Key Points
Unidirectional graft-versus-host vector 7/8 HLA mismatches have the same level of risk as bidirectional 7/8 mismatches.
For HLA homozygous recipients, a mismatch at the homozygous locus is preferred over a mismatch at the heterozygous loci.
Abstract
The impact of HLA homozygosity at mismatched (MM) loci on the outcome of 2687 myeloablative unrelated donor hematopoietic cell transplantations performed for malignant disease was evaluated among 4 groups: 7/8 bidirectional MM transplants (donor and recipient heterozygous MM, n = 1393), 7/8 host-versus-graft (HVG) vector MM (recipient homozygous, n = 112), 7/8 graft-versus-host (GVH) vector MM (donor homozygous, n = 119), and 8/8 matches (n = 1063). Multivariate analyses found 7/8 GVH (P = .001) and bidirectional MM groups (P < .0001) had significantly worse transplant-related mortality and overall and disease-free survival than the 8/8 match group, a difference not observed with the 7/8 HVG MM group (P > .01). The 3 7/8 groups differed only for grades III-IV acute GVH disease (GVHD), where HVG MM had less GVHD than the 7/8 bidirectional MM (hazard ratio [HR] 0.52, P = .0016) and GVH MM (HR 0.43, P = .0009) groups but not the 8/8 group (HR 0.83, P = .39). There were no differences between the 7/8 groups for relapse, chronic GVHD, neutrophil engraftment, or graft failure. GVH MM have the same risk as 7/8 bidirectional MM. 7/8 HVG MM confer a reduced risk of acute GVHD without an increased risk of disease relapse or graft failure compared with a 7/8 bidirectional MM.
Introduction
Recipients of hematopoietic stem cell transplantations are matched with their unrelated adult volunteer donors for HLA-A, HLA-B, HLA-C, and HLA-DRB1 high-resolution types (ie, 8/8 match) to optimize overall survival.1-3 Each allele mismatch reduces overall survival at 1 year by 9% to 10% (8/8 52%, 7/8 43%, 6/8 33%).1 For those patients without 8/8 matched donors, there is no consensus as to how homozygosity at an HLA locus should be handled in determining the degree of mismatch between a patient and a potential donor. Unidirectional mismatches may affect the rates of graft failure for a homozygous recipient receiving a graft from a heterozygous donor (ie, mismatch in the host-versus-graft [HVG] vector; R: A*02:01,*02:01 and D: A*02:01, *11:01) or graft-versus-host (GVH) disease for a homozygous donor (ie, mismatch in the GVH vector) (Table 1). If both donor and recipient are homozygous and mismatched (eg, A*02:01, *02:01 vs A*11:01, *11:01), the bidirectional mismatch may affect both outcomes.
Table 1.
Examples of HLA histocompatibility groups in study
| Donor (graft)* | Recipient (host)* | Match category† (A, B, C, DRB1) | Vector of mismatch | Guideline for 7/8 matching |
|---|---|---|---|---|
| A*02:01, A*02:01 | A*02:01, A*02:01 | 8/8 match | ||
| Homozygous | Homozygous | |||
| A*02:01, A*03:01 | A*02:01, A*02:01 | 7/8 mismatch | HVG | Less risk of GVHD compared with bidirectional mismatch |
| Heterozygous | Homozygous | |||
| A*02:01, A*02:01 | A*02:01, A*03:01 | 7/8 mismatch | GVH | Same risks as bidirectional mismatch |
| Homozygous | Heterozygous | |||
| A*01:01, A*02:01 | A*03:01, A*02:01 | 7/8 mismatch | Bidirectional (HVG and GVH) | |
| Heterozygous | Heterozygous |
Examples only; mismatches at all 4 loci, HLA-A, HLA-B, HLA-C, and HLA-DRB1, were included in study. Mismatched alleles are highlighted in bold text.
Match category assumes that alleles at all other loci (B, C, DRB1) are matched in these examples.
A study by the Seattle Transplant Group4 suggested that the risk of graft failure was increased if the recipient of a bone marrow graft was HLA homozygous at the mismatched class I locus (ie, in the HVG vector). Of the 471 donor-recipient pairs studied, primary graft failure was observed in 26 patients and secondary failure in 2. Of the 7 recipients homozygous at the mismatched class I locus, 5 exhibited graft failure, compared with 7 of the 98 heterozygous recipients (P value < .001). An increased percentage of homozygous recipients with multiple class I mismatches also showed more frequent graft failure compared with heterozygous recipients with multiple mismatches. Based on these data, the investigators suggested that mismatches at the recipient’s homozygous locus should be avoided.
This study reevaluated the outcome for unidirectional mismatches in recipients receiving either bone marrow or growth factor stimulated peripheral blood grafts for malignant diseases. The hypothesis tested was that unidirectional mismatches will have outcomes indistinguishable from 7/8 bidirectional mismatched transplantations. Comparisons in outcomes were also made to 8/8 matched transplantations.
Methods
Study population
The study included patients reported to the National Marrow Donor Program (NMDP) who received a transplant from an unrelated donor between 1988 and 2009. The study population consisted of recipients receiving their first marrow or peripheral blood stem cell unrelated donor transplantation for the treatment of acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), chronic myeloid leukemia (CML), or myelodysplastic syndrome (MDS). Early-stage disease was defined as AML or ALL in first complete remission, CML in first chronic phase, and MDS subtype refractory anemia. Intermediate-stage disease was defined as AML or ALL in second or subsequent complete remission and CML in accelerated phase or second chronic phase. Advanced-phase disease was defined as AML in first or higher relapse or primary induction failure, CML in blast phase, and MDS subtypes refractory anemia with excess blasts or in transformation.
All surviving recipients included in this analysis were retrospectively contacted and provided informed consent for participation in the NMDP research program, in accordance with the Declaration of Helsinki. Research was approved and conducted under the supervision of the NMDP Institutional Review Board. A modeling process was used, as previously described in Lee et al1 and Farag et al,5 to adjust for any bias introduced by the exclusion of nonconsenting survivors.
All HLA-typing was verified using DNA-based methods at high resolution, as previously described in Spellman et al.6 Cases were divided into 4 histocompatibility groups based on high-resolution typing at HLA-A, HLA-B, HLA-C, and HLA-DRB1 loci (Table 2). The comparison groups included (1) both donor and recipient with a single high-resolution mismatch (ie, mismatches altering the polypeptide sequence of the HLA antigen recognition site, including both allele and antigen mismatches) and both heterozygous at the mismatched (MM) locus (eg, A*02:01, *11:01 vs A*02:01, *24:02) (7/8 bidirectional MM); (2) the recipient homozygous for the mismatched locus (unidirectional 7/8 HVG), (3) the donor homozygous for the mismatched locus (7/8 GVH), and (4) 8/8 high resolution HLA-A, HLA-B, HLA-C, HLA-DRB1 matched transplants exhibiting homozygosity at 1 or more loci (8/8) (Table 1). This restriction was imposed for the 8/8 group to increase the comparability with the 7/8 groups of interest. A univariate analysis comparing outcomes between the 8/8 comparison group (n = 1063) and 8/8 matches where donor and recipient were heterozygous at the matched loci (n = 3619) noted no significant differences in overall survival, transplant-related mortality (TRM), disease-free survival, neutrophil engraftment, secondary graft failure, or acute and chronic GVH disease (GVHD). Significant differences were noted in the probability of relapse at 3 years (P = .003) and 5 years (P = .006), where 8/8 heterozygotes had a slightly greater likelihood of relapse (30% [95% confidence interval (CI) 29-32] for 8/8 heterozygotes vs 26% [95% CI 23-28] for 8/8) with 1 or more homozygous loci at 3 years. Unfortunately, the evaluation of bidirectional mismatches in homozygous recipients and donors could not be evaluated due to the low number of cases (3 total).
Table 2.
Characteristics of recipients receiving myeloablative first transplants for AML, ALL, CML, or MDS that are high-resolution typed for HLA-A, HLA-B, HLA-C, and HLA-DRB1 by HLA categories*,†
| Variable | 8/8‡ Matched N (%) | 7/8 HVG MM N (%) | 7/8 GVH MM N (%) | 7/8 Both Directions N (%) | P value |
|---|---|---|---|---|---|
| Number of patients | 1063 | 112 | 119 | 1393 | |
| Number of centers | 124 | 63 | 60 | 147 | |
| Recipient age, median (range), y | 38 (<1-70) | 34 (4-64) | 31 (<1-65) | 33 (<1-69) | <.0001 |
| Age at transplant, y | <.0001 | ||||
| 0-9 | 76 (7) | 8 (7) | 17 (14) | 141 (10) | |
| 10-19 | 105 (10) | 18 (16) | 16 (13) | 199 (14) | |
| 20-29 | 180 (17) | 16 (14) | 23 (19) | 250 (18) | |
| 30-39 | 209 (20) | 35 (31) | 22 (18) | 284 (20) | |
| 40-49 | 272 (26) | 19 (17) | 21 (18) | 317 (23) | |
| 50 and older | 221 (21) | 16 (14) | 20 (17) | 202 (15) | |
| Recipient race | <.0001 | ||||
| Caucasian | 955 (90) | 90 (80) | 106 (89) | 1121 (80) | |
| African American | 33 (3) | 11 (10) | 5 (4) | 92 (7) | |
| Asian/Pacific Islander | 22 (2) | 5 (4) | 3 (3) | 27 (2) | |
| Hispanic | 41 (4) | 6 (5) | 5 (4) | 133 (10) | |
| Native American | 3 (<1) | 0 | 0 | 6 (<1) | |
| Other | 9 (1) | 0 | 0 | 14 (1) | |
| Male sex | 609 (57) | 61 (54) | 58 (49) | 771 (55) | .31 |
| Karnofsky prior to transplant ≥90 | 717 (73) | 74 (72) | 74 (66) | 934 (71) | .42 |
| Disease at transplant | .003 | ||||
| AML | 399 (38) | 44 (39) | 43 (36) | 503 (36) | |
| ALL | 225 (21) | 24 (21) | 34 (29) | 378 (27) | |
| CML | 270 (25) | 32 (29) | 25 (21) | 362 (26) | |
| MDS | 169 (16) | 12 (11) | 17 (14) | 150 (11) | |
| Disease status at transplant | .96 | ||||
| Early | 762 (72) | 79 (71) | 83 (70) | 978 (70) | |
| Intermediate | 57 (5) | 8 (7) | 7 (6) | 86 (6) | |
| Advanced | 244 (23) | 25 (22) | 29 (24) | 329 (24) | |
| Graft type | .003 | ||||
| Bone marrow | 648 (61) | 76 (68) | 84 (71) | 942 (68) | |
| Peripheral blood stem cell | 415 (39) | 36 (32) | 35 (29) | 451 (32) | |
| In vivo T-cell depletion | .01 | ||||
| No | 850 (80) | 75 (67) | 92 (77) | 1069 (77) | |
| Yes | 213 (20) | 37 (33) | 27 (23) | 324 (23) | |
| GVHD prophylaxis | .001 | ||||
| Tacrolimus ± other | 558 (52) | 54 (48) | 51 (43) | 624 (45) | |
| Cyclosporine ± other | 505 (48) | 58 (52) | 68 (57) | 769 (55) | |
| Donor/recipient sex match | .004 | ||||
| Male/male | 436 (41) | 34 (30) | 40 (34) | 482 (35) | |
| Male/female | 269 (25) | 24 (21) | 35 (29) | 335 (24) | |
| Female/male | 173 (16) | 27 (24) | 18 (15) | 289 (21) | |
| Female/female | 185 (17) | 27 (24) | 26 (22) | 287 (21) | |
| Number of patients | 1063 | 112 | 119 | 1393 | |
| Donor/recipient CMV match | .02 | ||||
| Negative/negative | 354 (33) | 38 (34) | 42 (35) | 426 (31) | |
| Negative/positive | 334 (31) | 32 (29) | 37 (31) | 387 (28) | |
| Positive/negative | 136 (13) | 17 (15) | 23 (19) | 214 (15) | |
| Positive/positive | 190 (18) | 21 (19) | 15 (13) | 319 (23) | |
| Unknown | 49 (5) | 4 (4) | 2 (2) | 47 (3) | |
| Interval from diagnosis to transplant | 9 (<1-296) | 9 (<1-248) | 9 (<1-75) | 11 (<1-309) | .002 |
| HLA matching: HLA-A, HLA-B, HLA-C, and HLA-DRB1 | NA | ||||
| 8/8 matched | 1063 (100) | 0 | 0 | 0 | |
| HLA-A 1 mismatch (MM) | 0 | 34 (30) | 47 (39) | 420 (30) | |
| HLA-B 1 MM | 0 | 7 (6) | 9 (8) | 225 (16) | |
| HLA-C 1 MM | 0 | 57 (51) | 58 (49) | 617 (44) | |
| HLA-DRB1 1 MM | 0 | 14 (13) | 5 (4) | 131 (9) | |
| Donor age | <.0001 | ||||
| Donor age, median (range) | 34 (18-58) | 36 (20-56) | 38 (19-57) | 36 (19-61) | <.0001 |
| 18-29 | 352 (33) | 31 (28) | 22 (18) | 343 (25) | |
| 30-39 | 418 (39) | 37 (33) | 49 (41) | 520 (37) | |
| 40-49 | 235 (22) | 38 (34) | 37 (31) | 398 (29) | |
| 50 and older | 58 (5) | 6 (5) | 11 (9) | 132 (9) | |
| Donor race | <.0001 | ||||
| Caucasian | 925 (87) | 82 (73) | 98 (82) | 1063 (76) | |
| African American | 20 (2) | 7 (6) | 7 (6) | 90 (6) | |
| Asian/Pacific Islander | 22 (2) | 5 (4) | 5 (4) | 32 (2) | |
| Hispanic | 39 (4) | 7 (6) | 1 (1) | 125 (9) | |
| Native American | 7 (1) | 1 (1) | 0 | 16 (1) | |
| Other | 50 (5) | 10 (9) | 8 (7) | 67 (5) | |
| Year of transplant | .001 | ||||
| 1988-1995 | 137 (13) | 18 (16) | 18 (15) | 213 (15) | |
| 1996-2000 | 253 (24) | 26 (23) | 37 (31) | 376 (27) | |
| 2001-2005 | 356 (33) | 44 (39) | 39 (33) | 506 (36) | |
| 2006-2009 | 317 (30) | 24 (21) | 25 (21) | 298 (21) | |
| Median follow-up of survivors, mo (range) | 83 (3-244) | 60 (19-205) | 69 (12-157) | 72 (3-229) | .46§ |
NA, not available.
Data has been modeled to adjust for oversampling of deceased patients due to a lack of consent for NMDP cases before 2003.
Completeness index follows: Percent with 1-year follow-up: overall: 99%; percent with 3-year follow-up: overall: 95%; percent with 5-year follow-up: overall: 89%.
One or more locus is homozygous in the 8/8 matched category.
This figure is the log-rank P value.
The study evaluated the associations between histocompatibility groups and outcomes with overall survival and disease-free survival as primary end points, and acute GVHD, chronic GVHD, TRM, relapse, neutrophil engraftment, and graft failure as secondary end points. Overall survival considered death from any cause as the event, and surviving patients were censored at the date of last contact. Disease-free survival was defined as relapse or death from any cause, with patients who were alive and in complete remission censored at the time of last follow-up. The incidences of grades II-IV or III-IV acute GVHD were determined during the first 100 days after transplant and defined according to the Glucksberg scale.7 Chronic GVHD included limited and extensive conditions and was defined according to the Seattle criteria.8 TRM was defined as death during a continuous, complete remission. Relapse was defined as leukemia relapse or MDS recurrence. Neutrophil engraftment was defined as achieving an absolute neutrophil count greater than 500 for 3 consecutive measurements. Primary and secondary graft failures were considered together as a single outcome. Primary graft failure was defined as failure to achieve a post-nadir absolute neutrophil count of 500 cells per milliliter or donor peripheral blood T-cell chimerism of at least 5% (in the absence of peripheral blood T-cell chimerism, unsorted blood or marrow chimerism was used). Secondary graft failure was defined as initial donor engraftment followed by graft loss, evidenced by a persistent drop in the absolute neutrophil count to less than 500 cells per milliliter or loss of donor chimerism. Patients receiving second transplants for reasons other than relapse were also considered to have graft failure. Only those cases of graft failure that occurred within a year of the first transplant were included in the graft failure analysis. Events were summarized by the cumulative incidence estimate, with death as the competing risk.
Statistical methods
To compare pretransplant characteristics for discrete factors, the number of cases and their respective percentages were calculated and χ2 tests were performed to compare the 4 histocompatibility groups. For continuous factors, the medians and ranges were calculated and the Kruskal-Wallis test was used to analyze differences between the groups. Probabilities of disease-free survival were calculated using the Kaplan-Meier estimator. Estimated cumulative incidence was used to describe the probabilities for events with competing risks. These included engraftment, GVHD, relapse, and TRM. Comparisons of survival curves were done with the log-rank test.
Multivariate analyses were performed using the Cox proportional hazards model to compare the histocompatibility groups. Models were fit to determine which risk factors were related to a given outcome. All variables were tested for affirmation of the proportional hazards assumption. Factors violating the proportional hazards assumption were adjusted through stratification. A stepwise model building procedure was used to select risk factors for each outcome with a threshold of P ≤ .05 for entering into the models. Due to multiple comparisons, P < .01 was used to determine statistical significance for the main effect. All analyses were performed using SAS version 9.2 (SAS Institute, Inc).
Results
A total of 2687 recipients of unrelated donor hematopoietic stem cell transplantation were included in the analysis. The 3 HLA mismatched comparison groups (N = 1624) included 112 (6.9%) transplant pairs designated as 7/8 HVG MM, 119 (7.3%) as 7/8 GVH MM, and 1393 (85.8%) as 7/8 bidirectional MM (Table 2). The outcome of these transplantations was compared with the 8/8 matches (N = 1063) in which homozygosity was present at 1 or more of the 4 HLA loci. The characteristics of the population are described in Table 2. The 7/8 mismatched groups differed from the 8/8 matched reference group in the distribution of age (younger), race (more minorities), disease type (but not disease status), graft type (more bone marrow), T-cell depletion (more in 7/8), sex match, cytomegalovirus (CMV) match, donor age, donor race, and years of transplant. The majority of the HLA mismatches observed in the 3 7/8 histocompatibility groups were at the HLA-A and HLA-C loci.
Survival
Multivariate analysis was performed using the 8/8 matched group as the baseline. Overall survival was significantly associated with intermediate and advanced disease status (P < .0001) and non-Caucasian recipient race (P = .0103). After adjusting for significant covariates, significant differences were noted between the 8/8 matched group and the mismatched groups (P < .0001) (Table 3). Pairwise comparisons between the 8/8 group and the mismatched groups revealed differing effects. The 7/8 GVH MM (P = .0002) and 7/8 bidirectional groups (P < .0001) had significantly worse overall survival than the 8/8 match group, but this difference was not observed in a comparison of the 7/8 HVG MM group (HR 1.37, 95% CI 1.04-1.81, P = .03). No significant differences were observed among the 3 7/8 mismatched groups (P = .17).
Table 3.
Multivariate analysis summary: comparison with 8/8 matches and comparison among 7/8 groups
| Outcome | 8/8 matched | 7/8 HVG HR (95% CI), P value | 7/8 GVH HR (95% CI), P value | 7/8 bidirectional HR (95% CI), P value | 7/8 groups only P value* |
|---|---|---|---|---|---|
| Overall survival | 1.00 | 1.37 (1.04-1.81), .03 (NS) | 1.67 (1.27-2.18), .0002 | 1.29 (1.15-1.46), <.0001 | .17 (NS) |
| Disease-free survival | 1.00 | 1.38 (1.07-1.78), .013 (NS) | 1.53 (1.19-1.96), .001 | 1.35 (1.20-1.50), <.0001 | .60 (NS) |
| TRM | 1.00 | 1.44 (1.05-1.97), .025 (NS) | 1.82 (1.37-2.42), <.0001 | 1.56 (1.36-1.79), <.0001 | .46 (NS) |
| Relapse | 1.00 | 1.38 (0.97-1.95), .07 (NS) | 1.11 (0.76-1.63), .60 (NS) | 0.98 (0.84-1.16), .83 (NS) | .15 (NS) |
| Acute GVHD III-IV | 1.00 | 0.83 (0.55-1.27), .39 (NS) | 1.92 (1.40-2.62), <.0001 | 1.61 (1.38-1.88), <.0001 | .003 |
| Chronic GVHD | 1.00 | 0.82 (0.57-1.19), .30 (NS) | 0.98 (0.69-1.39), .90 (NS) | 1.02 (0.89-1.18), .77 (NS) | .51 (NS) |
| Neutrophil engraftment | 1.00 | 0.86 (0.67-1.10), .23 (NS) | 1.11 (0.87-1.40), .40 (NS) | 0.99 (0.89-1.09), .78 (NS) | .31 (NS) |
| Graft failure | 1.00 | 1.21 (0.43-3.40), .71 (NS) | 1.97 (0.88-4.41), .10 (NS) | 1.66 (1.12-2.45), .011 (NS) | .74 (NS) |
The “7/8 groups only” column represents the overall P value (2 df test) for the comparison of the 7/8 HVG MM, 7/8 GVH MM, and 7/8 bidirectional groups. The 7/8 groups are not considered significantly different if P > .01. All P values not meeting the P < .01 threshold for statistical significance are labeled “NS.”
Disease-free survival
Disease-free survival was significantly associated with recipient CMV positivity (P = .006) and increasing recipient age (P < .0001). After adjusting for significant covariates, significant differences were found between the 8/8 matched group and the mismatched groups (P < .0001) (Table 3). Pairwise comparisons between the 8/8 group and mismatched groups revealed differing effects similar to the analysis of overall survival. The 7/8 GVH MM and 7/8 bidirectional groups had significantly worse disease-free survival than the 8/8 match group. This difference did not reach statistical significance in the comparison between the 8/8 and 7/8 HVG MM groups (HR 1.38, 95% CI 1.07-1.78, P = .013). No significant difference was observed among the 3 7/8 mismatched groups (P = .60).
Transplant-related mortality
TRM was significantly associated with recipient CMV positivity (P = .008) and increasing donor age. After adjusting for significant covariates, significant differences were found between the 8/8 matched group and the mismatched groups (P < .0001) (Table 3). Pairwise comparisons between the 8/8 group and the mismatch groups revealed effects similar to the analysis of overall survival and disease-free survival. The 7/8 GVH and 7/8 bidirectional groups had significantly worse TRM compared with the 8/8 group. The 7/8 HVG group was not significantly different from the 8/8 group (HR 1.44, 95% CI 1.05-1.97, P = .025). No significant differences were observed among the 3 7/8 mismatched groups (P = .46).
Relapse
No significant associations were found with relapse, including the HLA match groups (P = .27).
Acute GVHD
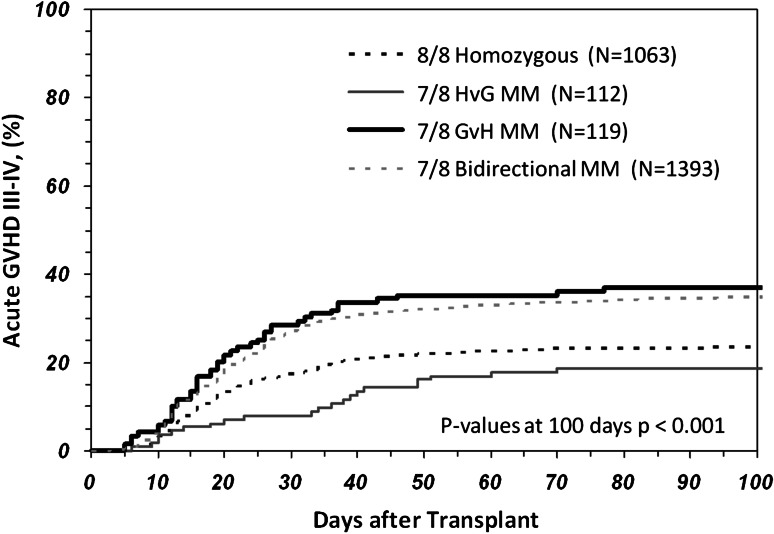
Acute GVHD grades III-IV was significantly associated with intermediate or advanced disease (P = .002), peripheral blood stem cell grafts (P = .003), non-Caucasian recipients (P = .02), male recipients (P = .005), and transplantations before 2001 (P < .0001). After adjusting for significant covariates, significant differences were noted between the 8/8 matched group and the 7/8 GVH MM (P < .0001) and 7/8 bidirectional mismatched (P < .0001) groups (Table 3). Acute GVHD III-IV was not significantly different between the 8/8 and 7/8 HVG mismatched groups (P = .39). A comparison of the 7/8 mismatched groups found that both the 7/8 GVH MM and 7/8 bidirectional mismatched groups had significantly higher rates of acute GVHD than the 7/8 HVG mismatched group (P = .003) (Figure 1). There was no significant difference between the 7/8 GVH and the 7/8 bidirectional mismatched groups in acute GVHD (P = .26).
Figure 1.
Cumulative incidence of acute GVHD grades III-IV during the first 100 days following a transplantation using an 8/8, 7/8 bidirectional MM, 7/8 GVH MM, or 7/8 HVG MM donor. Significant differences were observed between the 7/8 groups (P = .0001) and the 7/8 HVG group having a lower acute GVHD risk similar to the 8/8 group.
Chronic GVHD
Chronic GVHD was significantly associated with the use of in vivo T-cell depletion (P < .0001) and non-Caucasian recipients (P = .04). After adjusting for the significant covariates, no association was found with the HLA match group (P = .71).
Neutrophil engraftment
Neutrophil engraftment was significantly associated with the use of female donors into male recipients (P < .0001). After adjusting for the significant covariate, no association was found with the HLA match group (P = .49).
Graft failure
Graft failure was significantly associated with advanced disease (P = .008). After adjusting for the significant covariate, no significant difference was found between the 8/8 matched group and the mismatched groups for graft failure (P = .06). In addition, no significant differences were observed among the 3 7/8 mismatched groups (P = .74).
Causes of death
The primary causes of death, as reported by the centers in the various groups, were reviewed. The 7/8 bidirectional and GVH MM groups had slightly higher deaths attributed to GVHD, compared with the 8/8 and 7/8 HVG MM groups, 18.2% and 18.0% vs 14.9% and 14.3%, respectively. The 7/8 HVG MM group had more deaths attributed to graft rejection than the 8/8, 7/8 GVH MM, and 7/8 bidirectional groups, 9.5% vs 0.6%, 1.1%, and 1.6%, respectively. The 8/8 group had more deaths due to primary disease than the 7/8 groups, 32.2% vs 22.5% to 28.6%, respectively.
Power limitations
The 7/8 HVG and 7/8 GVH groups represented only 16% (8% per group) of all the 7/8 mismatches in the study population. To address concerns about power limitations in the data set, a power calculation based on the frequency of the 7/8 HVG and 7/8 GVH in the population and observed effect sizes was generated for the primary outcome of survival. The number of cases required to detect a difference between the 7/8 bidirectional group and 7/8 HVG or 7/8 GVH with 80% power is 19 890 or 13 833, respectively. The required sample sizes suggest that we need a much larger sample in order to detect the differences between bidirectional mismatches and GVH or HVG mismatch groups, and that any effects, if present, are not clinically significant.
Discussion
When no 8/8 matched donor exists for a patient, several options exist for a graft source including a 7/8 mismatched unrelated donor, umbilical cord blood, or a related haploidentical donor. While 7/8 mismatched unrelated donor transplantations have a 9% to 10% reduction in overall survival, this treatment may provide the best option for some patients.9,10 This study focused on developing guidelines for unrelated donor selection in the case where either the patient or a potential 7/8 matched donor is homozygous at the mismatched locus.
The major impact of homozygosity in the 7/8 mismatched groups is observed for acute GVHD. The 7/8 HVG MM group differs significantly from the 7/8 GVH MM and 7/8 bidirectional mismatch groups and is not significantly different from the 8/8 match group for acute GVHD. The associations of the histocompatibility groups with overall and disease-free survival are predominantly due to the impact of overall matching differences between 8/8 and 7/8, as previously observed in Lee et al.1 However, the decrease in the probability of acute GVHD in the 7/8 HVG MM group likely accounts for improved survival, as evidenced by the lack of a statistical difference from the 8/8 matched group for overall survival. The reduced acute GVHD observed in the 7/8 HVG MM grafts likely results from the absence of a mismatch from the perspective of the donor’s immune system, matching of the shared allele, and an apparent lack of a donor-derived immune response to its increased dose (ie, 2 allele copies in the homozygous recipient vs 1 in the donor).
It might be anticipated that a reduction in the incidence of GVHD in the 7/8 HVG MM grafts might result in an increased rate of relapse, but no significant association was observed. However, since overall and disease-free survival is not different for the 3 7/8 MM groups, a trend toward higher risk of relapse may be negating the positive impact of less GVHD for the HVG MM group.
The results of our study do not support previous observations. There was no impact on neutrophil engraftment or secondary graft failure observed with HLA homozygosity in a 7/8 unidirectional HVG MM, as previously noted by the Seattle group.4 The small sample size in the Seattle study, the difference in graft source (only bone marrow), the lower resolution of HLA typing used to define match categories, or differences in the immune competence of the recipients (CML only)11 may have given rise to these differences with our results. Our results regarding engraftment and graft failure are somewhat surprising in that one might expect that the 7/8 unidirectional mismatched grafts in which the recipient is homozygous and the donor heterozygous would result in immune recognition of the graft as foreign. The reported causes of death would appear to support the expected observation with the higher amount of death attributed to graft failure in the 7/8 HVG MM group (9.5% vs <2%). However, this did not come out in the statistical models of engraftment or graft failure as significant. Perhaps the disease status or conditioning of the recipient reduces the recipient’s ability to respond.
Other studies have also examined the role of HLA homozygosity in the selection of umbilical cord blood units, but with varying results.12,13 Because this stem cell source has a different cellular composition when compared with marrow or peripheral blood stem cell preparations, there may be differing effects of HLA matching on outcome than were observed in this study.
It is possible that engraftment failures observed in some homozygous recipients, as well as in bidirectional mismatched recipients, might be attributed primarily to the presence of preformed HLA-directed antibodies rather than de novo allorecognition of the graft.14-18 In fact, in solid organ transplantation, HLA homozygotes in general appear to be more sensitized to foreign HLA (Ilias Doxiadis, written communication). Evaluation of sensitization in the selection of a mismatched donor is used to reduce the likelihood of any potential immune response and should be considered, particularly for an HLA homozygous patient.
In summary, for unrelated donor selection, unidirectional mismatches in the GVH vector should be considered as the same level of risk as 7/8 bidirectional mismatches. For HLA homozygous recipients, the pool of potential donors can be expanded since there is no need to avoid a mismatch at the homozygous locus. In fact, HLA homozygous recipients receiving an HLA heterozygous graft will have a reduced risk of acute GVHD, and these 7/8 HVG MM donors might be preferred over a 7/8 mismatch at a heterozygous locus. These guidelines are summarized in Table 1.
Acknowledgments
This research was supported, in part, by funding from the Office of Naval Research (N00014-11-1-0590 and N00014-10-1-0199) (C.K.H.), and the National Cancer Institute (P30 CA51008) (L. Weiner). The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute of Allergy and Infectious Diseases; Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; contract HHSH234200637015C with the Health Resources and Services Administration (HRSA/DHHS); 2 grants (N00014-10-1-0204 and N00014-11-1-0339) from the Office of Naval Research; and grants from Allos, Inc, Amgen, Inc, Angioblast, Ariad, Be the Match Foundation, Blue Cross and Blue Shield Association, Buchanan Family Foundation, CaridianBCT, Celgene Corporation, CellGenix GmbH, Children’s Leukemia Research Association, Fresenius Biotech North America, Inc, Gamida Cell–Teva Joint Venture Ltd, Genentech, Inc, Genzyme Corporation, GlaxoSmithKline, HistoGenetics, Inc, Kiadis Pharma, The Leukemia & Lymphoma Society, The Medical College of Wisconsin, Merck & Co, Inc, Millennium: The Takeda Oncology Co, Milliman USA, Inc, Miltenyi Biotec, Inc, National Marrow Donor Program, OptumHealth Care Solutions, Inc, Osiris Therapeutics, Inc, Otsuka America Pharmaceutical, Inc, RemedyMD, Sanofi, Seattle Genetics, Sigma-Tau Pharmaceuticals, Soligenix, Inc, StemCyte, A Global Cord Blood Therapeutics Co, Stemsoft Software, Inc, Swedish Orphan Biovitrum, Tarix Pharmaceuticals, Teva Neuroscience, Inc, Therakos, Inc, and Wellpoint, Inc, as well as an anonymous donation to the Medical College of Wisconsin. The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, or any other agency of the US Government.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: C.K.H., A.W., and S.R.S. drafted the research plan; C.K.H., A.W., S.R.S., J.D., M. Aljurf, M. Askar, W.S., J.H., J.P., M.O., V.T., M.B., and S.J.L. critically revised the research plan; T.W., M.H., and J.U. performed statistics; C.K.H., A.W., S.R.S., J.D., M. Askar, W.S., J.H., J.P., M.O., M.B., and S.J.L. analyzed and interpreted data; C.K.H. and S.J.S. drafted the paper; and C.K.H., A.W., S.R.S., J.D., M. Aljurf, M. Askar, W.S., J.H., J.P., M.O., M.B., and S.J.L. critically revised the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stephen Spellman, Center for International Blood and Marrow Transplant Research, 3001 Broadway St NE, Minneapolis, MN 55413; e-mail: sspellma@nmdp.org.
References
- 1.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110(13):4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 2.Woolfrey A, Klein JP, Haagenson M, et al. HLA-C antigen mismatch is associated with worse outcome in unrelated donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2011;17(6):885–892. doi: 10.1016/j.bbmt.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spellman SR, Eapen M, Logan BR, et al. National Marrow Donor Program; Center for International Blood and Marrow Transplant Research. A perspective on the selection of unrelated donors and cord blood units for transplantation. Blood. 2012;120(2):259–265. doi: 10.1182/blood-2012-03-379032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petersdorf EW, Hansen JA, Martin PJ, et al. Major-histocompatibility-complex class I alleles and antigens in hematopoietic-cell transplantation. N Engl J Med. 2001;345(25):1794–1800. doi: 10.1056/NEJMoa011826. [DOI] [PubMed] [Google Scholar]
- 5.Farag SS, Bacigalupo A, Eapen M, et al. KIR Study Group, Center for International Blood and Marrow Transplantation Research. The effect of KIR ligand incompatibility on the outcome of unrelated donor transplantation: a report from the center for international blood and marrow transplant research, the European blood and marrow transplant registry, and the Dutch registry. Biol Blood Marrow Transplant. 2006;12(8):876–884. doi: 10.1016/j.bbmt.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Spellman S, Setterholm M, Maiers M, et al. Advances in the selection of HLA-compatible donors: refinements in HLA typing and matching over the first 20 years of the National Marrow Donor Program Registry. Biol Blood Marrow Transplant. 2008;14(suppl 9):37–44. doi: 10.1016/j.bbmt.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18(4):295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69(2):204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 9.Ballen KK, Koreth J, Chen YB, Dey BR, Spitzer TR. Selection of optimal alternative graft source: mismatched unrelated donor, umbilical cord blood, or haploidentical transplant. Blood. 2012;119(9):1972–1980. doi: 10.1182/blood-2011-11-354563. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs E, O’Donnell PV, Brunstein CG. Alternative transplant donor sources: is there any consensus? Curr Opin Oncol. 2013;25(2):173–179. doi: 10.1097/CCO.0b013e32835d815f. [DOI] [PubMed] [Google Scholar]
- 11.Geiger TL, Woodard P, Tong X, et al. Human leucocyte antigen alloimmunization after bone marrow transplantation: an association with chronic myelogenous leukaemia. Br J Haematol. 2002;117(3):634–641. doi: 10.1046/j.1365-2141.2002.03465.x. [DOI] [PubMed] [Google Scholar]
- 12.Stevens CE, Carrier C, Carpenter C, Sung D, Scaradavou A. HLA mismatch direction in cord blood transplantation: impact on outcome and implications for cord blood unit selection. Blood. 2011;118(14):3969–3978. doi: 10.1182/blood-2010-11-317271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanda J, Atsuta Y, Wake A, et al. HLA Working Group of the Japan Society for Hematopoietic Cell Transplantation. Impact of the direction of HLA mismatch on transplantation outcomes in single unrelated cord blood transplantation. Biol Blood Marrow Transplant. 2013;19(2):247–254. doi: 10.1016/j.bbmt.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 14.Spellman S, Bray R, Rosen-Bronson S, et al. The detection of donor-directed, HLA-specific alloantibodies in recipients of unrelated hematopoietic cell transplantation is predictive of graft failure. Blood. 2010;115(13):2704–2708. doi: 10.1182/blood-2009-09-244525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciurea SO, Thall PF, Wang X, et al. Donor-specific anti-HLA Abs and graft failure in matched unrelated donor hematopoietic stem cell transplantation. Blood. 2011;118(22):5957–5964. doi: 10.1182/blood-2011-06-362111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ottinger HD, Rebmann V, Pfeiffer KA, et al. Positive serum crossmatch as predictor for graft failure in HLA-mismatched allogeneic blood stem cell transplantation. Transplantation. 2002;73(8):1280–1285. doi: 10.1097/00007890-200204270-00016. [DOI] [PubMed] [Google Scholar]
- 17.Takanashi M, Atsuta Y, Fujiwara K, et al. The impact of anti-HLA antibodies on unrelated cord blood transplantations. Blood. 2010;116(15):2839–2846. doi: 10.1182/blood-2009-10-249219. [DOI] [PubMed] [Google Scholar]
- 18.Cutler C, Kim HT, Sun L, et al. Donor-specific anti-HLA antibodies predict outcome in double umbilical cord blood transplantation. Blood. 2011;118(25):6691–6697. doi: 10.1182/blood-2011-05-355263. [DOI] [PMC free article] [PubMed] [Google Scholar]