Abstract
Distinct subsets of nucleus accumbens (NAc) neurons differentially encode goal-directed behaviors for natural vs. drug rewards [R. M. Carelli et al. (2000) The Journal of Neuroscience, 20, 4255–4266], and the encoding of cocaine-seeking is altered following cocaine abstinence [J. A. Hollander & R. M. Carelli (2007) The Journal of Neuroscience, 27, 3535–3539]. Here, electrophysiological recording procedures were used to determine if the selective encoding of natural vs. cocaine reward by NAc neurons is: (i) maintained when the natural reinforcer is a highly palatable sweet tastant and (ii) altered by cocaine abstinence. Rats (n=14) were trained on a multiple schedule of sucrose reinforcement and cocaine self-administration (2–3 weeks) and NAc activity was recorded during the task before and after 30 days of cocaine abstinence. Of 130 cells recorded before abstinence, 82 (63%) displayed patterned discharges (increases or decreases in firing rate, termed phasic activity) relative to operant responding for sucrose or cocaine. As in previous reports, the majority of those cells displayed nonoverlapping patterns of activity during responding for sucrose vs. cocaine. Specifically, only 17 (21%) showed similar patterns of activity (i.e. overlapping activity) across the two reinforcer conditions. After abstinence, this pattern was largely maintained, 23 of 70 phasic cells (33%) were overlapping. However, cocaine abstinence altered the overall percentage of selectively active neurons across reinforcer conditions. Specifically, significantly more neurons became selectively activated during cocaine-directed behaviors than during sucrose-directed behaviors. The results indicate that, although the selective encoding of cocaine and natural rewards is maintained even with a highly palatable substance, 30 days of cocaine abstinence dynamically alters the overall population encoding of natural and drug rewards by NAc neurons.
Keywords: addiction, behavior, electrophysiology, rat, reward, self-administration
Introduction
The ability to seek and acquire natural rewards such as food and water is essential for survival. As such, the brain evolved a highly dynamic system to process information about natural reinforcers. It is often hypothesized that drugs of abuse exert their effects by ‘tapping into’ this system, causing aberrant reward processing and, ultimately, addiction (Wise, 1997). The nucleus accumbens (NAc) is a critical component of this system and has been implicated in processing information about both natural and drug rewards (Robinson & Berridge, 2000; Kelley, 2004). This structure also plays a key role in addiction, as the dopaminergic projection from the ventral tegmental area to the NAc is a crucial substrate for the reinforcing properties of abused drugs (DiChiara, 1995; Koob & Nestler, 1997; Kalivas & McFarland, 2003; Carlezon & Thomas, 2009).
Electrophysiological recordings show that NAc neurons display patterned discharges (increases or decreases in firing rate) relative to operant responding for both natural and drug reinforcers (Carelli & Deadwyler, 1994; Peoples & West, 1996; Carelli et al., 2000; Carelli, 2002; Nicola et al., 2004). However, different populations of NAc neurons selectively encode information about goal-directed behaviors for natural rewards (food/water) vs. intravenous cocaine (Carelli et al., 2000; Carelli & Ijames, 2001). Conversely, natural reinforcers activate largely the same population of neurons in the NAc (Carelli et al., 2000), even when one is highly palatable (Roop et al., 2002). These findings suggest that drugs and natural rewards activate a separate neural circuit in the NAc (Carelli et al., 2000).
However, the precise manner in which NAc neurons encode goal-directed behaviors for drug and natural rewards can be influenced by many factors, including the type of reinforcer and also the pattern of drug exposure (Hollander & Carelli, 2005; Hollander & Carelli, 2007). In human cocaine addicts, drug-taking behavior is often characterized by binges followed by periods of drug abstinence, increased craving, and relapse (Gawin, 1991). Further, animal studies revealed that cocaine abstinence leads to neuroadaptations in brain regions important for reward processing, including the NAc (Robinson et al., 2001; Lu et al., 2003; Conrad et al., 2008; Pickens et al., 2011). Importantly, the percentage of NAc neurons that encode goal-directed behaviors for cocaine, and cocaine-associated cues, is dramatically increased following 30 days of cocaine abstinence (Hollander & Carelli, 2005; Hollander & Carelli, 2007). It is therefore possible that drug abstinence may alter the differential processing of natural vs. drug rewards by NAc neurons.
The present study was completed with two primary objectives. First, we determined if the selective encoding by NAc neurons of natural vs. cocaine reward is maintained when the former is a highly palatable sweet tastant (i.e. sucrose), as opposed to less palatable food/water used in previous studies (Carelli et al., 2000; Carelli & Ijames, 2001). Second, we examined if the selective encoding by NAc neurons of cocaine-seeking and natural reward-seeking is altered by 30 days of cocaine abstinence. To this end, NAc neurons were recorded during a sucrose/cocaine multiple schedule before and after 30 days of cocaine abstinence.
Materials and methods
Animals
Male Sprague-Dawley rats (Harlan Sprague Dawley, Indianapolis, IN, USA; n=14) aged 90–120 days and weighing 260–350 g were used as subjects and individually housed with a 12/12 h light/dark cycle. Body weights were maintained at no less than 85% of pre-experimental levels by food restriction (10–15 g of Purina laboratory chow each day). Water was available ad libitum. This regimen was in place for the duration of the experiment, except during the post-operative recovery period when food was given ad libitum. All procedures were approved by the UNC Institutional Animal Care and Use Committee.
Surgery and behavioral training
All training was conducted in custom-made experimental chambers that consisted of a 43 × 43 × 53 cm Plexiglass chamber housed within a commercial sound-attenuated cubicle (Med Associates Inc., St Albans, VT, USA). One side of the chamber was equipped with two retractable levers, a corresponding cue light positioned above each lever, and a reward receptacle positioned between the two levers.
Rats were first trained to press one lever for sucrose (45 mg pellet) on a fixed-ratio 1 schedule of reinforcement. The start of the sucrose training session was signaled by the onset of the cue light positioned above the active lever and extension of the lever into the chamber. Lever depression resulted in delivery of a sucrose pellet to the reward receptacle, onset of a tone (65 dB, 2900 Hz, 20 s), and retraction of the lever (20 s). Rats underwent daily 30 min training sessions until they reached the criterion (at least 50 presses per session).
Rats were then prepared for extracellular recording in the NAc via implantation of microwire electrode arrays during the same surgery as catheter implantation using established procedures (Carelli et al., 2000; Hollander & Carelli, 2005). Each array was custom-designed (consisting of eight microwires each of 50 µm diameter and arranged in a 2 × 4 configuration) and purchased from a commercial source (NB Labs, Denison, TX, USA). Arrays were permanently implanted bilaterally into the NAc core or shell [AP, +1.7 mm; ML, ±1.3 mm for core and ±0.8 mm for shell; DV,−6.2 mm from brain, relative to bregma, level skull; Paxinos & Watson (2007)].
Following recovery from surgery, rats were trained to self-administer cocaine on a fixed-ratio 1 schedule of reinforcement during daily 2 h sessions. The start of the self-administration session was signaled by the onset of the cue light positioned above the active lever and extension of the lever into the chamber. The cocaine-associated lever was spatially distinct from the lever previously used during sucrose training. Lever depression resulted in intravenous cocaine delivery (0.33 mg/infusion, ~1 mg/kg/infusion, 6 s) via a computer-controlled syringe pump, onset of a different tone (65 dB, 800 Hz, 20 s), and retraction of the lever (20 s). The tones associated with cocaine vs. sucrose were counterbalanced across animals.
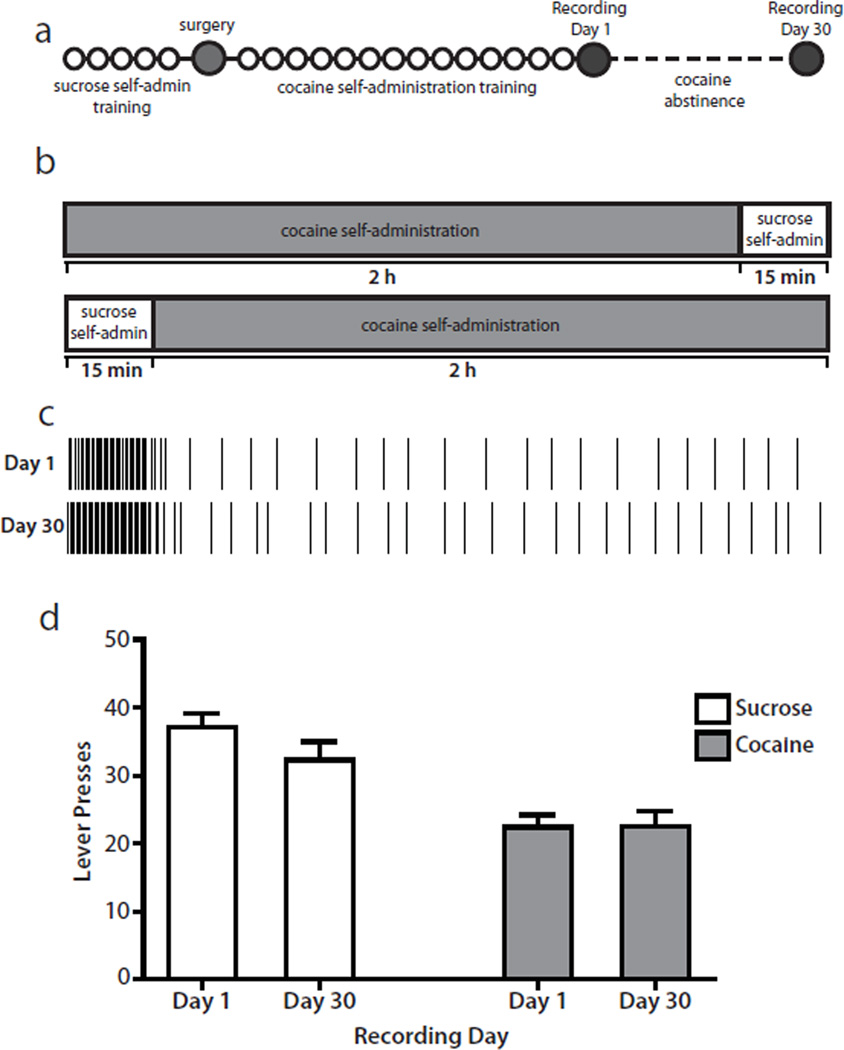
Following acquisition of cocaine self-administration (2–3 weeks), rats underwent electrophysiological recording (described below) during a multiple schedule of reinforcement for sucrose and cocaine. Specifically, rats had access to the sucrose-reinforced lever (fixed-ratio 1; 15 min) followed by a 20 s time-out period (no lever extended; dark chamber) and extension of the second cocaine-reinforced lever (fixed-ratio 1; 2 h). Illumination of a cue light above each lever signaled the phase (sucrose or cocaine) of the multiple schedule. The order of reinforcer availability (sucrose or cocaine) was varied across animals and recording days such that the same reinforcer was not always given first. In addition, the lever and tone associated with each reinforcer were counterbalanced across animals. Rats then underwent a 30 day abstinence period during which drug access was interrupted (rats remained in their home cages). After 30 days of abstinence, rats underwent a second recording session while performing an identical multiple schedule session for sucrose and cocaine. A timeline of the experimental design is shown in Fig. 1a. Figure 1b shows the multiple schedule session used on recording days. Some animals self-administered sucrose followed by cocaine, whereas others self-administered cocaine followed by sucrose.
Fig. 1.
Experimental design and behavior. (a) Diagram of the experimental timeline. Each circle represents 1 day. During sucrose self-administration training (~5 days), rats had access only to sucrose during daily 30 min sessions. During cocaine self-administration training (~14 days), rats had access only to cocaine during daily 2 h sessions. On recording Day 1 and Day 30, rats performed a sucrose/cocaine multiple schedule. Cocaine abstinence lasted for 30 days, during which time rats remained in their home cages without drug access. See Materials and methods for details. (b) Schematic diagram of the multiple schedule. On recording Day 1 and Day 30, rats had access to the sucrose-reinforced lever for 15 min followed by access to the cocaine-reinforced lever for 2 h. In a subset of animals, the order of the reinforcers was reversed so that cocaine self-administration preceded sucrose self-administration. See Materials and methods for details. (c) Example of a representative behavioral response pattern for one rat. Each vertical line indicates one lever press response. On Day 1 (top), the rat completed 36 responses on the sucrose lever and 24 responses on the cocaine lever. On Day 30 (bottom), the rat completed 42 responses on the sucrose lever and 30 responses on the cocaine lever. (d) Rats (n=14) displayed consistent numbers of lever press responses across recording sessions (Day 1 and Day 30) for both sucrose and cocaine.
Electrophysiological recordings
The electrophysiological procedures have been described in detail previously (Carelli & Deadwyler, 1994; Carelli et al., 2000; Hollander & Carelli, 2005; Roitman et al., 2005). Briefly, before the start of the session, rats were connected to a flexible recording cable attached to a commutator (Med Associates Inc.), which allowed virtually unrestrained movement within the chamber. NAc activity was recorded differentially between each active and the inactive (reference) electrode from the permanently implanted microwires. The inactive electrode was examined before the start of the session to verify the absence of neuronal spike activity and served as the differential electrode for other electrodes with cell activity. Online isolation and discrimination of neuronal activity were accomplished using a commercially available neurophysiological system (multi-channel acquisition processor, MAP System, Plexon, Dallas, TX, USA). Multiple window-discrimination modules and high-speed analog-to-digital signal processing in conjunction with computer software enabled the isolation of neuronal signals based on waveform analysis. The neurophysiological system incorporated an array of digital signal processors for continuous spike recognition. The digital signal processors provided a continuous parallel digital output of neuronal spike events to a Pentium computer. Another computer controlled the behavioral events of the experiment (Med Associates Inc.) and sent digital outputs corresponding to each event to the MAP box to be time-stamped along with the neural data. Principal component analysis of recorded waveforms was performed prior to each session and aided in the separation of multiple neuronal signals from the same electrode. A projection of waveform clusters was presented in a three-dimensional space, enabling manual selection of individual waveforms. Before each session, an individual template made up of many sampled waveforms was created for each cell isolated using principal component analysis. During the behavioral session, waveforms that matched this template were collected as the same neuron. Cell recognition and sorting were finalized after the experiment using the Offline Sorter program (Plexon), when neuronal data were further assessed based on principal component analysis of the waveforms, cell firing characteristics, and interspike intervals.
Data analysis
Changes in neuronal firing patterns relative to sucrose-reinforced or cocaine-reinforced responses were analyzed by constructing raster displays and peri-event histograms (PEHs) (bin width, 250 ms) surrounding each response using commercially available software (Neuroexplorer, Plexon). Cell firing was classified into one of three well-defined types of phasic neuronal firing patterns that occurred within seconds of the reinforced lever press response (Carelli & Deadwyler, 1994; Carelli et al., 2000; Hollander & Carelli, 2005; Jones et al., 2008). Specifically, cells were classified as type pre-response (PR) if they displayed an anticipatory increase in activity preceding the lever press, as type reinforcement–excitation (RFe) if they displayed an increase in firing rate immediately following a reinforced response, and as type reinforcement–inhibition (RFi) if they displayed a decrease in firing rate surrounding a reinforced response. Cells that showed no significant change in firing rate (increase and/or decrease) relative to a reinforced response were classified as nonphasic.
Individual units were classified as either type PR, RFe, or RFi if the firing rate was greater than or less than the 99.9% confidence interval projected from the baseline period (10 s before lever response) for at least two 250 ms time bins. This confidence interval was selected such that only robust responses were classified as phasic. Some cells in this analysis exhibited low baseline firing rates, and the 99.9% confidence interval included zero. When this was the case, inhibitions were assigned only if the number of consecutive 0 spike/s time bins surrounding the reinforced lever response was more than double the number of consecutive 0 spike/s time bins in the baseline period. Cells with extremely low firing rates (<0.1 spikes/s) or relatively high firing rates (>15 spikes/s) were probably not medium spiny neurons and were excluded from further analysis.
Cells were then classified based on their phasic activity across both reinforcers (sucrose and cocaine) during performance of the multiple schedule. Cells that displayed one of the three types of well-defined patterned discharges (type PR, RFe, or RFi) relative to sucrose-reinforced responding, but nonphasic activity relative to the cocaine-reinforced response were classified as ‘sucrose-selective’. Cells that displayed one of the three types of patterned discharges relative to cocaine-reinforced responding, but nonphasic activity relative to the sucrose-reinforced response were classified as ‘cocaine-selective’. Cells that displayed the same type of phasic activity to both reinforcers (e.g. type PR to both cocaine-reinforced and sucrose-reinforced responses) were classified as ‘overlapping’. Finally, cells showing different phasic patterns of activity during responding for cocaine vs. sucrose were classified as ‘differentially phasic’. Comparisons of the number of cells in each category were made across recording days using Fisher’s exact test. Comparisons of behavioral responding across recording days were accomplished with paired t-tests.
To quantify the strength of each neural correlate, signal-to-baseline (S:B) ratios were calculated as described previously (Hollander & Carelli, 2005; Jones et al., 2008). Specifically, the baseline epoch included the time period from -10 to -3 s before a reinforced lever press. The signal epoch included the 1 s time period surrounding the peak excitation (for type PR or RFe cells) or inhibition (for type RFi cells). S:B ratios were calculated by dividing the signal by the baseline. A value of 1 indicates no change (nonphasic activity), values greater than 1 indicate increases in firing rate, and values less than 1 indicate inhibitions in firing rate. Comparisons of S:B ratios were accomplished with unpaired t-tests.
Histology
Histological reconstruction of electrode positions was accomplished using established procedures (Carelli et al., 2000; Hollander & Carelli, 2005). After the experiment, rats were deeply anesthetized with a ketamine and xylazine mixture (100 and 20 mg/kg, respectively) and a 13.5 µA current was passed for 5 s through all recording wires. Rats were perfused with 10% formalin and 3% potassium ferracyanide and brains were removed, blocked, and sectioned (40 µm) throughout the rostral–caudal extent of the NAc. Sections were stained with thionin to aid with the identification of structures and location of the blue dot reaction product corresponding to the location of the marked electrode tip. To reconstruct electrode positions, serial sections were examined under a light microscope and the locations of all marked electrode tips were plotted for all subjects on coronal sections taken from the stereotaxic atlas of Paxinos and Watson (2007). Only neurons recorded from wires positioned in the NAc were used in the present study.
Results
Behavior
An example of behavioral responding during the multiple schedule for one representative animal is shown in Fig. 1c. Before abstinence (Day 1), the rat completed 36 sucrose-reinforced responses with an average inter-response interval (INT) of 23.60 ± 1.11 s and 24 cocaine-reinforced responses with an average INT of 4.99 ± 0.41 min. On Day 30, the same rat made 42 sucrose-reinforced responses with an average INT of 21.55 ± 0.32 s and 30 cocaine-reinforced responses with an average INT of 4.07 ± 0.32 min. Similar response patterns were observed across all animals (n=14). Specifically, during sucrose self-administration on Day 1, rats exhibited 37.14 ± 1.99 lever presses with an average INT of 30.50 ± 4.89 s. On Day 30, the same animals exhibited 32.29 ± 2.73 lever presses with an average INT of 45.40 ± 13.69 s. Cocaine self-administration responding on Day 1 was characterized by 22.43 ± 1.82 lever presses with an average INT of 6.00 ± 0.40 min. On Day 30, animals completed 22.54 ± 2.22 lever presses with an average INT of 5.68 ± 0.43 min. Importantly, behavioral response patterns during the multiple schedule were not altered by cocaine abstinence. Specifically, rats displayed a similar number of lever press responses during the first day of the multiple schedule (Day 1) and following 30 days of abstinence (Day 30) for both sucrose (t13=1.861; p>0.05) and cocaine (t12=0.075; p>0.05; Fig. 1d). Further, there was no significant difference in the average INT from Day 1 to Day 30 for either sucrose (t13=1.471; p>0.05) or cocaine (t12=0.9195; p>0.05). On Day 30, one animal was excluded due to mechanical problems. In sessions in which the cocaine self-administration phase preceded sucrose reinforcement, animals typically paused after the time-out phase for 30–40 min, and sucrose-reinforced responding was sometimes more erratic compared with sessions in which sucrose preceded cocaine.
Nucleus accumbens neurons exhibit three types of neuronal firing patterns relative to reinforced responding for sucrose reinforcement or intravenous cocaine
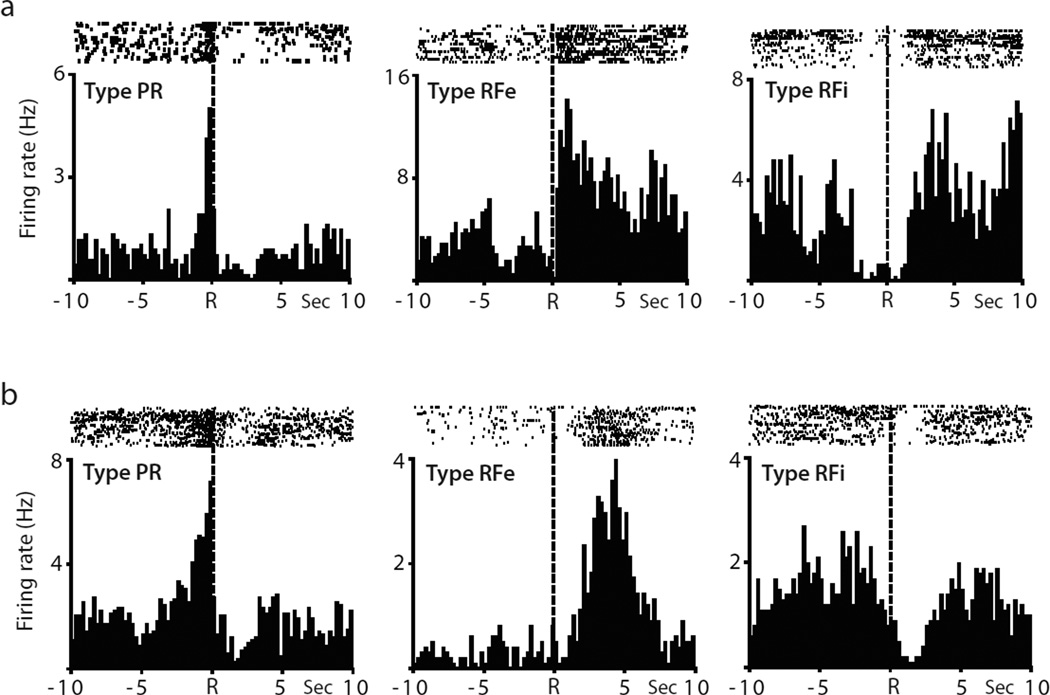
Independent of the abstinence conditions, three types of neuronal firing patterns (types PR, RFe and RFi) were recorded in the NAc during the multiple schedule relative to reinforced responding for either cocaine or sucrose. The rasters and PEHs in Fig. 2a show examples of the activity of representative phasically active neurons recorded during the cocaine self-administration phase of the multiple schedule. The raster displays and PEHs encompass a 20 s time interval surrounding the cocaine-reinforced response (represented by dashed line at time R). One neuron (left) exhibited an anticipatory increase in firing rate immediately before the response, classified as a type PR cell. Another neuron (middle) displayed an increase in firing rate immediately after the reinforced response, termed type RFe activity. The third neuron exhibited type RFi activity (right) showing a decrease in firing surrounding the reinforced response for intravenous cocaine.
Fig. 2.
Examples of individual NAc neurons showing one of the three types of patterned discharges (PR, RFe and RFi). (a) Example neurons recorded during cocaine self-administration. Raster displays and PEHs show the activity of each neuron recorded during a 20 s time period surrounding the cocaine-reinforced response (indicated by dashed line at time R). Individual cells exhibited type PR activity (left), type RFe activity (middle), or type RFi activity (right). (b) Example neurons recorded during sucrose self-administration.
Other neurons showed similar types of neuronal firing patterns relative to sucrose-reinforced responding. The rasters and PEHs in Fig. 2b show examples of representative phasically active neurons recorded during the sucrose self-administration phase of the multiple schedule. In this case, a neuron classified as a type PR cell (left) exhibited an anticipatory increase in firing rate immediately before the sucrose-reinforced lever press, whereas another cell exhibited type RFe activity (middle) characterized by an increase in firing rate after the response. A third neuron displayed type RFi cell activity (right) with a decrease in firing rate relative to the reinforced response.
Of all neurons recorded on Day 1, 63% (82 of 130 cells) displayed one of the three types of phasic activity described above, regardless of the reinforcer condition. On Day 30, 62% of recorded neurons (70 of 113 cells) exhibited one of the three types of patterned discharges noted above. Thus, there was no difference in the overall percentage of phasic cells from Day 1 to Day 30, independent of reinforcer type. Further, S:B ratios were not significantly different between cocaine and sucrose phasic responses that showed type PR activity (t33=0.6341; p>0.05), type RFe activity (t80=0.0995; p>0.05), or type RFi activity (t66=0.6205; p>0.05). That is, the magnitude of phasic responses (excitations or inhibitions) during cocaine self-administration was similar to the magnitude of phasic responses during sucrose self-administration.
Self-administration of cocaine prior to sucrose reduced the baseline firing rates of sucrose-responsive cells compared with when sucrose was self-administered first (from a mean of 5.330 ± 0.4268 Hz to 2.933 ± 0.3990 Hz; t150=2.709; p<0.01), consistent with cocaine’s actions in reducing overall firing rates previously reported by Peoples et al. (1998). However, the reinforcer order had no effect on the proportion of sucrose-responsive cells classified as type PR (p=0.7713, Fisher’s exact test), RFe (p=0.2826, Fisher’s exact test), or RFi (p=0.3592, Fisher’s exact test).
Populations of nucleus accumbens neurons exhibit differential firing properties relative to goal-directed behaviors for sucrose vs. cocaine prior to abstinence
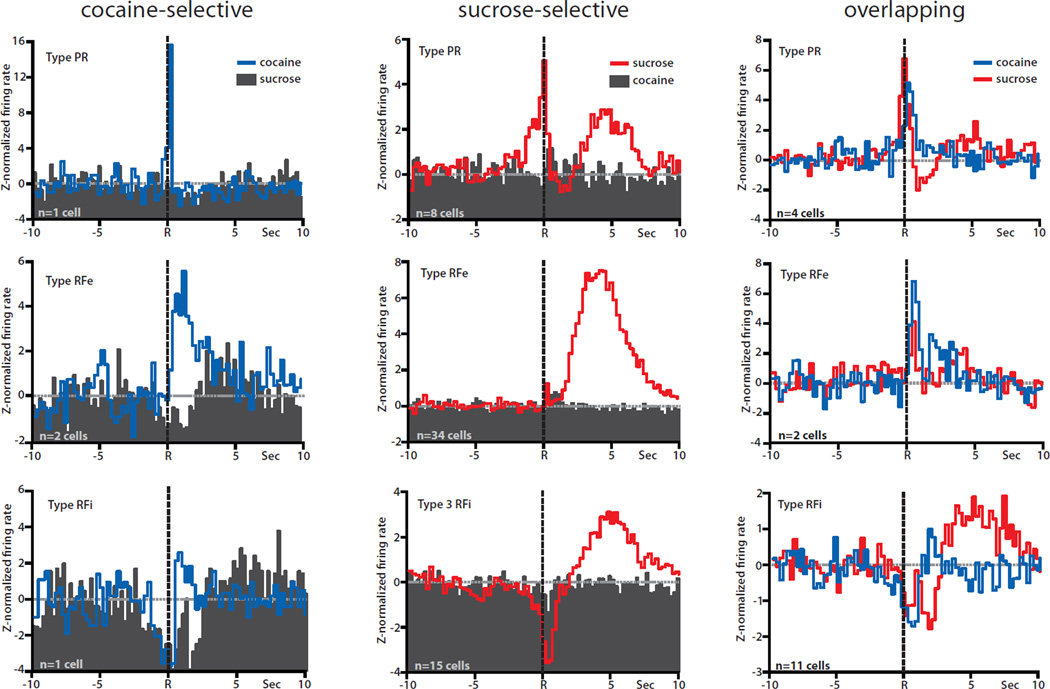
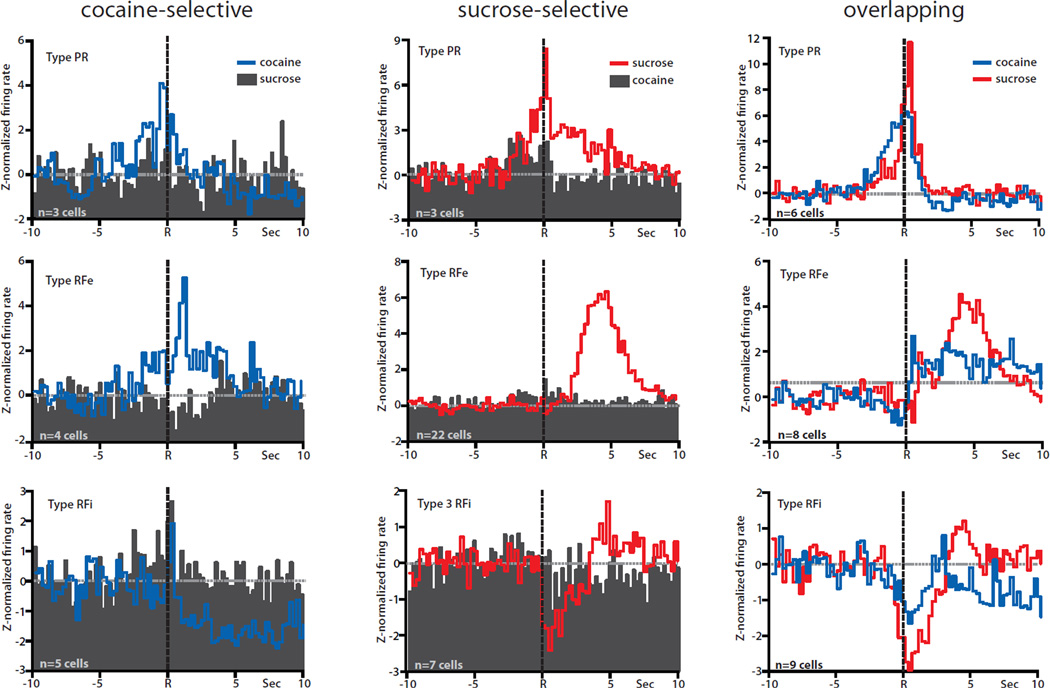
In previous studies we showed that distinct subsets of NAc neurons differentially encode information about goal-directed behavior for a natural (water/food) reward vs. cocaine self-administration using our multiple schedule design (Carelli et al., 2000; Carelli & Ijames, 2001; Carelli & Wondolowski, 2003). Therefore, the present study extended those findings by examining a natural reward/cocaine multiple schedule with the highly palatable natural reinforcer sucrose. Consistent with previous findings, distinct populations of NAc neurons differentially encoded information about lever press responding for cocaine or the palatable natural reward, sucrose, prior to abstinence. Specifically, as noted above, a total of 130 neurons were recorded during the multiple schedule for sucrose reinforcement and intravenous self-administration of cocaine on Day 1. Of 130 cells, 82 (63%) exhibited patterned discharges relative to the sucrose-reinforced or cocaine-reinforced response. Of 82 responsive neurons, 61 cells (74%) exhibited one of three types of patterned discharges (type PR, RFe, or RFi) relative to the sucrose or cocaine response during the multiple schedule, but not both. Only 17 cells (21%) showed similar patterned discharges relative to reinforced responding for sucrose and cocaine. Finally, 5% were classified as ‘differentially phasic’, exhibiting different types of phasic activity relative to sucrose-reinforced and cocaine-reinforced responding.
Figure 3 summarizes these findings and shows PEHs of normalized firing of all cocaine-selective (left), sucrose-selective (middle) and overlapping (right) neurons during the multiple schedule prior to abstinence (Day 1). Only four cells displayed one of the three types of phasic activity relative only to cocaine-reinforced responses (nonphasic activity relative to sucrose-reinforced responding), termed cocaine-selective (Fig. 3, left, blue lines). Of the four neurons, one cell was classified as type PR, two cells as type RFe and one cell as type RFi. In all cases, the same neurons were not responsive to sucrose (Fig. 3, left, black PEHs). Interestingly, a much larger number of neurons (n=57) displayed one of the three types of phasic firing patterns relative to responding for sucrose, but not cocaine, and were classified as sucrose-selective (Fig. 3, middle, red lines). Of 57 cells, 8 neurons were classified as type PR, 34 as type RFe and 15 as type RFi cells. The same neurons were not phasic to cocaine responding (Fig. 3, middle, black PEHs). Another population of neurons (n=17) exhibited similar patterns of phasic activity during responding for both cocaine and sucrose and were classified as overlapping (Fig. 3, right). A small subset of cells (n=4) showed different patterns of phasic activity during responding for cocaine vs. sucrose, and were classified as differentially phasic (not shown).
Fig. 3.
Population histograms of the three cell classifications (cocaine-selective, sucrose-selective, and overlapping) recorded prior to abstinence (Day 1), separated by type of phasic activity (PR, RFe, or RFi). Lever press responses are indicated by the dashed black line at time R in all PEHs. Averages were z-normalized, and the baseline is indicated by the dashed gray line at 0. Left column: population averages of cocaine-selective cells. The activity of the same neurons relative to cocaine responding (blue lines) vs. sucrose responding (filled histograms) plotted on the same graph. Normalized firing displayed one of the three well-defined patterns of phasic activity (PR, top; RFe, middle; RFi, bottom) relative to cocaine-reinforced responses but the same cells showed no change from baseline relative to sucrose-reinforced responses. Middle column: population averages of sucrose-selective cells. Normalized firing displayed one of the three patterns of phasic activity (PR, top; RFe, middle; RFi, bottom) relative to sucrose-reinforced responses (red lines), but no change from baseline relative to cocaine-reinforced responses (filled histograms). Right column: population averages of overlapping cells. Normalized firing displayed the same types of phasic activity (PR, top; RFe, middle; RFi, bottom) relative to both cocaine-reinforced (blue lines) and sucrose-reinforced (red lines) responses.
Cocaine abstinence shifts how nucleus accumbens neurons encode goal-directed actions for cocaine vs. sucrose reinforcement
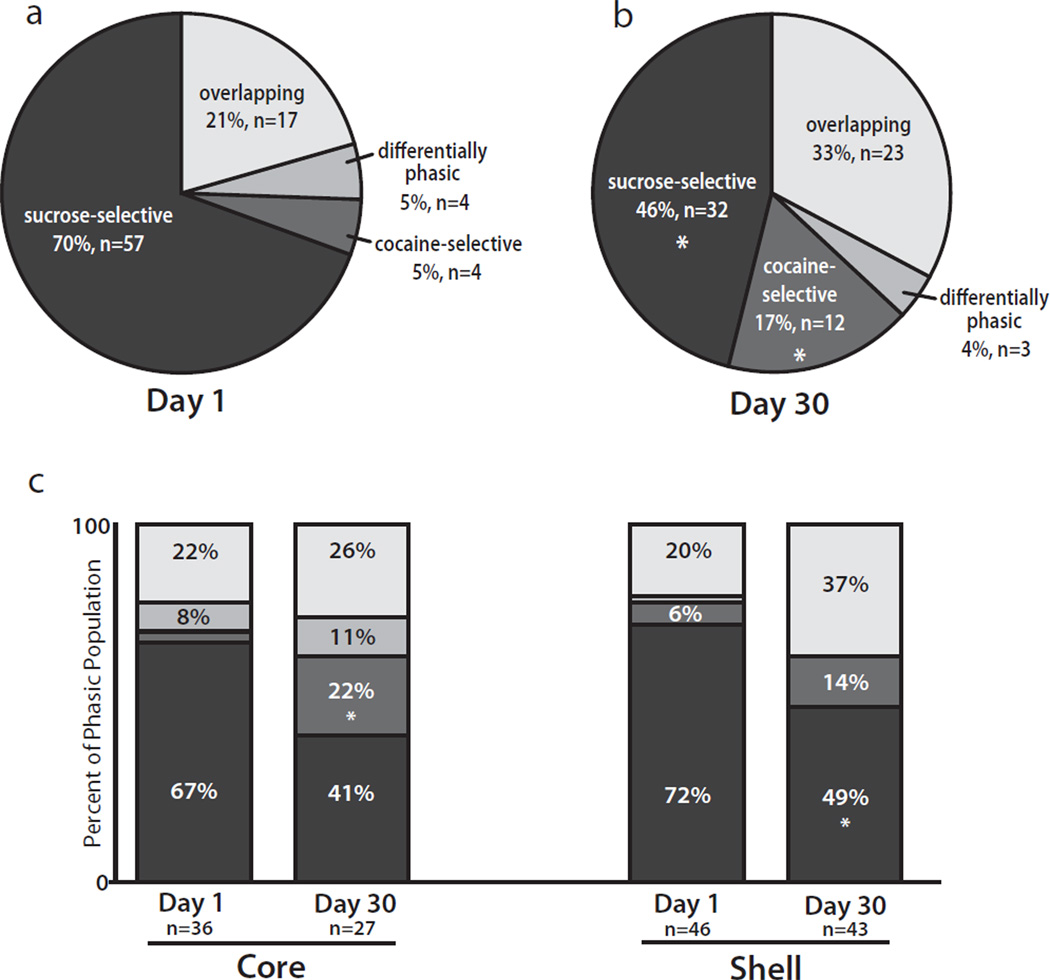
A major goal of the present study was to examine if 30 days of abstinence from cocaine altered how NAc neurons encode goal-directed actions for sucrose vs. cocaine reinforcement during the multiple schedule. To address this issue, the distribution of NAc patterned discharges was compared before and after 30 days of cocaine abstinence. Before abstinence, a high percentage of sucrose-selective cells (57 of 82 cells, 70%) was observed, whereas a relatively small percentage of cocaine-selective cells (4 of 82 cells, 5%) was recorded (Figs 3 and 5a). However, after abstinence (Figs 4 and 5b) there was an increase in the percentage of cocaine-selective cells (to 12 of 70 cells, 17%; p=0.017, Fisher’s exact test) and a decrease in the percentage of sucrose-selective cells (to 32 of 70 cells, 46%; p=0.005, Fisher’s exact test). Further, although a slight increase was observed in the percentage of overlapping cells following abstinence (from 17 of 82 cells, 21%, to 23 of 70 cells, 33%), this increase was not statistically significant (p=0.099, Fisher’s exact test). There was no change in the percentage of differentially phasic cells following abstinence (from 4 of 82 cells, 5%, to 3 of 70 cells, 4%; p=1.000, Fisher’s exact test). Finally, the percentage of nonphasic cells was not altered as a function of abstinence (from 48 of 130 cells, 37%, to 43 of 113 cells, 38%; p=0.8947, Fisher’s exact test). As above, reinforcer order had no effect on the percentage of cells classified as cocaine-selective (p=1.0000, Fisher’s exact test), sucrose-selective (p=0.0658, Fisher’s exact test), overlapping (p=0.1685, Fisher’s exact test), or differentially phasic (p=0.1388, Fisher’s exact test), and neural data from all animals were combined.
Fig. 5.
Distribution of phasic activity of NAc neurons during the sucrose/cocaine multiple schedule. (a) Breakdown of phasic activity of NAc neurons (core and shell, combined) recorded before cocaine abstinence. (b) Phasic activity of NAc neurons (core and shell, combined) recorded following 30 days of cocaine abstinence. After abstinence, the percentage of sucrose-selective cells significantly decreased, whereas the percentage of cocaine-selective cells significantly increased. *p<0.05. (c) Phasic activity of neurons in the NAc core vs. shell. In the core, the percentage of cocaine-selective cells significantly increased from Day 1 to Day 30. In the shell, the percentage of sucrose-selective cells significantly decreased from Day 1 to Day 30. *p<0.05.
Fig. 4.
Population histograms of the three cell classifications (cocaine-selective, sucrose-selective, and overlapping) recorded following 1 month of cocaine abstinence (Day 30). Same format as Fig. 3.
The abstinence-induced changes in neuronal firing noted above were differentially distributed across the core and shell subregions of the NAc (Fig. 5c). In the core, there was an increase in the percentage of cocaine-selective cells (from 1 of 36 cells, 3%, to 6 of 27 cells, 22%; p=0.036, Fisher’s exact test). There was also a trend toward a decrease in the percentage of sucrose-selective cells from Day 1 to Day 30 (from 24 of 36 cells, 67%, to 11 of 27 cells, 41%; p=0.0720, Fisher’s exact test). In the shell, there was a decrease in the percentage of sucrose-selective cells (from 33 of 46 cells, 72%, to 21 of 43 cells, 49%; p=0.032, Fisher’s exact test). Further, there was no change in the percentage of overlapping cells from Day 1 to Day 30 (from 9 of 46 cells, 20%, to 16 of 43 cells, 37%; p=0.0977, Fisher’s exact test). Abstinence had no effect on the percentage of cocaine-selective cells in the shell (p=0.3049, Fisher’s exact test). As above, the percentage of nonphasic cells was not altered by abstinence in either the core (from 23 of 59 cells, 39%, to 22 of 49 cells, 45%; p=0.5618, Fisher’s exact test) or the shell (from 25 of 71 cells, 35%, to 21 of 64 cells, 33%; p=0.8562, Fisher’s exact test).
The S:B ratios of cocaine-selective and sucrose-selective neurons were not altered by abstinence (cocaine-selective type RFe: t4=0.0337, p>0.05; sucrose-selective type PR: t9=0.6702, p>0.05; sucrose-selective type RFe: t54=0.1826, p>0.05; sucrose-selective type RFi: t20=0.8159, p>0.05). Abstinence had no effect on the S:B ratios of overlapping type RFe or RFi cells (sucrose type RFe: t8=1.525, p>0.05; cocaine type RFe: t8=1.752, p>0.05; sucrose type RFi: t18=0.6603, p>0.05; cocaine type RFi: t18=0.2667, p>0.05). However, the S:B ratios of the cocaine responses of type PR overlapping neurons increased following 1 month of abstinence (t8=3.466; p<0.01). This was not the case for the sucrose responses of these cells (t8=1.642; p>0.05). It is possible that this pattern also occurred for type PR responses in cocaine-selective neurons; however, as only one cell showed this type of activity on Day 1, no statistical comparisons could be made. Finally, abstinence had no effect on the baseline firing rates of recorded neurons (see Supporting Information Tables 1 and 2).
Histology
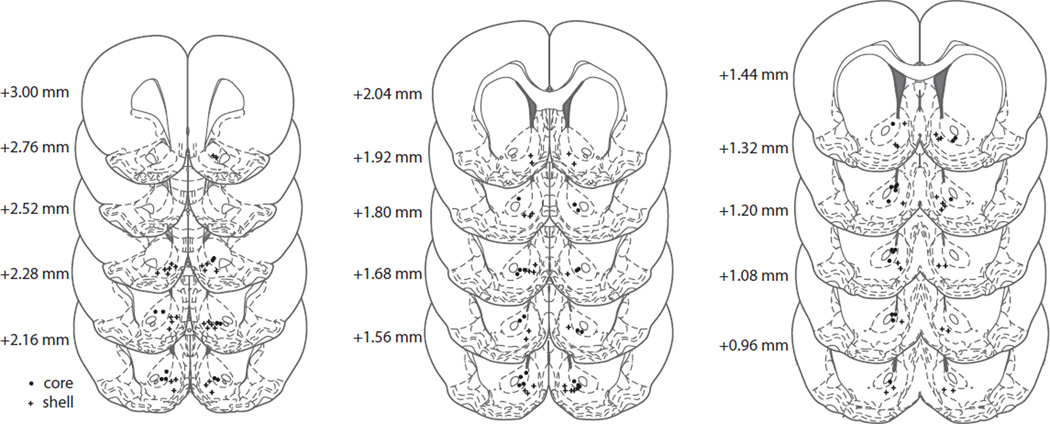
Histological reconstruction of electrode placement confirmed the location of recording wires in the NAc core or shell (Fig. 6). A total of 130 NAc neurons were recorded on Day 1 (core, n=59; shell, n=71), whereas 113 neurons were recorded after 30 days of abstinence (core, n=49; shell, n=64). Only data from electrode placements within the borders of the NAc, as depicted in the atlas of Paxinos and Watson (2007), were included in the analysis.
Fig. 6.
Schematic representation of electrode tip placements in the NAc core (dots) and shell (crosses). Numbers to the left of coronal sections indicate distance anterior to bregma (Paxinos & Watson, 2007).
Discussion
The present study was completed with two primary objectives. First, we examined if the selective encoding of goal-directed behaviors for cocaine vs. natural rewards would be maintained when the latter is a highly palatable tastant. Prior to abstinence, NAc neurons that encoded goal-directed behaviors for intravenous cocaine were largely separate from neurons activated during sucrose self-administration. These findings are consistent with earlier work (Carelli et al., 2000; Carelli & Ijames, 2001; Carelli & Wondolowski, 2003), but extend those findings by showing that this differential encoding is maintained even when the natural reinforcer is highly palatable. Second, we examined if 1 month of cocaine abstinence alters this predominantly selective encoding of cocaine-related and sucrose-related information by NAc neurons. Results revealed that after abstinence the majority of NAc cells (67%) displayed differential, nonoverlapping patterns of phasic activity relative to cocaine-reinforced vs. sucrose-reinforced responding. Further, there was a significant increase in the overall percentage of cells that were selective for cocaine-related information and a significant decrease in the percentage of cells that were selective for sucrose-related information. These findings may be relevant to the decrease in reinforcing properties of natural rewards often reported by human drug addicts (Gawin & Kleber, 1986; Gawin, 1991). Each of the primary findings of the present study is discussed in detail below.
Nucleus accumbens neurons differentially encode information about cocaine vs. the highly palatable natural reward sucrose prior to abstinence
As noted above, the first objective of this study was to examine if the selective encoding of goal-directed behaviors for cocaine vs. natural rewards (Carelli et al., 2000; Carelli & Ijames, 2001; Carelli & Wondolowski, 2003) is maintained when the latter is a highly palatable sweet tastant, as opposed to a more neutral food (Purina Lab chow) or water reinforcer. Differential encoding by NAc neurons was largely maintained when sucrose was the natural reinforcer in the multiple schedule. Interestingly, in the present study the percentage of overlapping neurons (21% before abstinence) was higher than that observed during a multiple schedule for cocaine and water (8% overlapping) or cocaine and food (7% overlapping) in our previous work (Carelli et al., 2000). Further, there was a greater percentage of neurons that were selective for sucrose-related information (70% in the present study) compared with neurons that were selective for water-related information (48% in the previous study). These differences may be related to the greater hedonic value of sucrose, compared with water.
However, our previous work also suggests that the percentage of NAc neurons that encode reinforcer-related information is not attributed solely to hedonics. For example, when animals performed a multiple schedule for water and sucrose, the majority of NAc neurons (65%) were similarly activated even though one was of greater hedonic value (Roop et al., 2002). Others have also shown that goal-directed behaviors for different types of abused drugs (cocaine and heroin) predominantly activate distinct subsets of NAc neurons (Chang et al., 1998), suggesting that the functional segregation of NAc neurons is sensitive not only to natural vs. drug rewards but also to different classes of abused drugs. Together, these findings suggest that the degree of overlap in populations of NAc neurons activated during goal-directed behaviors prior to abstinence is functionally complex and could be influenced by several interdependent factors including hedonics, reinforcer type (e.g. sweet tastant vs. food/water), and the inclusion of a drug reinforcer in the multiple schedule task.
Interestingly, sucrose-selective neurons (and, to a lesser extent, the sucrose responses of overlapping neurons) showed an increase in firing rate that peaked approximately 5 s after the lever press. This excitation is probably correlated with consumption of the sucrose pellet, suggesting that the particular features associated with a reinforcer (such as interoceptive effects or manner of delivery) can also influence NAc neural encoding.
The selective encoding of cocaine vs. the natural reward sucrose is largely maintained following 1 month of cocaine abstinence
A second objective of this study was to determine if 1 month of cocaine abstinence alters the predominantly selective encoding by NAc neurons of cocaine-related and sucrose-related information noted above. As previously reported, the percentage of NAc neurons phasically active during operant responding for cocaine, or during presentation of cocaine-related cues, is dramatically increased following 30 days of cocaine abstinence (Hollander & Carelli, 2005; Hollander & Carelli, 2007). Therefore, the current study was performed to determine if a specific population of NAc neurons account for the increase in cocaine-related information following abstinence. One possibility is that more NAc neurons exhibit overlapping phasic activity (i.e. similar types of phasic firing during cocaine and sucrose self-administration) after abstinence. This finding would indicate that cells that normally process information about highly palatable natural reinforcers are also recruited to process information about goal-directed actions for cocaine following abstinence. However, this was not the case; differential encoding of goal-directed behaviors for the two reinforcers was largely maintained following 1 month of abstinence. These results provide evidence that functionally segregated microcircuits exist in the NAc that selectively process specific types of reward-related information, and these microcircuits remain stable following 1 month of abstinence.
Further, the anatomical organization of the NAc lends support to this model of functional segregation. The classical view of the NAc as a limbic-motor integrator (Mogenson et al., 1980) is supported by anatomical studies that show that the NAc receives synaptic inputs from limbic areas including the ventral tegmental area, hippocampus, basolateral amygdala, and prefrontal cortex (Zahm & Brog, 1992; Brog et al., 1993). In turn, the NAc can guide motor output through connections with the ventral pallidum and lateral hypothalamus (Zahm, 1999). However, it is unlikely that the NAc as a whole sends a single integrated output to its target structures in order to initiate behavior. Theories of basal ganglia function suggest that the NAc is embedded in a larger system that is organized into several structurally and functionally discrete circuits that are essentially parallel in nature (Alexander et al., 1986; Alexander & Crutcher, 1990). Further, Pennartz et al. (1994) proposed that the NAc is composed of a collection of functionally heterogeneous ‘neuronal ensembles’ that are characterized by distinct afferent/efferent projections. Within this framework, unique sets of limbic inputs converge on specific ensembles of NAc neurons that then generate output to a particular set of target structures, inducing behavioral effects that are specifically linked with each ensemble. The present findings showing differential activation of discrete subsets of NAc neurons during goal-directed behavior for sucrose vs. cocaine even after abstinence support this view of NAc organization.
Further, the present study shows that cocaine abstinence differentially modulates cocaine-selective vs. overlapping populations of NAc neurons. We report that abstinence increases the percentage of cocaine-selective neurons but has no effect on the percentage of overlapping neurons. One interpretation of these results is that the abstinence-induced increase in phasic activity of NAc neurons previously reported (Hollander & Carelli, 2005; Hollander & Carelli, 2007) may primarily be the result of an increase in cocaine-selective cells. This finding has important implications for the functional state of the NAc following a period of cocaine abstinence.
Although overlapping cells probably represent neuronal ensembles that are able to encode behaviors associated with both drug and sucrose self-administration, cocaine-selective cells may represent ensembles activated specifically by drug-taking. It has been hypothesized that activation of drug-associated ensembles can induce drug-craving (O’Donnell, 2003). Thus, an increase in the percentage of cocaine-selective cells could lead to greater activation of drug-associated ensembles, potentiating drug-seeking behaviors relative to sucrose-seeking behaviors. In contrast, an increase in the percentage of overlapping cells would not bias behavior toward one outcome over the other.
Cocaine abstinence increases population encoding of cocaine-related information
In the present study, before abstinence there was a relatively high percentage of sucrose-selective cells (70%) and a low percentage of cocaine-selective cells (5%). The exact reasons for this predominance of sucrose-selective encoding are not clear at the present time. However, it is clear that 1 month of abstinence from cocaine alters this pattern of encoding of sucrose-related and cocaine-related information by distinct populations of NAc neurons. After 1 month of abstinence, there was an increase in the selective encoding of cocaine-related behaviors and a concomitant decrease in the selective encoding of sucrose-related behaviors. This switch was reflected in an increase in the percentage of cocaine-selective cells as well as a decrease in the percentage of sucrose-selective cells recorded in the NAc. These results could provide significant insight into the development of an addicted state.
Cocaine addicts going through withdrawal experience anhedonia, dysphoria, and an inability to perceive anything other than cocaine as potentially pleasurable (Gawin & Kleber, 1986). Furthermore, the intensity of these symptoms is associated with the patient’s degree of cocaine craving. Human imaging studies using positron emission tomography in cocaine addicts going through withdrawal have consistently demonstrated a reduction in striatal dopamine D2 receptor availability as well as a reduction in dopamine release in the striatum (Volkow et al., 1993; Volkow et al., 1997; Volkow et al., 1999). It is hypothesized that this hypodopaminergic activity in the striatum (including the NAc) could result in decreased activation of reward circuits by natural reinforcers, causing natural rewards to pale in comparison to drug rewards, thus leading to continued cocaine use as a means to compensate for this decreased reward sensitivity (Volkow et al., 1999; Volkow et al., 2010). The shift from primarily sucrose-related firing to more cocaine-associated discharges in the present study may therefore represent a neurophysiological correlate of this reduction in sensitivity to natural rewards relative to drug rewards following repeated cocaine administration and abstinence.
Although there is a relatively sparse encoding of cocaine-selective information in the present study, this finding may be functionally important. For example, histochemical studies by Hope and colleagues have shown that only a small subset (< 3%) of NAc neurons are selectively activated by cocaine in the drug-paired environment (Mattson et al., 2008; Koya et al., 2009). Even though sparsely distributed, these subsets of neurons have been shown to play a causal role in drug-associated behaviors. The selective inactivation of NAc neurons previously activated by cocaine in the drug-paired environment decreases context-specific cocaine-induced psychomotor sensitization (Koya et al., 2009). Thus, the activity of even a very small percentage of NAc neurons, as reported here, can have an impact on behavior.
It is possible that the decrease in the sucrose-selective population in the current study is a direct result of the increase in cocaine-selective cells following abstinence (i.e. previously sucrose-selective neurons may ‘shift’ to become cocaine-selective following abstinence). However, it is also possible that the increase in cocaine-selective neurons is a result of a new population of cells not previously activated. It is not possible to definitively determine if the same neurons were recorded on Day 1 and Day 30. Nevertheless, the present study shows that 1 month of cocaine abstinence increases the encoding of cocaine-specific information and decreases the sucrose-specific encoding of information.
Examination of nucleus accumbens activity in the core vs. shell
Here, abstinence-induced changes in cell firing were different within the core and shell subregions. Although the overall pattern of reduced sucrose encoding and increased cocaine encoding was seen in both the NAc core and shell, the increase in the percentage of cocaine-selective cells following abstinence was significant in the core but not the shell. Conversely, the decrease in the percentage of sucrose-selective cells following abstinence was significant in the shell but not the core. Considering the differences in afferent/efferent projections (Zahm & Brog, 1992; Zahm, 1999) and electrophysiological characteristics (Pennartz et al., 1992) between the core and shell, it is not surprising that there were differential changes in these subregions following abstinence. The larger increase in cocaine-selective cells in the core compared with the shell in this study may be related to the selective increase in phasic neurons in the core (but not the shell) following abstinence reported in our earlier studies (Hollander & Carelli, 2005; Hollander & Carelli, 2007). However, it is important to note that, although the increase in cocaine-selective cells was only significant in the core in the current study, there was also a tendency toward an increase in the shell. The earlier cocaine abstinence studies did not include a multiple schedule (animals only self-administered cocaine and no natural reinforcer was available); therefore, an interesting finding of the current study is that the performance of a sucrose/cocaine multiple schedule appears to recruit NAc shell neurons so that there is a change in both subregions of the NAc following 1 month of cocaine abstinence. However, given the relatively small core vs. shell sample size in the present study, further experiments may be necessary to draw definitive conclusions about the different roles that these subregions play in our paradigm.
Effects of cocaine abstinence on behavioral responding during the multiple schedule
Animals displayed consistent cocaine and sucrose self-administration behavioral response profiles across recording days. There was no effect of abstinence on either the number of lever press responses or INT for either reinforcer. These results are consistent with previous studies in which rats were allowed to resume self-administration of sucrose (Jones et al., 2008) or cocaine (Hollander & Carelli, 2005; Hollander & Carelli, 2007) following abstinence. Although cocaine abstinence has been shown to enhance motivation to obtain the drug as measured by an increase in operant responding after abstinence [termed ‘incubation of drug craving’; Grimm et al. (2001)], it is important to note that these experiments were performed under extinction conditions in which operant responding did not result in drug infusion. In the present study, rats were allowed to resume self-administration of cocaine after the abstinence period. Therefore, increases in operant responding were not predicted, consistent with similar experimental manipulations previously used in our laboratory (Hollander & Carelli, 2005; Hollander & Carelli, 2007). However, it is important to note that the animals also underwent a period of ‘sucrose abstinence’ as well as cocaine abstinence in the present study. Although others have documented an incubation of sucrose craving (Lu et al., 2004; Grimm et al., 2005), we have not previously observed this effect in our previous studies (Jones et al., 2008), or in the current study that incorporated the multiple schedule.
As discussed above, the present findings indicate that cocaine self-administration followed by a prolonged period of abstinence can lead to a disruption in reward processing such that encoding of cocaine is enhanced relative to the natural reward sucrose. This neural encoding mirrors the reduced sensitivity to natural rewards seen in cocaine addicts. In the present study, animals did not display a reduction in sucrose self-administration or an increase in cocaine self-administration following abstinence. However, given that the behavioral paradigm used here did not test operant responding under extinction conditions or involve a direct choice between cocaine and sucrose, it is not unexpected that animals would respond similarly before and after abstinence. Many studies that used behavioral paradigms that more directly examined hedonic processing or reward choice provide evidence that both animals (Aigner & Balster, 1978; Aston-Jones & Harris, 2004; Harris et al., 2007; Negus & Rice, 2008) and humans (Lubman et al., 2009) experience a decrease in sensitivity to natural reinforcers with repeated drug exposure. Furthermore, work from our laboratory has shown that cocaine experience can alter the hedonic value of a natural reinforcer that predicts access to drug self-administration (Wheeler et al., 2008; Wheeler et al., 2011). Therefore, although no overt changes in behavior were observed here during the sucrose/cocaine multiple schedule following abstinence, our findings are consistent with previous work showing that natural reinforcers become devalued as a consequence of repeated drug experience and abstinence.
Conclusions
Overall, the results of this study indicate that drugs of abuse such as cocaine do not simply ‘turn on’ the same brain reward circuits that have evolved to process information about natural reinforcers. Rather, cocaine activates a neural circuit in the NAc that is largely separate from the one engaged during goal-directed behaviors for natural rewards. Further, it appears that, following prolonged abstinence, normal reward processing is dysregulated and the encoding of drug-related information is potentiated at the cost of natural physiological rewards. It is possible that, with more drug exposure and extended or repeated periods of abstinence, these effects will become even greater, leading to the loss of control over drug-directed behaviors that is characteristic of the addicted state in humans.
Supplementary Material
Acknowledgements
This research was supported by DA029978 to C.M.C. and DA14339 to R.M.C. The authors thank Dr Robert Wheeler for help with electrophysiological and behavioral techniques, Jessica Briley and Laura Ciompi for assistance in running experiments, and Dr Michael Saddoris and Jon Sugam for helpful discussions of an earlier draft of this manuscript.
Abbreviations
- INT
inter-response interval
- NAc
nucleus accumbens
- PEH
peri-event histogram
- PR
pre-response
- RFe
reinforcement–excitation
- RFi
reinforcement–inhibition
- S:B
signal-to-baseline
References
- Aigner TG, Balster RL. Choice behavior in rhesus monkeys: cocaine versus food. Science. 1978;201:534–535. doi: 10.1126/science.96531. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends in Neurosciences. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Harris GC. Brain substrates for increased drug seeking during protracted withdrawal. Neuropharmacology. 2004;47:167–179. doi: 10.1016/j.neuropharm.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “Accumbens” part of the rat ventral striatum: Immunohistochemical detection of retrogradely transported fluoro-gold. The Journal of Comparative Neurology. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Carelli R, Deadwyler S. A comparison of nucleus accumbens neuronal firing patterns during cocaine self-administration and water reinforcement in rats. The Journal of Neuroscience. 1994;14:7735–7746. doi: 10.1523/JNEUROSCI.14-12-07735.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM. Nucleus accumbens cell firing during goal-directed behaviors for cocaine vs. ‘natural’ reinforcement. Physiology & Behavior. 2002;76:379–387. doi: 10.1016/s0031-9384(02)00760-6. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Ijames SG. Selective activation of accumbens neurons by cocaineassociated stimuli during a water/cocaine multiple schedule. Brain Research. 2001;907:156–161. doi: 10.1016/s0006-8993(01)02604-x. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Ijames SG, Crumling AJ. Evidence that separate neural circuits in the nucleus accumbens encode cocaine versus “natural” (water and food) reward. The Journal of Neuroscience. 2000;20:4255–4266. doi: 10.1523/JNEUROSCI.20-11-04255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, Wondolowski J. Selective encoding of cocaine versus natural rewards by nucleus accumbens neurons is not related to chronic drug exposure. The Journal of Neuroscience. 2003;23:11214–11223. doi: 10.1523/JNEUROSCI.23-35-11214.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Thomas MJ. Biological substrates of reward and aversion: A nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56(Supplement 1):122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Janak PH, Woodward DJ. Comparison of mesocorticolimbic neuronal responses during cocaine and heroin self-administration in freely moving rats. The Journal of Neuroscience. 1998;18:3098–3115. doi: 10.1523/JNEUROSCI.18-08-03098.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng L-J, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiChiara The role of dopamine in drug abuse viewed from the perspective of its role in motivation. Drug and Alcohol Dependence. 1995;38:95–137. doi: 10.1016/0376-8716(95)01118-i. [DOI] [PubMed] [Google Scholar]
- Gawin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251:1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers: clinical observations. Arch Gen Psychiatry. 1986;43:107–113. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Fyall AM, Osincup DP. Incubation of sucrose craving: effects of reduced training and sucrose pre-loading. Physiology & Behavior. 2005;84:73–79. doi: 10.1016/j.physbeh.2004.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation: Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Hummel M, Wimmer M, Mague SD, Aston-Jones G. Elevations of FosB in the nucleus accumbens during forced cocaine abstinence correlate with divergent changes in reward function. Neuroscience. 2007;147:583–591. doi: 10.1016/j.neuroscience.2007.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander JA, Carelli RM. Abstinence from cocaine self-administration heightens neural encoding of goal-directed behaviors in the accumbens. Neuropsychopharmacology. 2005;30:1464–1474. doi: 10.1038/sj.npp.1300748. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Carelli RM. Cocaine-associated stimuli increase cocaine seeking and activate accumbens core neurons after abstinence. The Journal of Neuroscience. 2007;27:3535–3539. doi: 10.1523/JNEUROSCI.3667-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JL, Wheeler RA, Carelli RM. Behavioral responding and nucleus accumbens cell firing are unaltered following periods of abstinence from sucrose. Synapse. 2008;62:219–228. doi: 10.1002/syn.20486. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology. 2003;168:44. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kelley A. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neuroscience & Biobehavioral Reviews. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Koob G, Nestler E. The neurobiology of drug addiction. J Neuropsychiatry Clin Neurosci. 1997;9:482–497. doi: 10.1176/jnp.9.3.482. [DOI] [PubMed] [Google Scholar]
- Koya E, Golden SA, Harvey BK, Guez-Barber DH, Berkow A, Simmons DE, Bossert JM, Nair SG, Uejima JL, Marin MT, Mitchell TB, Farquhar D, Ghosh SC, Mattson BJ, Hope BT. Targeted disruption of cocaineactivated nucleus accumbens neurons prevents context-specific sensitization. Nat Neurosci. 2009;12:1069–1073. doi: 10.1038/nn.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47(Supplement 1):214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Shaham Y, Hope BT. Molecular neuroadaptations in the accumbens and ventral tegmental area during the first 90 days of forced abstinence from cocaine self-administration in rats. Journal of Neurochemistry. 2003;85:1604–1613. doi: 10.1046/j.1471-4159.2003.01824.x. [DOI] [PubMed] [Google Scholar]
- Lubman DI, Yucel M, Kettle JWL, Scaffidi A, MacKenzie T, Simmons JG, Allen NB. Responsiveness to drug cues and natural rewards in opiate addiction: associations with later heroin use. Arch Gen Psychiatry. 2009;66:205–212. doi: 10.1001/archgenpsychiatry.2008.522. [DOI] [PubMed] [Google Scholar]
- Mattson BJ, Koya E, Simmons DE, Mitchell TB, Berkow A, Crombag HS, Hope BT. Context-specific sensitization of cocaine-induced locomotor activity and associated neuronal ensembles in rat nucleus accumbens. European Journal of Neuroscience. 2008;27:202–212. doi: 10.1111/j.1460-9568.2007.05984.x. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: Functional interface between the limbic system and the motor system. Progress in Neurobiology. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Negus SS, Rice KC. Mechanisms of withdrawal-associated increases in heroin selfadministration: pharmacologic modulation of heroin vs food choice in heroin-dependent rhesus monkeys. Neuropsychopharmacology. 2008;34:899–911. doi: 10.1038/npp.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM, Yun IA, Wakabayashi KT, Fields HL. Cue-evoked firing of nucleus accumbens neurons encodes motivational significance during a discriminative stimulus task. Journal of Neurophysiology. 2004;91:1840–1865. doi: 10.1152/jn.00657.2003. [DOI] [PubMed] [Google Scholar]
- O’Donnell P. Dopamine gating of forebrain neural ensembles. European Journal of Neuroscience. 2003;17:429–435. doi: 10.1046/j.1460-9568.2003.02463.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th edn. New York: Elsevier; 2007. [Google Scholar]
- Pennartz CMA, Dolleman-Van der Weel MJ, da Silva FHL. Differential membrane properties and dopamine effects in the shell and core of the rat nucleus accumbens studied in vitro. Neuroscience Letters. 1992;136:109–112. doi: 10.1016/0304-3940(92)90660-y. [DOI] [PubMed] [Google Scholar]
- Pennartz CMA, Groenewegen HJ, Lopes da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: An integration of behavioural, electrophysiological and anatomical data. Progress in Neurobiology. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Peoples LL, Uzwiak AJ, Guyette FX, West MO. Tonic inhibition of single nucleus accumbens neurons in the rat: A predominant but not exclusive firing pattern induced by cocaine self-administration sessions. Neuroscience. 1998;86:13–22. doi: 10.1016/s0306-4522(98)00116-x. [DOI] [PubMed] [Google Scholar]
- Peoples LL, West MO. Phasic firing of single neurons in the rat nucleus accumbens correlated with the timing of intravenous cocaine self-administration. The Journal of Neuroscience. 1996;16:3459–3473. doi: 10.1523/JNEUROSCI.16-10-03459.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends in Neurosciences. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive–sensitization view. Addiction. 2000;95:S91–S117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Gorny G, Mitton E, Kolb B. Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse. 2001;39:257–266. doi: 10.1002/1098-2396(20010301)39:3<257::AID-SYN1007>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Carelli RM. Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron. 2005;45:587–597. doi: 10.1016/j.neuron.2004.12.055. [DOI] [PubMed] [Google Scholar]
- Roop RG, Hollander JA, Carelli RM. Accumbens activity during a multiple schedule for water and sucrose reinforcement in rats. Synapse. 2002;43:223–226. doi: 10.1002/syn.10041. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang G-J. Imaging studies on the role of dopamine in cocaine reinforcement and addiction in humans. Journal of Psychopharmacology. 1999;13:337–345. doi: 10.1177/026988119901300406. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang G-J, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS, Tomasi D, Telang F, Baler R. Addiction: Decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain’s control circuit. BioEssays. 2010;32:748–755. doi: 10.1002/bies.201000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler RA, Aragona BJ, Fuhrmann KA, Jones JL, Day JJ, Cacciapaglia F, Wightman RM, Carelli RM. Cocaine cues drive opposing context-dependent shifts in reward processing and emotional state. Biological Psychiatry. 2011;69:1067–1074. doi: 10.1016/j.biopsych.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler RA, Twining RC, Jones JL, Slater JM, Grigson PS, Carelli RM. Behavioral and electrophysiological indices of negative affect predict cocaine selfadministration. Neuron. 2008;57:774–785. doi: 10.1016/j.neuron.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Wise R. Drug self-administration viewed as ingestive behaviour. Appetite. 1997;28:1–5. doi: 10.1006/appe.1996.0059. [DOI] [PubMed] [Google Scholar]
- Zahm DS. Functional-anatomical implications of the nucleus accumbens core and shell subterritories. Annals of the New York Academy of Sciences. 1999;877:113–128. doi: 10.1111/j.1749-6632.1999.tb09264.x. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Brog JS. On the significance of subterritories in the “accumbens” part of the rat ventral striatum. Neuroscience. 1992;50:751–767. doi: 10.1016/0306-4522(92)90202-d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.