Abstract
The hyper-IgE syndromes (HIES; originally named Job's syndrome) are a collection of primary immunodeficiency syndromes resulting in elevated serum IgE levels and typified by recurrent staphylococcal skin abscesses, eczema and pulmonary infections. The disorder has autosomal dominant and recessive forms. Autosomal dominant HIES has been shown to be mainly due to STAT3 mutations and additionally results in connective tissue, skeletal, vascular and dental abnormalities. Autosomal recessive HIES has been shown to be mainly due to mutations in DOCK8; these patients are more prone to viral skin infections instead. This review article discusses the common clinical features of the syndrome, the genetic mutations responsible and the pathogenesis of the disease, as well as treatments currently used.
Introduction
The hyper-IgE recurrent infection syndromes (HIES) comprise a group of primary immunodeficiency disorders that exhibit markedly elevated IgE levels, recurrent staphylococcal skin abscesses, eczema and pulmonary infections. Both autosomal dominant and autosomal recessive forms of the disorder have been described. Most autosomal dominant HIES (AD-HIES) have been found to be due to mutations in STAT3 (Signal transducer and activator of transcription 3; MIM#147060), whereas DOCK8 (Dedicator of cytokinesis 8) mutations have been identified in patients with autosomal recessive HIES (AR-HIES; MIM#243700). Patients with AD-HIES also exhibit distinct dental, skeletal and connective tissue abnormalities not found in patients with AR-HIES. The condition is thought to be rare, although the exact prevalence is unknown; approximately 200 cases have been described in the literature. STAT3 mutations have been found in many ethnic groups with an equal gender distribution.
So went Satan forth from the presence of the LORD, and smote Job with sore boils from the sole of his foot unto his crown'
The Book of Job, chapter 2, verse 7,
The Bible, King James Version, 1611
Davis and colleagues first described Job's syndrome in 1966 in their paper with two girls who had a triad of eczematoid dermatitis, and recurrent sinopulmonary and staphylococcal skin infections that distinctly lacked warmth, erythema or tenderness [1]. Subsequent to that, in 1972, Buckley and colleagues further characterised the syndrome, noting distinctive facial features and an elevation in IgE levels [2], thus leading to the use of the term Buckley's syndrome. Job's syndrome and Buckley's syndrome were subsequently found to represent the same disease [3], leading to its description as the hyper-IgE syndrome.
In 1999, the multi-system nature of HIES was further characterised by researchers at the NIH, who noted its autosomal dominant inheritance pattern [4]. Following this, in 2007, dominant-negative mutations in STAT3 were found to be responsible for the majority of cases of AD-HIES, thus linking the infectious and connective tissue disease abnormalities seen in the syndrome [5,6]. Subsequent research has resulted in a deeper understanding of the role of STAT3 in the pathogenesis and clinical features of the autosomal dominant form of the disease [7].
This review focuses mostly on AD-HIES, which occurs more frequently and is better described in the literature. The clinical features, genetics, pathophysiology and treatment of the condition are discussed in detail. AR-HIES is also touched upon, with reference to the similarities and differences compared to AD-HIES. In addition, other genetic diseases that also possess features of HIES are briefly described.
Autosomal dominant hyper-IgE syndrome
Clinical features
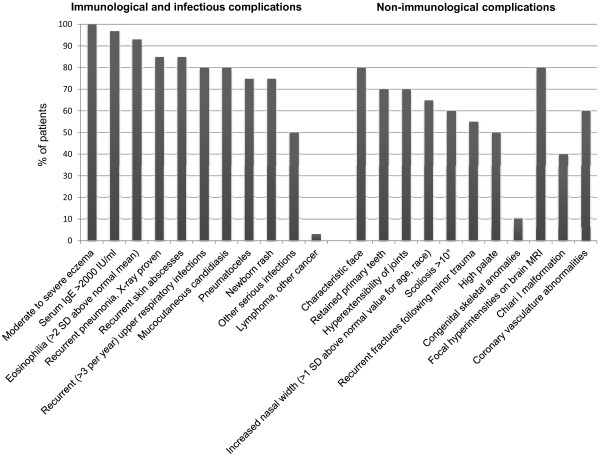
AD-HIES is a multi-system disease affecting immunological function, connective tissue and skeletal systems, dentition and vasculature. Figure 1 shows the frequency of 22 features in AD-HIES based on a cohort of 30 patients [4].
Figure 1.
Clinical features in AD-HIES (with approximate frequencies) [4]. MRI, magnetic resonance imaging; SD, standard deviation.
Immunological and infectious features
The most frequently found immunological abnormalities are eczematoid rashes, skin abscesses, respiratory infection, marked elevation in serum IgE, mucocutaneous candidiasis and eosinophilia.
The rash is usually present within a few weeks of life and can be found at birth. It is typically a pustular or eczematoid eruption on the face and scalp [8,9], and histologically, eosinophils are detected. The rash can resolve or progress to become eczematoid dermatitis. Similar to conventional eczema, the rash is also driven by Staphylococcus aureus, and improves with Staphylococcus clearance measures.
Boils and furuncles are almost invariably found in AD-HIES and are often not associated with signs of inflammation, resulting in the 'cold' abscesses in the original description of Job's syndrome [10].
Recurrent sinopulmonary infections represent another clinical hallmark in AD-HIES. Most patients have at least one episode of pneumonia, with more than 50% of patients having three or more episodes. The most common causative organism is S. aureus with Streptococcus pneumonia and Haemophilus influenzae less frequently implicated [4]. In addition, aberrant healing is frequently seen following pulmonary infection, with the development of pneumatoceles and bronchiectasis affecting up to 75% of patients. With the presence of parenchymal lung damage, the spectrum of pathogens then more closely resembles cystic fibrosis with Pseudomonas aeruginosa and non-tuberculous mycobacterial infection [11]. The pneumatoceles can also become occupied with moulds such as Aspergillus and Scedosporium [12]. The infection with Pseudomonas and moulds represents the major cause of mortality and morbidity in these patients [13]. Pneumocystis jiroveci infection has also been reported to occur in infancy prior to the development of bacterial lung disease [14,15].
Patients with AD-HIES also have increased susceptibility to fungal infection with up to 80% being affected with chronic mucocutaneous candidiasis. Focal extra-pulmonary infections with Cryptococcus and Histoplasma have also been infrequently described [16,17].
Non-immunological features
The multi-system abnormalities seen in AD- HIES confirm the widespread role played by STAT3 not only in the immune system, but also in the musculoskeletal, dental, craniofacial and vascular systems.
Characteristic facial features have been noted in AD-HIES and start becoming apparent in late childhood and early adolescence; these findings are almost universal by late adolescence. There is an asymmetric facies with prominent forehead and chin, increased inter-alar width, wide-set eyes, coarse skin and a high arched palate [4,18]. Craniosyntosis and Chiari I malformations have also been reported, although these are largely asymptomatic and do not usually require surgical intervention [19-21].
Musculoskeletal abnormalities found in AD-HIES include minimal trauma fractures, osteopenia, scoliosis and joint hyperextensibility [4]. About half of patients with AD-HIES develop minimal trauma fractures, mostly affecting the long bones and ribs. Many patients also have osteopenia, although the correlation between fractures and osteopenia is not very strong. Sixty percent of patients have scoliosis, which can be severe enough to warrant surgical intervention. Joint hyperextensibility occurs in 68% of patients and may account for the earlier occurrence of degenerative joint disease in this group of patients.
Abnormalities in the dentition are frequently seen in AD-HIES with approximately 70% of patients having delayed exfoliation of three or more primary teeth. Retention of primary teeth is thought to be due to reduced resorption of tooth roots resulting in failure of eruption of permanent teeth, although the mechanism underlying this abnormality is not known [22]. Dental extraction of primary teeth usually results in normal eruption of the permanent dentition. Other oral cavity abnormalities have also been described, including a high arched palate, central ridges and fissures of the palate and deep grooves on the tongue and buccal mucosa with multiple fissures [23].
More recently, vascular abnormalities, including tortuosity, dilatation and aneurysms of middle sized arteries, as well as lacunar infarcts, have been identified [13,19,24-26]. The report of a man with coronary artery aneurysms resulting in myocardial infarction led to more systematic evaluation of the coronary arteries [24,27]. Coronary artery aneurysms and tortuosity are commonly seen in AD-HIES. There was also an increased incidence of hypertension but not much atherosclerosis [28]. Cerebral artery aneurysms have also been reported and cerebral magnetic resonance imaging (MRI) has shown an increase in lacunar infarcts at a younger age as well as focal hyperintensities of indeterminate aetiology, although the clinical significance of this is uncertain [19]. The aetiology of the vascular abnormalities in HIES remains to be elucidated, although it is suspected to be due to the effects of STAT3 on vascular remodelling resulting in arterial fragility, rather than an inflammatory process. Murine data showing an increase in aneurysm severity and rupture after inhibition of STAT3 signalling or IL-17A blockade further supports this [26]. Dysregulation of transforming growth factor-β and matrix metallo-proteinases are thought to be involved, although this remains to be proven [26,28].
Patients with AD-HIES have a higher incidence of malignant disease, particularly non-Hodgkin's lymphoma [29-31]. Other malignancies reported include Hodgkin's lymphoma [31], and single case reports of squamous cell carcinoma of the vulva (related to human papilloma virus infection) [32] and pulmonary adenocarcinoma with liver, bone and spinal cord metastases [33]. It should be noted that in the majority of reported cases of malignancy, a molecular diagnosis of a STAT3 mutation was not made and other genetic mutations might have been responsible for the syndrome [31]. The increased risk of malignancy is potentially due to both increased susceptibility to infection (resulting in tumorigenesis) as well as aberrant function of STAT3, which has been shown to have roles in tumour development [31]. Autoimmune disease, including systemic lupus erythematosus, vasculitis, dermatomyositis and membranoproliferative glomerulonephritis, have also been described but only occur infrequently [34-38].
Laboratory findings
In keeping with the syndrome, marked elevations in serum IgE are usually present, with a serum IgE of >2,000 IU/ml being set as an arbitrary diagnostic level. However, as IgE levels only start to increase after birth, it is possible that the diagnostic threshold of >2,000 IU/ml may not be present in the very young. In addition, IgE levels may normalize or decrease in adulthood [4]. Using an age-adjusted value of ten times the age-appropriate level has been suggested in affected infants. The specificity of the IgE remains uncertain as well. Elevated anti-S. aureus and anti-Candida albicans IgE levels have been noted, but there is no known relationship with disease severity [39].
Eosinophilia is present in >90% of patients, and does not correlate with the elevation in IgE. White cell counts are usually within the normal range, although both elevation and chronic leukopenia with neutropenia have been reported [40]. Reduced levels of CD45RO+ central memory T cells and CD27+ memory B cells have also been noted [41-43]. The reduction of central memory T cells was thought to correlate with decreased ability to control latent varicella zoster and Epstein-Barr virus infection, with reduced T-cell memory responses to both viruses [43]. However, the significance of the reduction of memory B cells was unclear as there was no relationship between this and specific antibody production or infection history [42].
Genetics
Dominant negative mutations in STAT3 were identified as the cause of AD-HIES in 2007 [5,6]. Mutations were found mostly in the SH2 and DNA binding domains of STAT3 and were mostly missense mutations resulting in single amino acid changes or short in-frame deletions [5,6,44-47]. Despite the different functions of the affected domains, there does not seem to be a significant genotype-phenotype correlation [48]. There is, however, a slight increase in some of the non-immunological features in patients with SH2 mutations, including a high arched palate, widened inter-alar distance, upper respiratory tract infections and scoliosis [49]. It has been hypothesized that the increased frequency of upper respiratory tract infections might be due to anatomical, rather than immunological, differences.
Laboratory experiments have shown that mutated STAT3 exerts a dominant negative effect on wild-type STAT3 function. This is further supported by data showing that mice with a complete deletion of a single STAT3 allele are phenotypically normal. It should also be noted that STAT3 is necessary for in utero development as homozygous STAT3 knockout is embryologically lethal [50].
Pathogenesis
The identification of STAT3 mutations being the cause of AD-HIES has resulted in greater understanding of its role in both the immunologic and non-immunologic features of the disease, although there is much that is still not well understood. In broad terms, AD-HIES is a disease of both excess and too little inflammation, as evidenced by the florid purulence seen in pneumonias contrasted with the 'cold' abscesses.
STAT3 is a signal transduction protein that is integral in secretion or signalling of multiple cytokines, including IL-6, IL-10, IL-11, IL-17, IL -21, IL-22, IL-23, leukaemia inhibitory factor, oncostatin M, cardiotrophin-1, cardiotrophin-like cytokine and ciliary neurotrophic factor. The fact that this pathway involves both pro-inflammatory cytokines (for example, IL-6) and anti-inflammatory cytokines (for example, IL-10) accounts for both the excess and lack of inflammation seen.
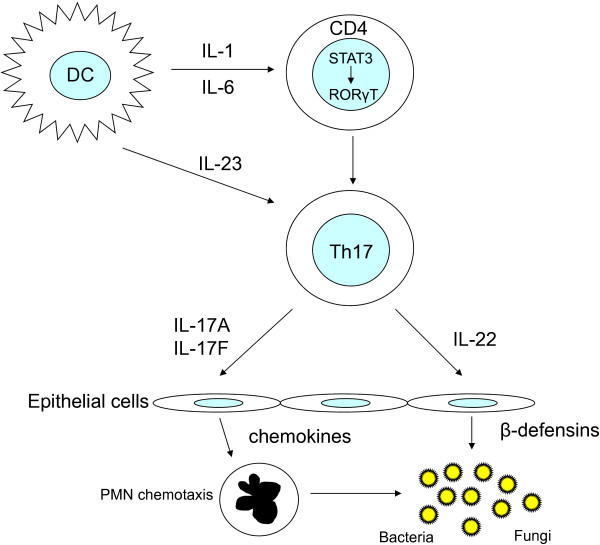
STAT3 mutations result in failure of differentiation of Th17 cells and subsequent failure of IL-17 secretion (Figure 2). This explains part of the increased susceptibility to infection seen in AD-HIES [44,47,51]. The susceptibility to mucocutaneous candidiasis due to defective IL-17 signalling has been shown in patients with auto-antibodies to IL-17 as well as mutations in IL-17F or the IL-17 receptor [52-54]. Abnormal IL-17 signalling in mice is associated with both candida and extracellular bacterial infections [55,56].
Figure 2.
Role of STAT3 and consequences of its dysfunction in the differentiation of Th17 cells and defence against infection. Secretion of IL-1 and IL-6 by dendritic cells (DCs) under appropriate conditions results in Th17 differentiation. IL-6 is a STAT3-dependent cytokine that activates the transcription factor retinoic acid-related RORγt. Th17 cells secrete IL-17A, IL17-F and IL-22. IL-17A and IL-17F stimulate epithelial cells to produce chemokines that recruit polymorphonuclear leukocytes (PMNs) for killing of pathogens by phagocytosis. IL-22 secretion triggers the production of defensins by epithelial cells for further defence against extracellular pathogens. Mutations in STAT3 result in failure of Th17 differentiation, which, in turn, leads to susceptibility to fungi and extracellular bacteria.
Th17 cells are known to also secrete IL-22, which is responsible for upregulating secretion of antimicrobial peptides like human beta defensins and CCL20 [57]. The production of these antimicrobial peptides from keratinocytes and lung epithelial cells (when stimulated by T cells) is reduced in AD-HIES patients [58]. These cell types have a far greater dependence on Th17 cytokines for their production of antibacterial peptides and chemokines, suggesting that the skin and lung infections in AD-HIES might be a result of deficient Th17 differentiation.
Craniosyntosis, delayed tooth eruption and supernumerary teeth have been shown to result from deficient IL-11 signalling as a result of homozygous missense mutations in IL-11RA (encoding interleukin 11 receptor, alpha) [59]. These mutations were shown to disrupt the ability of IL-11R alpha to activate STAT3-mediated signal transduction, hence resulting in clinical features seen in STAT3 deficiency.
In view of the abnormalities in tissue remodelling (evidenced by abnormal healing after pulmonary infection or surgery), matrix metalloproteinases (MMPs) have been investigated in patients with AD-HIES because of the role they play. STAT3 has been shown to have a role in the regulation of several MMPs [60-62]. In a study of 37 patients with AD-HIES, plasma MMP-8 and MMP-9 levels were found to be three times higher than in controls, in contrast to MMP-3 levels, which were only a third of those in the controls [63]. MMP-8 has been shown to be involved in acute lung inflammation [64], MMP-9 is associated with abdominal aortic aneurysms [65,66] and MMP-3 has a role in angiogenesis and fibrolysis, suggesting that they may be significant in the abnormalities seen in HIES.
Diagnosis
Based on the work done at the NIH, a HIES scoring system had originally been developed for genetic linkage studies [4,67]. AD-HIES was considered highly likely with a score of >40 and unlikely with a score of <20 points. A score between 20 and 40 gave an intermediate probability, and patients might have AD-HIES and could be followed over time to obtain more data, or could have another genetic form of HIES.
The most common differential diagnosis in a child with eczema and a significantly elevated IgE level is atopic dermatitis. With the discovery of STAT3 mutations and the finding that Th17 cells are reduced in this group of patients, further efforts were made to determine if this feature could be used to help improve diagnosis of STAT3 mutations [48,68]. These studies confirmed that in larger groups of patients with STAT3 mutations, Th17 cells were reduced and could potentially help with making the diagnosis. Further to this, an alternative scoring system (incorporating Th17 counts) to distinguish patients with and without STAT3 mutations has been suggested [48]. This scoring system divided patients into three categories: possible, with an IgE >1,000 IU/ml plus a weighted score of >30 of recurrent pneumonia, newborn rash, pathologic bone fractures, characteristic facies and high palate; probable, with these features and a lack of Th 17 cells or a definite family history of HIES; and definite, with these features and a dominant-negative heterozygous mutation in STAT3 [48].
However, testing for Th17 levels is a specialized test and may not be easily available, in which case testing for the STAT3 genetic mutation may be easier to perform in routine clinical practice. Although the scoring system represents a useful means for screening patients for genetic testing for STAT3 mutations, clinicians should not be put off from pursuing a molecular diagnosis in an individual patient on the basis of the diagnostic scores alone as the features of HIES can accumulate with time and more aggressive treatment can prevent the development of classical complications with time [48].
It should also be noted that there are other primary immunodeficiency disorders that can result in a clinical picture with eczematous rash, elevated IgE and recurrent infections. These include Omenn syndrome (MIM#603554, caused by hypomorphic mutations in RAG1, RAG2 and Artemis), Wiskott-Aldrich syndrome (MIM#301000, caused by WAS mutations), Wiskott-Aldrich syndrome 2 (MIM#614493, caused by WIPF1 mutations), immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX; MIM#304790, caused by FOXP3 mutations) and Netherton syndrome (MIM#256500, caused by SPINK5 mutations). However, these disorders have additional characteristics specific to the individual conditions.
Treatment
The principal goals of the management of HIES is aggressive treatment of infections and good skin care. As patients with HIES may lack the typical inflammatory features of infection, taking a good history, careful physical examination and appropriate imaging are necessary to pick up infections early.
The dermatitis in HIES is largely driven by ongoing infection, particularly S. aureus. Consequently, treatment of the skin includes bleach baths or chlorhexidine washes as well as prophylactic antibiotics (for example, co-trimoxazole, which targets S. aureus). The development of skin abscesses has reduced following the introduction of prophylactic antibiotics, although these sometimes require surgical intervention.
The other significant infectious problem is fungal infections. Chronic mucocutaneous candidiasis frequently occurs in the form of onychomycosis, and oral/vaginal thrush. Oral antifungal agents (for example, fluconazole) are generally effective in controlling the candidiasis, and if necessary, can be used for prophylaxis. In patients with fungal lung infections, anti-Aspergillus therapy (for example, itraconazole, voriconazole or posaconazole) can be used. Anti-Aspergillus prophylaxis is also considered in patients with pneumatoceles in view of the higher risk of developing fungal infection.
A further point of note is that the aberrant tissue healing following pulmonary infections can result in parenchymal abnormalities that allow colonisation with P. aeruginosa, fungal infections and non-tuberculous mycobacteria. Superinfection with these organisms represents the most challenging aspect of long-term management. Eradication of these organisms is difficult and the role of surgery for areas of parenchymal abnormality is uncertain. Pulmonary surgery appears to be associated with a greater risk of complications and should be carefully considered and only undertaken in a centre with particular experience in the disease.
Hypertension is often seen in HIES and should be treated aggressively due to the association with vascular abnormalities [28]. Defects in antibody production have also been reported in HIES, although these are variable [69]. These variable responses make it difficult to make blanket recommendations for immunoglobulin replacement therapy. There is also limited data suggesting improvement in some patients with high-dose immunoglobulin therapy [35,70], although some form of controlled trial is probably warranted. At present, it would seem reasonable to test vaccination responses and consider therapy in those who fail to respond.
Bone marrow transplantation has also been tried in AD-HIES, although its exact role remains to be clarified. The first patient transplanted was a 46 year old man with recurrent pneumonias who received a peripheral stem cell transplant for B cell lymphoma. However, he died 6 months following transplant with interstitial pneumonitis [71]. Subsequently, a second case involving a 7-year-old girl was reported [72]. She was transplanted to treat her severe HIES and her skin lesions improved. However, she developed recurrence of symptoms after 4 years. Her serum IgE also returned to pre-transplant levels. Interestingly, this occurred despite full donor engraftment in all lineages, suggesting that the reasons for recurrence may have been somatic or not just confined to the haematopoietic system.
More recently, two unrelated male children with sporadic STAT3 mutations were transplanted for highgrade non-Hodgkin's lymphoma [73]. At 10 and 14 years following transplantation, both patients were reported to be well with continued resolution of both immunological and non-immunological features. Of particular note, both osteoporosis and the characteristic facies improved following transplant. The successful transplant in these two individuals is significant as this potentially represents a means of preventing the long-term complications of chronic lung disease, vascular aneurysms and brain lesions.
Autosomal recessive hyper-IgE syndrome
Renner and colleagues [74] described a cohort of 13 patients from 6 consanguineous families that had features consistent with a diagnosis of HIES, including recurrent pneumonia and abscesses, eczema, elevated serum IgE and eosinophilia. However, these patients were different from those with AD-HIES in that they did not have the connective tissue and skeletal abnormalities typically seen, but had increased viral skin infection, more neurological symptoms and autoimmunity. The disease entity was designated as AR-HIES.
Subsequently, in 2009, mutations in the dedicator of cytokinesis-8 gene (DOCK8) were found to account for the majority of patients with AR-HIES [75,76]. Both homozygous and compound heterozygous mutations were reported and large deletions were frequent; and most of the individuals with DOCK8 mutations had absent or reduced levels of protein. DOCK8 belongs to the 11-member family of DOCK180 proteins, which are involved in cytoskeletal rearrangement allowing cell migration, adhesion and growth.
DOCK8 is a Cdc42-specific guanine nucleotide exchange factor (GEF) at the plasma membrane needed for spatial activation of Cdc42 at the leading edge of DCs during interstitial migration. Absence of DOCK8 results in failure of DC migration to lymph nodes and defective CD4+ T-cell priming [77]. In B cells, DOCK8 functions as an adaptor protein downstream of TLR9 and upstream of STAT3, driving B cell proliferation and immunoglobulin production [78]. DOCK8 deficiency impacts long-term memory of B cells as well as of virus-specific CD8+ T cells [79-81], which might explain the susceptibility to bacterial and persistent viral infections.
Clinically, patients with DOCK8 deficiency had features similar to AD-HIES, with elevated IgE levels, eosinophilia, eczema, recurrent sinopulmonary infections, staphylococcal skin abscesses, mucocutaneous candidiasis and increased frequency of malignant disease. Food allergies were also present in patients with DOCK8 deficiency (unlike AD-HIES). However, the connective tissue and skeletal abnormalities present in AD-HIES, such as retained dentition, characteristic facies and minimal trauma fractures, were much less frequent in DOCK8 deficiency. Notably, however, patients with DOCK8 deficiency were highly susceptible to viral skin infections, including severe Molluscum contagiosum infection, warts, herpes zoster and recurrent herpes simplex infections. There is higher mortality at a younger age in DOCK8 deficiency, with death often occurring before the age of 20. Other unusual features described in single patients include sclerosing cholangitis and colitis, granulomatous soft tissue lesions, primary central nervous system lymphoma and fatal metastatic leiomyosarcoma [82].
Several differentiating laboratory features have also been described in DOCK8 deficiency, compared to AD-HIES. Although both show elevated serum IgE levels and eosinophilia, patients with DOCK8 deficiency have reduced serum IgM levels as well as lymphopenia, mainly due to reductions in T cells, although normal levels are seen in some patients. Serum IgG and IgA levels as well as specific antibody production are variable, and abnormal lymphocyte proliferative responses, particularly in the CD8+ T cell compartment, have been noted [82].
Treatment
Broad treatment strategies in AR-HIES are similar to those in AD-HIES with good skin care, appropriate treatment and prophylaxis of staphylococcal skin infections, and prophylactic antimicrobial therapy for sinopulmonary infections. Allergic disease and asthma are more common in AR-HIES and require conventional treatment with inhaled corticosteroids and antihistamines. Specific antibody production in AR-HIES can be variable (despite normal IgG levels) and replacement immunoglobulin therapy has been used with anecdotal improvement in respiratory tract infections. Viral skin infections have unfortunately not improved with replacement immunoglobulin therapy. Widespread molluscum and human papilloma virus infection has been difficult to treat - standard therapies with salicylic acid, cryotherapy and imiquimod have had limited success; interferon-alpha has been used anecdotally with mixed results.
Haematopoeitic stem cell transplantation has been reported in DOCK8 deficiency in 12 patients to date [82-86]. In all individuals, resolution of recurrent infections (particularly viral skin infections with molluscum) and eczema occurred, although one individual continued to suffer from food allergies. Improvement in IgE levels as well as resolution of vasculitis were also reported. These initial results suggest that stem cell transplantation in AR-HIES may represent an excellent curative option given the high morbidity and mortality seen in the disease.
Other hyper-IgE syndromes
Several other defined single gene mutations have been described to result in syndromes with features of HIES as well as other abnormalities.
Mutations in Tyk2 (Tyrosine kinase 2; MIM#611521) were originally described in a single patient with AR-HIES who additionally suffered from susceptibility to Bacille Calmette-Guérin and salmonella, features more commonly associated with defects in the interferon-gamma/IL-12 pathway [87]. However, a second patient with Tyk2 mutations has been described with atypical mycobacterial and viral infections but without the pyogenic infections normally seen in HIES, suggesting that the occurrence of the HIES phenotype in Tyk2 deficiency may depend on other genetic loci as well [88].
Other genetic syndromes with features of HIES reported include the coexistence of HIES and Dubowitz syndrome (postnatal growth retardation, microcephaly and characteristic facies) [89]; HIES and pentasomy × [90]; and HIES and Saethre-Chotzen syndrome (acro-cephalosyndactyly, hypertelorism and ptosis due to mutations in TWIST) [91]. The common mechanisms between these syndromes and STAT3 and DOCK8 deficiency remain undefined at present.
Conclusion
With the identification of the molecular aetiologies of AD-HIES and AR-HIES, our understanding of these diseases and, in particular, the role of STAT3 and of DOCK8 in immune function has increased. In addition it would be expected that as time progresses, the role of these molecules in immune function as well as their contribution to the various non-immunologic features in HIES will be further delineated. This may shed further insight into common diseases like eczema, susceptibility to staphylococcal infection and idiopathic scoliosis. Furthermore, a better understanding of the STAT3 and DOCK8 pathways will also help in understanding the aetiology and pathogenesis in other, as yet undefined HIES syndromes.
Note
This article is part of the series on Adult immunodeficiency, edited by Hans-Hartmut Peter. Other articles in this series can be found at http://arthritis-research.com/series/immunodeficiency
Abbreviations
AD-HIES: autosomal dominant hyper-IgE syndrome; AR-HIES: autosomal recessive hyper-IgE syndrome; DOCK8: Dedicator of cytokinesis 8; HIES: hyper-IgE syndrome; IL: interleukin; MMP: matrix metalloproteinase; STAT: Signal transducer and activator of transcription; Tyk2: Tyrosine kinase 2.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Patrick FK Yong, Email: pyong@doctors.org.uk.
Alexandra F Freeman, Email: freemaal@mail.nih.gov.
Karin R Engelhardt, Email: k.engelhardt@ucl.ac.uk.
Steven Holland, Email: SHOLLAND@niaid.nih.gov.
Jennifer M Puck, Email: puckj@peds.ucsf.edu.
Bodo Grimbacher, Email: b.grimbacher@ucl.ac.uk.
References
- Davis SD, Schaller J, Wedgwood RJ. Job's Syndrome. Recurrent, "cold", staphylococcal abscesses. Lancet. 1966;14:1013–1015. doi: 10.1016/s0140-6736(66)90119-x. [DOI] [PubMed] [Google Scholar]
- Buckley RH, Wray BB, Belmaker EZ. Extreme hyperimmunoglobulinemia E and undue susceptibility to infection. Pediatrics. 1972;14:59–70. [PubMed] [Google Scholar]
- Hill HR, Ochs HD, Quie PG, Clark RA, Pabst HF, Klebanoff SJ, Wedgwood RJ. Defect in neutrophil granulocyte chemotaxis in Job's syndrome of recurrent "cold" staphylococcal abscesses. Lancet. 1974;14:617–619. doi: 10.1016/s0140-6736(74)91942-4. [DOI] [PubMed] [Google Scholar]
- Grimbacher B, Holland SM, Gallin JI, Greenberg F, Hill SC, Malech HL, Miller JA, O'Connell AC, Puck JM. Hyper-IgE syndrome with recurrent infections--an autosomal dominant multisystem disorder. N Engl J Med. 1999;14:692–702. doi: 10.1056/NEJM199903043400904. [DOI] [PubMed] [Google Scholar]
- Holland SM, DeLeo FR, Elloumi HZ, Hsu AP, Uzel G, Brodsky N, Freeman AF, Demidowich A, Davis J, Turner ML, Anderson VL, Darnell DN, Welch PA, Kuhns DB, Frucht DM, Malech HL, Gallin JI, Kobayashi SD, Whitney AR, Voyich JM, Musser JM, Woellner C, Schäffer AA, Puck JM, Grimbacher B. STAT3 mutations in the hyper-IgE syndrome. N Engl J Med. 2007;14:1608–1619. doi: 10.1056/NEJMoa073687. [DOI] [PubMed] [Google Scholar]
- Minegishi Y, Saito M, Tsuchiya S, Tsuge I, Takada H, Hara T, Kawamura N, Ariga T, Pasic S, Stojkovic O, Metin A, Karasuyama H. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;14:1058–1062. doi: 10.1038/nature06096. [DOI] [PubMed] [Google Scholar]
- Sowerwine KJ, Holland SM, Freeman AF. Hyper-IgE syndrome update. Ann N Y Acad Sci. 2012;14:25–32. doi: 10.1111/j.1749-6632.2011.06387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamlin SL, McCalmont TH, Cunningham BB, Esterly NB, Lai CH, Mallory SB, Mancini AJ, Tamburro J, Frieden IJ. Cutaneous manifestations of hyper-IgE syndrome in infants and children. J Pediatr. 2002;14:572–575. doi: 10.1067/mpd.2002.127503. [DOI] [PubMed] [Google Scholar]
- Eberting CL, Davis J, Puck JM, Holland SM, Turner ML. Dermatitis and the newborn rash of hyper-IgE syndrome. Arch Dermatol. 2004;14:1119–1125. doi: 10.1001/archderm.140.9.1119. [DOI] [PubMed] [Google Scholar]
- Erlewyn-Lajeunesse MD. Hyperimmunoglobulin-E syndrome with recurrent infection: a review of current opinion and treatment. Pediatr Allergy Immunol. 2000;14:133–141. doi: 10.1034/j.1399-3038.2000.00091.x. [DOI] [PubMed] [Google Scholar]
- Melia E, Freeman AF, Shea YR, Hsu AP, Holland SM, Olivier KN. Pulmonary nontuberculous mycobacterial infections in hyper-IgE syndrome. J Allergy Clin Immunol. 2009;14:617–618. doi: 10.1016/j.jaci.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinh DC, Sugui JA, Hsu AP, Freeman AF, Holland SM. Invasive fungal disease in autosomal-dominant hyper-IgE syndrome. J Allergy Clin Immunol. 2010;14:1389–1390. doi: 10.1016/j.jaci.2010.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman AF, Kleiner DE, Nadiminti H, Davis J, Quezado M, Anderson V, Puck JM, Holland SM. Causes of death in hyper-IgE syndrome. J Allergy Clin Immunol. 2007;14:1234–1240. doi: 10.1016/j.jaci.2006.12.666. [DOI] [PubMed] [Google Scholar]
- Freeman AF, Davis J, Anderson VL, Barson W, Darnell DN, Puck JM, Holland SM. Pneumocystis jiroveci infection in patients with hyper-immunoglobulin E syndrome. Pediatrics. 2006;14:e1271–e1275. doi: 10.1542/peds.2006-0311. [DOI] [PubMed] [Google Scholar]
- Garty BZ, Ben-Baruch A, Rolinsky A, Woellner C, Grimbacher B, Marcus N. Pneumocystis jirovecii pneumonia in a baby with hyper-IgE syndrome. Eur J Pediatr. 2010;14:35–37. doi: 10.1007/s00431-009-0973-5. [DOI] [PubMed] [Google Scholar]
- Jacobs DH, Macher AM, Handler R, Bennett JE, Collen MJ, Gallin JI. Esophageal cryptococcosis in a patient with the hyperimmunoglobulin E-recurrent infection (Job's) syndrome. Gastroenterology. 1984;14:201–203. [PubMed] [Google Scholar]
- Hutto JO, Bryan CS, Greene FL, White CJ, Gallin JI. Cryptococcosis of the colon resembling Crohn's disease in a patient with the hyperimmunoglobulinemia E-recurrent infection (Job's) syndrome. Gastroenterology. 1988;14:808–812. doi: 10.1016/0016-5085(88)90257-0. [DOI] [PubMed] [Google Scholar]
- Borges WG, Hensley T, Carey JC, Petrak BA, Hill HR. The face of Job. J Pediatr. 1998;14:303–305. doi: 10.1016/S0022-3476(98)70243-4. [DOI] [PubMed] [Google Scholar]
- Freeman AF, Collura-Burke CJ, Patronas NJ, Ilcus LS, Darnell D, Davis J, Puck JM, Holland SM. Brain abnormalities in patients with hyperimmunoglobulin E syndrome. Pediatrics. 2007;14:e1121–e1125. doi: 10.1542/peds.2006-2649. [DOI] [PubMed] [Google Scholar]
- Hoger PH, Boltshauser E, Hitzig WH. Craniosynostosis in hyper-IgEsyndrome. Eur J Pediatr. 1985;14:414–417. doi: 10.1007/BF00441793. [DOI] [PubMed] [Google Scholar]
- Smithwick EM, Finelt M, Pahwa S, Good RA, Naspitz CK, Mendes NF, Kopersztyk S, Spira TJ, Nahmias AJ. Cranial synostosis in Job's syndrome. Lancet. 1978;14:826. doi: 10.1016/s0140-6736(78)93028-3. [DOI] [PubMed] [Google Scholar]
- O'Connell AC, Puck JM, Grimbacher B, Facchetti F, Majorana A, Gallin JI, Malech HL, Holland SM. Delayed eruption of permanent teeth in hyperimmunoglobulinemia E recurrent infection syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;14:177–185. doi: 10.1067/moe.2000.103129. [DOI] [PubMed] [Google Scholar]
- Domingo DL, Freeman AF, Davis J, Puck JM, Tianxia W, Holland SM, Hart TC. Novel intraoral phenotypes in hyperimmunoglobulin-E syndrome. Oral Dis. 2008;14:73–81. doi: 10.1111/j.1601-0825.2007.01363.x. [DOI] [PubMed] [Google Scholar]
- Ling JC, Freeman AF, Gharib AM, Arai AE, Lederman RJ, Rosing DR, Holland SM. Coronary artery aneurysms in patients with hyper IgE recurrent infection syndrome. Clin Immunol. 2007;14:255–258. doi: 10.1016/j.clim.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young TY, Jerome D, Gupta S. Hyperimmunoglobulinemia E syndrome associated with coronary artery aneurysms: deficiency of central memory CD4+ T cells and expansion of effector memory CD4+ T cells. Ann Allergy Asthma Immunol. 2007;14:389–392. doi: 10.1016/S1081-1206(10)60887-3. [DOI] [PubMed] [Google Scholar]
- Chandesris MO, Azarine A, Ong KT, Taleb S, Boutouyrie P, Mousseaux E, Romain M, Bozec E, Laurent S, Boddaert N, Thumerelle C, Tillie-Leblond I, Hoarau C, Lebranchu Y, Aladjidi N, Tron F, Barlogis V, Body G, Munzer M, Jaussaud R, Suarez F, Clément O, Hermine O, Tedgui A, Lortholary O, Picard C, Mallat Z, Fischer A. Frequent and widespread vascular abnormalities in human signal transducer and activator of transcription 3 deficiency. Circ Cardiovasc Genet. 2012;14:25–34. doi: 10.1161/CIRCGENETICS.111.961235. [DOI] [PubMed] [Google Scholar]
- Alomar-Melero E, Martin TD, Janelle GM, Peng YG. An unusual giant right coronary artery aneurysm resembles an intracardiac mass. Anesth Analg. 2008;14:1161–1162. doi: 10.1213/ane.0b013e318181f74f. [DOI] [PubMed] [Google Scholar]
- Freeman AF, Avila EM, Shaw PA, Davis J, Hsu AP, Welch P, Matta JR, Hadigan C, Pettigrew RI, Holland SM, Gharib AM. Coronary artery abnormalities in Hyper-IgE syndrome. J Clin Immunol. 2011;14:338–345. doi: 10.1007/s10875-011-9515-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorin LJ, Jeha SC, Sullivan MP, Rosenblatt HM, Shearer WT. Burkitt's lymphoma developing in a 7-year-old boy with hyper-IgE syndrome. J Allergy Clin Immunol. 1989;14:5–10. doi: 10.1016/0091-6749(89)90471-5. [DOI] [PubMed] [Google Scholar]
- Leonard GD, Posadas E, Herrmann PC, Anderson VL, Jaffe ES, Holland SM, Wilson WH. Non-Hodgkin's lymphoma in Job's syndrome: a case report and literature review. Leuk Lymphoma. 2004;14:2521–2525. doi: 10.1080/10428190400004463. [DOI] [PubMed] [Google Scholar]
- Kumanovics A, Perkins SL, Gilbert H, Cessna MH, Augustine NH, Hill HR. Diffuse large B cell lymphoma in hyper-IgE syndrome due to STAT3 mutation. J Clin Immunol. 2010;14:886–893. doi: 10.1007/s10875-010-9452-z. [DOI] [PubMed] [Google Scholar]
- Clark TJ, Herod JJ, Kehoe S, Luesley DM. The development of invasive vulvar cancer in a patient with Job's syndrome, a rare immunodeficient condition. Br J Obstet Gynaecol. 1998;14:468–470. doi: 10.1111/j.1471-0528.1998.tb10137.x. [DOI] [PubMed] [Google Scholar]
- Oztop I, Demirkan B, Tarhan O, Kayahan H, Yilmaz U, Kargi A, Alakavuklar M. The development of pulmonary adenocarcinoma in a patient with Job's syndrome, a rare immunodeficiency condition. Tumori. 2004;14:132–135. doi: 10.1177/030089160409000126. [DOI] [PubMed] [Google Scholar]
- Min JK, Cho ML, Kim SC, Lee YS, Lee SH, Park SH, Hong YS, Cho CS, Kim HY. Hyperimmunoglobulin E-recurrent infection syndrome in a patient with juvenile dermatomyositis. Korean J Intern Med. 1999;14:95–98. doi: 10.3904/kjim.1999.14.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimata H. High-dose intravenous gamma-globulin treatment for hyperimmunoglobulinemia E syndrome. J Allergy Clin Immunol. 1995;14:771–774. doi: 10.1016/S0091-6749(95)70185-0. [DOI] [PubMed] [Google Scholar]
- Tanji C, Yorioka N, Kanahara K, Naito T, Oda H, Ishikawa K, Taguchi T. Hyperimmunoglobulin E syndrome associated with nephrotic syndrome. Intern Med. 1999;14:491–494. doi: 10.2169/internalmedicine.38.491. [DOI] [PubMed] [Google Scholar]
- Brugnoni D, Franceschini F, Airo P, Cattaneo R. Discordance for systemic lupus erythematosus and hyper IgE syndrome in a pair of monozygotic twins. Br J Rheumatol. 1998;14:807–808. doi: 10.1093/rheumatology/37.7.807b. [DOI] [PubMed] [Google Scholar]
- North J, Kotecha S, Houtman P, Whaley K. Systemic lupus erythematosus complicating hyper IgE syndrome. Br J Rheumatol. 1997;14:297–298. doi: 10.1093/rheumatology/36.2.297. [DOI] [PubMed] [Google Scholar]
- Berger M, Kirkpatrick CH, Goldsmith PK, Gallin JI. IgE antibodies to Staphylococcus aureus and Candida albicans in patients with the syndrome of hyperimmunoglobulin E and recurrent infections. J Immunol. 1980;14:2437–2443. [PubMed] [Google Scholar]
- Donabedian H, Gallin JI. The hyperimmunoglobulin E recurrent-infection (Job's) syndrome. A review of the NIH experience and the literature. Medicine (Baltimore) 1983;14:195–208. doi: 10.1097/00005792-198307000-00001. [DOI] [PubMed] [Google Scholar]
- Buckley RH. The hyper-IgE syndrome. Clin Rev Allergy Immunol. 2001;14:139–154. doi: 10.1385/CRIAI:20:1:139. [DOI] [PubMed] [Google Scholar]
- Speckmann C, Enders A, Woellner C, Thiel D, Rensing-Ehl A, Schlesier M, Rohr J, Jakob T, Oswald E, Kopp MV, Sanal O, Litzman J, Plebani A, Pietrogrande MC, Franco JL, Espanol T, Grimbacher B, Ehl S. Reduced memory B cells in patients with hyper IgE syndrome. Clin Immunol. 2008;14:448–454. doi: 10.1016/j.clim.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Siegel AM, Heimall J, Freeman AF, Hsu AP, Brittain E, Brenchley JM, Douek DC, Fahle GH, Cohen JI, Holland SM, Milner JD. A critical role for STAT3 transcription factor signaling in the development and maintenance of human T cell memory. Immunity. 2011;14:806–818. doi: 10.1016/j.immuni.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner ED, Rylaarsdam S, Anover-Sombke S, Rack AL, Reichenbach J, Carey JC, Zhu Q, Jansson AF, Barboza J, Schimke LF, Leppert MF, Getz MM, Seger RA, Hill HR, Belohradsky BH, Torgerson TR, Ochs HD. Novel signal transducer and activator of transcription 3 (STAT3) mutations, reduced T(H)17 cell numbers, and variably defective STAT3 phosphorylation in hyper-IgE syndrome. J Allergy Clin Immunol. 2008;14:181–187. doi: 10.1016/j.jaci.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma CS, Chew GY, Simpson N, Priyadarshi A, Wong M, Grimbacher B, Fulcher DA, Tangye SG, Cook MC. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med. 2008;14:1551–1557. doi: 10.1084/jem.20080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao H, Tóth B, Erdos M, Fransson I, Rákóczi E, Balogh I, Magyarics Z, Dérfalvi B, Csorba G, Szaflarska A, Megarbane A, Akatcherian C, Dbaibo G, Rajnavölgyi E, Hammarstróm L, Kere J, Lefranc G, Marodi L. Novel and recurrent STAT3 mutations in hyper-IgE syndrome patients from different ethnic groups. Mol Immunol. 2008;14:202–206. doi: 10.1016/j.molimm.2008.07.001. [DOI] [PubMed] [Google Scholar]
- de Beaucoudrey L, Puel A, Filipe-Santos O, Cobat A, Ghandil P, Chrabieh M, Feinberg J, von Bernuth H, Samarina A, Jannière L, Fieschi C, Stéphan JL, Boileau C, Lyonnet S, Jondeau G, Cormier-Daire V, Le Merrer M, Hoarau C, Lebranchu Y, Lortholary O, Chandesris MO, Tron F, Gambineri E, Bianchi L, Rodriguez-Gallego C, Zitnik SE, Vasconcelos J, Guedes M, Vitor AB, Marodi L. et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med. 2008;14:1543–1550. doi: 10.1084/jem.20080321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woellner C, Gertz EM, Schäffer AA, Lagos M, Perro M, Glocker EO, Pietrogrande MC, Cossu F, Franco JL, Matamoros N, Pietrucha B, Heropolitańska-Pliszka E, Yeganeh M, Moin M, Español T, Ehl S, Gennery AR, Abinun M, Breborowicz A, Niehues T, Kilic SS, Junker A, Turvey SE, Plebani A, Sanchez B, Garty BZ, Pignata C, Cancrini C, Litzman J, Sanal O. et al. Mutations in STAT3 and diagnostic guidelines for hyper-IgE syndrome. J Allergy Clin Immunol. 2010;14:424–432. doi: 10.1016/j.jaci.2009.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimall J, Davis J, Shaw PA, Hsu AP, Gu W, Welch P, Holland SM, Freeman AF. Paucity of genotype-phenotype correlations in STAT3 mutation positive Hyper IgE Syndrome (HIES) Clin Immunol. 2011;14:75–84. doi: 10.1016/j.clim.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Noguchi K, Shi W, Tanaka T, Matsumoto M, Yoshida N, Kishimoto T, Akira S. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc Natl Acad Sci USA. 1997;14:3801–3804. doi: 10.1073/pnas.94.8.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML, Davis J, Hsu A, Asher AI, O'Shea J, Holland SM, Paul WE, Douek DC. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;14:773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel A, Döffinger R, Natividad A, Chrabieh M, Barcenas-Morales G, Picard C, Cobat A, Ouachée-Chardin M, Toulon A, Bustamante J, Al-Muhsen S, Al-Owain M, Arkwright PD, Costigan C, McConnell V, Cant AJ, Abinun M, Polak M, Bougnéres PF, Kumararatne D, Marodi L, Nahum A, Roifman C, Blanche S, Fischer A, Bodemer C, Abel L, Lilic D, Casanova JL. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med. 2010;14:291–297. doi: 10.1084/jem.20091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne SK, Holland SM. Anti-cytokine autoantibodies explain some chronic mucocutaneous candidiasis. Immunol Cell Biol. 2010;14:614–615. doi: 10.1038/icb.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisand K, Bøe Wolff AS, Podkrajsek KT, Tserel L, Link M, Kisand KV, Ersvaer E, Perheentupa J, Erichsen MM, Bratanic N, Meloni A, Cetani F, Perniola R, Ergun- Longmire B, Maclaren N, Krohn KJ, Pura M, Schalke B, Ströbel P, Leite MI, Battelino T, Husebye ES, Peterson P, Willcox N, Meager A. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med. 2010;14:299–308. doi: 10.1084/jem.20091669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;14:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, Husain S, Kreindler JL, Dubin PJ, Pilewski JM, Myerburg MM, Mason CA, Iwakura Y, Kolls JK. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolls JK, McCray PB Jr, Chan YR. Cytokine-mediated regulation of antimicrobial proteins. Nat Rev Immunol. 2008;14:829–835. doi: 10.1038/nri2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minegishi Y, Saito M, Nagasawa M, Takada H, Hara T, Tsuchiya S, Agematsu K, Yamada M, Kawamura N, Ariga T, Tsuge I, Karasuyama H. Molecular explanation for the contradiction between systemic Th17 defect and localized bacterial infection in hyper-IgE syndrome. J Exp Med. 2009;14:1291–1301. doi: 10.1084/jem.20082767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieminen P, Morgan NV, Fenwick AL, Parmanen S, Veistinen L, Mikkola ML, van der Spek PJ, Giraud A, Judd L, Arte S, Brueton LA, Wall SA, Mathijssen IM, Maher ER, Wilkie AO, Kreiborg S, Thesleff I. Inactivation of IL11 signaling causes craniosynostosis, delayed tooth eruption, and supernumerary teeth. Am J Hum Genet. 2011;14:67–81. doi: 10.1016/j.ajhg.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroer N, Pahne J, Walch B, Wickenhauser C, Smola S. Molecular pathobiology of human cervical high-grade lesions: paracrine STAT3 activation in tumor-instructed myeloid cells drives local MMP-9 expression. Cancer Res. 2011;14:87–97. doi: 10.1158/0008-5472.CAN-10-2193. [DOI] [PubMed] [Google Scholar]
- Song Y, Qian L, Song S, Chen L, Zhang Y, Yuan G, Zhang H, Xia Q, Hu M, Yu M, Shi M, Jiang Z, Guo N. Fra-1 and Stat3 synergistically regulate activation of human MMP-9 gene. Mol Immunol. 2008;14:137–143. doi: 10.1016/j.molimm.2007.04.031. [DOI] [PubMed] [Google Scholar]
- Tsareva SA, Moriggl R, Corvinus FM, Wiederanders B, Schütz A, Kovacic B, Friedrich K. Signal transducer and activator of transcription 3 activation promotes invasive growth of colon carcinomas through matrix metalloproteinase induction. Neoplasia. 2007;14:279–291. doi: 10.1593/neo.06820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekhsaria V, Dodd LE, Hsu AP, Heimall JR, Freeman AF, Ding L, Holland SM, Uzel G. Plasma metalloproteinase levels are dysregulated in signal transducer and activator of transcription 3 mutated hyper-IgE syndrome. J Allergy Clin Immunol. 2011;14:1124–1127. doi: 10.1016/j.jaci.2011.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero PA, Knolle MD, Cala LF, Zhuang Y, Owen CA. Matrix metalloproteinase-8 inactivates macrophage inflammatory protein-1 alpha to reduce acute lung inflammation and injury in mice. J Immunol. 2010;14:1575–1588. doi: 10.4049/jimmunol.0900290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyo R, Lee JK, Shipley JM, Curci JA, Mao D, Ziporin SJ, Ennis TL, Shapiro SD, Senior RM, Thompson RW. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest. 2000;14:1641–1649. doi: 10.1172/JCI8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan-Palikhe P, Vikatmaa P, Lajunen T, Palikhe A, Lepäntalo M, Tervahartiala T, Salo T, Saikku P, Leinonen M, Pussinen PJ, Sorsa. Elevated MMP-8 and decreased myeloperoxidase concentrations associate significantly with the risk for peripheral atherosclerosis disease and abdominal aortic aneurysm. Scand J Immunol. 2010;14:150–157. doi: 10.1111/j.1365-3083.2010.02418.x. [DOI] [PubMed] [Google Scholar]
- Grimbacher B, Schäffer AA, Holland SM, Davis J, Gallin JI, Malech HL, Atkinson TP, Belohradsky BH, Buckley RH, Cossu F, Español T, Garty BZ, Matamoros N, Myers LA, Nelson RP, Ochs HD, Renner ED, Wellinghausen N, Puck JM. Genetic linkage of hyper-IgE syndrome to chromosome 4. Am J Hum Genet. 1999;14:735–744. doi: 10.1086/302547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimke LF, Sawalle-Belohradsky J, Roesler J, Wollenberg A, Rack A, Borte M, Rieber N, Cremer R, Maass E, Dopfer R, Reichenbach J, Wahn V, Hoenig M, Jansson AF, Roesen-Wolff A, Schaub B, Seger R, Hill HR, Ochs HD, Torgerson TR, Belohradsky BH, Renner ED. Diagnostic approach to the hyper-IgE syndromes: immunologic and clinical key findings to differentiate hyper-IgE syndromes from atopic dermatitis. J Allergy Clin Immunol. 2010;14:611–617. doi: 10.1016/j.jaci.2010.06.029. [DOI] [PubMed] [Google Scholar]
- Sheerin KA, Buckley RH. Antibody responses to protein, polysaccharide, and phi X174 antigens in the hyperimmunoglobulinemia E (hyper-IgE) syndrome. J Allergy Clin Immunol. 1991;14:803–811. doi: 10.1016/0091-6749(91)90126-9. [DOI] [PubMed] [Google Scholar]
- Wakim M, Alazard M, Yajima A, Speights D, Saxon A, Stiehm ER. High dose intravenous immunoglobulin in atopic dermatitis and hyper-IgE syndrome. Ann Allergy Asthma Immunol. 1998;14:153–158. doi: 10.1016/S1081-1206(10)62802-5. [DOI] [PubMed] [Google Scholar]
- Nester TA, Wagnon AH, Reilly WF, Spitzer G, Kjeldsberg CR, Hill HR. Effects of allogeneic peripheral stem cell transplantation in a patient with job syndrome of hyperimmunoglobulinemia E and recurrent infections. Am J Med. 1998;14:162–164. doi: 10.1016/S0002-9343(98)00200-9. [DOI] [PubMed] [Google Scholar]
- Gennery AR, Flood TJ, Abinun M, Cant AJ. Bone marrow transplantation does not correct the hyper IgE syndrome. Bone Marrow Transplant. 2000;14:1303–1305. doi: 10.1038/sj.bmt.1702446. [DOI] [PubMed] [Google Scholar]
- Goussetis E, Peristeri I, Kitra V, Traeger-Synodinos J, Theodosaki M, Psarra K, Kanariou M, Tzortzatou-Stathopoulou F, Petrakou E, Fylaktou I, Kanavakis E, Graphakos S. Successful long-term immunologic reconstitution by allogeneic hematopoietic stem cell transplantation cures patients with autosomal dominant hyper-IgE syndrome. J Allergy Clin Immunol. 2010;14:392–394. doi: 10.1016/j.jaci.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Renner ED, Puck JM, Holland SM, Schmitt M, Weiss M, Frosch M, Bergmann M, Davis J, Belohradsky BH, Grimbacher B. Autosomal recessive hyperimmunoglobulin E syndrome: a distinct disease entity. J Pediatr. 2004;14:93–99. doi: 10.1016/S0022-3476(03)00449-9. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Davis JC, Dove CG, Su HC. Genetic, clinical, and laboratory markers for DOCK8 immunodeficiency syndrome. Dis Markers. 2010;14:131–139. doi: 10.3233/DMA-2010-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt KR, McGhee S, Winkler S, Sassi A, Woellner C, Lopez-Herrera G, Chen A, Kim HS, Lloret MG, Schulze I, Ehl S, Thiel J, Pfeifer D, Veelken H, Niehues T, Siepermann K, Weinspach S, Reisli I, Keles S, Genel F, Kutukculer N, Camcioğlu Y, Somer A, Karakoc-Aydiner E, Barlan I, Gennery A, Metin A, Degerliyurt A, Pietrogrande MC, Yeganeh M. et al. Large deletions and point mutations involving the dedicator of cytokinesis 8 (DOCK8) in the autosomal-recessive form of hyper-IgE syndrome. J Allergy Clin Immunol. 2009;14:1289–1302. doi: 10.1016/j.jaci.2009.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada Y, Tanaka Y, Terasawa M, Pieczyk M, Habiro K, Katakai T, Hanawa- Suetsugu K, Kukimoto-Niino M, Nishizaki T, Shirouzu M, Duan X, Uruno T, Nishikimi A, Sanematsu F, Yokoyama S, Stein JV, Kinashi T, Fukui Y. DOCK8 is a Cdc42 activator critical for interstitial dendritic cell migration during immune responses. Blood. 2012;14:4451–4461. doi: 10.1182/blood-2012-01-407098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabara HH, McDonald DR, Janssen E, Massaad MJ, Ramesh N, Borzutzky A, Rauter I, Benson H, Schneider L, Baxi S, Recher M, Notarangelo LD, Wakim R, Dbaibo G, Dasouki M, Al-Herz W, Barlan I, Baris S, Kutukculer N, Ochs HD, Plebani A, Kanariou M, Lefranc G, Reisli I, Fitzgerald KA, Golenbock D, Manis J, Keles S, Ceja R, Chatila TA, Geha RS. DOCK8 functions as an adaptor that links TLR-MyD88 signaling to B cell activation. Nat Immunol. 2012;14:612–620. doi: 10.1038/ni.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall KL, Chan SS, Ma CS, Fung I, Mei Y, Yabas M, Tan A, Arkwright PD, Al Suwairi W, Lugo Reyes SO, Yamazaki-Nakashimada MA, Garcia-Cruz Mde L, Smart JM, Picard C, Okada S, Jouanguy E, Casanova JL, Lambe T, Cornall RJ, Russell S, Oliaro J, Tangye SG, Bertram EM, Goodnow CC. DOCK8 deficiency impairs CD8 T cell survival and function in humans and mice. J Exp Med. 2011;14:2305–2320. doi: 10.1084/jem.20110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall KL, Lambe T, Goodnow CC, Cornall RJ. The essential role of DOCK8 in humoral immunity. Dis Markers. 2010;14:141–150. doi: 10.3233/DMA-2010-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe T, Crawford G, Johnson AL, Crockford TL, Bouriez-Jones T, Smyth AM, Pham TH, Zhang Q, Freeman AF, Cyster JG, Su HC, Cornall RJ. DOCK8 is essential for T-cell survival and the maintenance of CD8+ T-cell memory. Eur J Immunol. 2011;14:3423–3435. doi: 10.1002/eji.201141759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanal O, Jing H, Ozgur T, Ayvaz D, Strauss-Albee DM, Ersoy-Evans S, Tezcan I, Turkkani G, Matthews HF, Haliloglu G, Yuce A, Yalcin B, Gokoz O, Oguz KK, Su HC. Additional diverse findings expand the clinical presentation of DOCK8 deficiency. J Clin Immunol. 2012;14:698–708. doi: 10.1007/s10875-012-9664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatz SA, Benninghoff U, Schütz C, Schulz A, Hönig M, Pannicke U, Holzmann KH, Schwarz K, Friedrich W. Curative treatment of autosomal-recessive hyper-IgE syndrome by hematopoietic cell transplantation. Bone Marrow Transplant. 2011;14:552–556. doi: 10.1038/bmt.2010.169. [DOI] [PubMed] [Google Scholar]
- Bittner TC, Pannicke U, Renner ED, Notheis G, Hoffmann F, Belohradsky BH, Wintergerst U, Hauser M, Klein B, Schwarz K, Schmid I, Albert MH. Successful long-term correction of autosomal recessive hyper-IgE syndrome due to DOCK8 deficiency by hematopoietic stem cell transplantation. Klin Padiatr. 2010;14:351–355. doi: 10.1055/s-0030-1265135. [DOI] [PubMed] [Google Scholar]
- Barlogis V, Galambrun C, Chambost H, Lamoureux-Toth S, Petit P, Stephan JL, Michel G, Fischer A, Picard C. Successful allogeneic hematopoietic stem cell transplantation for DOCK8 deficiency. J Allergy Clin Immunol. 2011;14:420–422. doi: 10.1016/j.jaci.2011.03.025. [DOI] [PubMed] [Google Scholar]
- Metin A, Tavil B, Azık F, Azkur D, Ok-Bozkaya I, Kocabas C, Tunc B, Uckan D. Successful bone marrow transplantation for DOCK8 deficient hyper IgE syndrome. Pediatr Transplant. 2012;14:398–399. doi: 10.1111/j.1399-3046.2011.01641.x. [DOI] [PubMed] [Google Scholar]
- Minegishi Y, Saito M, Morio T, Watanabe K, Agematsu K, Tsuchiya S, Takada H, Hara T, Kawamura N, Ariga T, Kaneko H, Kondo N, Tsuge I, Yachie A, Sakiyama Y, Iwata T, Bessho F, Ohishi T, Joh K, Imai K, Kogawa K, Shinohara M, Fujieda M, Wakiguchi H, Pasic S, Abinun M, Ochs HD, Renner ED, Jansson A, Belohradsky BH. et al. Human tyrosine kinase 2 deficiency reveals its requisite roles in multiple cytokine signals involved in innate and acquired immunity. Immunity. 2006;14:745–755. doi: 10.1016/j.immuni.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Kilic SS, Hacimustafaoglu M, Boisson-Dupuis S, Kreins AY, Grant AV, Abel L, Casanova JL. A patient with tyrosine kinase 2 deficiency without hyper-IgE syndrome. J Pediatr. 2012;14:1055–1057. doi: 10.1016/j.jpeds.2012.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniades K, Hatzistilianou M, Pitsavas G, Agouridaki C, Athanassiadou F. Co-existence of Dubowitz and hyper-IgE syndromes: a case report. Eur J Pediatr. 1996;14:390–392. doi: 10.1007/BF01955268. [DOI] [PubMed] [Google Scholar]
- Boeck A, Gfatter R, Braun F, Fritz B. Pentasomy × and hyper IgE syndrome: co-existence of two distinct genetic disorders. Eur J Pediatr. 1999;14:723–726. doi: 10.1007/s004310051187. [DOI] [PubMed] [Google Scholar]
- Boeck A, Kosan C, Ciznar P, Kunz J. Saethre-Chotzen syndrome and hyper IgE syndrome in a patient with a novel 11 bp deletion of the TWIST gene. Am J Med Genet. 2001;14:53–56. doi: 10.1002/ajmg.10007. [DOI] [PubMed] [Google Scholar]