholar]
- Kindahl H, Edqvist LE, Granstrom E, Bane A. Release of prostaglandin-F2alpha as reflected by 15-Keto-13,14-Dihydroprostaglandin F2alpha in peripheral-circulation during normal luteolysis in heifers. Prostaglandins. 1976;11:871–878. doi: 10.1016/0090-6980(76)90194-5. [DOI] [PubMed] [Google Scholar]
- Knickerbocker JJ, Wiltbank MC, Niswender GD. Mechanisms of luteolysis in domestic livestock. Domest Anim Endocrinol. 1988;5:91–107. doi: 10.1016/0739-7240(88)90011-2. [DOI] [PubMed] [Google Scholar]
- Kreitmann O, Hodgen GD. Low tubal ovum transfer: an alternative to in vitro fertilization. Fertil Steril. 1980;34:375–378. doi: 10.1016/s0015-0282(16)45011-9. [DOI] [PubMed] [Google Scholar]
- Kreitmann O, Nixon WE, Hodgen GD. Induced corpus luteum dysfunction after aspiration of the preovulatory follicle in monkeys. Fertil Steril. 1981;35:671–675. doi: 10.1016/s0015-0282(16)45563-9. [DOI] [PubMed] [Google Scholar]
- Lenton EA, Landgren BM, Sexton L. Normal variation in the length of the luteal phase of the menstrual cycle - Indentification of the short luteal phase. Br J Obstet Gynaecol. 1984;91:685–689. doi: 10.1111/j.1471-0528.1984.tb04831.x. [DOI] [PubMed] [Google Scholar]
- Magnus V. Ovariets betydning for svangerskabet med soerligt hensyn til corpus luteum. Norsk Mag Loegevidensk. 1901;62:1138–1142. [Google Scholar]
- Malpighi M. Praeclarissimo & eruditissimo Viro D. Jocobo Sponsio Medicinae doctor, & Lugdenensi anatomicl Accuatatissimo. Philos Trans R Soc London. 1685;14:639–640. [Google Scholar]
- Mann GE, Lamming GE. Timing of prostaglandin F-2 alpha release episodes and oxytocin receptor development during luteolysis in the cow. Anim Reprod Sci. 2006;93:328–336. doi: 10.1016/j.anireprosci.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Mapletoft RJ, Delcampo MR, Ginther OJ. Local venoarterial pathway for uterine-induced luteolysis in cows. Proc Soc Exp Biol Med. 1976;153:289–294. doi: 10.3181/00379727-153-39530. [DOI] [PubMed] [Google Scholar]
- McCracken JA, Custer EE, Lamsa JC. Luteolysis: a neuroendocrine-mediated event. Physiol Rev. 1999;79:263–323. doi: 10.1152/physrev.1999.79.2.263. [DOI] [PubMed] [Google Scholar]
- McNeilly AS, Crow WJ, Fraser HM. Suppression of pulsatile LH secretion by GnRH antagonist does not affect episodic progesterone secretion or corpus luteum function in ewes. J Reprod Fertil. 1992;96:865–874. doi: 10.1530/jrf.0.0960865. [DOI] [PubMed] [Google Scholar]
- Meidan R, Girsh E, Blum O, Aberdam E. In vitro differentiation of bovine theca and granulosa cells into small and large luteal-like cells - Morphological and functional characteristics. Biol Reprod. 1990;43:913–921. doi: 10.1095/biolreprod43.6.913. [DOI] [PubMed] [Google Scholar]
- Meidan R, Levy N, Kisliouk T, Podlovny L, Rusiansky M, Klipper E. The yin and yang of corpus luteum-derived endothelial cells: balancing life and death. Domest Anim Endocrinol. 2005;29:318–328. doi: 10.1016/j.domaniend.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Milvae RA, Alila HW, Bushmich SL, Hansel W. Bovine corpus luteum function after removal of granulosa cells from the preovulatory follicle. Domest Anim Endocrinol. 1991;8:439–443. doi: 10.1016/0739-7240(91)90012-9. [DOI] [PubMed] [Google Scholar]
- Moor RM, Rowson LEA. Local maintenance of the corpus luteum in sheep with embryos transferred to various isolated portions of the uterus. J Reprod Fertil. 1966a;12:539–550. doi: 10.1530/jrf.0.0120539. [DOI] [PubMed] [Google Scholar]
- Moor RM, Rowson LEA. Local uterine mechanisms affecting luteal function in the sheep. J Reprod Fertil. 1966b;11:307–310. doi: 10.1530/jrf.0.0110307. [DOI] [PubMed] [Google Scholar]
- Morris D, Diskin M. Effect of progesterone on embryo survival. Animal. 2008;2:1112–1119. doi: 10.1017/S1751731108002474. [DOI] [PubMed] [Google Scholar]
- Moudgal NR. Corpus luteum of the nonhuman primate. Adv Vet Sci Comp Med. 1984;28:343–366. doi: 10.1016/b978-0-12-039228-5.50016-4. [DOI] [PubMed] [Google Scholar]
- Mussard ML, Burke CR, Behke EJ, Gasser CL, Day ML. Influence of premature induction of a luteinizing hormone surge with gonadotropin-releasing hormone on ovulation, luteal function, and fertility in cattle. J Anim Sci. 2007;85:937–943. doi: 10.2527/jas.2006-592. [DOI] [PubMed] [Google Scholar]
- Niswender GD, Juengel JL, McGuire WJ, Belfiore CJ, Wiltbank MC. Luteal function: the estrous-cycle and early-pregnancy. Biol Reprod. 1994;50:239–247. doi: 10.1095/biolreprod50.2.239. [DOI] [PubMed] [Google Scholar]
- Niswender GD, Juengel JL, Silva PJ, Rollyson MK, McIntush EW. Mechanisms controlling the function and life span of the corpus luteum. Physiol Rev. 2000;80:1–29. doi: 10.1152/physrev.2000.80.1.1. [DOI] [PubMed] [Google Scholar]
- O'Hara L, Scully S, Maillo V, Kelly AK, Duffy P, Carter F, Forde N, Rizos D, Lonergan P. Effect of follicular aspiration just before ovulation on corpus luteum characteristics, circulating progesterone concentrations and uterine receptivity in single-ovulating and superstimulated heifers. Reproduction. 2012;143:673–682. doi: 10.1530/REP-11-0505. [DOI] [PubMed] [Google Scholar]
- Ohara A, Mori T, Taii S, Ban C, Narimoto K. Functional differentiation in steroidogenesis of 2 types of luteal cells isolated from mature human corpora lutea of menstrual cycle. J Clin Endocrinol Metab. 1987;65:1192–1200. doi: 10.1210/jcem-65-6-1192. [DOI] [PubMed] [Google Scholar]
- Oshea JD, Rodgers RJ, Docchio MJ. Cellular composition of the cyclic corpus luteum of the cow. J Reprod Fertil. 1989;85:483–487. doi: 10.1530/jrf.0.0850483. [DOI] [PubMed] [Google Scholar]
- Oshea JD, Rodgers RJ, McCoy K, Docchio MJ. Ultrastructural cytology of the cyclic corpus luteum of the cow. Acta Anat. 1990;138:154–165. doi: 10.1159/000146933. [DOI] [PubMed] [Google Scholar]
- Parkinson TJ, Turvey A, Jenner LJ. A morphometric analysis of the corpus luteum of the cow during the estrous cycle and early pregnancy. Theriogenology. 1994;41:1115–1126. doi: 10.1016/s0093-691x(05)80034-2. [DOI] [PubMed] [Google Scholar]
- Pate JL. Cellular components involved in luteolysis. J Anim Sci. 1994;72:1884–1890. doi: 10.2527/1994.7271884x. [DOI] [PubMed] [Google Scholar]
- Pate JL. Involvement of immune cells in regulation of ovarian function. J Reprod Fertil Suppl. 1995;49:365–377. [PubMed] [Google Scholar]
- Pate JL, Keyes PL. Immune cells in the corpus luteum: friends or foes? Reproduction. 2001;122:665–676. doi: 10.1530/rep.0.1220665. [DOI] [PubMed] [Google Scholar]
- Peters KE, Bergfeld EG, Cupp AS, Kojima FN, Mariscal V, Sanchez T, Wehrman ME, Grotjan HE, Hamernik DL, Kittok RJ, Kinder JE. Luteinizing hormone has a role in development of fully functional corpora lutea (CL) but is not required to maintain CL function in heifers. Biol Reprod. 1994;51:1248–1254. doi: 10.1095/biolreprod51.6.1248. [DOI] [PubMed] [Google Scholar]
- Peters MW, Pursley JR. Timing of final GnRH of the ovsynch protocol affects ovulatory follicle size, subsequent luteal function, and fertility in dairy cows. Theriogenology. 2003;60:1197–1204. doi: 10.1016/s0093-691x(03)00120-1. [DOI] [PubMed] [Google Scholar]
- Prenant A. La valeur morphologique du corps jaune. son action physilogique et therapeutique possible. Rev Genet Sci Pures Appl. 1898;9:646–654. [Google Scholar]
- Retamales I, Carrasco I, Troncoso JL, Heras JL, Devoto L, Vega M. Morphofunctional study of human luteal cell subpopulations. Hum Reprod. 1994;9:591–596. doi: 10.1093/oxfordjournals.humrep.a138555. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Oshea JD. Purification, morphology, and progesterone production and content of 3 cell types isolated from the corpus luteum of the sheep. Aust J Biol Sci. 1982;35:441–455. [PubMed] [Google Scholar]
- Rodgers RJ, Oshea JD, Bruce NW. Quantitation of cells in the ovine corpus luteum. J Anat. 1983;136:645–646. [Google Scholar]
- Rodgers RJ, Oshea JD, Bruce NW. Morphometric analysis of the cellular composition of the ovine corpus luteum. J Anat. 1984;138:757–770. [PMC free article] [PubMed] [Google Scholar]
- Rothchild I. The regulation of the mammalian corpus luteum. Recent Prog Horm Res. 1981;37:183–298. doi: 10.1016/b978-0-12-571137-1.50009-8. [DOI] [PubMed] [Google Scholar]
- Sanders SL, Stouffer RL, Brannian JD. Androgen production by monkey luteal cell subpopulations at different stages of the menstrual cycle. J Clin Endocrinol Metab. 1996;81:591–596. doi: 10.1210/jcem.81.2.8636273. [DOI] [PubMed] [Google Scholar]
- Sanders SL, Stouffer RL. Localization of steroidogenic enzymes in macaque luteal tissue during the menstrual cycle and simulated early pregnancy: immunohistochemical evidence supporting the two-cell model for estrogen production in the primate corpus luteum. Biol Reprod. 1997;56:1077–1087. doi: 10.1095/biolreprod56.5.1077. [DOI] [PubMed] [Google Scholar]
- Sangsritavong S, Combs DK, Sartori R, Armentano LE, Wiltbank MC. High feed intake increases liver blood flow and metabolism of progesterone and estradiol-17 beta in dairy cattle. J Dairy Sci. 2002;85:2831–2842. doi: 10.3168/jds.S0022-0302(02)74370-1. [DOI] [PubMed] [Google Scholar]
- Sartori R, Rosa GJM, Wiltbank MC. Ovarian structures and circulating steroids in heifers and lactating cows in summer and lactating and dry cows in winter. J Dairy Sci. 2002;85:2813–2822. doi: 10.3168/jds.S0022-0302(02)74368-3. [DOI] [PubMed] [Google Scholar]
- Sartori R, Haughian JM, Shaver RD, Rosa GJM, Wiltbank MC. Comparison of ovarian function and circulating steroids in estrous cycles of Holstein heifers and lactating cows. J Dairy Sci. 2004;87:905–920. doi: 10.3168/jds.S0022-0302(04)73235-X. [DOI] [PubMed] [Google Scholar]
- Sawyer HR, Abel JH, McClellan MC, Schmitz M, Niswender GD. Secretory granules and progesterone secretion by ovine corpora lutea in vitro. Endocrinology. 1979;104:476–486. doi: 10.1210/endo-104-2-476. [DOI] [PubMed] [Google Scholar]
- Sawyer HR, Niswender KD, Braden TD, Niswender GD. Nuclear changes in ovine luteal cells in response to PGF2-alpha. Domest Anim Endocrinol. 1990;7:229–237. doi: 10.1016/0739-7240(90)90029-y. [DOI] [PubMed] [Google Scholar]
- Schams D, Berisha B. Regulation of corpus luteum function in cattle: an overview. Reprod Domest Anim. 2004;39:241–251. doi: 10.1111/j.1439-0531.2004.00509.x. [DOI] [PubMed] [Google Scholar]
- Shirasuna K, Asahi T, Sasaki M, Shimizu T, Miyamoto A. Distribution of arteriolovenous vessels, capillaries and enos expression in the bovine corpus luteum during the estrous cycle: a possible implication of different sensitivity by luteal phase to PGF(2 alpha) in the increase of luteal blood flow. J Reprod Dev. 2010;56:124–130. doi: 10.1262/jrd.09-106o. [DOI] [PubMed] [Google Scholar]
- Silva PJ, Juengel JL, Rollyson MK, Niswender GD. Prostaglandin metabolism in the ovine corpus luteum: catabolism of prostaglandin F-2 alpha (PGF(2 alpha)) coincides with resistance of the corpus luteum to PGF(2 alpha) Biol Reprod. 2000;63:1229–1236. doi: 10.1095/biolreprod63.5.1229. [DOI] [PubMed] [Google Scholar]
- Silvia WJ, Lewis GS, McCracken JA, Thatcher WW, Wilson L., Jr Hormonal regulation of uterine secretion of prostaglandin F2 alpha during luteolysis in ruminants. Biol Reprod. 1991;45:655–663. doi: 10.1095/biolreprod45.5.655. [DOI] [PubMed] [Google Scholar]
- Smith MF, McIntush EW, Smith GW. Mechanisms associate with corpus luteum development. J Anim Sci. 1994;72:1857–1872. doi: 10.2527/1994.7271857x. [DOI] [PubMed] [Google Scholar]
- Southee JA, Hunter MG, Law AS, Haresign W. Effect of hysterectomy on the short life cycle corpus luteum produced after GnRH-induced ovulation in the anestrous ewe. J Reprod Fertil. 1988;84:149–155. doi: 10.1530/jrf.0.0840149. [DOI] [PubMed] [Google Scholar]
- Stouffer RL. Progesterone as a mediator of gonadotrophin action in the corpus luteum: beyond steroidogenesis. Hum Reprod Update. 2003;9:99–117. doi: 10.1093/humupd/dmg016. [DOI] [PubMed] [Google Scholar]
- Summers PM, Wennink CJ, Hodges JK. Cloprostenol induced luteolysis in the marmoset monkey (Callithrix, Jacchus) J Reprod Fertil. 1985;73:133–138. doi: 10.1530/jrf.0.0730133. [DOI] [PubMed] [Google Scholar]
- Takasaki A, Tamura I, Kizuka F, Lee L, Maekawa R, Asada H, Taketani T, Tamura H, Shimamura K, Morioka H, Sugino N. Luteal blood flow in patients undergoing GnRH agonist long protocol. J Ovarian Res. 2011;4:2. doi: 10.1186/1757-2215-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torquato S, Stillinger FH. Jammed hard-particle packings: from Kepler to Bernal and beyond. Rev Mod Phys. 2010;82:2633–2672. [Google Scholar]
- Townson DH, Pate JL. Regulation of prostaglandin synthesis by interleukin-1-beta in cultured bovine luteal cells. Biol Reprod. 1994;51:480–485. doi: 10.1095/biolreprod51.3.480. [DOI] [PubMed] [Google Scholar]
- Tsai SJ, Wiltbank MC, Bodensteiner KJ. Distinct mechanisms regulate induction of messenger ribonucleic acid for prostaglandin (PG) G/H synthase-2, PGE (EP(3)) receptor, and PGF(2 alpha) receptor in bovine preovulatory follicles. Endocrinology. 1996;137:3348–3355. doi: 10.1210/endo.137.8.8754761. [DOI] [PubMed] [Google Scholar]
- Tsai SJ, Wiltbank MC. Prostaglandin F-2 alpha induces expression of prostaglandin G/H synthase-2 in the ovine corpus luteum: a potential positive feedback loop during luteolysis. Biol Reprod. 1997;57:1016–1022. doi: 10.1095/biolreprod57.5.1016. [DOI] [PubMed] [Google Scholar]
- Tsai SJ, Wu MH, Chuang PC, Chen HM. Distinct regulation of gene expression by prostaglandin F-2 alpha (PGF(2 alpha)) is associated with PGF(2 alpha) resistance or susceptibility in human granulosa-luteal cells. Mol Hum Reprod. 2001;7:415–423. doi: 10.1093/molehr/7.5.415. [DOI] [PubMed] [Google Scholar]
- Vargyas J, Kletzky O, Marrs RP. The effet of laparoscopic follicular aspiration on ovarian steroidogenesis during the early preimplantation period. Fertil Steril. 1986;45:221–225. doi: 10.1016/s0015-0282(16)49158-2. [DOI] [PubMed] [Google Scholar]
- Vasconcelos JLM, Sartori R, Oliveira HN, Guenther JG, Wiltbank MC. Reduction in size of the ovulatory follicle reduces subsequent luteal size and pregnancy rate. Theriogenology. 2001;56:307–314. doi: 10.1016/s0093-691x(01)00565-9. [DOI] [PubMed] [Google Scholar]
- Vasconcelos JLM, Sangsritavong S, Tsai SJ, Wiltbank MC. Acute reduction in serum progesterone concentrations after feed intake in dairy cows. Theriogenology. 2003;60:795–807. doi: 10.1016/s0093-691x(03)00102-x. [DOI] [PubMed] [Google Scholar]
- Webley GE, Hodges JK, Given A, Hearn JP. Comparison of the luteolytic action of GnRH antagonist and cloprostenol, and the ability of hCG and melatonin to override their luteolytic effects in the marmoset monkey. J Endocrinol. 1991;128:121–129. doi: 10.1677/joe.0.1280121. [DOI] [PubMed] [Google Scholar]
- Webley GE, Michael AE, Abayasekara DRE. The relationship between the production and the anti-gonadotrophic action of prostaglandin F(2 alpha) in luteal cells from the marmoset monkey (Callithrix jacchus) in the early and mid-luteal phase. Gen Comp Endocrinol. 2010;166:436–442. doi: 10.1016/j.ygcen.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Wiltbank JN, Casida LE. Alteration of ovarian activity by hysterectomy. J Anim Sci. 1956;15:134–140. [Google Scholar]
- Wiltbank MC, Dysko RC, Gallagher KP, Keyes PL. Relationship between blood-flow and steroidogenesis in the rabbit corpus-luteum. J Reprod Fertil. 1988;84:513–520. doi: 10.1530/jrf.0.0840513. [DOI] [PubMed] [Google Scholar]
- Wiltbank MC, Gallagher KP, Dysko RC, Keyes PL. Regulation of blood-flow to the rabbit corpus-luteum - effects of estradiol and human chorionic-gonadotropin. Endocrinology. 1989;124:605–611. doi: 10.1210/endo-124-2-605. [DOI] [PubMed] [Google Scholar]
- Wiltbank MC, Diskin MG, Flores JA, Niswender GD. Regulation of the corpus-luteum by protein Kinase-C.2. Inhibition of lipoprotein-stimulated steroidogenesis by prostaglandin-F2-alpha. Biol Reprod. 1990;42:239–245. doi: 10.1095/biolreprod42.2.239. [DOI] [PubMed] [Google Scholar]
- Wiltbank MC, Diskin MG, Niswender GD. Differential actions of 2nd messenger systems in the corpus-luteum. J Reprod Fertil. 1991;43:65–75. [PubMed] [Google Scholar]
- Wiltbank MC, Belfiore CJ, Niswender GD. Steroidogenic enzyme-activity after acute activation of protein-kinase (Pk) a and Pkc in ovine small and large luteal cells. Mol Cell Endocrinol. 1993;97:1–7. doi: 10.1016/0303-7207(93)90205-x. [DOI] [PubMed] [Google Scholar]
- Wiltbank MC. Cell-types and hormonal mechanisms associated with mid-cycle corpus-luteum function. J Anim Sci. 1994;72:1873–1883. doi: 10.2527/1994.7271873x. [DOI] [PubMed] [Google Scholar]
- Wiltbank MC, Ottobre JS. Regulation of intraluteal production of prostaglandins. Reprod Biol Endocrinol. 2003;1:91. doi: 10.1186/1477-7827-1-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltbank MC, Lopez H, Sartori R, Sangsritavong S, Gumen A. Changes in reproductive physiology of lactating dairy cows due to elevated steroid metabolism. Theriogenology. 2006;65:17–29. doi: 10.1016/j.theriogenology.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Wiltbank MC, Souza AH, Carvalho PD, Bender RW, Nascimento AB. Improving fertility to timed artificial insemination by manipulation of circulating progesterone concentrations in lactating dairy cattle. Reprod Fertil Dev. 2012;24:238–243. doi: 10.1071/RD11913. [DOI] [PubMed] [Google Scholar]
- Wulff C, Wilson H, Rudge JS, Wiegand SJ, Lunn SF, Fraser HM. Luteal angiogenesis: prevention and intervention by treatment with vascular endothelial growth factor Trap(A40) J Clin Endocrinol Metab. 2001;86:3377–3386. doi: 10.1210/jcem.86.7.7662. [DOI] [PubMed] [Google Scholar]
- Xu J, Stouffer RL, Searles RP, Hennebold JD. Discovery of LH-regulated genes in the primate corpus luteum. Mol Human Reprod. 2005;11:151–159. doi: 10.1093/molehr/gah157. [DOI] [PubMed] [Google Scholar]
- Zeleznik AJ. In vivo responses of the primate corpus luteum to luteinizing hormone and chorionic gonadotropin. Proc Natl Acad Sci USA. 1998;95:11002–11007. doi: 10.1073/pnas.95.18.11002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeleznik AJ, Somers JP. Regulation of the primate corpus luteum: cellular and molecular perspectives. Trends Endocrinol Metab. 1999;10:189–193. doi: 10.1016/s1043-2760(98)00145-3. [DOI] [PubMed] [Google Scholar]
- Zeleznik AJ. Modifications in gonadotropin signaling: a key to understanding cyclic ovarian function. J Soc Gynecol Invest. 2001;8:S24–S25. doi: 10.1016/s1071-5576(00)00101-5. (abstract) [DOI] [PubMed] [Google Scholar]