Abstract
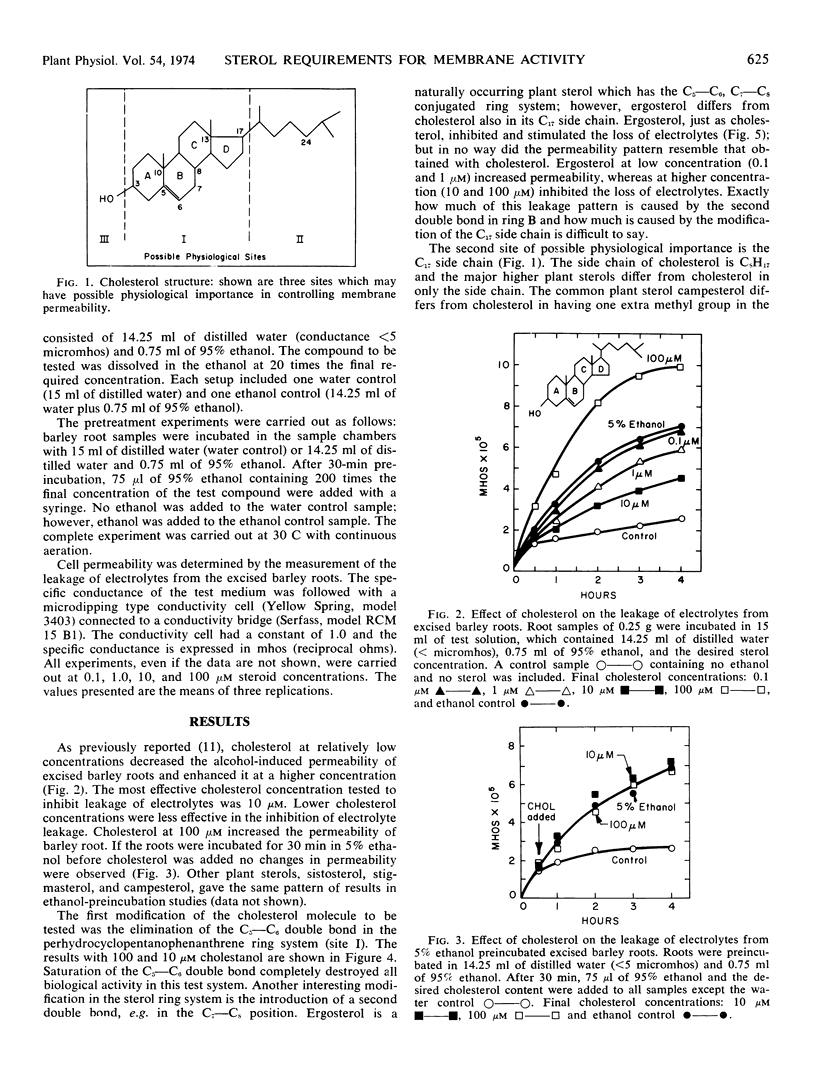
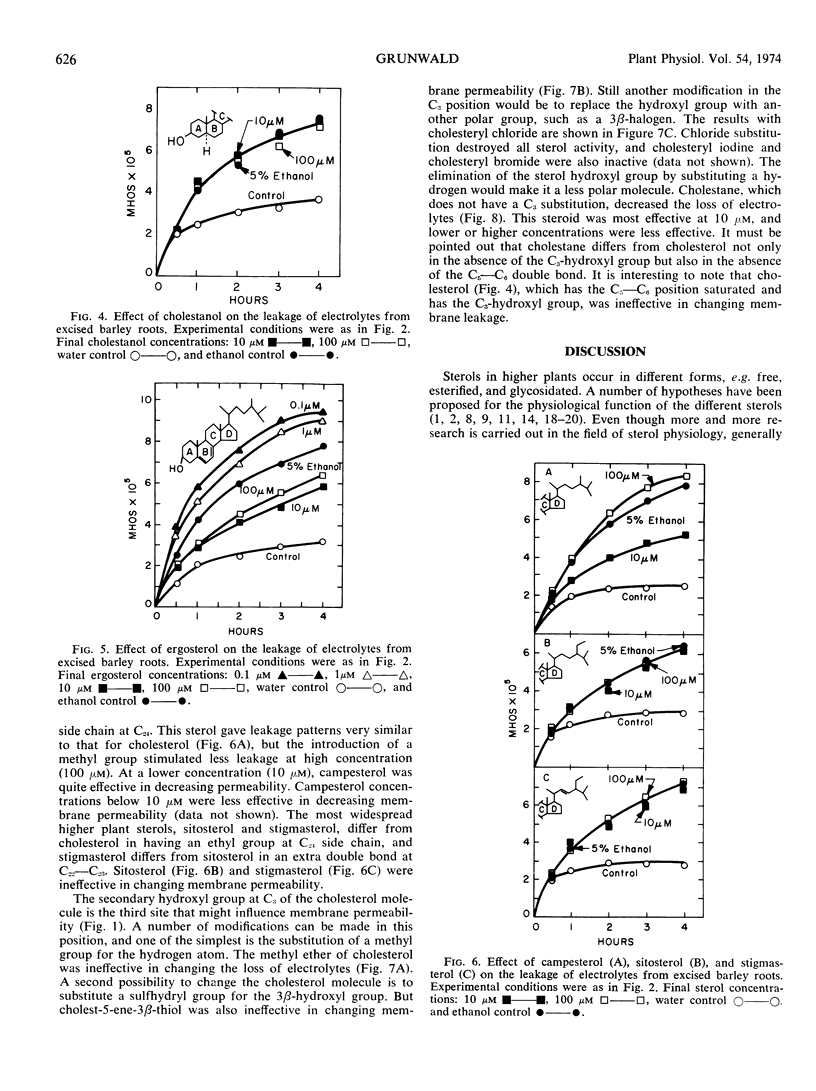
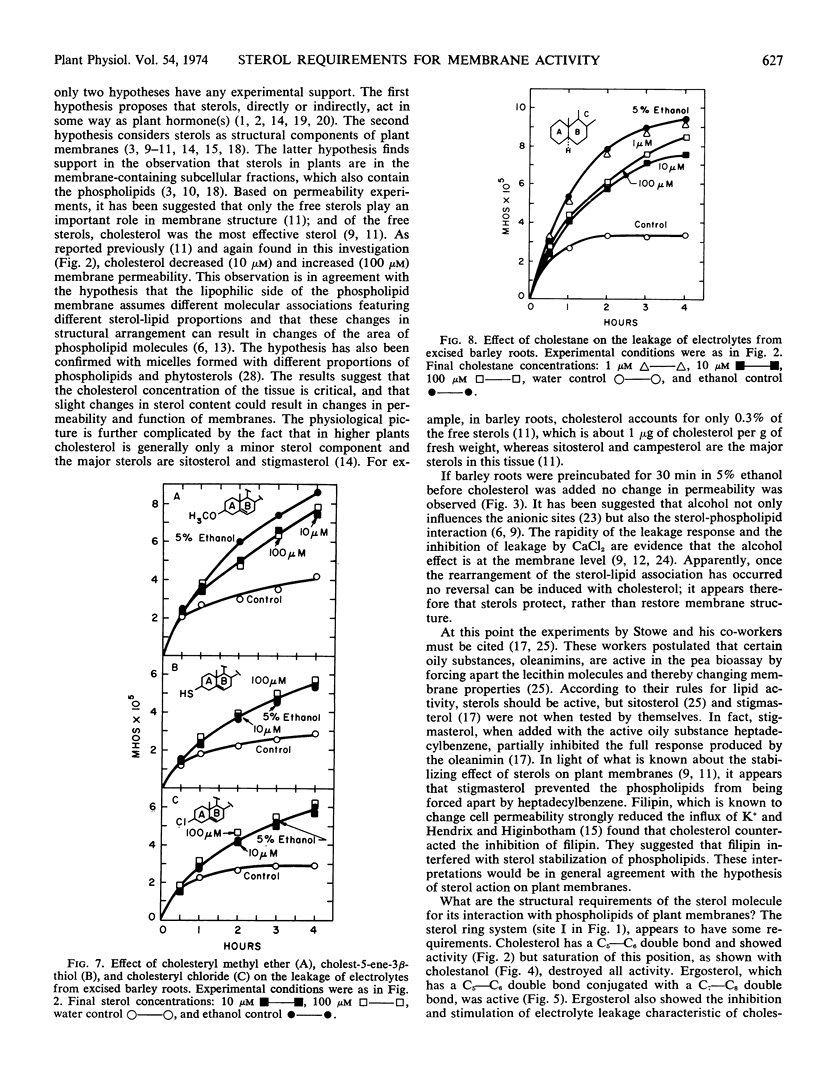
Various sterols and related steroids were tested for their ability to influence ethanol-induced electrolyte leakage from Hordeum vulgare roots. Cholesterol had the greatest influence and, depending on concentration, it stimulated or inhibited the loss of electrolyte. Cholesterol, however, was ineffective if the roots were pretreated with ethanol. These data suggest that sterols protect rather than restore membrane structure. First, modifications in the cholesterol perhydrocyclopentanophenanthrene ring system suggest that at least one double bond is required for membrane activity. Second, increasing the bulkiness of the C17 side chain of cholesterol, as shown with campesterol, stigmasterol, and sitosterol, decreased its activity. Apparently for maximum effectiveness the sterol molecule should have a relatively flat configuration. Third, the C3-hydroxyl group is required for membrane activity since cholesteryl methyl ether, cholest-5-ene-3β-thiol and cholesteryl halogens were without activity. Exception to the foregoing rule was cholestane which was slightly active but which has neither a C3-hydroxyl group nor a double bond in the ring system.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner J., Heftmann E., Zeevaart J. A. Suppression of Floral Induction by Inhibitors of Steroid Biosynthesis. Plant Physiol. 1963 Jan;38(1):81–88. doi: 10.1104/pp.38.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt R. D., Benveniste P. Isolation and identification of sterols from subcellular fractions of bean leaves (Phaseolus vulgaris). Biochim Biophys Acta. 1972 Sep 1;282(1):85–92. doi: 10.1016/0005-2736(72)90313-6. [DOI] [PubMed] [Google Scholar]
- Bruckdorfer K. R., Demel R. A., De Gier J., van Deenen L. L. The effect of partial replacements of membrane cholesterol by other steroids on the osmotic fragility and glycerol permeability of erythrocytes. Biochim Biophys Acta. 1969 Jul 15;183(2):334–345. doi: 10.1016/0005-2736(69)90089-3. [DOI] [PubMed] [Google Scholar]
- Deenen L. L. Phospholipide. Beziehungen zwischen ihrer chemischen Struktur und Biomembranen. Naturwissenschaften. 1972 Nov;59(11):485–491. doi: 10.1007/BF00609812. [DOI] [PubMed] [Google Scholar]
- FINEAN J. B. Phospholipid-cholesterol complex in the structure of myelin. Experientia. 1953 Jan 15;9(1):17–19. doi: 10.1007/BF02147697. [DOI] [PubMed] [Google Scholar]
- Grunwald C. Effect of sterols on the permeability of alcohol-treated red beet tissue. Plant Physiol. 1968 Apr;43(4):484–488. doi: 10.1104/pp.43.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald C. Effects of free sterols, steryl ester, and steryl glycoside on membrane permeability. Plant Physiol. 1971 Nov;48(5):653–655. doi: 10.1104/pp.48.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald C. Sterol distribution in intracellular organelles isolated from tobacco leaves. Plant Physiol. 1970 Jun;45(6):663–666. doi: 10.1104/pp.45.6.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HECHTER O., LESTER G. Cell permeability and hormone action. Recent Prog Horm Res. 1960;16:139–186. [PubMed] [Google Scholar]
- Hsia J. C., Long R. A., Hruska F. E., Gesser H. D. Steroid-phosphatidylcholine interactions in oriented multibilayers--a spin label study. Biochim Biophys Acta. 1972 Dec 1;290(1):22–31. doi: 10.1016/0005-2736(72)90048-x. [DOI] [PubMed] [Google Scholar]
- Iwata T., Stowe B. B. Probing a Membrane Matrix Regulating Hormone Action: II. The Kinetics of Lipid-Induced Growth and Ethylene Production. Plant Physiol. 1973 Apr;51(4):691–701. doi: 10.1104/pp.51.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp R. J., Mercer E. I. Studies on the sterols and sterol esters of the intracellular organelles of maize shoots. Biochem J. 1968 Nov;110(1):119–125. doi: 10.1042/bj1100119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopcewicz J. Influence of estrogens on the auxins content in plants. Naturwissenschaften. 1970 Jan;57(1):48–48. doi: 10.1007/BF00593574. [DOI] [PubMed] [Google Scholar]
- Long R. A., Hruska F., Gesser H. D., Hsia J. C., Williams R. Membrane condensing effect of cholesterol and the role of its hydroxyl group. Biochem Biophys Res Commun. 1970 Oct 23;41(2):321–327. doi: 10.1016/0006-291x(70)90506-1. [DOI] [PubMed] [Google Scholar]
- Montfoort A., van Golde L. M., van Deenen L. L. Molecular species of lecithins from various animal tissues. Biochim Biophys Acta. 1971 Mar 16;231(2):335–342. doi: 10.1016/0005-2760(71)90147-0. [DOI] [PubMed] [Google Scholar]
- Siegel S. M., Daly O. Regulation of betacyanin efflux from beet root by poly-L-lysine, ca-ion and other substances. Plant Physiol. 1966 Nov;41(9):1429–1434. doi: 10.1104/pp.41.9.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel S. M., Halpern L. A. THE EFFECT OF BRANCHING AT C-1 ON THE BIOLOGICAL ACTIVITY OF ALCOHOLS. Proc Natl Acad Sci U S A. 1964 May;51(5):765–768. doi: 10.1073/pnas.51.5.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe B. B., Dotts M. A. Probing a membrane matrix regulating hormone action: I. The molecular length of effective lipids. Plant Physiol. 1971 Nov;48(5):559–565. doi: 10.1104/pp.48.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood A., Paleg L. G. The influence of gibberellic Acid on the permeability of model membrane systems. Plant Physiol. 1972 Jul;50(1):103–108. doi: 10.1104/pp.50.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]



