Abstract
Purpose
This study investigated the cytokine profile in bladder tissue and urine of painful bladder syndrome/interstitial cystitis (PBS/IC) patients.
Methods
Multiplex analysis of 23 cytokines was performed with a multiple antigen bead assay (Luminex 100 IS) on cold cup bladder biopsy and urine specimens collected during cystoscopy with hydrodistention (HD) under general anesthesia from 10 PBS/IC patients (ICS definition). Collected tissue specimens and urine from pre-HD and post-HD (mean 27 days) were compared to banked urine and tissue specimens (n = 10) collected from control subjects without PBS/IC symptoms.
Results
Univariate comparison of bladder tissue levels found significant elevation of IL-16, IL-18, CTACK, ICAM-1, MCP-3, SCGFβ, TRAIL, and VCAM-1 in PBS/IC relative to controls. Multivariate analysis revealed VCAM-1 and ICAM-1 were responsible for the discrimination of both tissue and urine of PBS/IC from controls. Urine levels of MCP-3 and TRAIL were significantly reduced a month after HD in concert with improvement in standardized measures of clinical symptoms (pain, urgency, and frequency (PUF) overall score [mean 25.8 ± 5.5 vs. 20.3 ± 7, p = 0.04] and symptom score [mean 18.2 ± 3.2 vs. 12.2 ± 5.9; p = 0.009]). Post-HD urine levels of MCSF(r = 0.88; p = 0.003), MCP-3 (r = 0.81; p = 0.01), SDF1α (r = 0.82; p = 0.01), and IL-18 (r = 0.64; p = 0.08) positively correlated with improved symptom scores.
Conclusions
These results indicate significant elevation of cytokines in PBS/IC bladder tissue relative to controls. Significant reduction in post-HD urine levels of MCP-3 and TRAIL relative to pre-HD in PBS/IC was associated with clinical improvement (as measured by PBS/IC symptom scores) to qualify them as biomarker candidates.
Keywords: Cytokines, Interstitial cystitis, Urine, Bladder, Biomarker
Introduction
Although the etiology of PBS/IC is not fully understood, bladder inflammation associated with production of inflammatory cytokines has been proposed as a potential cause of pathogenesis of the disease [1, 2]. In previous studies, cytokines and chemokines such as interleukin-2 (IL-2), IL-6, IL-8, CXC chemokines, and tumor necrosis factor-α (TNF-α) were shown to be increased in bladder tissues and/or urine from PBS/IC patients, suggesting that these cytokines might represent specific markers of PBS/IC [1, 2].
None of the previous studies in this area have simultaneously measured the levels of cytokines in bladder tissue as well as urine collected from PBS/IC patients to address the lingering doubts on the probable source of disease-associated proteins in urine [1, 2]. In the present study, we compared the cytokine levels in the tissues and urine obtained from PBS/IC patients versus controls. In addition, we also examined the relationships between temporal changes in urine levels after hydrodistension with symptom scores of PBS/IC patients in order to objectively assess treatment response.
Materials and methods
Patient enrollment
The study was conducted after prior approval from the University of Pittsburgh Institutional Review Board. Between July 2008 and July 2009, patients meeting the criteria for the ICS definition of PBS/IC were enrolled in the study after informed consent. Patients of other causative diseases such as infection, malignancy, or calculi of the urinary tract were excluded, and enrolled PBS/IC patients were scheduled for bladder biopsy and hydrodistention (HD). PUF scores were obtained from each patient pre- and post-HD using a standardized questionnaire [3].
Tissue acquisition
Cystoscopy was performed under general anesthesia, and collected urine was immediately placed on ice. The bladder was then gradually filled with saline solution by gravity from a height of 80 cm at a speed of 50–100 ml/min. The bladder was considered to be filled maximally when the drip infusion of the solution stopped by gravity. The fluid infusion was then turned off, kept for approximately 5 min, and the solution in the bladder was gradually drained. This distention procedure was repeated for a total of 3 times. Following HD, two cold cup bladder biopsies were obtained from the non-bleeding sites at the posterior bladder wall. Biopsied specimens were flash-frozen in liquid nitrogen and kept at −80°C until further analysis.
PBS/IC patients were seen at a follow-up visit, and a post-HD urine specimen was collected. Urine specimens were centrifuged at 3,500 rpm for 5 min, and the supernatant material was flash-frozen in liquid nitrogen and kept at −80°C until use. Control bladder tissue specimens were obtained from organ donors following death from either stroke or trauma, whereas control urine specimens were obtained from patients with asymptomatic small renal masses prior to surgery or intervention.
Multiplex analysis
Frozen tissue was suspended in lysis buffer (2.66% Tris (hydroxymethyl) aminomethane HCl, 0.985% Tris (hydroxymethyl) aminomethane, 0.5 mM. phenylmethylsulfonyl fluoride, 1 M leupeptin, 1 M pepstatin A, and 0.3 M aprotinin), homogenized with a polytron-type homogenizer on ice and incubated for 30 min at 4°C. Samples were centrifuged at 10,000g for 10 min at 4°C.
We used Bio-Plex Pro Human Cytokine 23-plex Assay Kit (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer’s instructions on automated immunoassay analyzer (Luminex TM 100 IS System, Austin, TX) as described in our earlier study [4]. Four groups of proteins were determined in our study: (1) cytokines, including interleukin-1α (IL-1α), IL-3, IL-12, IL-18, interferon-α2 (INF-α2), leukemia inhibitory factor (LIF), macrophage colony-stimulating factor (M-CSF), macrophage migration inhibitory factor (MIF), stem cell factor (SCF), tumor necrosis factor-β (TNF-β), TNF-related apoptosis-inducing ligand (TRAIL), vascular cell adhesion protein (VCAM-1), inter-cellular adhesion molecule 1 (ICAM-1), and IL-2 receptor α (IL-2Rα); (2) CC chemokines including cutaneous T-cell-attracting chemokine (CTACK/CCL27), monocyte chemotactic protein-3 (MCP-3/CCL7); (3) CXC chemokines including stromal cell-derived factor-1 (SDF-1/CXCL12), growth-related oncogene (CXCL1), monokine induced by gamma interferon (MIG/CXCL9); and (4) growth factors such as β-nerve growth factor (β-NGF), stem cell growth factor-β (SCGFβ), and hepatocyte growth factor (HGF).
The cytokine/chemokine/growth factor concentrations were normalized to protein concentration for tissue lysates and creatinine concentration for urine. The creatinine in urine was measured using the previously published high-performance liquid chromatography method [5].
Statistical analysis
Univariate and multivariate statistics were used to analyze continuous variables between various groups. Paired comparison of pre- and post-HD PBS/IC patients and their unpaired comparison with asymptomatic controls were done using non-parametric Mann–Whitney U test. Correlations between cytokine levels and symptom scores were assessed by both Pearson’s r (parametric) and Spearman’s r (non-parametric) correlation test. A multivariate statistical tool, principal component analysis (PCA), was used to visually assess discrimination between patients and controls using the cytokines identified by univariate data analysis. Univariate urine cytokine data are expressed as median 25 and 75 percentile levels, and differences were considered significant at p value less than 0.05.
Results
Urine and tissue cytokine levels of PBS/IC patients versus controls
We identified 10 PBS/IC patients (9 women and 1 man) that met the inclusion and exclusion criteria, all of whom consented for participation in this study. The mean age was not significantly different in PBS/IC patients (mean age: 47.5 years) versus control tissue donors (mean age: 52 years), but the difference in age was significant in PBS/IC versus control urine cohorts (mean age: 64 years). Because 90% of PBS/IC patients were female, the control cohorts were intentionally matched for this demographic.
Univariate data analysis
All 23 cytokines, chemokine, and growth factors were detectable in both PBS/IC and control tissues (Table 1). Significant elevation in the expression of IL-16, IL-18, SCGFβ, CTACK, TRAIL, ICAM-1, MCP-3, and VCAM-1 were detected in the PBS/IC bladder tissue compared to control bladder tissue collected from organ donors. No significant difference was noticed in the comparison of PBS/IC patients with asymptomatic control patients for the urine levels of any proteins (Table 2).
Table 1.
Comparison of multiplex cytokine analysis of bladder tissue lysates from IC patient biopsies and control (banked) specimens
| Cytokine | IC bladder tissue (pg/mg protein) (n = 10) | Control bladder tissue (pg/mg protein) (n = 10) | p value (unpaired Mann– Whitney U test) |
|---|---|---|---|
| IL-1a | 7.8 ± 4.8 | 4.9 ± 3.4 | NS |
| IL-2Rα | 50.0 ± 40 | 22.6 ± 20 | NS |
| IL-3 | 241.0 ± 206 | 143.4 ± 141 | NS |
| IL-12 | 154.9 ± 138 | 70.9 ± 74 | NS |
| IL-16 | 2,094.5 ± 1,815 | 190.0 ± 203 | 0.004* |
| IL-18 | 79.4 ± 74 | 18.1 ± 21 | 0.02* |
| CTACK | 146.0 ± 95 | 68.4 ± 61 | 0.04* |
| GRO | 130.8 ± 101 | 74.9 ± 47 | NS |
| HGF | 3,809.2 ± 5,850 | 963.5 ± 478 | NS |
| ICAM-1 | 3,419.7 ± 3,091 | 1,026.2 ± 1,090 | 0.03* |
| IFNα-2 | 45.4 ± 22 | 30.1 ± 9 | NS |
| LIF | 15.4 ± 23 | 19.3 ± 12 | NS |
| MCP-3 | 24.7 ± 15 | 12.7 ± 8.5 | 0.04* |
| MCSF | 70.7 ± 48 | 40.1 ± 27 | NS |
| MIF | 8,060.8 ± 5,372 | 8,740.4 ± 2,820 | NS |
| MIG | 6,107 ± 12,590 | 44.2 ± 45 | NS |
| βNGF | 9.3 ± 7.6 | 4.7 ± 4.6 | NS |
| SCF | 56.5 ± 44 | 26.4 ± 23 | NS |
| SCGFβ | 790.2 ± 590 | 347.4 ± 249 | 0.04* |
| SDF1α | 98.4 ± 72 | 49.3 ± 32 | NS |
| TNF-β | 11.4 ± 9.7 | 8.6 ± 6.7 | NS |
| TRAIL | 758.6 ± 652 | 230.9 ± 265 | 0.02* |
| VCAM-1 | 4,463.9 ± 3,315 | 889.6 ± 916 | 0.004* |
Significant difference between control and IC patients, p < 0.05
Table 2.
Distribution of urine cytokine levels in controls, IC pre- and IC post-hydrodistention subgroups stratified by interquartile intervals
| Cytokine | Control urine samples (pg cytokine/mg creatinine) (n = 10)
|
Pre-HD urine IC patients (pg cytokine/mg creatinine) (n = 10)
|
Post-HD urine IC patients (pg cytokine/mg creatinine) (n = 8)
|
p value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 25% | Median | 75% | 25% | Median | 75% | 25% | Median | 75% | ||
| IL-1α | 1.226 | 3.47 | 12.57 | 1.615 | 3.244 | 5.277 | 2.54 | 3.21 | 3.866 | NS |
| IL-2Rα | 33.75 | 106 | 176.5 | 60.64 | 126.4 | 180.4 | 44.77 | 125.4 | 162.4 | NS |
| IL-3 | 15.09 | 144 | 459.9 | 85.6 | 150.9 | 323.3 | 60.76 | 86.31 | 101 | 0.05* |
| IL-12 | 14.05 | 53.55 | 210.5 | 36.75 | 54.4 | 146.9 | 23.4 | 29.32 | 44.81 | NS |
| IL-16 | 5.047 | 18.57 | 170.3 | 6.869 | 12.37 | 132.9 | 7.784 | 10.31 | 12.56 | NS |
| IL-18 | 3.444 | 11.53 | 35.8 | 3.801 | 8.553 | 11.94 | 3.686 | 6.164 | 9.25 | NS |
| CTACK | 11.17 | 115.5 | 275.7 | 29.44 | 38.91 | 269 | 4.262 | 28.64 | 67.82 | NS |
| GRO | 21.89 | 83.59 | 1,119 | 36.54 | 68.12 | 164.4 | 29.97 | 60.26 | 66.96 | NS |
| HGF | 714.2 | 2,633 | 7,633 | 1,337 | 1,678 | 4,154 | 1,241 | 1,757 | 2,174 | NS |
| ICAM-1 | 391.4 | 2,302 | 6,038 | 1,046 | 1,833 | 3,813 | 696.2 | 1,006 | 1,639 | NS |
| IFNα-2 | 4.44 | 19.42 | 65.49 | 7.707 | 14.84 | 38.7 | 7.372 | 9.273 | 16.93 | NS |
| LIF | 5.577 | 11.3 | 67.71 | 1.563 | 10.05 | 13.68 | 5.638 | 6.857 | 10.79 | NS |
| MCP-3 | 17.46 | 35.39 | 50.17 | 5.84 | 23.88 | 39.19 | 3.723 | 5.233 | 14.01 | 0.025* |
| MCSF | 68.17 | 197.3 | 603.8 | 62.88 | 105.2 | 267.9 | 82.11 | 103.4 | 139.7 | NS |
| MIF | 823.8 | 2,422 | 10,430 | 433 | 1,917 | 5,223 | 666 | 1,196 | 2,184 | NS |
| MIG | 39.34 | 51.63 | 675.8 | 49.14 | 144.1 | 2,644 | 57.89 | 104.6 | 475.3 | NS |
| βNGF | 0.7624 | 2.713 | 16.25 | 1.064 | 1.908 | 6.598 | 1.021 | 1.378 | 1.706 | NS |
| SCF | 12.24 | 48.1 | 161.1 | 12.94 | 19.65 | 134.1 | 12.78 | 15.18 | 20.74 | NS |
| SCGFβ | 290.3 | 739.2 | 1,869 | 114.5 | 533.5 | 1,102 | 118.5 | 204.9 | 1,061 | NS |
| SDF1α | 8.459 | 219.8 | 1,310 | 23.32 | 61.87 | 128.9 | 27.33 | 53.17 | 68.28 | NS |
| TNF-β | 4.439 | 7.823 | 14.67 | 4.333 | 6.82 | 9.81 | 4.063 | 4.387 | 6.807 | NS |
| TRAIL | 58.44 | 178.8 | 818.9 | 87.32 | 235.2 | 744.5 | 64.76 | 110.3 | 135.9 | 0.05* |
| VCAM-1 | 392.9 | 3,820 | 13,591 | 349.4 | 1,301 | 6,973 | 323.4 | 1,868 | 4,321 | NS |
Significant difference between IC pre- and IC post-hydrodistention groups
Multivariate data analysis
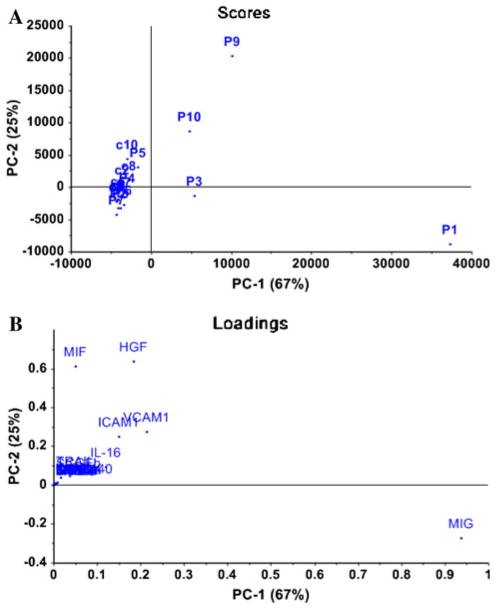
PCA was applied separately to cytokine dataset from tissue and urine of PBS/IC and controls, which confirmed the elevated tissue levels of IL-16, VCAM-1, and ICAM-1 cytokines in PBS/IC patients as discriminatory versus controls (Fig. 2b) as previously identified in Table 1. PCA of urine data from Table 2 led to the identification of two components that together explained 80% of the total variance with 57% by PC1 and 23% by PC2.
Fig. 2.
Principal component analysis of cytokine profiles in tissue obtained from 10 PBS/IC patients (identified P1–P10) and 10 controls (identified by c1–c10) according to the first two components computed using PCA as determined by PCA scores (a) and loadings plots determined by relative levels of 23 cytokines (b). a Control patients demonstrated a similar trend in mapping position and sufficiently deviated from PBS/IC patients. b The mapping position of controls and PBS/IC patients was mainly influenced by three cytokines (IL-16, VCAM-1, and ICAM-1), which contributed equally to the variance of PC1 and PC2. PC1, which explains 67% of overall variance in the dataset, was largely influenced by increased tissue levels of MIG, and variance of PC2 was excessively influenced by tissue levels of MIF and HGF
Effect of HD on urine cytokine levels of PBS/IC patients
During cystoscopic observation, no ulcerative lesions were seen in any of 10 PBS/IC patients, and after HD, glomerulations with petechial hemorrhage from the mucosal surface were observed in all patients. Urine specimens from 8 patients were available at both pre-HD and post-HD stage. Urine specimens were collected at a mean of 27 days post-HD (range: 15–64 days). Post-HD urine levels of MCP-3, IL-3, and TRAIL were significantly decreased compared to pre-HD levels (Table 2). In addition, pre-HD data of 4 PBS/IC patients (P1, P6, P7, and P10) mapped separately from post-HD data in agreement with paired comparison in Table 2. Consistent with the PCA analysis of tissue cytokine data (Fig. 2b), the main contributors to the discrimination of urine cytokine data were also identified to be VCAM-1 and ICAM-1 (data not shown).
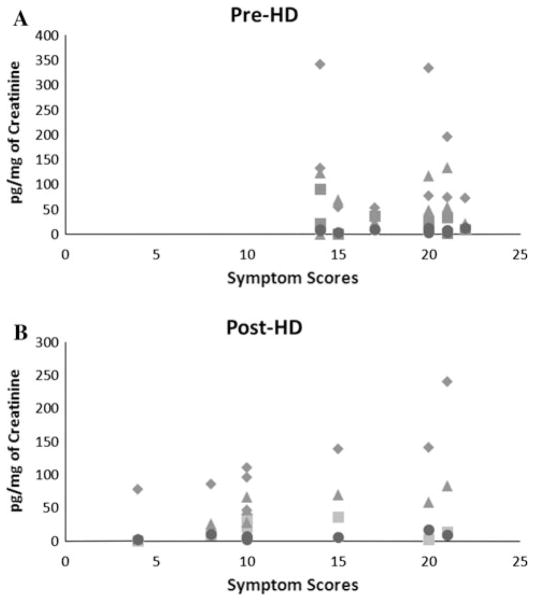
Significant improvement was seen at post-HD with regard to reduction in symptom scores with reduced overall (25.8 ± 5.5 vs. 20.3 ± 7, p = 0.04) and symptoms scores (18.2 ± 3.2 vs. 12.2 ± 5.9; p = 0.009) compared to pre-HD stage. Post-HD symptom scores in PBS/IC patients showed linear correlation with lower urine levels of MCSF (r = 0.88; p = 0.003), MCP-3 (r = 0.81; p = 0.01), SDF1α (r = 0.82; p = 0.01), and IL-18 (r = 0.64; p = 0.08) (Fig. 1b). Correlation with rest of the 19 cytokines at post-HD stage was either absent or not significant. In contrast, none of the 23 cytokines showed any correlation with symptom scores measured at pre-HD stage (Fig. 1a).
Fig. 1.
Correlation of urinary cytokines and symptoms scores of PBS/IC patient’s post-HD. a Symptom scores of PBS/IC patient skewed to higher sides at pre-HD stage. b Low symptom scores in PBS/IC patients post-HD showed linear correlation with urine levels of MCSF filled diamond (r = 0.88; p = 0.003), MCP-3 filled square (r = 0.81; p = 0.01), SDF1α filled triangle (r = 0.82; p = 0.01), and IL-18 filled circle (r = 0.64; p = 0.08)
Discussion
In this study, we analyzed the urine and tissue proteome of PBS/IC relative to asymptomatic controls by univariate and multivariate analysis. The variation from the heterogenous nature of PBS/IC precluded complete spatial separation of PBS/IC tissue data from controls (Fig. 2a). PCA analysis identified 3 cytokines as chief contributors to the discrimination of PBS/IC tissue from controls (i.e., IL-16, ICAM-1, and VCAM-1) (Fig. 2b), which were also elevated on unpaired comparison with controls (Table 1). The identification of VCAM-1 and ICAM-1 as the primary contributor of variance in urine data demonstrated that variance in tissue and urine data originates from same source.
The subjective improvement of hydrodistended PBS/IC patient was associated with objective changes in urinary proteome. Post-HD symptom scores showed linear correlation with urine levels of MCSF, MCP-3, SDF1α, and IL-18, which justify further investigation of their role in PBS/IC. Symptom scores being skewed to a higher side at pre-HD stage masked any correlation with cytokine levels. Apparently, the low symptom score post-HD indicates that HD does effect the perception of pain and distress in the cohort of treated patients.
Paired comparison of pre- and post-HD urine revealed a significant decrease in levels of MCP-3, IL-3, and TRAIL after HD (Table 2) [3]. The important role of MCP-3 in inflammatory process of PBS/IC is highlighted considering its elevated tissue levels in PBS/IC patients (Table 1), significant decrease in post-HD urine (Table 2), and positive association of post-HD urine levels with symptom scores measured at a mean of 27 days (Fig. 1). A previous preclinical study from our group described the role of another MCP family member MCP-1 in the progression of cyclophosphamide-induced cystitis [6].
Chemokines from MCP family are known to provoke mast cell activation and has chemotactic activity for monocytes that differentiate into macrophages at site of inflammation [7, 8] which can secrete other chemokines that act as a chemoattractant for neutrophils [9]. TNF-related apoptosis-inducing ligand (TRAIL) is a death ligand known to be expressed on neutrophils and mast cells [10].
Neutrophils infiltrating into bladder (both by releasing their preformed soluble TRAIL and their membrane–associated TRAIL) in PBS/IC [11] can participate in TRAIL-associated apoptosis by binding to the death receptors DR4 (TRAIL-RI) and DR5 (TRAIL-RII), which have previously been shown to be localized in bladder biopsy samples of PBS/IC [12]. Significantly higher intensity score of DR4 in bladder tissue of PBS/IC [12] is in agreement with elevated MCP-3 reported here (Fig. 1; Tables 1 and 2) and with the attraction of monocytes by DR4 via the RhoGTPase signaling pathway [13].
Control of neutrophil death is central to the resolution of inflammation [14] and therefore the post-HD reduction in TRAIL is consistent with post-HD symptom improvement and probable resolution of inflammation (Table 2). These finding justify TRAIL as an attractive biomarker candidate for PBS/IC and, interestingly TRAIL is not associated with urinary tract infection [15].
Prior studies have also noted that cystoscopy with HD has a therapeutic effect on PBS/IC symptoms although the effect is usually short-lived [16]. Chai et al. showed a reduction in antiproliferative factor and an increase in heparin binding epithelial growth factor after HD [17], and Liu et al. reported post-HD reduced NGF associated with lower pain [18]. However, in this study, the elevation of bladder tissue or urine NGF levels were not detected in PBS/IC patients compared with controls, possibly due to the small number of patients.
We acknowledge the limitations of this study are the small sample size, which is a direct result of the rarity of biopsy in PBS/IC patients during the accrual period for this study. Error of high dimensionality in PCA analysis cannot be excluded here, which is introduced by the number of subjects in this study being lower than the number of variables (cytokines) investigated. Future studies, specifically looking at MCP-3 and TRAIL in urine specimens of PBS/IC patients pre- and post-HD, need to be performed to validate these findings with larger sample sizes.
Conclusions
These results indicate significant elevation of cytokines in PBS/IC bladder tissue relative to controls. Significant reduction in post-HD urine levels of MCP-3 and TRAIL relative to pre-HD in PBS/IC was associated with clinical improvement (as measured by PBS/IC symptom scores) to qualify them as biomarker candidates. Further larger-scale studies are warranted to map the cytokine profile in PBS/IC and confirm these findings.
Acknowledgments
This study was supported by the Samuel Wilan Research Fund for Interstitial Cystitis, NIH grants (DK57267 and DK88836) and Department of Defense grant PR110326.
Footnotes
Conflict of interest None.
References
- 1.Erickson DR, Xie SX, Bhavanandan VP, Wheeler MA, Hurst RE, Demers LM, et al. A comparison of multiple urine markers for interstitial cystitis. J Urol. 2002;167:2461–2469. [PubMed] [Google Scholar]
- 2.Ogawa T, Homma T, Igawa Y, Seki S, Ishizuka O, Imamura T, et al. CXCR3 binding chemokine and TNFSF14 over expression in bladder urothelium of patients with ulcerative interstitial cystitis. J Urol. 2010;183:1206–1212. doi: 10.1016/j.juro.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Brewer ME, White WM, Klein FA, Klein LM, Waters WB. Validity of pelvic pain, urgency, and frequency questionnaire in patients with interstitial cystitis/painful bladder syndrome. Urology. 2007;70:646–649. doi: 10.1016/j.urology.2007.06.1089. [DOI] [PubMed] [Google Scholar]
- 4.Tyagi P, Barclay D, Zamora R, Yoshimura N, Peters K, Vodovotz Y, et al. Urine cytokines suggest an inflammatory response in the overactive bladder: a pilot study. Int Urol Nephrol. 2010;42:629–635. doi: 10.1007/s11255-009-9647-5. [DOI] [PubMed] [Google Scholar]
- 5.George SK, Dipu MT, Mehra UR, Singh P, Verma AK, Ramgaokar JS. Improved HPLC method for the simultaneous determination of allantoin, uric acid and creatinine in cattle urine. J Chromatogr, B: Anal Technol Biomed Life Sci. 2006;832:134–137. doi: 10.1016/j.jchromb.2005.10.051. [DOI] [PubMed] [Google Scholar]
- 6.Smaldone MC, Vodovotz Y, Tyagi V, Barclay D, Philips BJ, Yoshimura N, et al. Multiplex analysis of urinary cytokine levels in rat model of cyclophosphamide-induced cystitis. Urology. 2009;73:421–426. doi: 10.1016/j.urology.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Z, Cherryholmes G, Mao A, Marek C, Longmate J, Kalos M, et al. High plasma levels of MCP-1 and eotaxin provide evidence for an immunological basis of fibromyalgia. Exp Biol Med (Maywood) 2008;233:1171–1180. doi: 10.3181/0712-RM-328. [DOI] [PubMed] [Google Scholar]
- 8.Bouchelouche K, Alvarez S, Andersen L, Nordling J, Horn T, Bouchelouche P. Monocyte chemoattractant protein-1 production by human detrusor smooth muscle cells. J Urol. 2004;171:462–466. doi: 10.1097/01.ju.0000090192.36436.d5. [DOI] [PubMed] [Google Scholar]
- 9.Tyagi P, Killinger K, Tyagi V, Nirmal J, Peters K. Urinary chemokines as Non-Invasive predictors of Ulcerative interstitial cystitis. J Urol. 2012;187:1275–1279. doi: 10.1016/j.juro.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 11.Dodd LG, Tello J. Cytologic examination of urine from patients with interstitial cystitis. Acta Cytol. 1998;42:923–927. doi: 10.1159/000331969. [DOI] [PubMed] [Google Scholar]
- 12.Kutlu O, Akkaya E, Koksal IT, Bassorgun IC, Ciftcioglu MA, Sanlioglu S, et al. Importance of TNF-related apoptosis-inducing ligand in pathogenesis of interstitial cystitis. Int Urol Nephrol. 2010;42:393–399. doi: 10.1007/s11255-009-9632-z. [DOI] [PubMed] [Google Scholar]
- 13.Wei W, Wang D, Shi J, Xiang Y, Zhang Y, Liu S, et al. Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) induces chemotactic migration of monocytes via a death receptor 4-mediated RhoGTPase pathway. Mol Immunol. 2010;47:2475–2484. doi: 10.1016/j.molimm.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 14.McGrath EE, Marriott HM, Lawrie A, Francis SE, Sabroe I, Renshaw SA, et al. TNF-related apoptosis-inducing ligand (TRAIL) regulates inflammatory neutrophil apoptosis and enhances resolution of inflammation. J Leukoc Biol. 2011;90:855–865. doi: 10.1189/jlb.0211062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosevear HM, Lightfoot AJ, O’Donnell MA, Griffith TS. The role of neutrophils and TNF-related apoptosis-inducing ligand (TRAIL) in bacillus Calmette-Guerin (BCG) immunotherapy for urothelial carcinoma of the bladder. Cancer Metastasis Rev. 2009;28:345–353. doi: 10.1007/s10555-009-9195-6. [DOI] [PubMed] [Google Scholar]
- 16.Ottem DP, Teichman JM. What is the value of cystoscopy with hydrodistension for interstitial cystitis? Urology. 2005;66:494–499. doi: 10.1016/j.urology.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Chai TC, Zhang CO, Shoenfelt JL, Johnson HW, Jr, Warren JW, Keay S. Bladder stretch alters urinary heparin-binding epidermal growth factor and antiproliferative factor in patients with interstitial cystitis. J Urol. 2000;163:1440–1444. [PubMed] [Google Scholar]
- 18.Liu HT, Tyagi P, Chancellor MB, Kuo HC. Urinary nerve growth factor level is increased in patients with interstitial cystitis/bladder pain syndrome and decreased in responders to treatment. BJU Int. 2009;104:1476–1481. doi: 10.1111/j.1464-410X.2009.08675.x. [DOI] [PubMed] [Google Scholar]