Abstract
The Muscular Dystrophy Surveillance Tracking and Research Network (MD STARnet) is a multisite collaboration to determine the prevalence of childhood-onset Duchenne/Becker muscular dystrophy and to characterize health care and health outcomes in this population. MD STARnet uses medical record abstraction to identify patients with Duchenne/Becker muscular dystrophy born January 1, 1982 or later who resided in one of the participating sites. Critical diagnostic elements of each abstracted record are reviewed independently by ≥4 clinicians and assigned to 1 of 6 case definition categories (definite, probable, possible, asymptomatic, female, not Duchenne/Becker muscular dystrophy) by consensus. As of November 2009, 815 potential cases were reviewed. Of the cases included in analysis, 674 (82%) were either “definite” or “probable” Duchenne/Becker muscular dystrophy. These data reflect a change in diagnostic testing, as case assignment based on genetic testing increased from 67% in the oldest cohort (born 1982–1987) to 94% in the cohort born 2004–2009.
Keywords: Duchenne muscular dystrophy, Becker muscular dystrophy, diagnostic testing, dystrophin
Introduction
Duchenne/Becker muscular dystrophy is the most common form of childhood-onset muscular dystrophy and is caused by deficiency or absence of dystrophin. The full-length dystrophin gene was sequenced in 19871 and has 79 exons and over 2.2 million base pairs. In 1988, the protein’s amino acid sequence was deduced from cDNA,2 and shortly thereafter, immunostaining showed that dystrophin is localized to the intracellular sarcolemma.3 Advances in understanding Duchenne/Becker muscular dystrophy have led to more specific and accurate diagnostic testing and improved accuracy of genetic counseling.
To date, few population-based longitudinal studies of Duchenne/Becker muscular dystrophy have been conducted. The MD STARnet is a collaboration of 6 US sites funded through cooperative agreements with the Centers for Disease Control and Prevention to identify and follow all Duchenne/Becker muscular dystrophy patients born January 1, 1982 or later in defined geographical areas. Information about diagnosis, disease course, and treatment is abstracted annually from medical records. Chart review data are supplemented by voluntary participation in telephone interviews or mailed surveys. In addition to determining population-based prevalence estimates, the MD STARnet cohort provides a unique opportunity to examine a number of questions about this chronic disease, including clinical care, management outcomes, access to health care, quality of life, and impact on the family. In this paper, we present the methods used by the MD STARnet to assign a case to a diagnostic category. We also report changes in clinical data used for case assignment over time.
Methods
The original sites for the MD STARnet included the states of Iowa, Colorado, and Arizona, and western New York State. The state of Georgia was added to the MD STARnet in 2005 and Hawaii in 2008. The objective of the MD STARnet is to identify all patients with Duchenne/Becker muscular dystrophy born on or after January 1, 1982 who ever resided in one of these geographic areas.
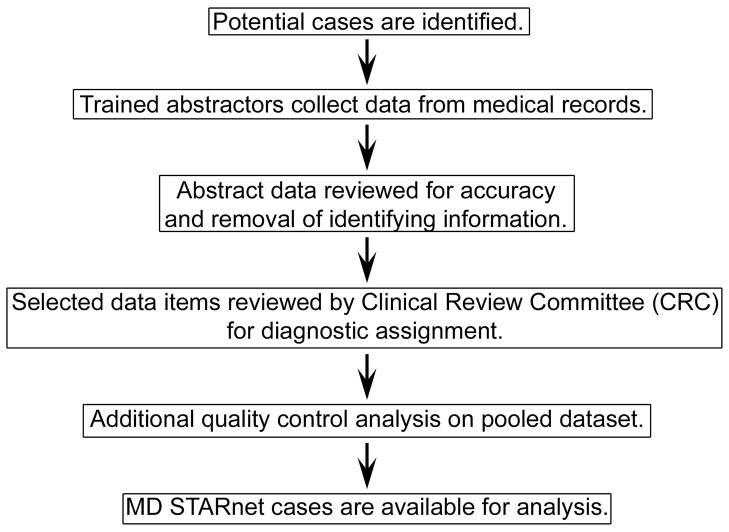
Details of the surveillance methods used by MD STARnet have been published4 and Figure 1 summarizes the approach to case review and data collection. Briefly, each participating site obtains permission for case finding and medical record abstraction either through institutional review board approval or by state-mandated public health reporting. Trained abstractors identify potential cases from multiple sources. Common sources of potential Duchenne/Becker muscular dystrophy cases include neuromuscular and outpatient neurology clinic records, hospital discharge databases (ICD9 code 359.1 diagnoses), hospital records, and self-report by families in response to advertisements. When a potential case is identified, an MD STARnet abstractor uses structured methods to record information from the medical records at one or more sites of care. At each MD STARnet site, abstracts are subjected to a computerized quality control check, and then reviewed for completeness. After local review, the abstracted data are collected centrally at the MD STARnet Data Coordination Center at the University of Iowa as individual, de-identified records.
Figure 1.
Summary of MD STARnet approach to case abstraction, review, and pooling.
At the Data Coordination Center, a subset of the abstracted elements for each potential case is sent monthly to the MD STARnet Clinical Review Committee for assignment of a case category. The Clinical Review Committee consists of a clinician from each site with experience in diagnosis and treatment of patients with muscular dystrophy. Data items used by the Clinical Review Committee are those critical to diagnosis of Duchenne/Becker muscular dystrophy, and include symptoms and age at onset, creatine kinase value, results of dystrophin mutation analysis testing, muscle biopsy reports, and family history. After review of these items, each Clinical Review Committee member independently assigns the case to one of 6 case categories. Table 1 provides details of the case category definitions. If all committee members agree on a case, this assignment is used in future analyses. If committee members are discordant in assigning a case category, the case is reviewed and discussed by at least 4 members. If consensus cannot be reached, the case is returned to the abstractor to find additional information. If no additional information is available, the case is assigned the lowest agreed-upon case category, with “definite” the highest level of confidence, followed by “probable,” “intermediate,” and “possible” as the lowest.
Table 1.
Case Definitions for MD STARnet
| Case | Definition |
|---|---|
| Definite | A “definite” case has documented clinical symptoms referable to a dystrophinopathy and direct support of the diagnosis by at least one of these criteria:
|
| Probable | A “probable” case has an elevated CK, a family history consistent with an X-linked muscular dystrophy, and documented clinical symptoms referable to a dystrophinopathy. Cases that have abnormal dystrophin results on muscle biopsy but lack the data required in the definition of ‘definite’ cases, above, are called “probable.” |
| Possible | A “possible” case has an elevated CK and documented clinical symptoms referable to a dystrophinopathy, but no muscle biopsy data, dystrophin mutation analysis, or family history to support the diagnosis. Cases that do not have CK information available in the clinical record, and who do not meet criteria for “definite” case, are called “possible.” |
| Asymptomatic | Asymptomatic individuals are those who have no clinical symptoms referable to a dystrophinopathy but who have laboratory results and/or a positive family history that will likely result in the development of a dystrophinopathy phenotype. |
| Female | Females are included in MD STARnet if they have onset of clinical symptoms referable to a dystrophinopathy appearing before age 21 years and either
|
| Not DBMD | Available information indicates case is definitely not Duchenne/Becker muscular dystrophy. |
Abbreviations: CK, creatine kinase
Medical record abstraction recurs annually. If new diagnostic information is found, the case returns to the Clinical Review Committee for review and possible reassignment of case status. For example, if a pathological dystrophin mutation is reported on a “possible” case, re-review would result in assignment as a “definite” case.
After the first 8 months of case reviews, Clinical Review Committee members became concerned that the diagnostic criteria for cases with case categorization based primarily on muscle biopsy were not sufficiently stringent, and that forms of limb girdle muscular dystrophy could meet the criteria for “definite” cases.5 The case definitions were revised and the current definitions shown in Table 1 were adopted in October 2005.
Presented here are the proportion of cases in each category tallied by birth year intervals and the diagnostic test used for the assignment of “definite” and “probable” cases. The present analysis uses data from all sites except Hawaii.
Results
As of November 2009, the MD STARnet Clinical Review Committee had reviewed 815 cases. Twelve lacked sufficient information for classification and 8 were classified as not Duchenne/Becker muscular dystrophy; these are excluded from analysis. The remaining 795 case category assignments were: 613 (77%) “definite,” 61 (8%) “probable,” 104 (13%) “possible,” 11 (1%) “asymptomatic,” and 6 (1%) “female.”
The Clinical Review Committee has reviewed 210 cases more than once, either for quality control (see below) or because new information was abstracted. Of the 815 unique cases reviewed, 340 (42%) have been non-consensus in at least one cycle of review, and assigned a case category by consensus.
After adoption of the current case category definitions in October 2005, cases were re-reviewed using the new criteria. The changes adopted in October 2005 only affected cases that were categorized based on muscle biopsy; therefore, the Data Coordination Center identified all previously reviewed “definite” or “probable” cases that had abnormal muscle biopsy results and normal or absent DNA testing. Seventy-three cases (69 “definite” and 4 “probable”) were identified and re-reviewed. Of these, 16 (21.9%) were assigned a different case category. Most cases (n = 15) were changed from “definite” to “probable,” with the remaining case changing from “probable” to “definite.”
To determine consistency of case category assignment, the Data Coordination Center randomly selected 24 cases (approximately 5% of reviewed cases at that time) for re-review in February 2006. The initial case assignments were deleted and the cases were submitted to the Clinical Review Committee along with that month’s new cases. Of the 24 cases, 22 (92%) were given the same case category on both reviews, and 2 were assigned a lower category on re-review. For one discordant case, the category assignment was based on the muscle biopsy results of a maternal relative. With the adoption of the more stringent category definition discussed above, the muscle biopsy report on the relative met criteria for a “probable” rather than “definite” case. The second discordant case was diagnosed prenatally in 1990. On initial review, the Clinical Review Committee accepted the prenatal diagnosis results as definitive and classified the case as “definite.” On re-review, committee members noted that the method used for prenatal diagnosis (mutation analysis vs. linkage) was unclear from the abstracted data; therefore, the case was re-classified from “definite” to “probable.”
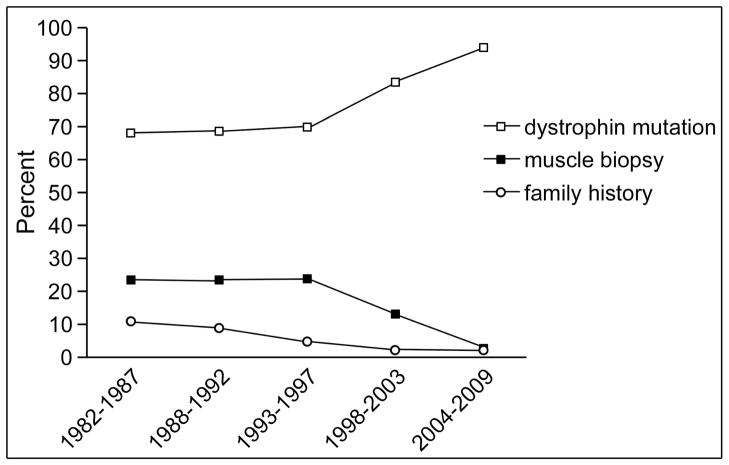
Across birth intervals, the proportion of cases assigned as “definite” increased from 63% to 86% (Table 2). Most of this increase occurred in birth cohorts from 1982–1992. We also determined which clinical data were used to assign a case as “definite” or “probable” by year of birth. The percentage of cases assigned to “definite” or “probable” based on dystrophin mutation in the proband or a family member increased steadily, from 67% of the oldest 5-year cohort, born 1982–1987, to 94% in the youngest cohort, born 2004–2009 (Figure 2). It is important to note that this does not reflect the method by which the diagnosis was originally made, but reflects the percentage of the population who had a mutation identified at the time of case review by the Clinical Review Committee.
Table 2.
Case Assignment by Birth Year Intervals
| Definite (%) | Probable (%) | Possible (%) | Asymptomatic (%) | Female (%) | |
|---|---|---|---|---|---|
| 1982–1987 | 120 (63) | 22 (12) | 41 (22) | 4 (2) | 2 (1) |
| 1988–1992 | 177 (75) | 22 (9) | 35 (15) | 0 (0) | 3 (1) |
| 1993–1997 | 157 (86) | 9 (5) | 16 (9) | 1 (1) | 0 (0) |
| 1998–2003 | 129 (85) | 7 (5) | 11 (7) | 3 (2) | 1 (1) |
| 2004–2009 | 30 (86) | 1 (3) | 1 (3) | 3 (9) | 0 (0) |
| All years (795) | 613 (77) | 61 (8) | 104 (13) | 11 (1) | 6 (1) |
Figure 2.
Data used for case assignment by birth year intervals. The diagnostic test (on either proband or a maternal family member) used to assign diagnostic classification for ‘definite’ and “probable” cases was identified through review of Clinical Review Committee reports. The graph shows change in the percent of cases assigned by dystrophin mutation, muscle biopsy, or X-linked family history in 5-year intervals. There is a steady increase in percentage of cases with dystrophin mutations, particularly for those born after 1997 (predicted diagnosis in 2002).
Discussion
The MD STARnet dataset is a unique resource for determining prevalence of and collecting population-based data for patients with Duchenne/Becker muscular dystrophy.6 It is important to be rigorous in the definitions used to classify cases for the MD STARnet so that the utility of this data set will stand the test of time. Diagnostic criteria for the diagnosis of Duchenne and Becker muscular dystrophy have been published,7 and we adapted these for case assignment based on retrospective chart review. We believe that the MD STARnet case definitions along with the review protocols described here allow clear understanding of the population being studied. We expect that most clinical analyses using the MD STARnet dataset will use cases assigned to the “definite” and “probable” categories.
The data reported here illustrate that MD STARnet data can demonstrate changes in clinical practice, reflecting technological advances in genetic testing. Similar results and patterns were seen in a detailed analysis of mutations reported in the MD STARnet population.8 Deletion/duplication testing began in 19889 and sequencing for point mutations was first commercially available in 2001.10 Among “definite” cases in the MD STARnet population, case assignment was based in genetic test results (in proband or a family member) in 67% of cases born 1982–1997 (corresponding to diagnosis before ~2002, assuming diagnosis at age 5 years). This is consistent with the published data showing that 60–70% of Duchenne/Becker muscular dystrophy patients have a deletion or duplication.11 The MD STARnet data show a clear increase in the proportion of the population for whom genetic test results were available, and a parallel decline in muscle-biopsy-based diagnosis for the cohorts born after 1998 (corresponding to diagnosis after 2003). Although the youngest cohort reported here is small, 94% of patients have a genetic diagnosis, again consistent with other reports that 90% to 95% of Duchenne/Becker muscular dystrophy patients have a mutation detectable with current genetic testing.11
The importance of the MD STARnet dataset extends beyond the chart abstraction used in the data presented here. In addition to the annual medical record review, “definite” and “probable” cases are invited to participate in voluntary interviews and surveys to explore aspects of disease that cannot be studied through medical record abstraction. Conducting research studies with the unique MD STARnet population, for which the total potential population is defined by surveillance, allows evaluation of the impact of any selection bias in the voluntary research cohorts.
MD STARnet data can be used to identify gaps in medical care. For example, Ciafaloni, et al, used the MD STARnet dataset to study the time course from earliest symptoms to diagnosis of muscular dystrophy.12 Cases were limited to those with onset of symptoms before age 7 years who were not known to have a family history of Duchenne muscular dystrophy at the time of diagnosis. There were approximately 2.5 years between first symptoms and a diagnosis of Duchenne muscular dystrophy. Parents did not report concerns to medical professionals for approximately one year. There was another delay of approximately one year in response of medical professionals to parental concerns. This important information has resulted in formation of a task force to raise awareness of the need for early identification and diagnostic testing for children with signs of neuromuscular disease (The National Task Force for Early Identification of Childhood Neuromuscular Disorders).
In summary, the MD STARnet has compiled a multiple-source, population-based dataset for the study of Duchenne/Becker muscular dystrophy. The case classification definitions and procedures developed by the MD STARnet has produced a well-characterized cohort that forms the basis for future analyses of health care and health outcomes for individuals with Duchenne/Becker muscular dystrophy. In addition, employment of the clinical review process in MD STARnet will provide experience of benefit to future surveillance systems that focus on specific genetic conditions or birth defects.
Acknowledgments
Work was carried out in each of the MD STARnet sites, with writing completed at the University of Iowa. The study was funded by the Centers for Disease Control and Prevention under a Cooperative Agreement for Surveillance and Epidemiologic Research of Duchenne and Becker Muscular Dystrophy (U01 DD000189). It was presented in part at the Neurobiology of Disease in Children Symposium: Muscular Dystrophy, in conjunction with the 38th Annual Meeting of the Child Neurology Society, Louisville, Kentucky, October 14, 2009 supported by grants from the National Institutes of Health (5R13NS040925-09), the National Institutes of Health Office of Rare Diseases Research, the Muscular Dystrophy Association, and the Child Neurology Society. The authors thank Melanie Fridl Ross, MSJ, ELS, for editing this manuscript. The authors also acknowledge with appreciation the work of the abstractors, local reviewers, data managers and other MD STARnet personnel, without whom this work would not be possible.
References
- 1.Koenig M, Hoffman EP, Bertelson CJ, et al. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987;50:509–517. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- 2.Koenig M, Monaco AP, Kunkel LM. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988;53:219–228. doi: 10.1016/0092-8674(88)90383-2. [DOI] [PubMed] [Google Scholar]
- 3.Bonilla E, Samitt CE, Miranda AF, et al. Duchenne muscular dystrophy: deficiency of dystrophin at the muscle cell surface. Cell. 1988;54:447–452. doi: 10.1016/0092-8674(88)90065-7. [DOI] [PubMed] [Google Scholar]
- 4.Miller LA, Romitti PA, Cunniff C, et al. The muscular Dystrophy Surveillance Tracking and Research Network (MD STARnet): surveillance methodology. Birth Defects Res A Clin Mol Teratol. 2006;76:793–797. doi: 10.1002/bdra.20279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz M, Hertz JM, Sveen ML, Vissing J. LGMD2I presenting with a characteristic Duchenne or Becker muscular dystrophy phenotype. Neurology. 2005;64:1635–1637. doi: 10.1212/01.WNL.0000157654.59374.E5. [DOI] [PubMed] [Google Scholar]
- 6.Prevalence of Duchenne/Becker muscular dystrophy among males aged 5–24 years - four states, 2007. MMWR Morb Mortal Wkly Rep. 2009;58:1119–22. [PubMed] [Google Scholar]
- 7.Jennekens FG, ten Kate LP, de Visser M, Wintzen AR. Diagnostic criteria for Duchenne and Becker muscular dystrophy and myotonic dystrophy. Neuromuscul Disord. 1991;1:389–391. doi: 10.1016/0960-8966(91)90001-9. [DOI] [PubMed] [Google Scholar]
- 8.Cunniff C, Andrews J, Meaney FJ, et al. Mutation analysis in a population-based cohort of boys with Duchenne or Becker muscular dystrophy. J Child Neurol. 2009;24:425–430. doi: 10.1177/0883073808324770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chamberlain JS, Gibbs RA, Ranier JE, et al. Deletion screening of the Duchenne muscular dystrophy locus via multiplex DNA amplification. Nucleic Acids Res. 1988;16:11141–11156. doi: 10.1093/nar/16.23.11141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendell JR, Buzin CH, Feng J, et al. Diagnosis of Duchenne dystrophy by enhanced detection of small mutations. Neurology. 2001;57:645–650. doi: 10.1212/wnl.57.4.645. [DOI] [PubMed] [Google Scholar]
- 11.Dent KM, Dunn DM, von Niederhausern AC, et al. Improved molecular diagnosis of dystrophinopathies in an unselected clinical cohort. Am J Med Genet A. 2005;134:295–298. doi: 10.1002/ajmg.a.30617. [DOI] [PubMed] [Google Scholar]
- 12.Ciafaloni E, Fox DJ, Pandya S, et al. Delayed diagnosis in duchenne muscular dystrophy: data from the Muscular Dystrophy Surveillance, Tracking, and Research Network (MD STARnet) J Pediatr. 2009;155:380–385. doi: 10.1016/j.jpeds.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]