Abstract
Lysine biosynthesis in seedlings of barley (Hordeum vulgare L. var. Emir) was studied by direct injection of the following precursors into the endosperm of the seedlings: acetate-1-14C; acetate-2-14C; pyruvate-1-14C; pyruvate-2-14C; pyruvate-3-14C; alanine-1-14C; aspartic acid-1-14C; aspartic acid-2-14C; aspartic acid-3-14C; aspartic acid-4-14C; α-aminoadipic acid-1-14C; and α, ε-diaminopimelic acid-1-(7)-14C. The distribution of activity in the individual carbon atoms of lysine in the different biosynthetic experiments was determined by chemical degradation. The incorporation percentages and labeling patterns obtained are in agreement with the occurrence of the diaminopimelic acid pathway. The results do not fit the incorporation percentages and labeling patterns expected if the α-aminoadipic acid pathway was operating. However, the results show that barley seedlings are able to convert a small part of the α-aminoadipic acid administered directly to lysine.
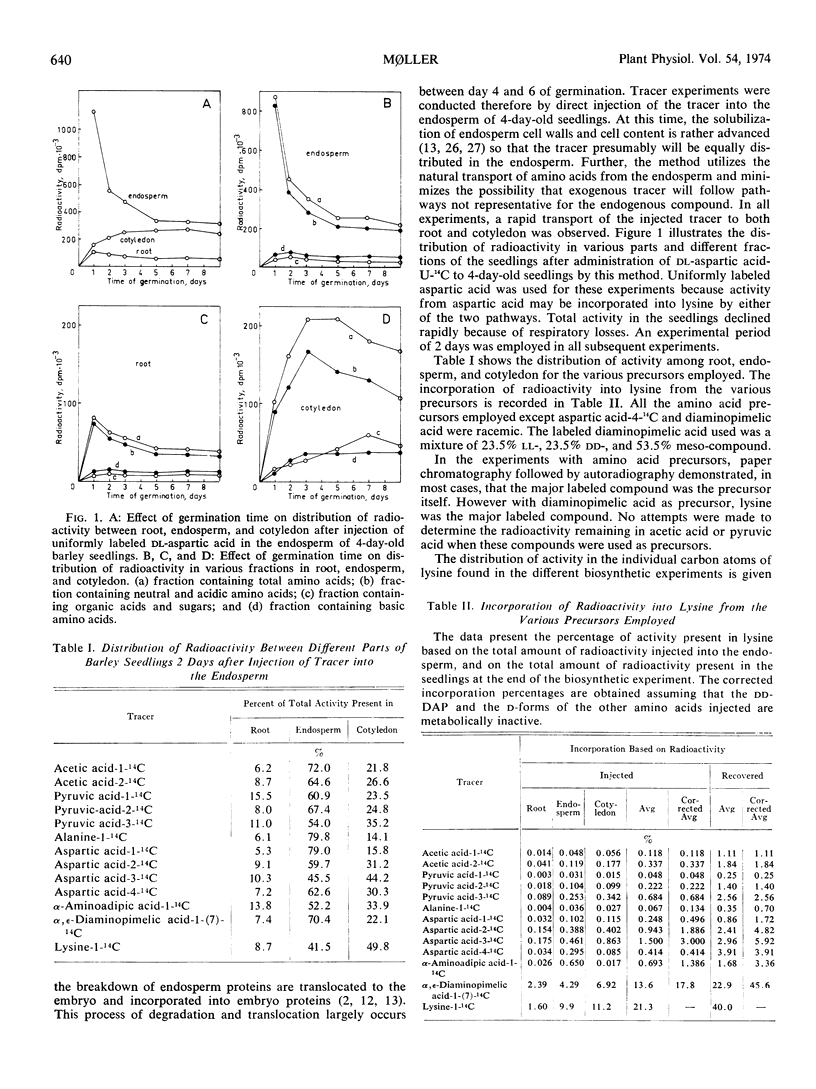
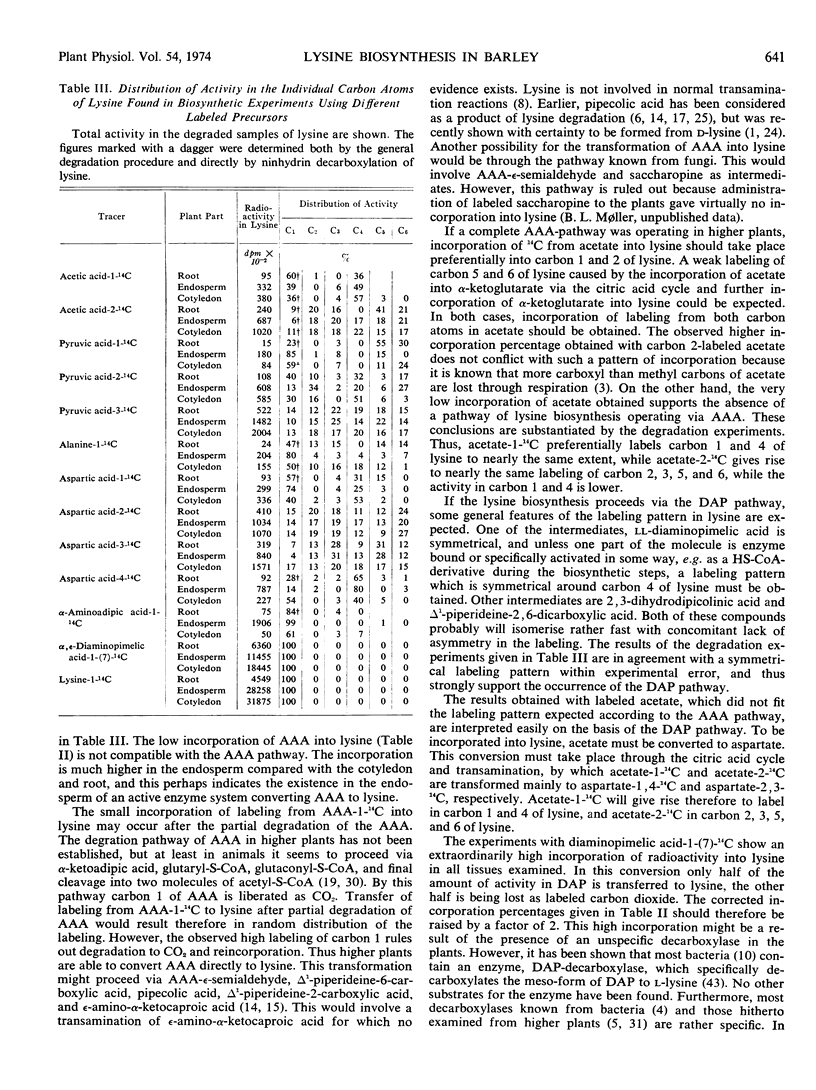
The labeling pattern of lysine was found to be symmetrical around carbon 4. This indicates that the biosynthetic pathway proceeds via a symmetrical intermediate like ll-α, ε-diaminopimelic acid, or includes compounds as 2, 3-dihydrodipicolinic acid or Δ1-piperideine-2, 6-dicarboxylic acid which probably isomerise with concomitant lack of asymmetry in the labeling. The percentages of incorporation show that both the mesoand ll-forms of α, ε-diaminopimelic acid are metabolically convertible to lysine in seedlings of barley.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BILINSKI E., MCCONNELL W. B. Studies on wheat plants using C14 compounds. III. The utilization of acetate for amino acid biosynthesis. Can J Biochem Physiol. 1957 Jun;35(6):357–363. [PubMed] [Google Scholar]
- BOULANGER P., OSTEUX R. Les destinées de la lysine dans les règnes animal et végétal. C R Hebd Seances Acad Sci. 1954 Aug 2;239(5):458–460. [PubMed] [Google Scholar]
- CLARK I., RITTENBERG D. The metabolic activity of the alpha-hydrogen atom of lysine. J Biol Chem. 1951 Apr;189(2):521–528. [PubMed] [Google Scholar]
- CUMMINGS M. M., HUDGINS P. C. Chemical constituents of pine pollen and their possible relationship to sarcoidosis. Am J Med Sci. 1958 Sep;236(3):311–317. [PubMed] [Google Scholar]
- GILVARG C. Biosynthesis of diaminopimelic acid. Fed Proc. 1960 Dec;19:948–952. [PubMed] [Google Scholar]
- Higashino K., Tsukada K., Lieberman I. Saccharopine, a product of lysine breakdown by mammalian liver. Biochem Biophys Res Commun. 1965 Jul 26;20(3):285–290. doi: 10.1016/0006-291x(65)90361-x. [DOI] [PubMed] [Google Scholar]
- LOWY P. H. The conversion of lysine to pipecolic acid by Phaseolus vulgaris. Arch Biochem Biophys. 1953 Nov;47(1):228–229. doi: 10.1016/0003-9861(53)90457-3. [DOI] [PubMed] [Google Scholar]
- Larsen P. O. A convenient method for liquid scintillation counting of barium carbonate-14C. Int J Appl Radiat Isot. 1973 Oct;24(10):612–613. doi: 10.1016/0020-708x(73)90133-6. [DOI] [PubMed] [Google Scholar]
- Leistner E., Gupta R. N., Spenser I. D. A general method for the determination of precursor configuration in biosynthetic precursor-product relationships. Derivation of pipecolic acid from D-lysine, and of piperidine alkaloids from L-lysine. J Am Chem Soc. 1973 Jun 13;95(12):4040–4047. doi: 10.1021/ja00793a035. [DOI] [PubMed] [Google Scholar]
- Medvedev A. I., Kretovich V. L. Dva puti biosinteza lizina u vysshikh rastenii. Biokhimiia. 1966 Jul-Aug;31(4):659–665. [PubMed] [Google Scholar]
- Meghal S. K., Cheung H. S., O'Neal R. M., Koeppe R. E. Metabolism of DL-lysine-2- and -6-14C in rats and dogs. J Biol Chem. 1966 Jun 10;241(11):2622–2625. [PubMed] [Google Scholar]
- Shimura Y., Vogel H. J. Diaminopimelate decarboxylase of Lemna perpusilla: partial purification and some properties. Biochim Biophys Acta. 1966 May 5;118(2):396–404. doi: 10.1016/s0926-6593(66)80048-6. [DOI] [PubMed] [Google Scholar]
- Tiwari H. P., Spenser I. D. Precursors of mimosine in Mimosa pudica. Can J Biochem. 1965 Oct;43(10):1687–1691. doi: 10.1139/o65-186. [DOI] [PubMed] [Google Scholar]
- Turner J. C. Triton X-100 scintillant for carbon-14 labelled materials. Int J Appl Radiat Isot. 1968 Jul;19(7):557–563. doi: 10.1016/0020-708x(68)90065-3. [DOI] [PubMed] [Google Scholar]
- Vogel H. J. ON BIOCHEMICAL EVOLUTION: LYSINE FORMATION IN HIGHER PLANTS. Proc Natl Acad Sci U S A. 1959 Dec;45(12):1717–1721. doi: 10.1073/pnas.45.12.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE P. J., KELLY B. PURIFICATION AND PROPERTIES OF DIAMINOPIMELATE DECARBOXYLASE FROM ESCHERICHIA COLI. Biochem J. 1965 Jul;96:75–84. doi: 10.1042/bj0960075. [DOI] [PMC free article] [PubMed] [Google Scholar]



