Abstract
Aims
The ability of dietary enrichment with monounsaturated (MUFA), n-3, or n-6 polyunsaturated fatty acids (PUFA) to reverse glucose intolerance and vascular dysfunction resulting from excessive dietary saturated fatty acids is not resolved. We hypothesized that partial replacement of dietary saturated fats with n-3 PUFA enriched menhaden oil (MO) would provide greater improvement in glucose tolerance and vascular function compared to n-6 enriched safflower oil (SO) or MUFA-enriched olive oil (OO).
Material and Methods
We fed mice a high saturated fat diet (60% kcal from lard) for 12 weeks before substituting half the lard with MO, SO or OO for an additional 4 weeks. At the end of 4 weeks, we assessed glucose tolerance, insulin signaling and reactivity of isolated pressurized gracilis arteries.
Results
After 12 weeks of saturated fat diet, body weights were elevated and glucose tolerance abnormal compared to mice on control diet (13% kcal lard). Diet substituted with MO restored basal glucose levels, glucose tolerance, and indices of insulin signaling (phosphorylated Akt) to normal whereas restoration was limited for SO and OO substitutions. Although dilation to acetylcholine was reduced in arteries from mice on HF, OO and SO diets compared to normal diet, dilation to acetylcholine was fully restored and constriction to phenylephrine reduced in MO fed mice compared to normal.
Conclusion
We conclude that short term enrichment of an ongoing high fat diet with n-3 PUFA rich MO but not MUFA rich OO or n-6 PUFA rich SO reverses glucose tolerance, insulin signaling, and vascular dysfunction.
Introduction
Excess body weight and obesity are major risk factors for a multitude of cardiovascular diseases. Although there is agreement that reducing total dietary saturated fats is beneficial, the optimal nutrient replacement for saturated fats (mono- or polyunsaturated fats) is unclear (1). While consumption of monounsaturated (MUFA) and/or poly-unsaturated fatty acids (PUFA) improves cholesterol levels and insulin sensitivity compared to diets high in saturated fats, the effects of dietary substitutes on cardio-vascular endpoints are not resolved. Randomized controlled trials have not consistently demonstrated benefits of PUFAs and MUFAs in prevention or reversal of cardiovascular disease and intake of n-6 PUFAs alone without a concomitant increase in n-3 PUFAs not only fails to reduce cardiovascular risk, but may increase risk (2, 3). Although selected individuals may benefit from replacement of dietary saturated fats with MUFAs, there is increasing evidence that PUFAs are a better substitute since they improve lipid metabolism and insulin sensitivity (4, 5).
Long chain PUFAs are subcategorized into omega-3 (n-3) and omega-6 (n-6) fatty acids based on the position of the double bond nearest the methyl end of the acyl chain. Although both n-3 and n-6 PUFAs are precursors of eicosanoids that serve as signaling molecules, the major eicosanoid metabolites and their functional endpoints differs. Eicosanoids derived from n-6 PUFAs promote inflammation and development of atherosclerosis whereas eicosanoids derived from n-3 PUFAs are anti-inflammatory and protect against atherosclerosis (6, 7). Differences in study designs, experimental models and endpoints complicate decisions for the optimal dietary composition to reduce cardiovascular risk. A direct comparison of diets enriched in n-3 and n-6 PUFAs versus MUFAs on glucose metabolism and vascular reactivity has not been reported.
To date few studies have addressed the ability of dietary fatty acids to reverse established glucose intolerance and vascular dysfunction. The majority of studies have assessed dietary fatty acids in a preventative role but the ability to reverse existing metabolic dysfunction may be more clinically relevant. The mouse model of diet-induced obesity allows us to address the ability of fatty acid composition to reverse the effects of a high saturated fat diet. Mice on diets enriched in saturated fats (45–60%) exhibit many of the elements of the metabolic syndrome including obesity, altered glucose utilization, and insulin resistance, all preludes to development of type 2 diabetes (8–11). For these studies, we utilized C57BL/6 mice on a diabetogenic diet to examine the ability of specific dietary fatty acid compositions to reverse the effects of a high saturated fat diet on glucose tolerance and vascular function. We hypothesized that a diet enriched in n-3 PUFAs rather than n-6 PUFAs or MUFA provides the optimal substitute for reversing the effects of high saturated fat diet on glucose utilization and vascular dysfunction.
Experimental Procedures
Animal model
Animal protocols were approved by the Animal Care and Use Committee of the Iowa City Veterans Affairs Health Care System and complied with the “Guiding Principles for Research Involving Animals and Human Beings.” Male C57BL6/J mice (12 weeks) obtained from Jackson Laboratories were fed a normal mouse diet (Control, Con, 13% kcal fat, Teklab, 7001) or high saturated fat diet (HF, lard, 60% kcal fat, Research Diets, D12492) for 12 weeks. At 12 weeks, half of the kcal derived from lard was replaced with menhaden oil (MO, enriched in n-3 PUFA, Research Diets, New Brunswick, NJ, D10122003), safflower oil (SO, enriched in n-6 PUFA, Research Diets, D10122002), or olive oil (OO, enriched in MUFA, Research Diets, D10122001) for an additional 4 weeks (diet composition, Supplement, Table 1). Protein and carbohydrate levels were lower in all high fat diets compared to the control diet but equivalent in all high fat diets. Prior to terminal study glucose tolerance tests were performed. For terminal study, mice were euthanized with sodium pentobarbital (150 mg/kg ip), weighed and tissues and serum were obtained for measurement of epididymal fat pad mass, vascular reactivity, protein expression, and lipid analyses. Total free fatty acids (FFA), cholesterol (CHO), and triglycerides were measured using a colorimetric assay (Roche Diagnostics, Mannheim, Germany; Bio Vision, Mountain View, CA, ALPCO Diagnostics, Salem, NH, respectively). Levels of leptin and insulin were measured using an antibody based detection approach and a Luminex system (12). Liver samples were embedded in Tissue-Tek O.C.T. compound (Sakura Finetek, Torrance, CA), sectioned (10 µm thickness) and stained with oil red O to determine area of triglyceride and lipid deposition by NIH Image.
Glucose Tolerance Test
Glucose tolerance tests were performed in mice after 12 weeks of high fat diet only and after the additional 4 weeks of modified diets. Prior to terminal study, glucose tolerance tests were performed on conscious mice following an overnight fast. Blood was collected from the tail prior to and following injection of glucose (2 g/kg ip, 15, 30, 45, 60, 90 and 120 minutes). Glucose levels were measured using glucose oxidase test strips and a hand-held Glucometer (Lifescan Inc., Milpitas, CA).
Western immunoblotting
As an index of insulin activation, levels of phosphorylated Akt were measured in soleus and EDL from separate groups of mice 15 minutes after injection of insulin (5 units) into the inferior vena cava during terminal pentobarbital anesthesia. Soleus and EDL were homogenized on ice in buffer (CelLytic M, Sigma-Aldrich) containing protease and phosphatase inhibitors. Equal amounts of lysate protein were separated by SDS PAGE and probed for total Akt and Ser473 phosphorylated Akt (Cell Signaling Technology, Danvers, MA, #9272 and 9271). Membranes were incubated with secondary HRP-conjugated antibodies and immuno-reactivity was visualized with enhanced chemiluminescence. Blots were digitized and phosphorylated Akt was normalized to total Akt (NIH Image).
Serum and tissue lipid analysis
At the terminal study, liver and EDL samples were obtained to analyze fatty acid composition using gas-liquid chromatography (13, 14). Lipids were extracted from tissues with a 2:1 (vol/vol) mixture of chloroform and methanol followed by phase separation with a solution of 154 mM NaCl and 4 mM HCl. Fatty acid composition of liver and EDL were measured after the lipid fraction was transesterified in 14% boron trifluoride in methanol and the fatty acid methyl esters extracted into heptane before separation by gas-liquid chromatography (13, 14). Individual fatty acids peaks as % of total fatty acids were identified by comparison to known fatty acid standards.
Isolated microvascular reactivity
The gracilis muscle was removed and placed in ice cold Krebs buffer (mmol/L: NaCl 118, KCl 4.7, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25, glucose 11). Gracilis arteries (~110–180 µm in diameter) were isolated, cannulated onto glass micropipettes filled with Krebs in an organ chamber and secured with suture. Warmed (37°C), oxygenated (20% O2, 5% CO2, and 75% N2) Krebs buffer was continuously circulated through the chamber. Vessels were pressurized to 60 mmHg and equilibrated for 45–60 min prior to study. Vessel images were projected on a video monitor using a digital camera and lumen diameter measured with an electronic dimension analyzer. Artery segments were submaximally contracted (50–60% of maximal) with thromboxane mimetic, U46619 (0.05–0.1 µg/ml) before responses to ACh (01 nM to 10 µM) and SNP (1 nM to 10 µM) were measured. Constriction to KCl (25–75 mM) and phenylephrine (1 nM to 10 µM) were measured.
Statistical analysis
Data are presented as mean ±SEM. In all data, ‘n’ represents numbers of mice per group. Responses of a single artery from each animal were measured. Functional responses of arteries were compared by repeated measures analysis of variance followed by Bonferroni. Other data were compared using ANOVA with Tukey’s HSD or unpaired Student’s t test using GraphPad Prism. Significance was defined as p<0.05.
Results
Effect of 12 weeks high saturated fat diet on body and fat weights and glucose homeostasis
Body weight and epididymal fat pad mass were greater in mice on high saturated fat diet for 12 weeks compared to age-matched mice on normal fat diet (body weight: 45.3 ± 1.9 g* versus 33.8 ± 0.7 g; epididymal fat: 872 ± 91 mg* versus 341 ± 55 mg, n=9 per group, *p<0.05 versus normal diet). Although fasting basal blood glucose of mice on high fat diet was not significantly different from mice on normal diet (normal diet 6.88 ± 0.28 mmol/L versus high fat 8.13 ± 0.33 mmol/L), following a bolus injection, clearance of blood glucose was significantly impaired in these mice compared to mice on normal diet (Supplement, Figure 1).
Effect of substitution of saturated fats with MUFA and PUFAs on body weight and adipose tissue mass
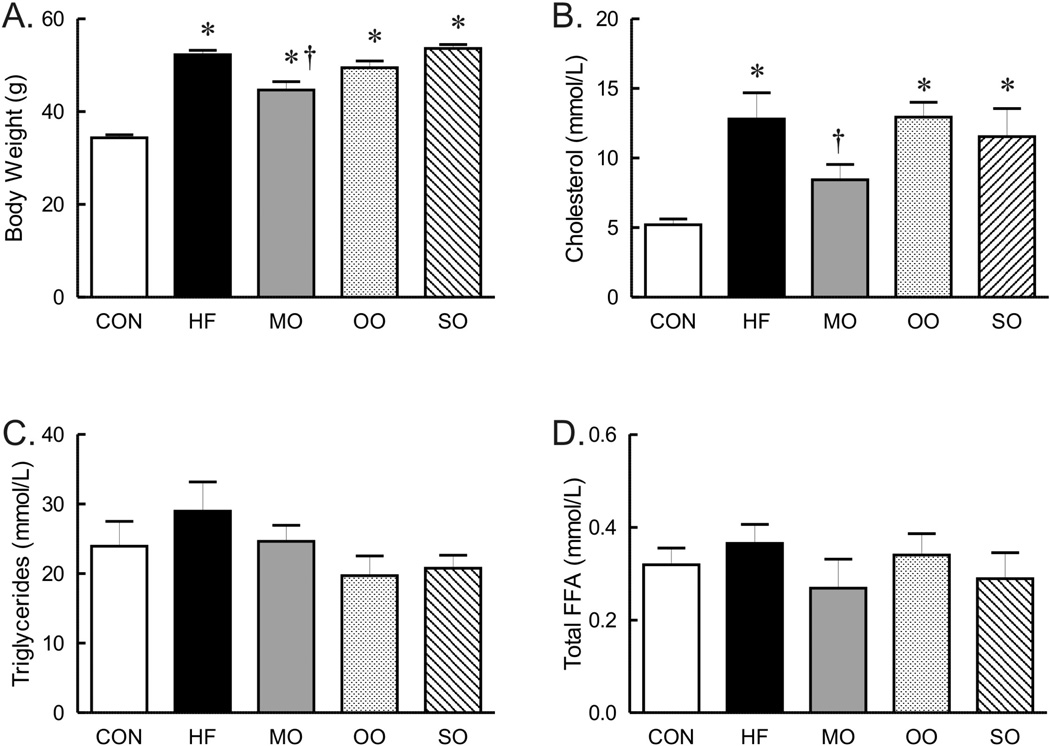
After 12 weeks of high saturated fat diet, half of the saturated fats in the diet was replaced with n-3 enriched menhaden oil (MO), n-6 enriched safflower oil (SO) or monounsaturated fat n-9 enriched olive oil (OO) for an additional 4–8 weeks. During this short term substitution of dietary fats, all mice on high fat diets continued to gain more weight compared to the control fat diet (Figure 1A). The weight of mice fed diets substituted with olive oil or safflower oil was similar to the weight of mice fed the high saturated fat diet whereas weight of mice on menhaden oil was less than mice on saturated fats only (Figure 1A). However, it should be noted that body weight of mice on menhaden oil was not different from mice on olive oil but was significantly less than mice on safflower oil (Figure 1A). The effect of diet on white epididymal adipose tissue mass only partially correlated with body weight. Epididymal fat pad mass was significantly greater in mice on high fat and menhaden oil diets but not in mice on olive oil or safflower substituted diets (control 478 ± 70 mg, HF 799 ± 41 mg*, MO 933 ±149 g*, OO 674 ± 44 mg, SO 737 ± 51 mg, *p<0.05 vs control). Thus, dietary fat composition greatly modulated weight gain and epididymal adipose tissue mass, but not in a congruent fashion.
Figure 1.
Body weight (A, n=17 per group except olive oil with n=14) and serum cholesterol (B), triglycerides (C) and total free fatty acids (Total FFA, C, n=6–8 per group) in mice on control (CON), high saturated fat (HF), and menhaden oil (MO), olive oil, and safflower oil (SO) substituted diets. * p < 0.05 versus control diet, † p < 0.05 versus HF.
Effect of diet on serum and tissue free fatty acids
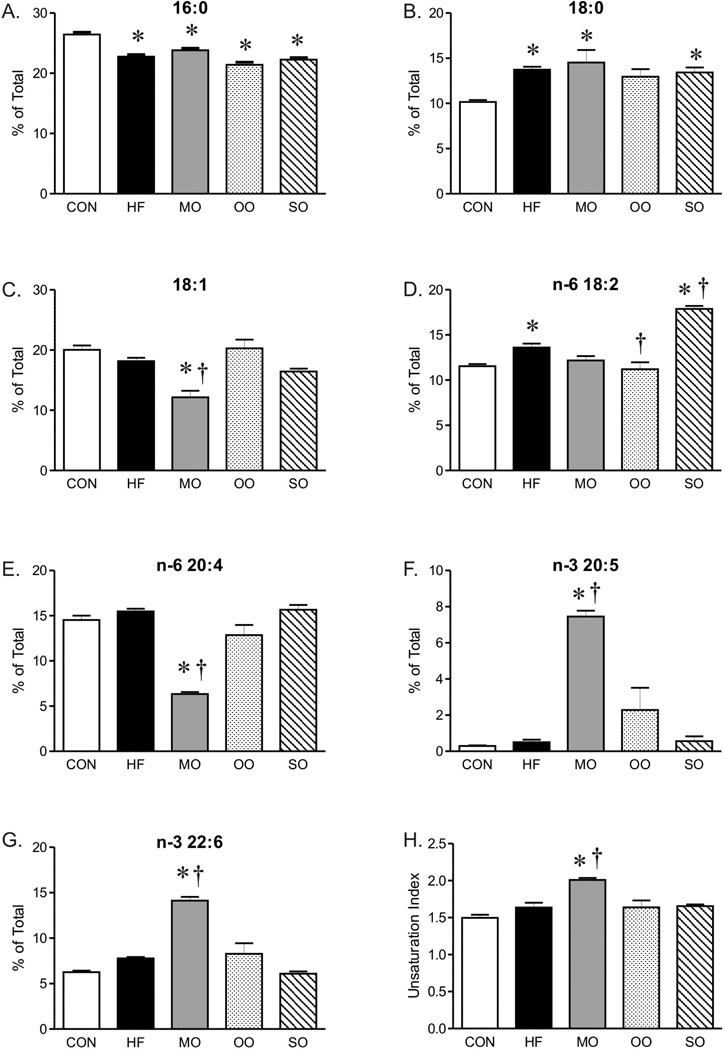
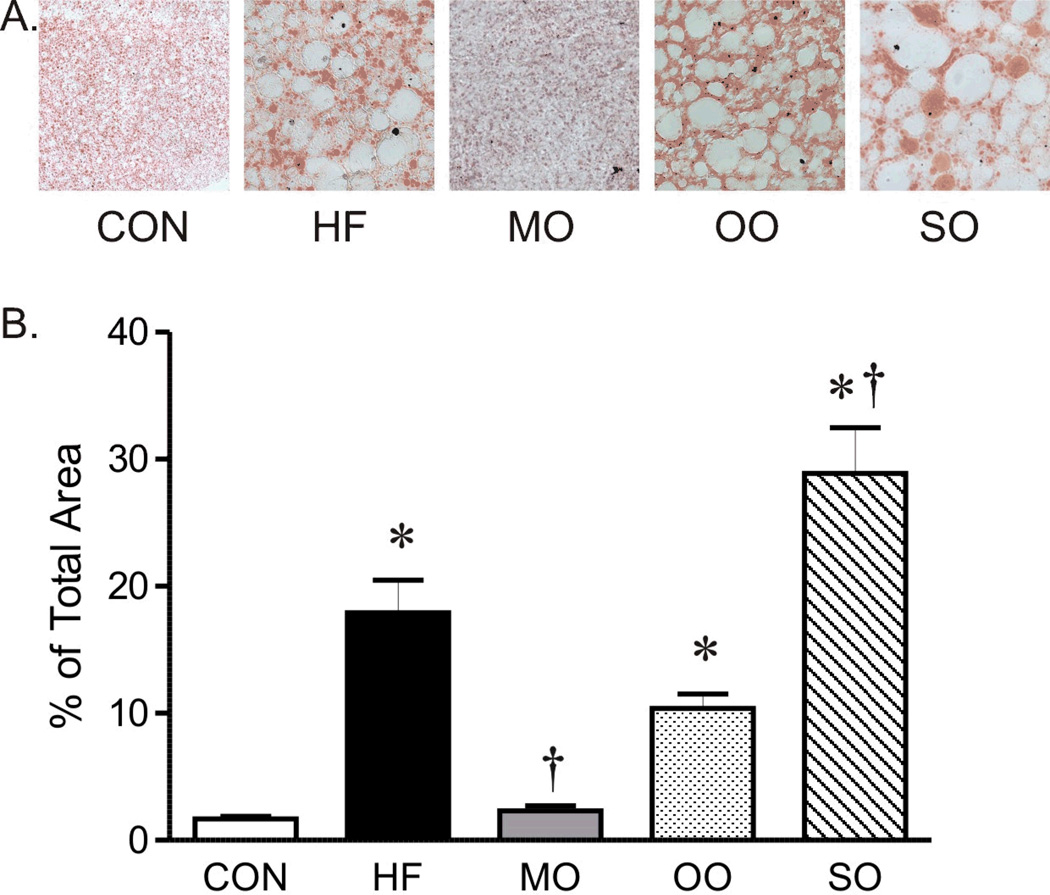
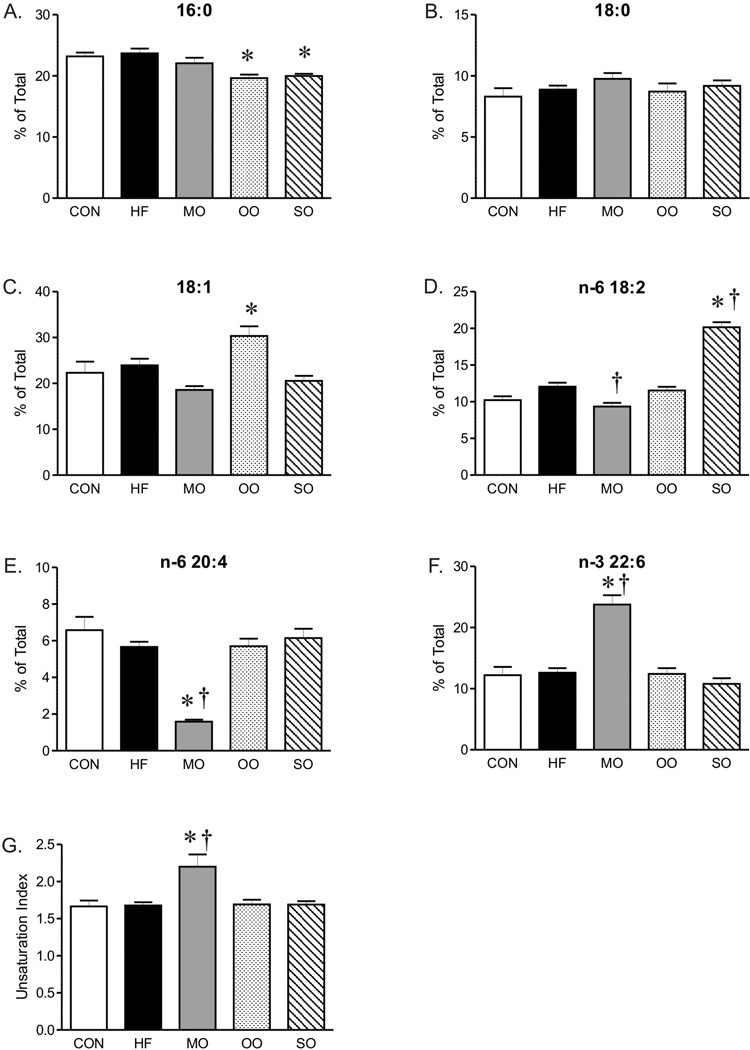
The serum cholesterol of mice on high saturated fat diet was almost three fold greater than mice on normal diet without a significant effect on triglyceride or total fatty acid composition (Figure 1B, C and D). Although total serum fatty acid composition did not change, the fatty acid composition of liver was affected by the diet high in saturated fats. Analysis of diets by gas chromatography showed similar fatty acid composition (as % of total) in control and high saturated fat diets (Supplement, Table 2), however, the fatty acid composition of livers of mice on high saturated fat diet was shifted to higher levels of 18-carbon fatty acids (18:0 and 18:2) while levels of 16:0 saturated fat decreased (Figure 2). The unsaturation index of livers was not affected by the high saturated fat diet (Figure 2H). This change in liver fatty acid content was accompanied by a marked increase in hepatic steatosis within livers of mice on saturated fat diet compared to mice on normal diet (Figure 3), although indices of liver dysfunction were not elevated (aspartate and aspartate transaminase, data not shown). In contrast to liver, the diet high in saturated fats had no effect on fatty acid composition of EDL (Figure 4).
Figure 2.
Fatty acid composition (% of Total) and unsaturation index of liver from mice on control (CON), high fat (HF), and menhaden oil (MO), olive oil (OO) and safflower oil (SO) substituted diets. Mean ± SEM; * p < 0.05 versus CON; † p < 0.05 versus HF, n=7–8 per group.
Figure 3.
Representative section of oil red O staining (40X, A) and area of oil red O staining (% of total area, B) of liver from mice on control diet (CON), high saturated fat diet (HF), and menhaden oil (MO), olive oil (OO), and safflower oil (SO) substituted diets. Mean ± SEM, * p < 0.05 versus CON, † p < 0.05 versus HF, n=7–8 per group.
Figure 4.
Fatty acid composition (% of Total) and unsaturation index of EDL from mice on control (CON), high fat (HF), and menhaden oil (MO), olive oil (OO) and safflower oil (SO) substituted diets. Levels of 20:5 were below detectable limits in EDL. Mean ± SEM; * p < 0.05 versus CON; † p < 0.05 versus HF, n=6–7.
Similar to mice on high saturated fat diet, triglycerides and total fatty acid content were not different in mice on diets substituted with menhaden oil, olive oil, or safflower oil compared to normal chow (Figure 1C and D). Substitution of just half of the saturated fat with menhaden oil decreased cholesterol levels compared to mice on the diet with saturated fats alone (Figure 1B). The diet enriched in menhaden oil was higher in EPA (20:5 n-3 PUFA) and DHA (22:6 n-3 PUFA) (Supplement, Table 2) which significantly impacted fatty acid composition of both liver and EDL of mice on the diet. Both liver and EDL of mice on menhaden oil had significantly higher levels of n-3 DHA (22:6, Figures 2G and 4F) but only liver had appreciable increases in n-3 EPA (20:5, Figure 2F). Levels of n-3 EPA were low in EDL of all groups (data not shown). Levels of 18:1 and 20:4 n-6 were lower in liver only of mice on menhaden oil diet (Figure 2C and E). These changes in liver and EDL fatty acid composition resulted in an increased unsaturation index (Figures 2H and 4G) and a marked reduction in hepatic steatosis in mice on menhaden oil enriched diet (Figure 3). The area of lipid vacuoles within the liver was fully restored to normal after short term substitution of saturated fatty acid with menhaden oil (Figure 3).
In contrast to menhaden oil, substitution of saturated fats with MUFA enriched olive oil or n-6 PUFA enriched safflower oil had no effect on the increased cholesterol levels (Figure 1B). The olive oil substituted diet which is higher in oleic acid MUFA (18:1, Supplement, Table 2), decreased liver but not EDL 18:2 compared to high saturated fat diet alone (Figures 2D and 4D). The safflower oil substituted diet, enriched in n-6 linoleic acid (18:2, Supplement Table 2), increased liver and EDL linoleic acid composition (18:2, Figures 2D and 4D) but did not increase arachidonic acid levels (20:4). Neither olive oil nor safflower oil significantly affected the unsaturation index of liver or EDL (Figures 2H and 4G) nor did they reverse the hepatic steatosis found in animals on the high saturated fat diet (Figure 3). In fact, safflower oil increased hepatic steatosis (Figure 3B). Thus, only menhaden oil substitution improved the abnormalities in fatty acid deposition in liver resulting from a high saturated fat diet.
Effects of dietary fats on glucose tolerance
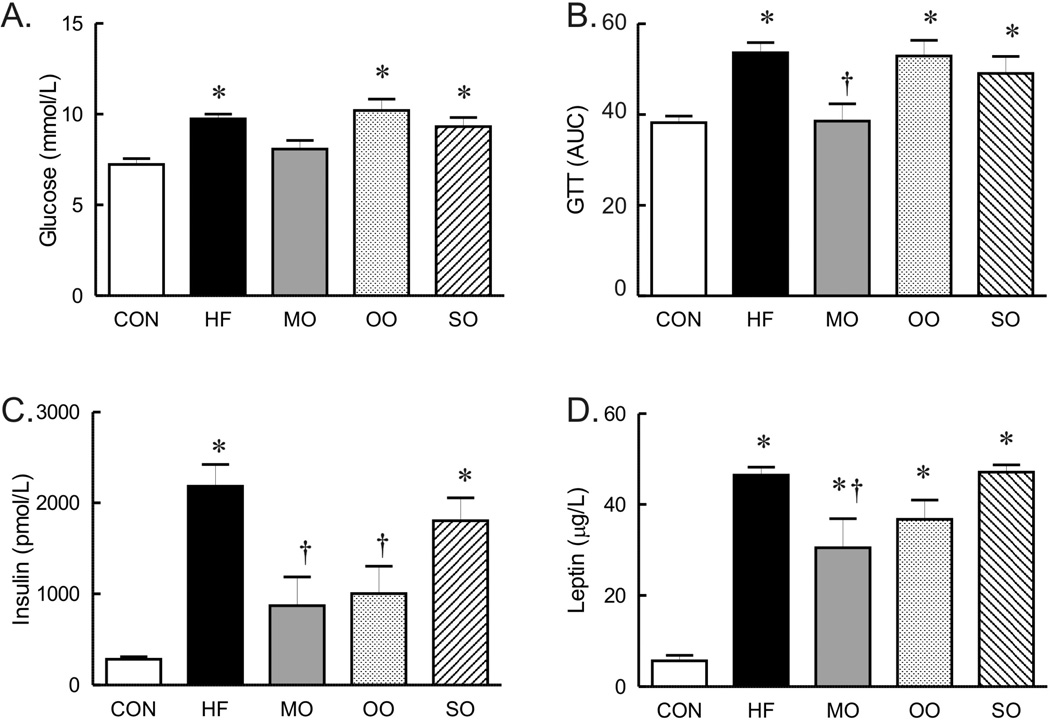
To compare the effects of short term dietary fat substitution on glucose homeostasis and indices of insulin signaling, glucose tolerance tests and serum levels of insulin, leptin and adiponectin were compared. Whereas the fasting glucose level was not significantly higher in mice on high saturated fat diet for only 12 weeks, by 16 weeks, fasting glucose in mice on the high saturated fat diet was significantly higher than mice on normal diet (Figure 5A). Similar to mice on the high saturated fat diet for 12 weeks, glucose tolerance was abnormal in mice on the saturated fat diet for the additional 4 weeks (Figure 5B). Fasting glucose levels and glucose tolerance were similarly elevated in mice on olive oil and safflower oil diets (Figure 5A and B). Fasting glucose levels and glucose tolerance tests were restored to normal levels only in mice on the diet substituted with menhaden oil for 4 weeks (Figure 5A and B).
Figure 5.
Fasting glucose levels (n=14 per group), GTT curves (AUC area under the curves for each, n=14 per group) and serum insulin (n=4–6 per group)and leptin (n=7–8 per group) in mice on control (CON), high saturated fat (HF), and menhaden oil (MO), olive oil (OO), and safflower oil (SO) substituted diets. Mean ± SEM, * p < 0.05 versus control diet, † p < 0.05 versus HF.
The abnormal glucose regulation was associated with markedly increased insulin levels in (non-fasting) mice on high saturated fat and safflower oil substituted diets compared to control (Figure 5C) suggestive of insulin resistance. Insulin levels in mice on menhaden oil and olive oil substituted diets were significantly lower than mice on high saturated fat diet and tended to be higher than mice on normal diet though the difference was not significant (Figure 5C). Leptin levels (non-fasting) were elevated in all high fat diet groups compared to normal diet suggestive of insulin resistance (15) although levels in the menhaden oil group were significantly lower than mice on high saturated fat diet (Figure 5D). Lastly, adiponectin was elevated in all groups on high fat diet but only significant in mice on menhaden and olive oil substituted diets (µg/ml Con 19.5 ± 0.8, HF 24.5 ± 0.3, MO 29.4 ± 0.8*, OO 25.0 ± 0.5*, SO 23.1 ± 0.4, *p<0.05 vs Con, n=7–8 per group).
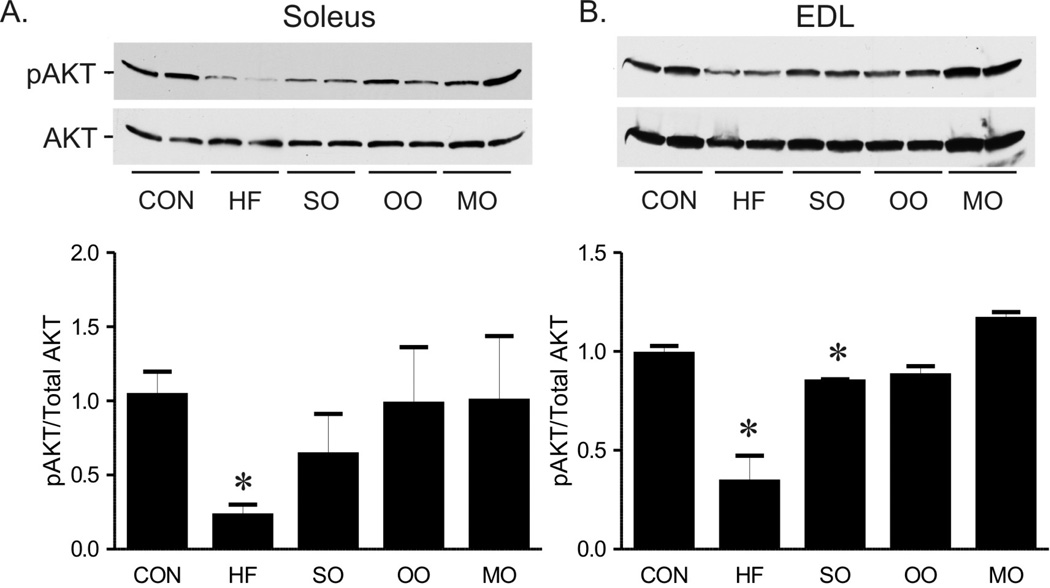
Phosphorylated Akt, an index of Akt activation, a critical node of intracellular insulin signaling, was at least 50% lower in both soleus and EDL from mice on high saturated fat diet compared to mice on normal diet (Figure 6). Consistent with other indices of insulin sensitivity, levels of phosphorylated Akt in EDL and soleus muscles were fully restored in mice on menhaden oil (Figure 6). Phosphorylated Akt was significantly reduced in EDL and tended to be lower in soleus in mice on safflower oil (Figure 6). Phosphorylated Akt was restored in soleus and EDL in mice on olive oil (Figure 6). In summary, all indices of insulin signaling and sensitivity were restored to normal by short term substitution of half the saturated fat content with menhaden oil, whereas substitution with either MUFA or n-6 PUFA restored some but not all indices of insulin signaling and sensitivity to normal.
Figure 6.
Representative (top) and mean phosphorylated AKT (bottom) compared to total AKT in soleus (A) and EDL (B) of mice on control (CON), high saturated fat (HF), and safflower oil (SO), olive oil, and menhaden oil (MO) substituted diets. Mean ± SEM p < 0.05 versus control diet, n=3 per group except MO where n=2.
Effect of dietary fatty acid composition on responses of gracilis arteries
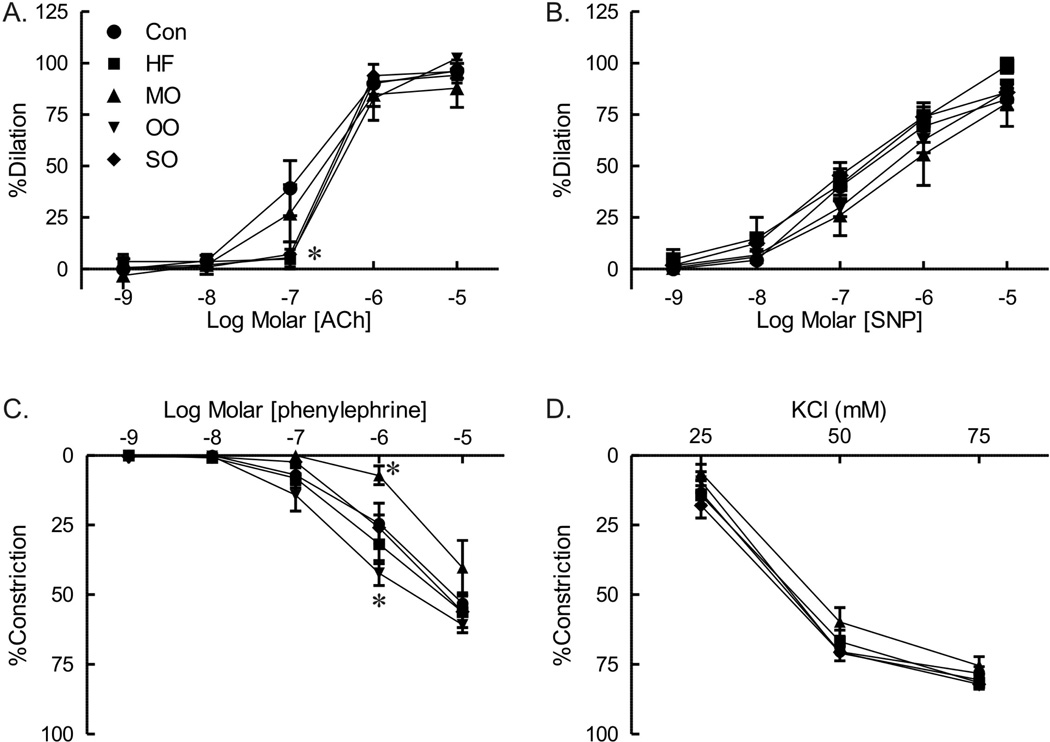
To determine whether short term modification of dietary fats with n-3 PUFA that reversed insulin resistance also improved vascular function in skeletal muscle, responses of isolated gracilis arteries from mice on different modified fat diets were compared. There was a modest reduction in dilation to acetylcholine of arteries from mice fed high saturated fat diet (Figure 7A). Substitution of a portion of the saturated fats with menhaden oil fully restored dilation of gracilis arteries to acetylcholine (Figure 7A). Responses of arteries from mice on both olive oil and safflower oil were impaired and not different from mice on high saturated fats (Figure 7A). There was no difference in dilation of arteries to nitroprusside among the groups (Figure 7B).
Figure 7.
Responses to acetylcholine (ACh, A), sodium nitroprusside (SNP, B), phenylephrine (C.), and KCl (D) of gracilis arteries from mice on normal diet (CON), high saturated fat diet (HF), menhaden oil substituted diet (MO), olive oil (OO) and safflower oil (SO) substituted diets. Mean ± SEM p < 0.05 versus CON, n=9 per group except menhaden oil with n=6.
Although a diet high in saturated fat had no effect on constriction of arteries to phenylephrine (Figure 7C), constriction was affected by diets with menhaden oil. There was marked reduction in constriction to phenylephrine of arteries from menhaden oil fed mice whereas responses of arteries from olive oil fed mice were increased (Figure 7C). Constriction to KCl was similar in all groups (Figure 7D). Thus, substitution of a portion of saturated fats in the diet with n-3 enriched PUFAs derived from menhaden oil but not MUFA or n-6 enriched PUFAs rapidly improves glucose tolerance, insulin signaling and vascular reactivity in skeletal muscle.
Discussion
Although numerous studies have demonstrated vascular dysfunction in mice on high saturated fat diets (8, 16, 17), no studies to date have simultaneously compared the ability of specific dietary fatty acids to reverse established metabolic and vascular dysfunction after their development. This study demonstrated several novel findings. First, glucose utilization was restored to normal levels with short term substitution of just a portion of the saturated fats in the diet with n-3 PUFA enriched menhaden oil. Glucose tolerance was abnormal in mice on high saturated fat diet for 12 weeks. Despite continued high fat diet, substitution of half of the saturated fats with menhaden oil was sufficient to markedly improve glucose tolerance after only 4 weeks whereas olive oil and safflower oil substitutions were not as effective. Second, substitution of saturated fats in the diet with menhaden oil improved indices of impaired insulin sensitivity and metabolism in mice on high fat diet. Cholesterol and insulin levels as well as lipid deposition in liver were all reduced in mice on menhaden oil compared to mice on high saturated fats. Insulin signaling assessed by levels of phosphorylated Akt was fully restored to normal levels in skeletal muscle of mice on menhaden oil. Third, associated with the restoration of insulin signaling and glucose tolerance was an improvement in dilation in response to acetylcholine of gracilis arteries from mice on menhaden oil. Although dilation to acetylcholine was modestly impaired in gracilis arteries from mice on the high saturated fat diet, responses were improved only in arteries from mice on menhaden oil. In addition, constriction to phenylephrine was reduced in arteries from mice on menhaden oil. Thus, substitution of a portion of the saturated fats in the diet to an n-3 PUFA while still maintaining the same amount of total fats, restored glucose tolerance and vascular function in mice.
The findings in mice on menhaden oil, markedly contrast with results from mice on n-6 PUFA enriched safflower oil and MUFA enriched olive oil substituted diets, which have been proposed as substitutes for saturated fatty acids. Mice on safflower oil and olive oil substituted diets had equivalent weight gain, increased cholesterol levels, glucose intolerance, and increased leptin as mice on the saturated fat diet. Although olive oil reduced insulin levels, tended to reduce liver lipid deposits and restored Akt activation in EDL, glucose tolerance and dilation of gracilis arteries of mice on olive oil diet were still impaired. The effects of safflower oil in the diet were even more divergent. Body weight, levels of cholesterol, insulin and leptin, and lipid deposits in the liver were no different or even greater than mice on saturated fats alone. Dilation to acetylcholine of arteries from olive oil and safflower oil fed mice was not different from responses of arteries from mice on the saturated fat diet. Thus, diets enriched in n-3 PUFAs restored insulin sensitivity and vascular function in a relatively short time whereas neither occurred with either n-6 PUFAs or MUFAs.
Beneficial effects of dietary n-3 PUFAs
The association between diets enriched in n-3 PUFAs and a reduction in the incidence in cardiovascular disease was noted more than 30 years ago (6). The failure of clinical trials of dietary fish oil to reduce angioplasty-associated restenosis or to promote atherosclerotic regression while reducing development and progression of early vascular disease suggest that further study into the metabolic effects of n-3 PUFAs are needed. The protective effects of diets enriched in n-3 PUFAs may include a reduction in arterial wall lipid deposition, inflammation, cell proliferation and plaque vulnerability (6, 18). The major n-3 components of fish oil eicosapentaenoic acid (20:5 EPA) and docosahexaenoic acid (22:6 DHA) can serve as alternative substrates for cyclooxygenase and lipoxygenase promoting generation of 3-series prostaglandins and thromboxanes and 5-series leukotrienes, respectively. The biological function of these metabolites is more favorable than products of the preferred substrate n-6 polyunsaturated arachidonic acid (6).
Summary
In summary, this is the first study to compare in the same animals the ability of diets enriched in the MUFA rich olive oil, n-3 PUFA rich menhaden oil and n-6 PUFA rich safflower oil to modify glucose tolerance and vascular function in a model of high saturated fat induced obesity. Only the diet enriched in n-3 PUFAs restored glucose tolerance and insulin signaling to normal levels following a relatively short duration of substitution of a portion of the saturated fats in a high saturated fat diet. Although olive oil or safflower oil improved some indices of insulin signaling, the diet duration may not have been sufficient to reverse the impaired glucose tolerance and to improve vascular function. These studies lay the groundwork for future studies to examine the mechanisms involved and the optimal duration of dietary substitution for improving cardiovascular endpoints.
Supplementary Material
Acknowledgements
These studies were supported by resources and the use of facilities at the Department of Veterans Affairs Iowa City Health Care System, Iowa City, IA 52246 and by grants from the National Institutes of Health awarded to Andrew W. Norris (DK081548) and Mark A. Yorek (DK073990) and VA Merit Awards to Kathryn G. Lamping (5I0BX000543-03), Mark A. Yorek (01BX001680-01) and Christine L. Oltman (1I01BX000857-01). The contents of this manuscript are the sole responsibility of the authors and do not necessarily represent the official views of the granting agencies.
References
- 1.Zelman K. The great fat debate: a closer look at the controversy-questioning the validity of age-old dietary guidance. J Am Diet Assoc. 2011 May;111(5):655–658. doi: 10.1016/j.jada.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 2.Degirolamo C, Rudel LL. Dietary monounsaturated fatty acids appear not to provide cardioprotection. Curr Atheroscler Rep. 2010 Nov;12(6):391–396. doi: 10.1007/s11883-010-0133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gillingham LG, Harris-Janz S, Jones PJ. Dietary monounsaturated fatty acids are protective against metabolic syndrome and cardiovascular disease risk factors. Lipids. 2011 Mar;46(3):209–228. doi: 10.1007/s11745-010-3524-y. [DOI] [PubMed] [Google Scholar]
- 4.Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2010;7(3):e1000252. doi: 10.1371/journal.pmed.1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramsden CE, Hibbeln JR, Majchrzak SF, Davis JM. n-6 fatty acid-specific and mixed polyunsaturate dietary interventions have different effects on CHD risk: a meta-analysis of randomised controlled trials. Br J Nutr. 2010 Dec;104(11):1586–1600. doi: 10.1017/S0007114510004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abeywardena MY, Head RJ. Longchain n-3 polyunsaturated fatty acids and blood vessel function. Cardiovasc Res. 2001 Dec;52(3):361–371. doi: 10.1016/s0008-6363(01)00406-0. [DOI] [PubMed] [Google Scholar]
- 7.Wan JB, Huang LL, Rong R, Tan R, Wang J, Kang JX. Endogenously decreasing tissue n-6/n-3 fatty acid ratio reduces atherosclerotic lesions in apolipoprotein E-deficient mice by inhibiting systemic and vascular inflammation. Arterioscler Thromb Vasc Biol. 2010 Dec;30(12):2487–2494. doi: 10.1161/ATVBAHA.110.210054. [DOI] [PubMed] [Google Scholar]
- 8.Molnar J, Yu S, Mzhavia N, Pau C, Chereshnev I, Dansky HM. Diabetes induces endothelial dysfunction but does not increase neointimal formation in high-fat diet fed C57BL/6J mice. Circ Res. 2005 Jun 10;96(11):1178–1184. doi: 10.1161/01.RES.0000168634.74330.ed. [DOI] [PubMed] [Google Scholar]
- 9.Schreyer SA, Wilson DL, LeBoeuf RC. C57BL/6 mice fed high fat diets as models for diabetes-accelerated atherosclerosis. Atherosclerosis. 1998 Jan;136(1):17–24. doi: 10.1016/s0021-9150(97)00165-2. [DOI] [PubMed] [Google Scholar]
- 10.Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 1988 Sep;37(9):1163–1167. doi: 10.2337/diab.37.9.1163. [DOI] [PubMed] [Google Scholar]
- 11.Kim F, Pham M, Maloney E, Rizzo NO, Morton GJ, Wisse BE, et al. Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arterioscler Thromb Vasc Biol. 2008 Nov;28(11):1982–1988. doi: 10.1161/ATVBAHA.108.169722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davidson EP, Coppey LJ, Calcutt NA, Oltman CL, Yorek MA. Diet-induced obesity in Sprague-Dawley rats causes microvascular and neural dysfunction. Diabetes Metab Res Rev. 2010 May;26(4):306–318. doi: 10.1002/dmrr.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yorek M, Strom D, Spector A. Effect of membrane polyunsaturation on carrier-mediated transport in culutred retinoblastoma cells: alterations in taurine uptake. J Neurochem. 1984;42(1):254–261. doi: 10.1111/j.1471-4159.1984.tb09726.x. [DOI] [PubMed] [Google Scholar]
- 14.Yorek M, Bohnker R, Dudley D. Comparative utilization of n-3 polyunsaturated fatty acids by cultured human Y-79 retinoblastoma cells. Biochim Biophys Acta. 1984;795(2):277–285. doi: 10.1016/0005-2760(84)90076-6. [DOI] [PubMed] [Google Scholar]
- 15.Askari H, Tykodi G, Liu J, Dagogo-Jack S. Fasting plasma leptin level is a surrogate measure of insulin sensitivity. J Clin Endocrinol Metab. 2010 Aug;95(8):3836–3843. doi: 10.1210/jc.2010-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayasi R, Akamine EH, Davel AP, Rodrigues MA, Carvalho CR, Rossoni LV. Oxidative stress and inflammatory mediators contribute to endothelial dysfunction in high-fat diet-induced obesity in mice. J Hypertens. 2010 Oct;28(10):2111–2119. doi: 10.1097/HJH.0b013e32833ca68c. [DOI] [PubMed] [Google Scholar]
- 17.Symons JD, McMillin SL, Riehle C, Tanner J, Palionyte M, Hillas E, et al. Contribution of insulin and Akt1 signaling to endothelial nitric oxide synthase in the regulation of endothelial function and blood pressure. Circ Res. 2009 May 8;104(9):1085–1094. doi: 10.1161/CIRCRESAHA.108.189316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sudheendran S, Chang CC, Deckelbaum RJ. N-3 vs. saturated fatty acids: effects on the arterial wall. Prostaglandins Leukot Essent Fatty Acids. 2010 Apr-Jun;82(4–6):205–209. doi: 10.1016/j.plefa.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.