Abstract
The assimilatory nitrate reductase (NADH: nitrate oxidoreductase, E.C. 1.6.6.2.) from the marine diatom Thalassiosira pseudonana, Hasle and Heimdal, has been purified 200-fold and characterized. The regulation of nitrate reductase in response to various conditions of nitrogen nutrition has been investigated.
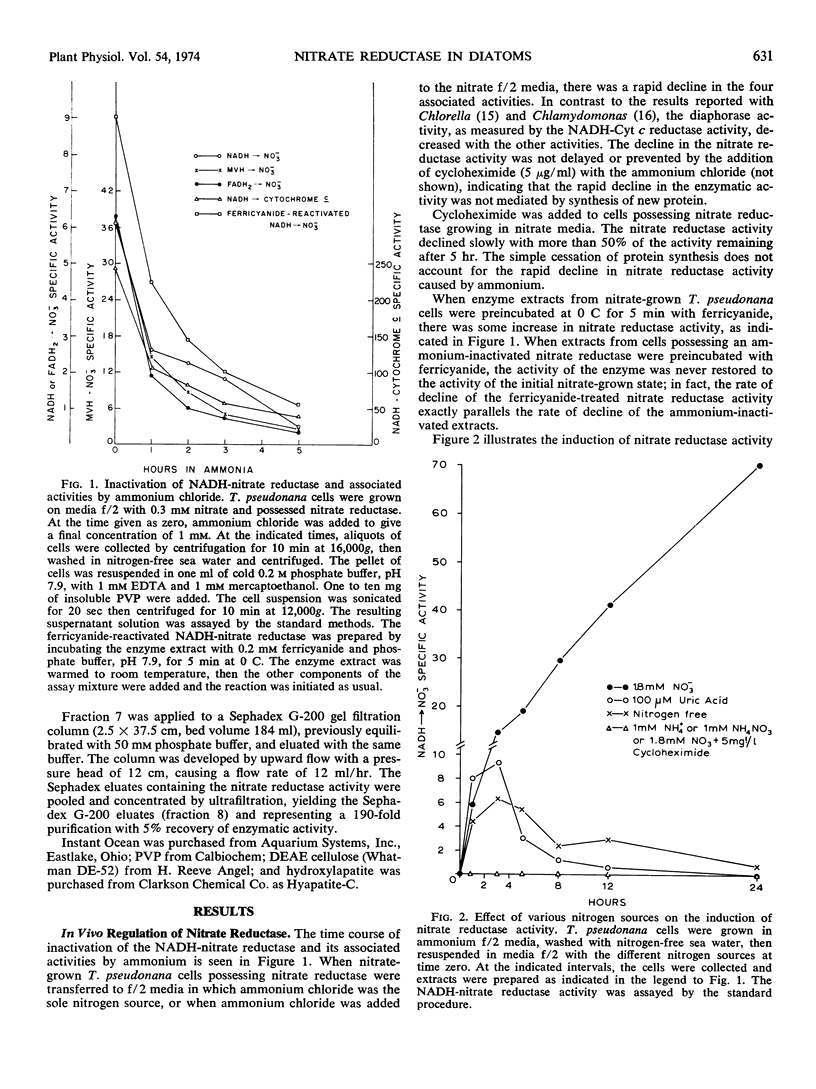
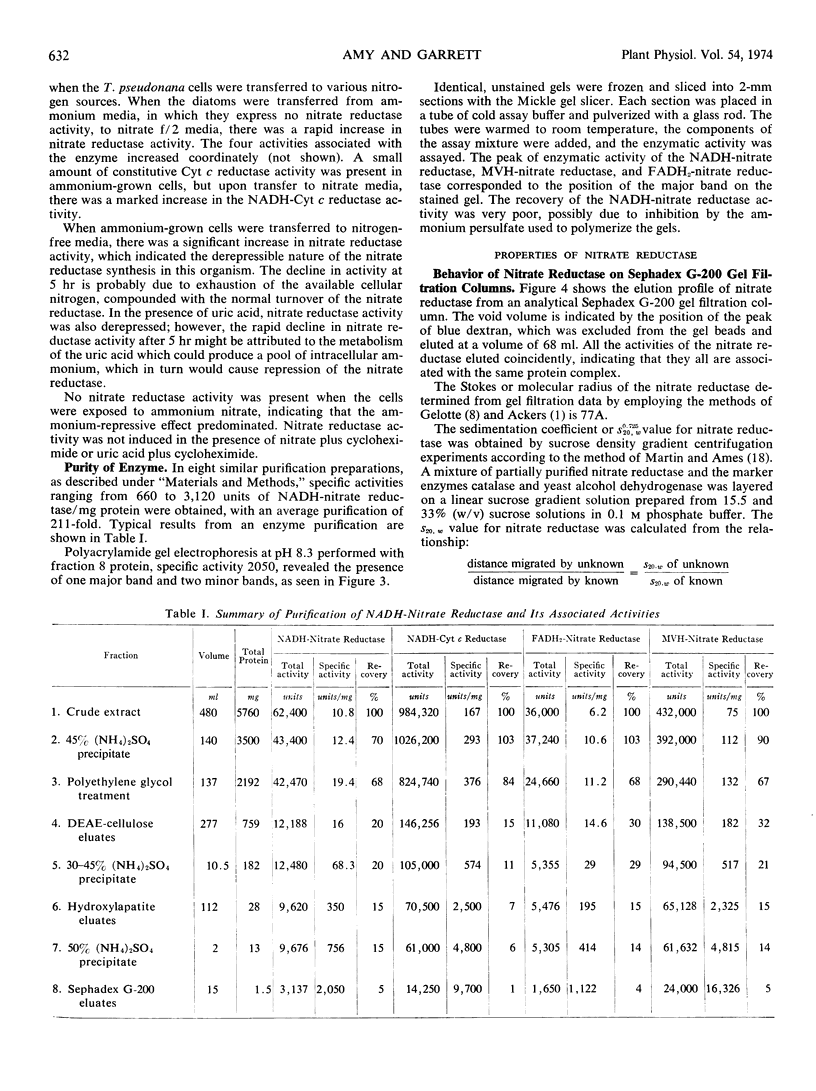
Nitrate reductase activity is repressed by the presence of ammonium in vivo, and its synthesis is derepressed when ammonium is absent. The derepression process is sensitive to cycloheximide and apparently requires protein synthesis. Repression of enzyme activity by ammonium is neither inhibited nor delayed by the presence of cycloheximide. In vitro, ammonium does not inhibit enzyme activity.
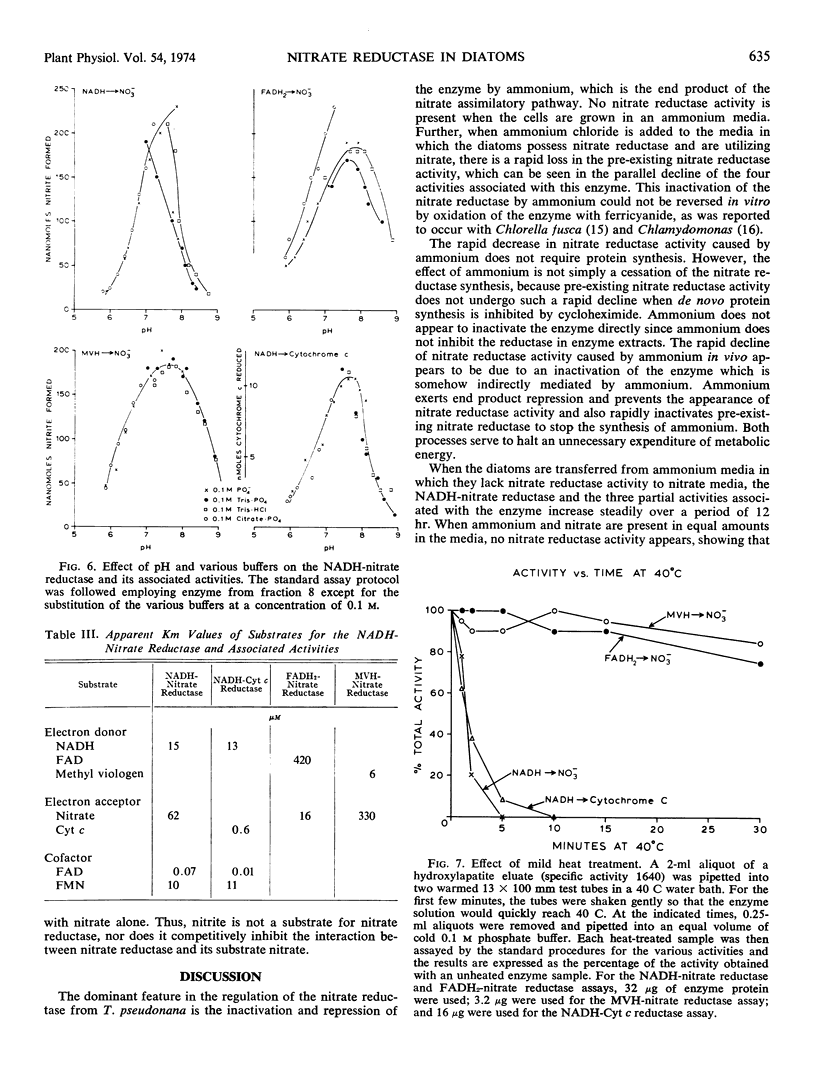
NADH is the physiological electron donor for the enzyme in a flavin-dependent reaction. Spectral studies have indicated the presence of a b-type cytochrome associated with the enzyme. It is possible to observe enzymatic oxidation-reduction reactions which represent partial functions of the over-all electron transport capacity of this enzyme. Nitrate reductase will accept electrons from artificial electron donors such as reduced methyl viologen in a flavin-independent reaction. Further, dithionitereduced flavin adenine dinucleotide can donate electrons to the enzyme to reduce nitrate to nitrite. Finally, the nitrate reductase will exhibit a diaphorase activity and reduce the artificial electron acceptor mammalian cytochrome c in flavin-adeninedinucleotide-dependent reaction.
Inhibition studies with potassium cyanide, sodium azide, and o-phenanthroline have yielded indirect evidence for metal component (s) of the enzyme.
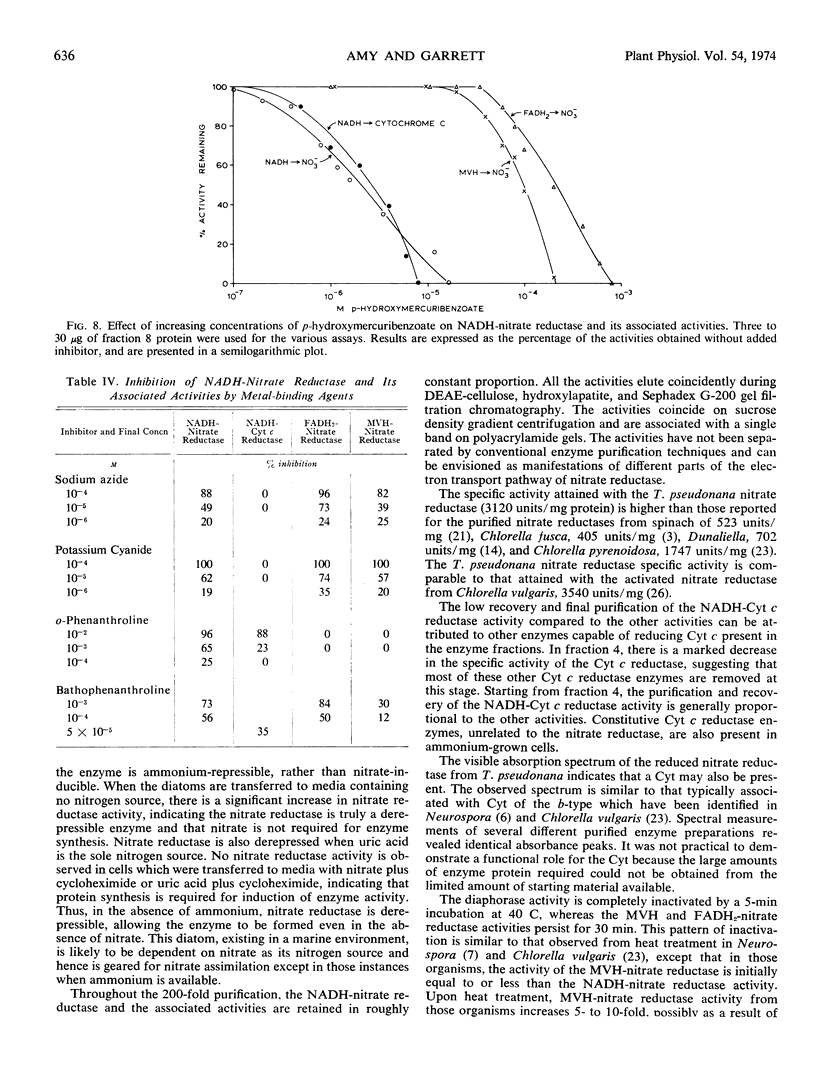
The inhibition of the NADH-requiring enzyme activities by p-hydroxymercuribenzoate has shown that an essential sulfhydryl group is involved in the initial portion of the electron transport. Heat treatment exerts an effect similar to the p-hydroxymercuribenzoate inhibition; namely, the NADH-requiring activities are rapidly inactivated, whereas the terminal nitrate-reducing activities are relatively stable to heat.
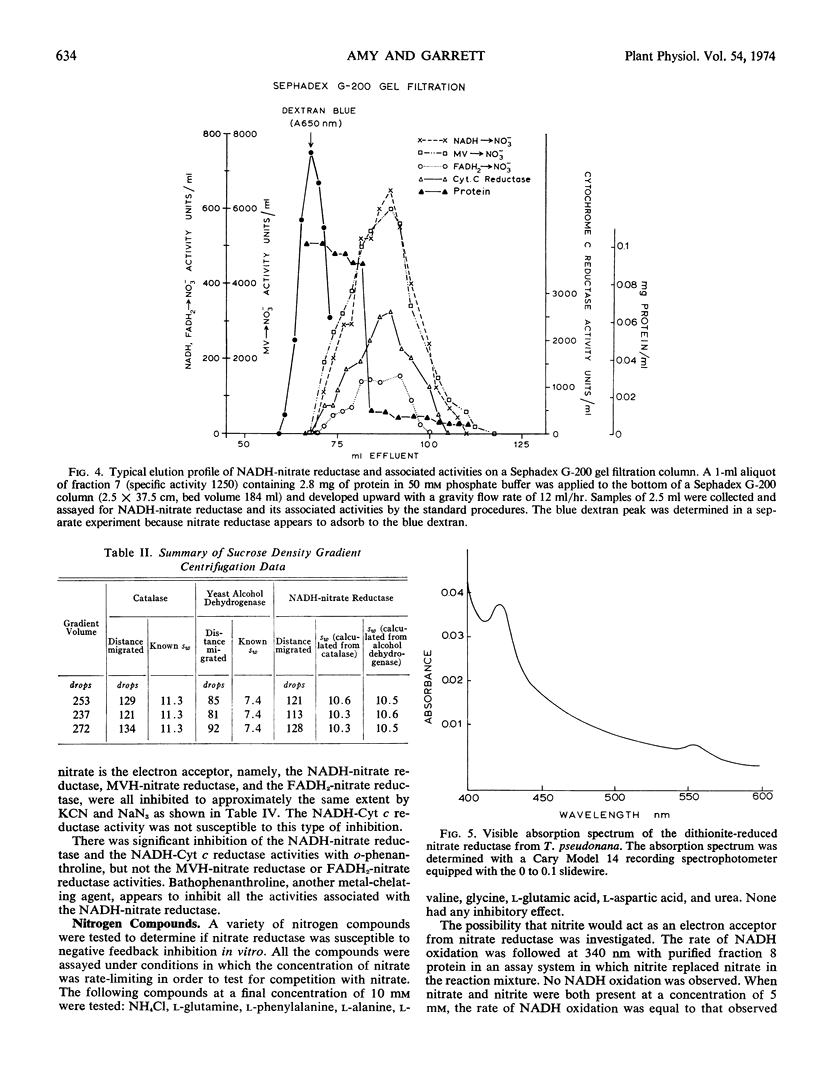
The T. pseudonana nitrate reductase molecule has the hydrodynamic properties of an ellipsoid with a frictional coefficient of 1.69 and a molecular weight of 330,000.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACKERS G. K. MOLECULAR EXCLUSION AND RESTRICTED DIFFUSION PROCESSES IN MOLECULAR-SIEVE CHROMATOGRAPHY. Biochemistry. 1964 May;3:723–730. doi: 10.1021/bi00893a021. [DOI] [PubMed] [Google Scholar]
- CLARKE J. T. SIMPLIFIED "DISC" (POLYACRYLAMIDE GEL) ELECTROPHORESIS. Ann N Y Acad Sci. 1964 Dec 28;121:428–436. doi: 10.1111/j.1749-6632.1964.tb14214.x. [DOI] [PubMed] [Google Scholar]
- GUILLARD R. R., RYTHER J. H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can J Microbiol. 1962 Apr;8:229–239. doi: 10.1139/m62-029. [DOI] [PubMed] [Google Scholar]
- Garrett R. H., Nason A. Further purification and properties of Neurospora nitrate reductase. J Biol Chem. 1969 Jun 10;244(11):2870–2882. [PubMed] [Google Scholar]
- Garrett R. H., Nason A. Involvement of a B-type cytochrome in the assimilatory nitrate reductase of Neurospora crassa. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1603–1610. doi: 10.1073/pnas.58.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepper L., Hageman R. H. The occurrence of nitrate reductase in apple leaves. Plant Physiol. 1969 Jan;44(1):110–114. doi: 10.1104/pp.44.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Losada M., Paneque A., Aparicio P. J., Vega J. M., Cárdenas J., Herrera J. Inactivation and repression by ammonium of the nitrate reducing system in chlorella. Biochem Biophys Res Commun. 1970 Mar 27;38(6):1009–1015. doi: 10.1016/0006-291x(70)90340-2. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- NASON A. Symposium on metabolism of inorganic compounds. II. Enzymatic pathways of nitrate, nitrite, and hydroxylamine metabolisms. Bacteriol Rev. 1962 Mar;26:16–41. doi: 10.1128/br.26.1.16-41.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paneque A., Del Campo F. F., Ramírez J. M., Losada M. Flavin nucleotide nitrate reductase from spinach. Biochim Biophys Acta. 1965 Sep 27;109(1):79–85. doi: 10.1016/0926-6585(65)90092-0. [DOI] [PubMed] [Google Scholar]
- Ryther J. H., Dunstan W. M. Nitrogen, phosphorus, and eutrophication in the coastal marine environment. Science. 1971 Mar 12;171(3975):1008–1013. doi: 10.1126/science.171.3975.1008. [DOI] [PubMed] [Google Scholar]
- Schloemer R. H., Garrett R. H. Partial Purification of the NADH-Nitrate Reductase Complex from Chlorella pyrenoidosa. Plant Physiol. 1973 Mar;51(3):591–593. doi: 10.1104/pp.51.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Solomonson L. P., Vennesland B. Properties of a nitrate reductase of Chlorella. Biochim Biophys Acta. 1972 Jun 23;267(3):544–557. doi: 10.1016/0005-2728(72)90183-1. [DOI] [PubMed] [Google Scholar]
- Vennesland B., Jetschmann C. The nitrate reductase of Chlorella pyrenoidosa. Biochim Biophys Acta. 1971 Mar 10;227(3):554–564. doi: 10.1016/0005-2744(71)90006-4. [DOI] [PubMed] [Google Scholar]




