Dexamethasone suppresses ER stress in inflammatory bowel disease by promoting correct protein folding and ER-associated degradation.
Abstract
Endoplasmic reticulum (ER) stress in intestinal secretory cells has been linked with colitis in mice and inflammatory bowel disease (IBD). Endogenous intestinal glucocorticoids are important for homeostasis and glucocorticoid drugs are efficacious in IBD. In Winnie mice with intestinal ER stress caused by misfolding of the Muc2 mucin, the glucocorticoid dexamethasone (DEX) suppressed ER stress and activation of the unfolded protein response (UPR), substantially restoring goblet cell Muc2 production. In mice lacking inflammation, a glucocorticoid receptor antagonist increased ER stress, and DEX suppressed ER stress induced by the N-glycosylation inhibitor, tunicamycin (Tm). In cultured human intestinal secretory cells, in a glucocorticoid receptor-dependent manner, DEX suppressed ER stress and UPR activation induced by blocking N-glycosylation, reducing ER Ca2+ or depleting glucose. DEX up-regulated genes encoding chaperones and elements of ER-associated degradation (ERAD), including EDEM1. Silencing EDEM1 partially inhibited DEX’s suppression of misfolding-induced ER stress, showing that DEX enhances ERAD. DEX inhibited Tm-induced MUC2 precursor accumulation, promoted production of mature mucin, and restored ER exit and secretion of Winnie mutant recombinant Muc2 domains, consistent with enhanced protein folding. In IBD, glucocorticoids are likely to ameliorate ER stress by promoting correct folding of secreted proteins and enhancing removal of misfolded proteins from the ER.
Inflammatory bowel disease (IBD) is characterized by an aberrant or exaggerated immune response against the intestinal microflora influenced by genetic and environmental factors. Ulcerative colitis (UC) and, to a lesser degree, Crohn’s disease colitis are characterized by the loss of goblet cells, a thinner mucus layer, presence of crypt abscesses, and distortion of mucosal glands (Dvorak et al., 1980; Trabucchi et al., 1986). Recent studies suggest that defects in the intestinal epithelial secretory cells leading to an aberrant mucosal barrier could be involved in the pathogenesis of IBD (Heazlewood et al., 2008; Kaser et al., 2008; Wei et al., 2012).
The major macromolecular component of intestinal mucus is the mucin glycoprotein MUC2, which is synthesized by secretory goblet cells (McGuckin et al., 2009). N-glycosylation and formation of numerous disulfide bonds, which are necessary for dimerization and folding of MUC2, take place in the endoplasmic reticulum (ER), which is the initial site for synthesis and posttranslational modification of secreted and transmembrane proteins (Marciniak and Ron, 2006). MUC2 is a likely candidate for misfolding in the ER, because of its large size (>5,000 aa), high disulfide content, and homo-oligomerization. Impaired ER function caused by factors such as inhibition of posttranslational modifications, altered ER Ca2+, increased protein synthesis, viral infection, temperature shock and energy depletion can lead to accumulation of unfolded or misfolded proteins in the ER, initiating ER stress.
ER stress has been linked to a spectrum of human diseases including neurodegenerative diseases, developmental disorders, cancer, diabetes, cystic fibrosis, and infectious and inflammatory diseases (Nanua and Yoshimura, 2004; Medigeshi et al., 2007; Deng et al., 2008; Maeda et al., 2009; Hosoi and Ozawa, 2010). Recently the accumulation of MUC2 precursor and molecular evidence of ER stress in intestinal secretory cells have been linked to intestinal inflammation and the pathogenesis of IBD (Heazlewood et al., 2008; Kaser et al., 2008). ER stress in intestinal secretory cells could promote inflammation by diminishing the efficacy of the mucosal barrier via reduced synthesis and secretion of mucins and antimicrobial molecules, and by initiating inflammatory signaling in stressed intestinal secretory cells (McGuckin et al., 2010).
Several murine models link intestinal ER stress with inflammation. Mis-sense mutations in Muc2 in the N-ethyl-N-nitrosourea mutants Winnie and Eeyore lead to Muc2 misfolding in the ER resulting in ER stress and to spontaneous TH17 dominant intestinal inflammation akin to human UC (Heazlewood et al., 2008; Eri et al., 2011). Mice deficient in the mucin-specific, ER-resident protein disulfide isomerase (PDI), anterior gradient 2 (Agr2) show complete shutdown of mucin biosynthesis by goblet cells, accompanied by ER stress and spontaneous intestinal inflammation (Park et al., 2009). Intestinal deficiency in the ER-resident enzyme fatty acid synthase results in loss of palmitoylation of Muc2, Muc2 misfolding, ER stress, reduced mucin production, and inflammation (Wei et al., 2012). In response to protein misfolding, cells activate the unfolded protein response (UPR), which maintains a healthy ER via restoration of correct protein folding, degradation of terminally misfolded proteins, and inhibition of polypeptide translation (Kaufman, 2002; Schröder and Kaufman, 2005; Vembar and Brodsky, 2008). The ER chaperones glucose-regulating peptide (GRP) 78, calnexin, and calreticulin assist nascent glycoproteins to fold correctly and subsequently exit the ER (Kamimoto et al., 2006; Malhotra and Kaufman, 2007). GRP78 remains associated with the UPR pathway-initiating molecules inositol-requiring enzyme (IRE)1-α/β and protein kinase RNA-like ER kinase (PERK), and with activating transcription factor (ATF)6-α/β under normal physiological conditions (Kaufman, 2002). During ER stress, GRP78 is sequestered from the UPR-transducing molecules to the misfolded proteins, resulting in activation of the UPR (Xue et al., 2005b). Mice with an inadequate UPR, such as the intestinal-specific Xbp1 deletion (Kaser et al., 2008), Ire1β (Ern2) knockout (Bertolotti et al., 2001), and Woodrat mice (hypomorphic for Mbtps1; Brandl et al., 2009), are more susceptible to intestinal inflammation. Together, these murine models show that in intestinal secretory cells either increased protein misfolding or an inappropriate UPR to the normal level of misfolding results in intestinal inflammation.
Glucocorticoid drugs are immunosuppressive agents widely used to treat inflammatory disorders, including IBD (Barnes, 1998), but are also endogenously produced in the intestine where they are an important component of homeostasis (Cima et al., 2004; Mueller et al., 2006). The mechanism of glucocorticoids is attributed to pleiotropic effects of the glucocorticoid receptor (GR) on multiple transcriptional pathways. Steroid-activated GR binds to glucocorticoid-responsive elements, resulting in modulation of antiinflammatory transcriptional pathways such as NF-κB, Annexin1, and MAPK (Lasa et al., 2002; Martens et al., 2005). GR can also decrease the expression of proinflammatory genes directly by protein–protein interactions (Martens et al., 2005). Although, glucocorticoids have been reported to inhibit ER stress in epithelial cells (Fujii et al., 2006; Shang et al., 2011), the mechanism of action was not demonstrated. In this study, we demonstrate that glucocorticoids ameliorate ER stress in intestinal secretory cells by promoting correct protein folding and enhancing degradation of misfolded proteins.
RESULTS
Glucocorticoids suppress ER stress and restore mature Muc2 biosynthesis in Winnie mice
Biosynthesis of Muc2 involves C-terminal dimerization and N-glycosylation in the ER, followed by O-glycosylation in the Golgi and N-terminal oligomerization, thus forming large polymers that are stored in granules in the thecae before secretion. We have previously shown that the missense mutation in the N-terminal D3-domain of Muc2 in Winnie mice results in altered oligomerization and inappropriate assembly of some Muc2 molecules, leading to accumulation of non–O-glycosylated misfolded Muc2 precursor in the ER (Heazlewood et al., 2008). Mis-folding results in ER vacuolization, smaller goblet cell thecae and reduced secretion of mature Muc2 that appears to correlate with goblet cell age.
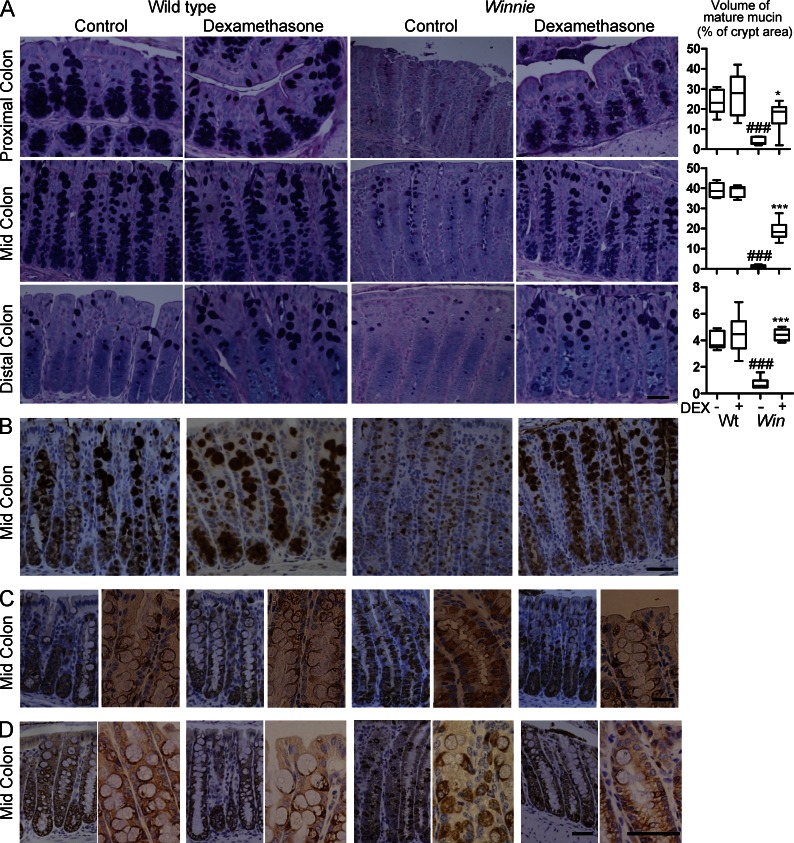
To explore the influence of glucocorticoids on intestinal ER stress and ER stress–mediated inflammation, Winnie and C57BL/6 WT mice were treated with dexamethasone (DEX) for 28 d and colonic tissue was analyzed for morphometric changes in goblet cells, inhibition of ER stress, and inflammation. Alcian blue (AB)/periodic acid Schiff (PAS) staining was used to determine the amount of fully glycosylated mature mucin within the goblet cell secretory granules that stain with AB due to the presence of negatively charged sugars. Volumetric analysis showed a significant decrease in the amount of mature glycosylated mucin in Winnie compared with WT mice, as reported previously (Heazlewood et al., 2008). Although DEX treatment did not affect mucin production in WT mice, in Winnie mice DEX significantly restored the production of O-glycosylated mucins (Fig. 1 A). In the proximal and mid colon of mice there are two distinct goblet cell lineages: a shorter lived surface lineage and a longer lived basal lineage, whereas the distal colon contains only the short-lived surface lineage (Altmann, 1983). In the distal colon, DEX restored mucin production to the levels seen in WT mice, whereas in the proximal and mid colon, restoration was less complete due to incomplete recovery of mucin production in the basal long-lived goblet cell lineage (Fig. 1 A). Small intestinal goblet cells are also affected in Winnie mice and recovered with DEX treatment (not depicted).
Figure 1.
Glucocorticoids restore mucin biosynthesis in intestinal goblet cells with a mucin folding defect. WT C57BL/6 (WT) and Winnie (Win) mice at 4 wk of age were treated with DEX (20 ng/g body weight i.p. daily for 28 d) or vehicle (control) and intestinal tissue sampled (n = 7–8/group, single experiment). (A) Representative AB/PAS stained proximal, mid and distal colon tissue sections from control and DEX-treated WT and Winnie mice. The volume of stored O-glycosylated mucin in each intestinal region as a percentage of total crypt volume is shown at the right. Box plots with whiskers show median, quartiles and the maximum range. Kruskal-Wallis nonparametric analysis, Dunn’s multiple comparison #, WT versus Win *, Win versus DEX-treated Win; *, P < 0.05; ###,***, P < 0.001. (B) Cellular localization of Muc2 in representative mid colon tissue sections of control and DEX-treated WT and Winnie mice was determined by immunohistochemistry with the mM2.2 antibody reactive with the precursor and mature forms of Muc2. (C) Accumulation of Muc2 precursor in representative mid colon tissue sections in control and DEX-treated WT and Winnie mice was assessed by immunohistochemistry with an antibody reactive with the precursor but not the mature form of Muc2. (D) Abundance of the Agr2 protein disulfide isomerize involved in Muc2 biosynthesis in representative mid colon tissue sections in control and DEX-treated WT and Winnie mice was assessed by immunohistochemistry. Bars, 50 µm.
To investigate the influence of DEX on Muc2 biosynthesis, immunohistochemical analysis was performed using an antibody that stains both the precursor and the fully O-glycosylated Muc2, and a Muc2 precursor antibody that does not react with the O-glycosylated mucin. These antibodies revealed that (a) the increase in AB-positive O-glycosylated mucin in DEX-treated Winnie mice was accompanied by an increase in thecal Muc2 (Fig. 1 B), and (b) this was accompanied by a decrease in Muc2 precursor in the ER region of goblet cells (Fig. 1 C). Consistent with the AB staining, in the proximal and mid colon, the decrease in Muc2 precursor accumulation was less than in the distal colon. The recovery of mucin production is thus explained by decreased misfolding of Muc2 rather than an increase in another mucin. To further analyze the effect on misfolding, we stained for the ER PDI Agr2, which is coexpressed with Muc2 and required for Muc2 biosynthesis (Park et al., 2009). Although the precise role for Agr2 in Muc2 biosynthesis is not demonstrated, PDIs are involved in disulphide bond formation and Muc2 has 215 cysteines, and forms disulphide-linked oligomers. Agr2 accumulated in the same region of Winnie goblet cells as the Muc2 precursor, and accumulation was diminished by DEX treatment (Fig. 1 D).
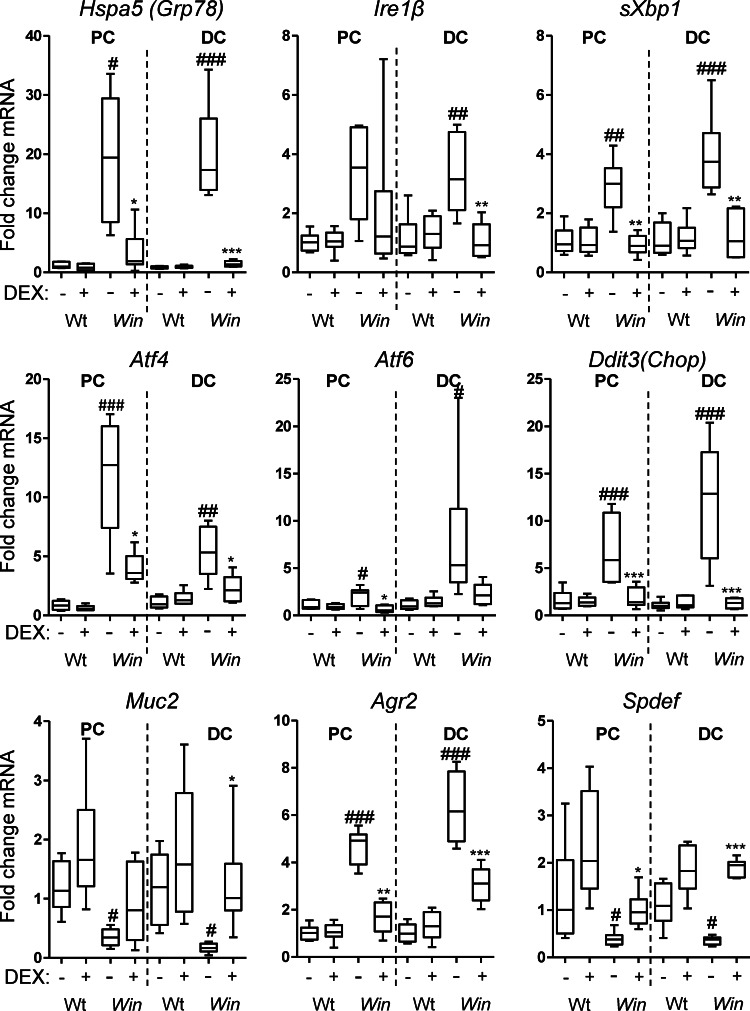
Restoration of mucin biosynthesis and reduction of misfolded Muc2 precursor suggested that glucocorticoids alleviate ER stress. mRNA for the chaperone and key regulator of ER stress, Grp78, was up-regulated 18- and 24-fold in the Winnie proximal and distal colon, respectively (Fig. 2). Splicing of Xbp1 mRNA, which directly measures Ire1 endonuclease activity, was increased in the Winnie colon, as was mRNA expression of Ire1β, Atf4, and Ddit3 (Chop), all of which are consistent with ER stress and activation of the UPR (Fig. 2). All of these markers decreased significantly, returning to near normal levels in Winnie mice treated with DEX, whereas these genes were unaffected in treated WT mice (Fig. 2).
Figure 2.
Glucocorticoids reduce ER stress and activation of the UPR and restore goblet cell gene expression in Winnie mice. mRNA expression of ER chaperones (Grp78), UPR signaling molecules (splicing of Xbp1, Ire1β), UPR transcription factors (Atf4, Atf6, and Ddit3 [Chop]), and goblet cell–related genes (Muc2, Agr2, and Spdef) was determined by qRT-PCR in the proximal and distal colons from control and DEX-treated WT and Winnie (Win) mice in the experiment described in Fig. 1. mRNA levels were corrected to β-actin and are presented as fold changes in expression relative to the mean expression in vehicle treated WT mice. Statistics: Box plots with whiskers show median, quartiles, and the maximum range. Kruskal-Wallis nonparametric analysis, Dunn’s multiple comparison #, WT versus Win, *, Win versus DEX-treated Win; #,*, P < 0.05; ##,**, P < 0.01; ###,***, P < 0.001.
DEX treatment decreased ER stress while promoting mature mucin production. Therefore, we examined the expression of mRNA for Muc2, Agr2, and the goblet cell transcription factor Spdef (Park et al., 2007; Gregorieff et al., 2009; Noah et al., 2010). Spdef and Muc2 were significantly decreased in Winnie colons consistent with diminished goblet cell differentiation/maturation or premature apoptosis. In contrast, Agr2 was up-regulated 4.3- and 6.7-fold in the proximal and distal regions of the colon, respectively (Fig. 2). Agr2 is regulated by Spdef during goblet cell maturation (Gregorieff et al., 2009; Noah et al., 2010), so increasing Agr2 in the face of decreasing Spdef implies that there is a UPR-regulated increase of Agr2 in response to mucin misfolding. DEX increased Muc2 and Spdef mRNA close to levels seen in WT mice and diminished the increase in Agr2, suggesting that the recovery in mature mucin after glucocorticoid treatment is accompanied by decreased ER stress, decreased activation of the UPR, and increased goblet cell maturation and mucin biosynthesis.
Glucocorticoids suppress intestinal inflammation in Winnie mice
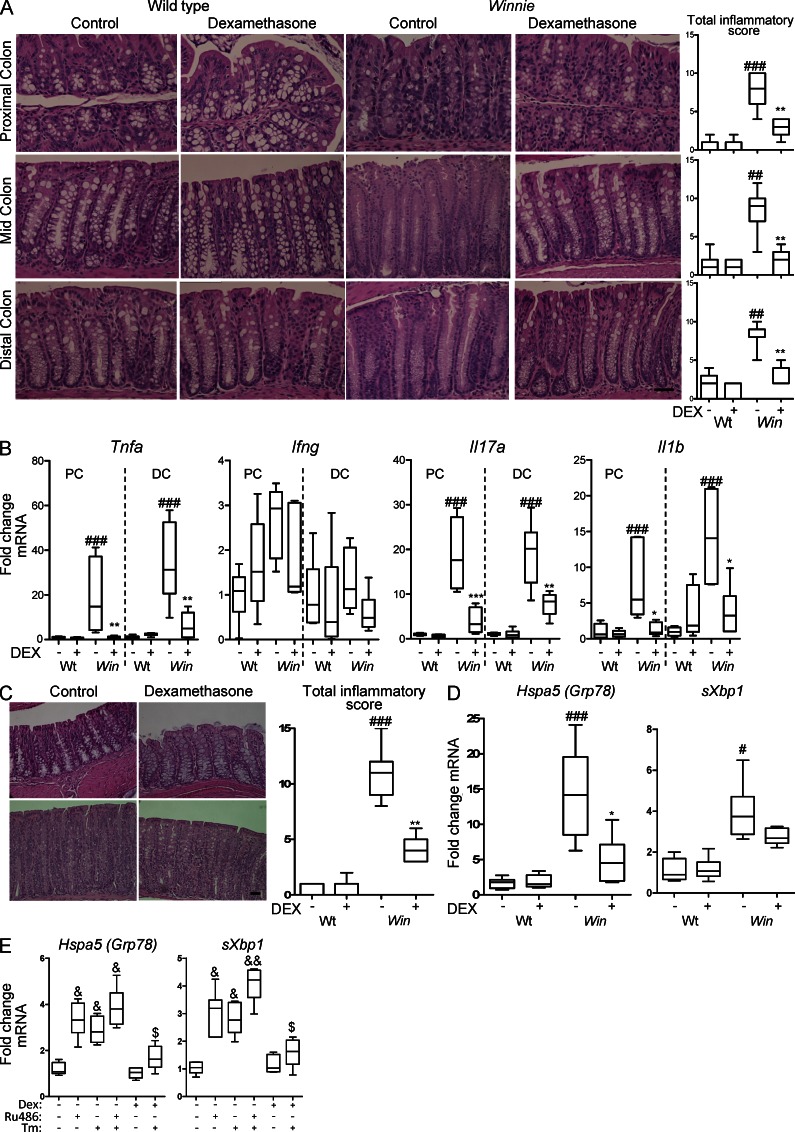
Inflammation in Winnie is characterized by activation of innate and adaptive immunity, with increased production of proinflammatory cytokines, an influx of immune cells and elongation of colonic crypts (Heazlewood et al., 2008; Eri et al., 2011). Glucocorticoids suppress inflammatory responses (Barnes, 1998) and inflammatory factors could enhance ER stress (Hasnain et al., 2012). Because the influence of DEX on Muc2 biosynthesis could be via direct effects on ER stress in goblet cells and/or mediated indirectly via suppression of inflammation, we assessed molecular and histological markers of inflammation. In DEX-treated Winnie mice, total inflammatory scores decreased in the proximal, mid, and distal colon, whereas DEX had no effect on WT mice (Fig. 3 A). Colonic mRNA expression of innate (Il1β, Tnfα), TH1 (Ifnγ, Tnfα), and TH17 (Il17a) cytokines was assessed. Tnfα, Il17a, and Il1β were significantly elevated in Winnie mice, but there was no change in Ifnγ (Fig. 3 B). After DEX treatment, cytokine gene expression returned to the levels seen in WT mice in the proximal colon, and decreased substantially in the distal colon (Fig. 3 B). Winnie mesenteric lymph node (MLN) leukocytes secreted more TNF, IFN-γ, and IL-17A, and DEX inhibited the secretion of T cell cytokines in leukocytes from Winnie and WT mice (not depicted), confirming that DEX suppressed production of inflammatory cytokines. Thus, the decreased ER stress and restored mucin production after DEX treatment could result from suppression of inflammation.
Figure 3.
Glucocorticoids suppress intestinal inflammation in Winnie mice and also suppress basal ER stress in the noninflamed intestine. (A) Representative hematoxylin and eosin–stained proximal, mid, and distal colon tissue sections from control and DEX-treated WT and Winnie (Win) mice in the experiment described in Fig. 1. The total intestinal inflammatory scores for each region of the colon are shown at the right. Bar, 50 µm. (B) Tnfα, Ifnγ, Il17a, and Il1β mRNA levels measured by qRT-PCR in the proximal and distal colon of control and DEX-treated WT and Win mice corrected to β-actin and presented as fold changes in expression relative to the mean expression of vehicle-treated WT mice. (C) WT and Win mice were treated with DEX (100 ng/g body weight) or vehicle i.p. daily for 5 d. Representative hematoxylin and eosin–stained mid-colon tissue sections and inflammation scores are shown. Bar, 50 µm. (D) mRNA expression of Grp78, and sXbp1 measured by qRT-PCR as in B in the experiment in C. (E) WT mice were administered DEX (20 ng/g body weight i.p. daily), RU-38486 (100 ng/g i.p. daily) or vehicle (control) for 5 d ± Tm (400 ng/g i.p.) 16 h before sampling the intestine and assessing Grp78 and sXbp1 as in D; n = 7–8/group, single experiment. Statistics: box plots with whiskers show median, quartiles, and the maximum range. Kruskal-Wallis nonparametric analysis, Dunn’s multiple comparison: #, WT versus Win; *, Win versus DEX-treated Win; &, versus untreated; $, versus Tm; #,*,&,$, P < 0.05; ##,**,&&, P < 0.01; ###,***, P < 0.001.
We assessed the efficacy of higher dose DEX over a shorter time course of 5 d. Consistent with the 4-wk treatment, acute treatment with DEX decreased intestinal inflammation (Fig. 3 C) and restored mucin production in apical goblet cells (not depicted) and substantially decreased Grp78 and sXbp1 mRNA in the Winnie colon (Fig. 3 D), suggesting that DEX might have a direct effect on ER stress. However, this acute treatment with DEX also reduced histological inflammation, colonic inflammatory cytokine expression, and the secretion of cytokines from cultured MLN leukocytes (unpublished data). This experiment could not differentiate between direct effects of the glucocorticoid on ER stress and indirect effects via inflammation. Therefore, to assess the influence of glucocorticoids in the noninflamed intestine we treated WT mice with the glucocorticoid receptor antagonist RU38486 for 5 d. This resulted in increased intestinal Grp78 and sXbp1 mRNA (Fig. 3 E), demonstrating that endogenous glucocorticoids suppress ER stress in the absence of a misfolding trigger or inflammation. Furthermore, ER stress induced in WT mice by 12-h treatment with the N-glycosylation inhibitor, tunicamycin (Tm), was almost completely inhibited by prior administration of DEX (Fig. 3 E), showing that exogenous glucocorticoids inhibit intestinal misfolding-induced ER stress in the absence of inflammation.
Glucocorticoids suppress ER stress in intestinal epithelial cells in vitro
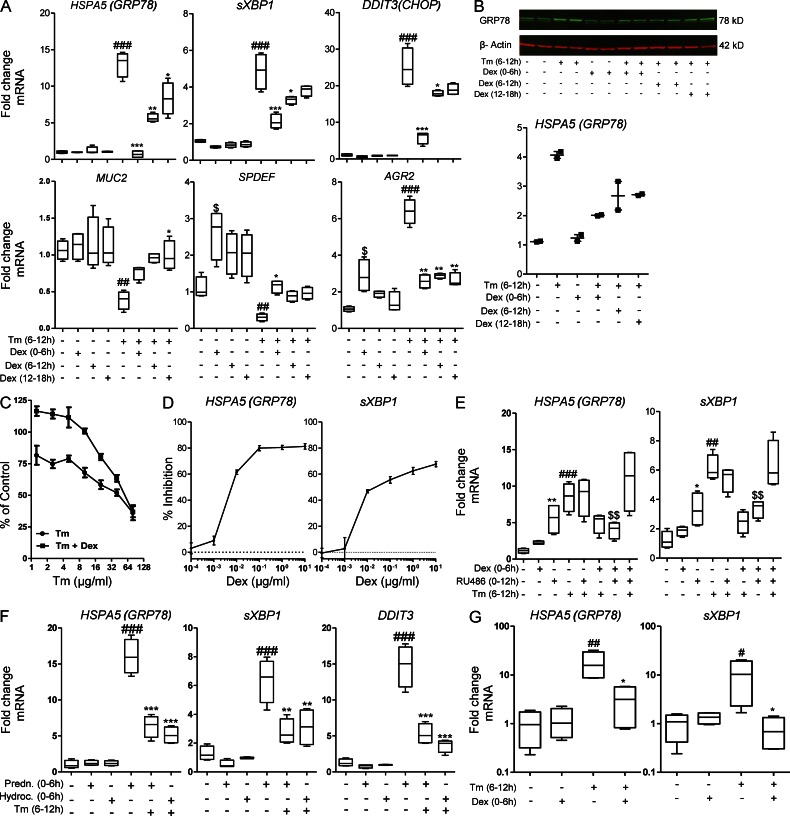
To determine the mechanisms by which glucocorticoids suppress ER stress, experiments were conducted in cultured human LS174T colonic adenocarcinoma cells. LS174T cells show goblet cell differentiation and secretion of major intestinal mucins, primarily MUC2. ER stress was induced by inhibiting N-glycosylation with Tm (MUC2 has 29 N-glycosylation sites). Tm-induced ER stress was demonstrated by up-regulation of GRP78 at both the mRNA and protein level, increased splicing of XBP1 mRNA, and increased production of CHOP mRNA (Fig. 4 A). Exposure to DEX before, during, or after Tm significantly decreased the expression of these three genes (Fig. 4 A) and GRP78 protein (Fig. 4 B) indicating that DEX directly inhibits ER stress in these intestinal epithelial cells. Pretreatment with DEX was most effective, suggesting that DEX up-regulates genes involved in alleviating ER stress induced by protein misfolding. DEX also protected Tm-treated LS174T cells from apoptosis at all but very high concentrations of Tm (Fig. 4 C). A titration experiment showed that DEX suppressed ER stress in a dose-dependent manner at concentrations >1 ng/ml (2.5 nM; Fig. 4 D).
Figure 4.
Glucocorticoids abrogate the Tm-induced activation of the UPR in human colonic epithelial cells. LS174T cells were treated with DEX (10 µg/ml) for 6 h before, during, or after exposure to Tm (10 µg/ml) for 6 h. (A) mRNA expression of UPR genes GRP78, sXBP1, CHOP, and goblet cell–related genes MUC2, SPDEF, and AGR2 were measured by qRT-PCR. The expression of these were normalized to β-actin. (B) Western blot analysis of GRP78 with densitometric analysis of GRP78 protein levels relative to β-actin on the right. (C) LS174T cells were treated with DEX (10 µg/ml) for 6 h before a range of concentrations of Tm for 24 h and mitochondrial activity was assessed by MTT assay presented as the percentage of untreated cells; n = 4/condition; representative from two experiments. (D) Dose–response curve of DEX inhibition of GRP78 and sXBP1 mRNA expression after exposure to Tm. The mRNA expression was analyzed by qRT-PCR and normalized to β-actin. (E) Treatment of LS174T cells with RU38486 (1 µg/ml) for 2 h before DEX (100 ng/ml), was performed to inhibit glucocorticoid receptors. The UPR response induced by Tm was determined by qRT-PCR as in A. (F) Effect of 2.5 µM hydrocortisone or prednisolone on Tm induced ER stress in LS174T cells; qRT-PCR as in A. (G) Human colonic crypts in culture were treated with DEX (10 µg/ml) for 8 h before exposure to Tm (10 µg/ml) for 12 h, and mRNA expression of GRP78 and sXBP1 was assessed as in A. Statistics: A, C, E: n = 4 in an experiment representative of 2–3 individual experiments; D, F, G: n = 4 single experiment. Box plots with whiskers show median, quartiles and the maximum range. Kruskal-Wallis nonparametric analysis, Dunn’s multiple comparison: #, versus control; *, versus Tm; $, versus Tm + DEX + RU38486; *,#, P < 0.05; **,##,$$, P < 0.01; ***,###, P < 0.001.
Suppression of Tm-induced ER stress by DEX was inhibited by RU38486 (Fig. 4 E), and interestingly, RU38486 increased ER stress in the absence of Tm. This implies that autocrine glucocorticoid signaling suppresses ER stress in LS174T cells, analogous to the finding in RU38486-treated mice (Fig. 3 E). Alternative glucocorticoids, prednisolone, and hydrocortisone, also inhibited Tm-induced increased expression of GRP78, sXBP1, and CHOP (Fig. 4 F). DEX also inhibited ER stress in HT29 colon cancer cells (not depicted). To confirm that this phenomenon occurs in nontransformed cells, we established primary human colonic crypt cultures. DEX inhibited Tm-induced up-regulation of GRP78 and sXBP1 in the cultured crypts confirming suppression of ER stress in normal colonic epithelial cells (Fig. 4 G).
To further explore the mechanism by which glucocorticoids alleviate ER stress in LS174T cells we analyzed the mRNA expression of MUC2, SPDEF, and AGR2 (Fig. 4 A). Tm treatment significantly inhibited SPDEF and MUC2 mRNA showing that Tm-induced ER stress diminishes goblet cell maturation and mucin biosynthesis. In Tm-treated cells, DEX restored the expression of MUC2 and SPDEF, but decreased the expression of the PDI, AGR2, consistent with less ER stress and diminished activation of the UPR. The amelioration of ER stress without activation of the UPR by DEX in colonic epithelial cells suggests that DEX inhibits ER stress by either (a) increasing removal and degradation of the terminally misfolded proteins from the ER or (b) enhancing correct folding of proteins.
Glucocorticoid modulation of ER stress is independent of NF-κB
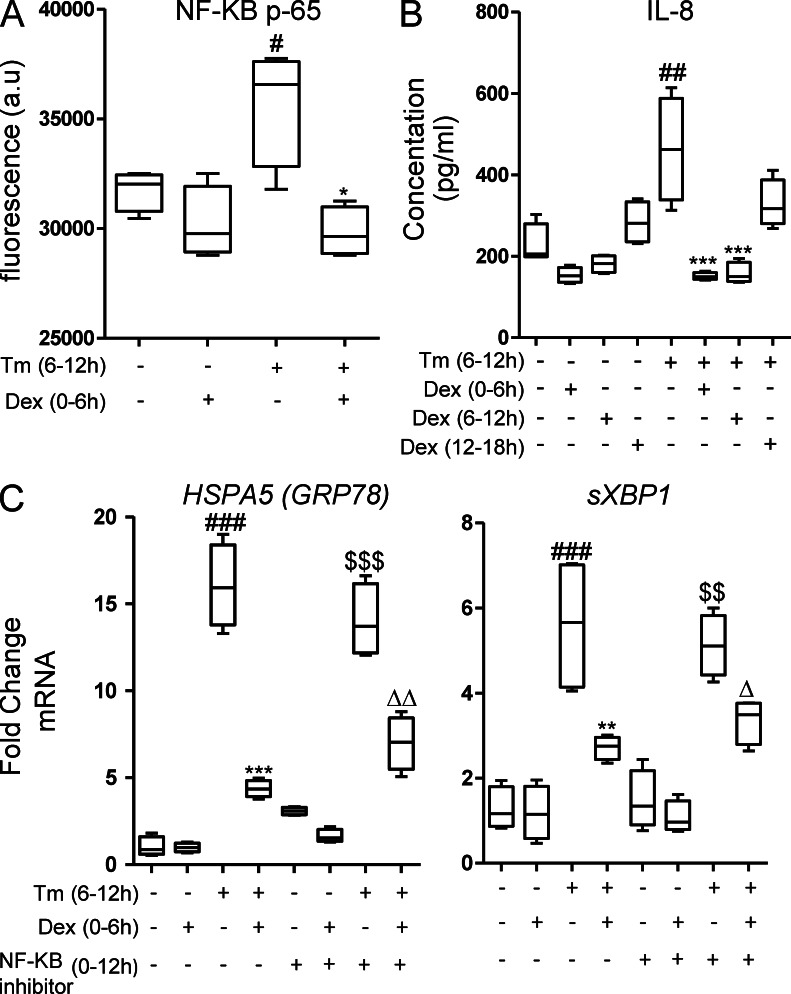
Many of the antiinflammatory effects of glucocorticoids can be attributed to inhibition of NF-κB, and protein misfolding can activate NF-κB via the UPR. As expected, induction of ER stress in LS174T cells with Tm resulted in activation of NF-κB (Fig. 5 A) and production of the NF-κB–induced inflammatory chemokine IL-8 (Fig. 5 B). However, pharmacological inhibition of NF-κB actually caused a mild increase in ER stress in LS174T cells and did not suppress Tm-induced ER stress (Fig. 5 C). Furthermore, co-treatment with the NF-κB inhibitor reduced rather than improved the DEX inhibition of Tm-induced ER stress (Fig. 5 C), showing that inhibition of ER stress by glucocorticoids is independent of their inhibition of NF-κB.
Figure 5.
Glucocorticoid inhibition of ER stress is independent of suppression of NF-κB. (A) NF-κB activation was assessed by measuring NF-κBp65 after treating LS174T cells with Tm ± DEX (10 µg/ml). (B) IL-8 was measured in culture supernatants from LS174T cells treated with Tm ± DEX. (C) mRNA expression of GRP78 and sXBP1 in the presence of the NF-κB inhibitor BAY11-7085 (20 µM). Statistics: n = 4 in a single experiment; Box plots with whiskers show median, quartiles, and the maximum range. Kruskal-Wallis nonparametric analysis, Dunn’s multiple comparison: #, versus control; *, versus Tm; $, versus BAY11; Δ, versus BAY11 + Tm; #,*,Δ, P < 0.05; ##,**,$$,ΔΔ, P < 0.01; ###,***,$$$, P < 0.001.
Inhibition of ER stress by glucocorticoids is partially dependent on enhanced ERAD
Like many other complex secretory proteins, it is likely that a significant proportion of MUC2 misfolds during biosynthesis (Austin, 2009). Molecular chaperones of the folding cycle play a very important role in assisting and monitoring correct protein folding. If proteins attain terminally illegitimate conformations then they are subjected to removal from the ER and subsequent degradation by a process known as ER-associated degradation (ERAD; Olivari and Molinari, 2007). Elimination of terminally misfolded soluble proteins like MUC2 requires cleavage of N-linked glycans by mannosidases such as the ER degradation–enhancing α-mannosidase–like lectins (EDEMs). Removal through the Sec61 translocon pore is assisted by molecular chaperones like OS9 on the ER side and VCP in the cytosol. We hypothesized that DEX was inhibiting ER stress by up-regulating components of the ERAD pathway and enhancing removal of terminally misfolded MUC2, and thereby reducing stress in the ER, averting activation of the UPR and permitting continued MUC2 synthesis. To test this hypothesis, we examined DEX modulation of the expression of ERAD pathway members and assessed the importance of this pathway using siRNA knockdown of EDEM1.
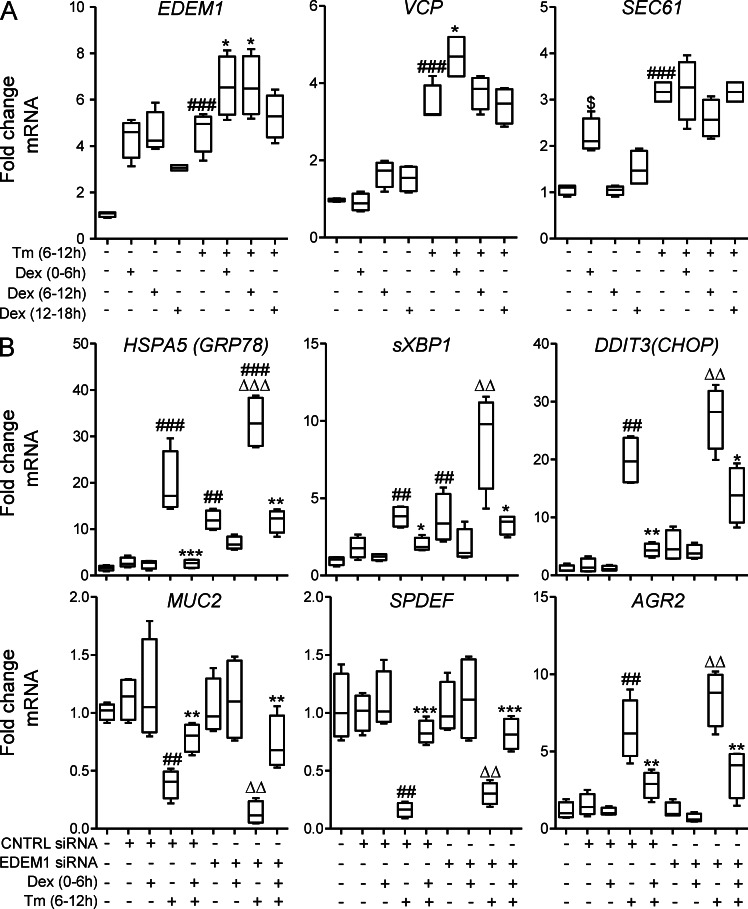
To identify direct modulation of ERAD genes, we treated LS174T cells with DEX before, during, or after induction of ER stress. In the absence of Tm, DEX up-regulated EDEM1 mRNA by 4.9-fold (Fig. 6 A). Tm also increased EDEM1 by 5.1-fold, but when pretreated with DEX, expression was higher (7.6-fold greater than control) showing that DEX modulates EDEM1 independently of the UPR. In a different pattern, SEC61 and VCP were slightly up-regulated by DEX alone, but to a greater degree by Tm alone and remained high when cells were co-treated with Tm and DEX. These results show that DEX modulates components of the ERAD pathway; therefore, to test our hypothesis that DEX inhibits ER stress via boosting ERAD, we knocked down EDEM1 by siRNA. siRNA transfection decreased EDEM1 mRNA expression by ≥75% in LS174T cells. EDEM1 knockdown up-regulated the expression of GRP78 and sXBP1 14.6- and 4.6-fold, respectively, showing that inhibiting ERAD in intestinal secretory cells induces ER stress, which is consistent with a substantial basal rate of misfolding (Fig. 6 C). Furthermore, Tm-induced ER stress was greater in the absence of EDEM1, demonstrating that EDEM1 is important in disposal of misfolded proteins in both the basal state and during exacerbated misfolding (Fig. 6 C). DEX was less able to inhibit the expression of UPR-regulated genes GRP78, sXBP1, and CHOP in cells with EDEM1-silenced versus control siRNA (Fig. 6 C). Expression of MUC2, SPDEF, and AGR2 were not modulated by EDEM1 knockdown. Collectively, these results demonstrate that DEX up-regulates components of the ERAD pathway and that, although enhanced ERAD partially explains the mechanism by which the steroid inhibits ER stress, other ERAD-independent (or at least EDEM1-independent) mechanisms also appear to contribute.
Figure 6.
Glucocorticoids increase expression of proteins involved in ERAD contributing to the inhibition of ER stress. (A) LS174T cells were treated with DEX (10 µg/ml) for 6 h ± Tm (5 µg/ml) for 6 h, as shown. mRNA expression of the molecular chaperones involved in the ERAD pathway (EDEM1, SEC61, and VCP) were measured by qRT-PCR. The expression was normalized to β-actin. (B) EDEM1 was silenced in LS174T cells by EDEM1 siRNA treatment for 48 h. mRNA expression of GRP78, sXBP1, CHOP, MUC2, SPDEF, and AGR2 were measured in EDEM1-silenced LS174T cells, treated with DEX (10 µg/ml) for 6 h, and treated with Tm (5 µg/ml) for 6 h. The expression of these genes was normalized to β-actin. Statistics: n = 4, representative of 2–3 experiments; box plots with whiskers show median, quartiles, and the maximum range. Kruskal-Wallis nonparametric analysis, Dunn’s multiple comparison: (A) #, versus control; *, versus Tm; (B) #, versus control; *, versus Tm and same siRNA; Δ, versus EDEM1 siRNA alone; #,*, P < 0.05; ##,**,ΔΔ, P < 0.01; ###,***,ΔΔΔ, P < 0.001.
Glucocorticoids promote correct folding of proteins in the ER
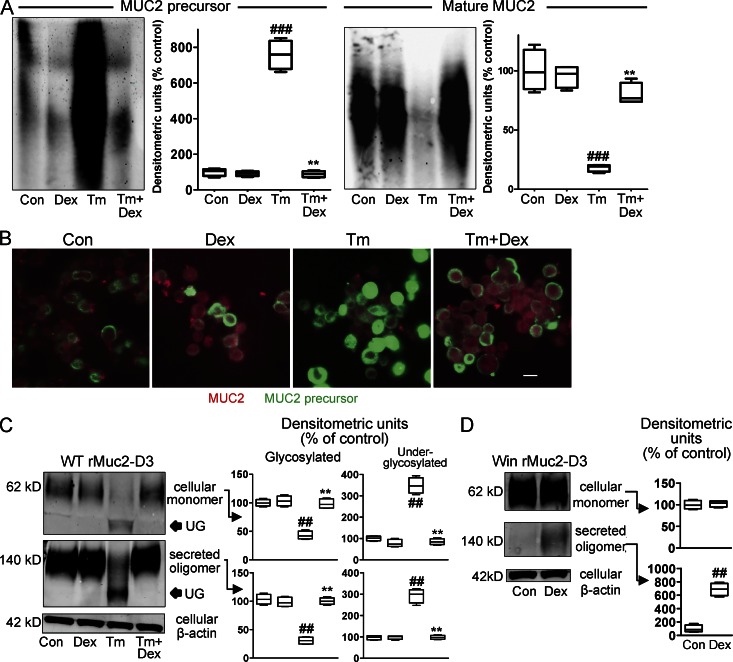
Because DEX does not depend solely on ERAD to inhibit ER stress, we hypothesized it might promote correct protein folding under adverse conditions. Therefore, we used antibodies reactive with the MUC2 precursor and mature MUC2 to evaluate MUC2 misfolding in LS174T cells. Tm resulted in accumulation of the MUC2 precursor (>7-fold increase), accompanied by >80% reduction in production of mature MUC2 (Fig. 7 A). Treatment with DEX reversed this, with disappearance of the precursor and restoration of mature mucin with the relative abundance of precursor and mature mucin indistinguishable from nontreated control cells (Fig. 7 A). Similarly, immunofluorescence staining of DEX-treated cells showed restoration of mature mucin production and reversal of the Tm-induced precursor accumulation (Fig. 7 B). These observations show that DEX corrects folding of MUC2 and progression through the secretory pathway despite inhibition of N-glycosylation by Tm.
Figure 7.
Glucocorticoids ameliorate ER stress by enhancing correct folding and secretion of proteins. (A and B) LS174T cells were treated with DEX (10 µg/ml) for 6 h, followed by treatment with Tm (5 µg/ml) for 6 h. (A) Proteins were reduced and separated by agarose gel electrophoresis and detected by Western blotting with the 4F1 antibody (precursor) and the Muc2 (H300) antibody (mature mucin). Representative from four replicates analyzed by densitometry and expressed as percentage of untreated control cells. Note that Mr cannot be estimated using agarose gels. (B) Immunofluorescence staining and confocal microscopy with the same antibodies as in A; MUC2 precursor (green), mature mucin (red); bar, 10 µm. (C) MKN45 cells were transfected with a plasmid encoding the rMuc2-D3 WT protein and treated with DEX (10 µg/ml) for 6 h, followed by 6-h treatment with Tm (10 µg/ml). The lysates and secretions from transfections were analyzed by PAGE/Western blotting. Recombinant proteins were detected with the M2 anti-FLAG antibody, and no reactivity was seen with untransfected cells. Representative from four replicates analyzed by densitometry after correction for β-actin in the same lane and expressed as percentage of untreated control cells. (D) MKN45 cells were transfected with a plasmid encoding rMuc2-D3 with the Win mutation. Lysates and secretions were analyzed as in (C). Statistics: A, C, D: representative blot shown, densitometry from 4 distinct experimental replicates run on the same set of Western blots; box plots with whiskers show median, quartiles, and the maximum range. Kruskal-Wallis nonparametric analysis, Dunn’s multiple comparison: #, versus control; *, versus Tm; ##,**, P < 0.01; ###, P < 0.001.
To provide further evidence that DEX promotes correct folding, cDNAs encoding the WT and Winnie Muc2 N-terminal D3 domain were expressed in MKN45 gastric cells, which lack endogenous MUC2. The D3 domain, which is mutated in Winnie, is involved in N-terminal oligomerization of Muc2, and we have previously shown inappropriate oligomerization and retention in the secretory pathway of Winnie recombinant D3 domain (Heazlewood et al., 2008). First, induction of ER stress by Tm in cells transfected with the WT D3 domain resulted in a major reduction (57%) in the amount of the precursor monomer in the ER and the appearance of a faster migrating form likely to be a form with fewer N-glycans. This was accompanied by a 70% reduction in the secretion of appropriately glycosylated plus some underglycosylated oligomer. These changes were completely reversed by treatment with DEX, consistent with successful progression through the secretory pathway (Fig. 7 C). Second, the Winnie D3 mutation almost completely abolished secretion, but DEX treatment resulted in a 6.9-fold increase in the secretion of the Winnie D3 oligomer (Fig. 7 D), suggesting that DEX promotes factors involved in correct folding and exit from the ER in secretory cells.
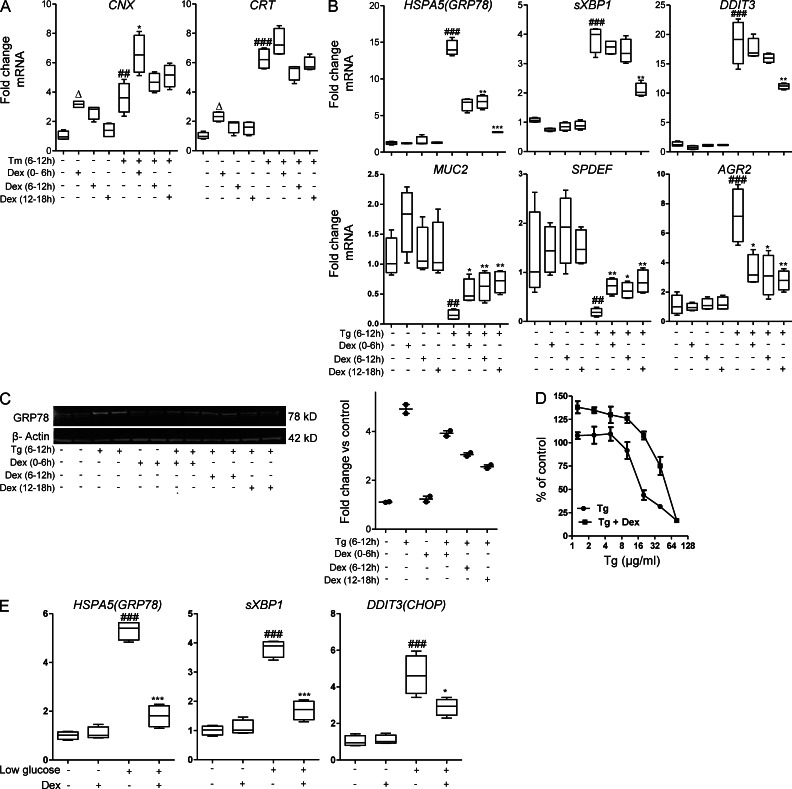
Calnexin and calreticulin are ER chaperones for glycoproteins and integral components of the folding cycle in which attempts are made to refold misfolded proteins (Hebert et al., 1995). Genes encoding these chaperones were up-regulated by DEX alone, Tm alone, and by the combination of Tm and DEX (Fig. 8 A), thus providing a mechanism by which DEX-initiated changes in gene expression could promote correct mucin folding.
Figure 8.
Glucocorticoids up-regulate Ca2+-dependent chaperones and inhibit ER stress induced by chelation of Ca2+ or by ATP depletion. (A) In LS174T cells treated with DEX (10 µg/ml) for 6 h, followed by treatment with Tm (5 µg/ml) for 6 h, mRNA expression of ER-resident glycoprotein chaperones involved in the folding cycle (calnexin, Cnx and calreticulin, Crt) was determined by qRT-PCR. mRNA levels were corrected to β-actin and are presented as fold changes in expression relative to the mean expression in untreated control cells. (B) LS174T cells were treated with thapsigargin (Tg; 5 µM) for 6 h. Cells were treated with DEX (10 µg/ml) for 6 h before, during, or after exposure to Tg; qRT-PCR as in A; n = 4, representative of three individual experiments. (C) Western blot analysis of GRP78 with densitometric analysis of GRP78 protein levels relative to β-actin on the right in the experiment in A. (D) LS174T cells were treated with DEX (10 µg/ml) for 6 h before a range of concentrations of Tg for 24 h and mitochondrial activity assessed by MTT assay presented as the percentage of untreated cells. (E) LS174T cells were grown in media with low glucose (5.5 mM) for 6 h ± DEX (10 µg/ml). mRNA expression of GRP78, sXBP1, and CHOP were measured by qRT-PCR. Statistics: A, B, D, and E: n = 4 in an experiment representative of 2–3 individual experiments; C: representative Western blot from 2 distinct experiments, each with n = 2; A, B, E: box plots with whiskers show median, quartiles, and the maximum range; Kruskal-Wallis nonparametric analysis, Dunn’s multiple comparison: #, versus control; *, versus Tg; *, P < 0.05; ##,**, P < 0.01; ###,***, P < 0.001; E: versus control, ###, P < 0.001; versus low glucose; *, P < 0.05; ***, P < 0.001.
Calnexin and calreticulin were first isolated as Ca2+-binding proteins; the ER is the major intracellular calcium store; and depletion of ER Ca2+ induces ER stress (Lodish and Kong, 1990). Therefore, to identify if the inhibition of ER stress by DEX is dependent on Ca2+, we treated LS174T cells with thapsigargin (Tg), which depletes ER calcium. Tg induced ER stress and up-regulated mRNA expression of GRP78, sXBP1, CHOP, and AGR2 by 13.1-, 4.0-, 19.4-, and 6.8-fold, respectively (Fig. 8 A), whereas the mRNA expression of MUC2 and SPDEF were significantly reduced. DEX treatment before, simultaneously and following Tg treatment, suppressed ER stress, but the temporal efficacy was opposite to that with Tm. Posttreatment with DEX was the most effective in inhibiting Tg-induced ER stress and activation of UPR and also restored MUC2 and SPDEF mRNA expression (Fig. 8 B), whereas pretreatment was the most effective for Tm-induced ER stress (Fig. 4 A). Posttreatment with DEX was also most effective in decreasing Tg-induced GRP78 protein (Fig. 8 C). The inability of DEX to effectively protect cells when Ca2+ is disturbed suggests that suppression of ER stress by glucocorticoids is dependent on maintenance of ER Ca2+, which is consistent with a role for the Ca2+-dependent chaperones, CNX and CRT, and/or other Ca2+-dependent proteins. Glucocorticoids have been reported to block Ca2+ channels in the ER and thereby inhibit leakage of Ca2+ from the ER (De et al., 2011). As with Tm, DEX provided protection from Tg-induced cell death for all but the highest concentrations of Tg (Fig. 8 D).
Depletion of intracellular ATP by reducing glucose in the media induces ER stress (Fujii et al., 2006). We also demonstrated that DEX inhibits this form of ER stress in LS174T cells (Fig. 8 E).
DISCUSSION
In this study we demonstrated that glucocorticoids directly ameliorate ER stress in intestinal secretory cells occurring as a result of protein misfolding in vitro and in vivo. To our knowledge, this is the first study to demonstrate direct inhibition of ER stress by glucocorticoids in vivo, and to show the mechanism of action is via GR-driven enhancement of protein folding and degradation of misfolded proteins. These mechanisms are independent of the well documented NF-κB-mediated anti-inflammatory action of the steroids on leukocytes which was evident in DEX-treated Winnie mice. Although one could argue the repression of ER stress by glucocorticoids in Winnie mice could be due to inhibition of inflammation, we demonstrated that endogenous and exogenous glucocorticoids inhibit ER stress in the noninflamed intestine in vivo, and prevent accumulation of misfolded proteins in intestinal goblet cells cultured in vitro.
Glucocorticoids significantly inhibited ER stress in vitro when protein misfolding and ER stress was induced by inhibition of N-glycosylation, disturbance of intracellular Ca2+, depletion of glucose or inhibition of ERAD. This shows that modulation of ER stress by glucocorticoids is potentially relevant to many forms of human disease involving protein misfolding and ER stress. This study also enhances our basic understanding of ER functioning. Although the current understanding is that the UPR is the master regulator of components of the folding cycle and ERAD (Shang and Lehrman, 2004), we demonstrate that glucocorticoids can modulate these pathways independently of the UPR and help prepare the ER for biosynthetic challenges before induction of ER stress. Thus glucocorticoids are likely to act at multiple levels to prevent inflammation in the intestine.
Glucocorticoids up-regulated chaperones involved in correct protein folding, calnexin and calreticulin, and components of ERAD including EDEM’s, suggesting regulation of a suite of genes, which can improve the capability of the ER of secretory cells to produce complex proteins with limited need for activation of the UPR. These changes were accompanied by a reduction in misfolding and increased secretion of mature protein in the face of inhibition of N-glycosylation or misfolding mutations. While restoration of protein secretion could be achieved by reducing the threshold of ER quality control mechanisms which block exit of misfolded proteins from the ER, we saw no evidence for secretion of misfolded protein aggregates in the presence of the glucocorticoids in our experimental systems.
Several previous studies provide support for a protective effect of glucocorticoids on ER stress. Glucose depletion-induced ER stress in human embryonic kidney 293 cells was inhibited by DEX facilitating synthesis of nephrin, analogous to the recovery in MUC2 production we observed in Tm-treated intestinal cells. Diminished ER stress was attributed to increased ATP production based on a correlation with DEX-increased ATP levels but without direct testing of that mechanism (Fujii et al., 2006). Our study demonstrated that glucocorticoids, differentially orchestrated the transcription of components of the folding cycle and molecules involved in ERAD independently of activation of the UPR. Our experiments in glucose-replete media show that DEX suppressed three different forms of ER stress, making it unlikely an increase in cellular ATP explains the suppression. Interestingly in 293 cells DEX could not suppress Ca2+ ionophore-induced ER stress (Fujii et al., 2006), whereas we found DEX could suppress thapsigargin-induced ER stress. In another study in which LPS induced mild ER stress in lung NCI-H292 epithelial cells, DEX inhibition of ER stress was attributed to decreased MUC5AC production but without direct testing of this mechanism (Shang et al., 2011). In contrast to the decreased production of MUC5AC, we observed DEX facilitated correct folding and secretion of MUC2 in Tm-stressed LS174T cells, facilitated secretion of MUC2 D3-domain proteins carrying the Winnie mutation, and that DEX also recovered mucin biosynthesis in Winnie mice. DEX has also recently been shown to inhibit IRE1β and splicing of XBP1, and enhance amylase secretion in AR42J exocrine pancreatic cells, which is consistent with our findings (Cross et al., 2012). All of these previous reports of diminished ER stress with DEX are compatible with our proposed mechanism of action via facilitation of folding and boosting ERAD.
Functional maturity of the intestine is facilitated by endogenous local intestinal glucocorticoids (Gartner et al., 2002; Solomon et al., 2001). Hydrocortisone (HC) effects differentiation and proliferation of fetal small intestinal and colonic epithelial cell proliferation and maturation (Arsenault and Ménard, 1985). Colonic and small intestinal epithelial cells express glucocorticoid receptors and synthesize glucocorticoids (Atanasov et al., 2008; Modica et al., 2010; Mueller et al., 2006). Glucocorticoid production by intestinal epithelial cells, is dependent on nuclear receptor liver receptor homologue-1 (LRH-1; Mueller et al., 2006). Germ line haploinsufficiency for Lrh-1 reduces local glucocorticoid synthesis and predisposes mice to TNBS-induced intestinal inflammation (Coste et al., 2007). In support of a role for local glucocorticoid synthesis in IBD, UC, and Crohn’s disease patients have lower intestinal LRH-1 expression and glucocorticoid concentrations (Coste et al., 2007). We have now shown by inhibiting glucocorticoid signaling in mice with RU38486 that endogenous glucocorticoids suppress ER stress in the noninflamed intestine and that protection from misfolding-induced ER stress can be boosted by exogenous glucocorticoids. Interestingly, we also demonstrated inhibition of endogenous glucocorticoids by RU38486 induces ER stress in LS174T cells, despite these cells being shown to have low endogenous LRH-1 (Sidler et al., 2011). Facilitation of protein folding and minimizing ER stress in secretory cells by local glucocorticoids is likely to be an important component of maintaining intestinal homeostasis.
Although DEX directly inhibits ER stress in vitro independently of suppression of NF-κB, it is possible that the DEX-mediated inhibition of inflammation in leukocytes has indirect suppressive effects on ER stress in vivo. Inflammatory factors produced by leukocytes could modulate ER stress. For example, TNF initiates ER stress in murine fibrosarcoma cells attributed to increased production of reactive oxygen species (Xue et al., 2005a). Similarly, TNF and IFN-γ increase GRP78 in intestinal enterocyte-like Caco2 cells (Hao et al., 2012). In Winnie mice, DEX inhibited TNF and IFN-γ production by MLN T cells, and the mRNA expression of these genes in the colon, which could also contribute to the diminished ER stress. DEX also enhances FOXP3 expression and IL10 production by T regulatory cells (Chen et al., 2006; Xie et al., 2009), and IL-10 inhibits ER stress in intestinal cells (Shkoda et al., 2007; Hasnain et al., 2013). These observations indicate that modulation of local intestinal cytokine levels by glucocorticoids can indirectly inhibit the propagation of ER stress and pathophysiology in the intestinal secretory cells in synergy with the direct effects we have demonstrated.
In glucocorticoid-treated Winnie mice, the complete recovery of goblet cell mucin production in the apical lineage versus incomplete restoration in the basal lineage is likely to be caused by the longer life span of the basal cells contributing to the progressive build-up of misfolded proteins and more severe ER stress. ER stress in Winnie and LS174T cells resulted in decreased expression of both SPDEF and MUC2 mRNA consistent with down-regulation of these genes by the UPR. SPDEF is expressed during goblet cell maturation, stays expressed in fully differentiated cells, and regulates MUC2. Glucocorticoids increased the expression of SPDEF but not MUC2 mRNA in nonstressed cells, and abolished the decrease in both genes induced by Tm. The UPR-mediated decrease in SPDEF and MUC2 is likely to contribute significantly to decreased mucin production in the ER-stressed intestine. This suggests that the UPR does not only regulate protein translation but also modulates transcription factors required for secretory cell maturation. Deficiency of the ER-resident enzyme, fatty-acid synthase (FAS), decreases s-palmitoylation of MUC2 and induces ER stress in both LS174T cells and in murine intestinal goblet cells, resulting in colitis with a phenotype similar to Winnie (Wei et al., 2012). Glucocorticoids increase FAS expression and s-palmitoylation of proteins in nonintestinal cells (Xu et al., 1995) providing another mechanism by which steroids could enhance intestinal mucus production. All of these observations suggest that glucocorticoids are important at multiple stages in the intestine from development, maturation of epithelial cells, to maintenance of a functional mucosal barrier, and that treatment of all these functions are potentially important for inducing and maintaining remission in IBD.
In this study, we have demonstrated that glucocorticoids very effectively reduce ER stress in the intestine. Although systemic and, to a lesser extent, topical glucocorticoid therapy is widely used to control inflammation in IBD, long-term use is restricted by pleiotropic physiological side effects. Because glucocorticoids directly suppress ER stress in epithelial cells, luminal delivery of glucocorticoids such as budesonide with low systemic activity, should be an effective route of administration, as demonstrated in clinical trials in UC (Manguso and Balzano, 2007), and perhaps should be considered as a component of maintenance therapy for patients in remission. Overexpression of LRH-1 significantly increases the expression of steroidogenic enzymes and the synthesis of cortisol in intestinal cells (Sidler et al., 2011). Boosting endogenous production of LRH-1 or boosting steroid synthesizing enzymes downstream of LRH-1 will increase the endogenous production of the local intestinal cortisol and could be used to maintain epithelial cell homeostasis and maintain long term remission. Identification of all the links between glucocorticoid receptor-induced transcription and improved ER folding and ERAD could reveal additional targets for therapy, possibly via intermediary transcription factors, which could avoid unwanted off-target effects of glucocorticoids. In summary, judicious glucocorticoid modulation of ER stress has important therapeutic ramifications for a wide range of ER stress–mediated diseases and for the therapeutic use of glucocorticoid drugs.
MATERIALS AND METHODS
Cell culture and drug treatments.
The human colon carcinoma cell line LS174T (American Type Culture Collection) and the human gastric carcinoma cell line MKN45 (Institute of Physical and Chemical Research Cell Bank) were cultured in DMEM high and RPMI 1640 medium, respectively, supplemented with 10% FCS, 2 mM l-glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin (Invitrogen), and maintained at 37°C in 5% CO2. Cells were cultured in the presence of DEX, Tm, Tg, cycloheximide (CHX), mifepristone (RU38486; Sigma-Aldrich), and NF-κB inhibitor (BAY11-7085; Sigma-Aldrich) at the concentrations and times indicated in the figure legends. Chemicals were dissolved in DMSO or PBS and appropriate vehicle controls were used in all experiments. Human colonic crypts from noninflamed colon from a patient undergoing surgery for diverticular disease were cultured using techniques adapted from Jung et al. (2011). Crypts (400 crypts/50 µl Matrigel) were cultured in Wnt3a-conditioned medium and ADF 1:1 with 10 mM Glutamax, Hepes, N-2 (1×; Invitrogen), B-27 without retinoic acid (1×; Invitrogen), 10 mM Nicotinamide (Sigma-Aldrich), 1 mM N-Acetyl-L-cysteine (Sigma-Aldrich), 50 ng/ml RSPO1 (Acrobiosystems), human EGF (Invitrogen), 100 ng/ml human Noggin (PeproTech), 1 µg/ml gastrin (Sigma-Aldrich), 500 nM LY2157299 (Axon MedChem), 10 µM SB202190 (Sigma-Aldrich), 0.01 µM PGE2 (Sigma-Aldrich), and 10 µM DAPT (Sigma-Aldrich; gamma-secretase inhibitor to induce goblet cell differentiation).
Mice and drug treatments.
WT C57BL/6 (purchased from the Animal Resource Authority, Western Australia) and the mutant Winnie with a single missense mutation in Muc2 on a C57BL/6 background (Heazlewood et al., 2008) bred in-house were housed in a PC2 specific pathogen-free animal facility. All animal experimentation was approved by the University of Queensland Animal Ethics Committee. Equal numbers of male and female mice were injected i.p with DEX, RU38486 or Tm at the doses and times indicated in the figure legends.
Morphometric analysis of stored mucin in intestinal goblet cells.
Quantification of the volume of goblet cell thecae containing fully O-glycosylated mucins was performed in AB and PAS-stained paraffin sections of intestine. O-glycosylated mucins stain blue/purple with AB, whereas the non–O-glycosylated precursor stains magenta. The morphometric analysis was performed using ImagePro Plus 3.0 software. Three different regions from the proximal, mid, and distal colon, containing four to seven longitudinally sectioned crypts, were analyzed in each animal. The software was programmed to determine the area occupied by the blue-stained goblet cell thecae in each crypt and express that as a ratio of the area (equivalent to relative volume) of the crypt.
Assessment of intestinal inflammation.
Histological scoring of mucosal epithelial damage and inflammatory infiltration in H&E-stained intestinal tissue sections was performed as previously described (Heazlewood et al., 2008). Leukocytes were harvested from the MLNs and 2 × 106 leucocytes were cultured in RPMI 1640 containing 10% FCS and stimulated with 50 ng/ml of PMA and 750 ng/ml ionomycin for 48 h. MLN culture supernatants were assayed for inflammatory cytokines IL-1β, TNF, IFN-γ (BD), and IL-17 (R&D systems) according to the manufacturer’s instructions.
RNA isolation and gene expression analysis.
RNA from colonic tissue and cultured cells was extracted in TRIzol (Invitrogen) and purified using a RNeasy mini kit (QIAGEN). RNA quality and quantity was assessed using a NANODROP1000 spectrophotometer (Thermo Fisher Scientific), and 1 µg was reverse transcribed to cDNA by using a iScript cDNA synthesis kit (Bio-Rad Laboratories). Quantitative PCR with primers spanning exons was performed using Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen) on a Rotor-Gene 6000 and analyzed using Corbett Rotorgene version 6 software (Corbett LifeScience). Expression of each gene was normalized to the housekeeping gene, β-actin, using the Pfaffl equation and expressed relative to the mean of a relevant control group.
Antibodies, Western blotting, immunohistochemistry, and immunofluorescence.
Antibodies were sourced as follows: mouse Muc2 C-terminal reactive polyclonal (Heazlewood et al., 2008), mouse Muc2 precursor antibody (a gift from G. Hansson, University of Gothenburg, Gothenburg, Sweden), human MUC2 (Santa Cruz Biotechnology, Inc.; clone sc15334), human MUC2 nonglycosylated VNTR peptide repeat (4F1; Heazlewood et al., 2008), GRP78 (Santa Cruz Biotechnology, Inc.; polyclonal N-20), anti-FLAG (Sigma-Aldrich; clone M2), β-actin (Novus Biologicals; clone AC-15), and AGR2 (ab22208; Abcam). Standard immunohistochemical procedures were performed on intestinal sections to detect Muc2, Muc2 precursor, and Agr2 with the MACH4 HRP polymer detection system (Biocare Medical). Standard immunofluorescence staining for MUC2, MUC2 precursor (detected with anti–rabbit Alexa Fluor 633 and anti–mouse Alexa Fluor 488; Invitrogen) and DAPI (Invitrogen) was analyzed by multi-tracking on a confocal microscope (LSM510; Carl Zeiss). Agarose gel electrophoresis was conducted to separate MUC2 and MUC2 precursor, and Western blotting performed with detection by dual label infrared fluorescence on an Odyssey instrument (Li-Cor).
siRNA silencing of EDEM1.
On-target plus siRNA specific for EDEM1, as well as scrambled control siRNA were chemically synthesized (Thermo Fisher Scientific) as a mixture of four siRNAs targeting different regions. LS174T cells with a confluence of 70–80% were transfected with 200 nM of EDEM1 or scrambled control siRNA using RNAi-MAX (Life Technologies) according to the manufacturer’s instructions. The level of knockdown of the EDEM1 was determined by qRT-PCR.
Expression of recombinant truncated Muc2 N-terminal proteins.
MKN45 cells were transfected with WT and Winnie rMUC2-D3 plasmid encoding the human MUC2 D3-domain with Lipofectamine 2000 (Invitrogen) in serum-free conditions as previously described (Heazlewood et al., 2008). After 6 h, FCS was added to 10% and experiments conducted 48 h after transfection. For assessment of secretions, culture media was replaced with serum-free media for 24 h before collecting and concentrating the supernatant through a 10-kD centrifugal membrane filter (Millipore).
NF-κBp65 phosphorylation assay.
All reagents were supplied in the SureFire NF-κBp65 (p-Ser536) assay kit (PerkinElmer). The assay was performed in 384-well white Proxiplates according to the manufacturer’s instructions. In brief, cells were harvested and lysed in the AlphaScreen SureFire lysis buffer and protease inhibitors and PhosSTOP (Roche). The detection mix was added to 4 µl lysate containing equal amounts of total proteins. The detection mix consisted of reaction buffer, dilution buffer, activation buffer, acceptor beads, and donor beads (prepared in a 40:20:10:1:1 ratio) prepared under low light conditions. The plate was incubated at least 4 h at room temperature in the dark. Plates were read on the EnVision plate reader (Perkin Elmer).
Statistical analysis.
All statistical analyses were performed using Prism v5.01 (GraphPad Software). For normally distributed data a Student’s t test or an ANOVA with an appropriate post-hoc test was used to compare groups. For analyses where a normal distribution could not be demonstrated, including where the number of replicates was low, the nonparametric Kruskal-Wallis test with Dunn’s multiple comparison tests were used and data were presented as box plots. The sample size and statistical analysis used are detailed in each figure legend.
Acknowledgments
Thu Tran and Rachel Adams are acknowledged for their technical assistance. Thanks are extended to Prof David Ron and Prof John Prins for useful discussions and comments on the manuscript.
This research was supported by National Health and Medical Research Council (NHMRC) Project Grant 488809. I. Das is supported by Griffith University Postgraduate Research Scholarship and Griffith University International Postgraduate Research Scholarship scholarships. T. Florin and M. McGuckin are supported by NHMRC Practitioner and Senior Research Fellowships, respectively.
The authors have no competing financial interests to declare.
Footnotes
Abbreviations used:
- AB
- Alcian blue
- DEX
- dexamethasone
- EDEM
- ER degradation–enhancing α-mannosidase–like lectin
- ERAD
- ER-associated degradation
- GR
- glucocorticoid receptor
- IBD
- inflammatory bowel disease
- MLN
- mesenteric lymph node
- PAS
- periodic acid Schiff
- PDI
- protein disulfide isomerase
- Tg
- thapsigargin
- Tm
- tunicamycin
- UC
- ulcerative colitis
- UPR
- unfolded protein response
References
- Altmann G.G. 1983. Morphological observations on mucus-secreting nongoblet cells in the deep crypts of the rat ascending colon. Am. J. Anat. 167:95–117 10.1002/aja.1001670109 [DOI] [PubMed] [Google Scholar]
- Arsenault P., Ménard D. 1985. Influence of hydrocortisone on human fetal small intestine in organ culture. J. Pediatr. Gastroenterol. Nutr. 4:893–901 10.1097/00005176-198512000-00008 [DOI] [PubMed] [Google Scholar]
- Atanasov A.G., Leiser D., Roesselet C., Noti M., Corazza N., Schoonjans K., Brunner T. 2008. Cell cycle-dependent regulation of extra-adrenal glucocorticoid synthesis in murine intestinal epithelial cells. FASEB J. 22:4117–4125 10.1096/fj.08-114157 [DOI] [PubMed] [Google Scholar]
- Austin R.C. 2009. The unfolded protein response in health and disease. Antioxid. Redox Signal. 11:2279–2287 10.1089/ars.2009.2686 [DOI] [PubMed] [Google Scholar]
- Barnes P.J. 1998. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin. Sci. 94:557–572 [DOI] [PubMed] [Google Scholar]
- Bertolotti A., Wang X.Z., Novoa I., Jungreis R., Schlessinger K., Cho J.H., West A.B., Ron D. 2001. Increased sensitivity to dextran sodium sulfate colitis in IRE1β-deficient mice. J. Clin. Invest. 107:585–593 10.1172/JCI11476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl K., Rutschmann S., Li X., Du X., Xiao N., Schnabl B., Brenner D.A., Beutler B. 2009. Enhanced sensitivity to DSS colitis caused by a hypomorphic Mbtps1 mutation disrupting the ATF6-driven unfolded protein response. Proc. Natl. Acad. Sci. USA. 106:3300–3305 10.1073/pnas.0813036106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Oppenheim J.J., Winkler-Pickett R.T., Ortaldo J.R., Howard O.M. 2006. Glucocorticoid amplifies IL-2-dependent expansion of functional FoxP3(+)CD4(+)CD25(+) T regulatory cells in vivo and enhances their capacity to suppress EAE. Eur. J. Immunol. 36:139–149 10.1002/eji.200535219 [DOI] [PubMed] [Google Scholar]
- Cima I., Corazza N., Dick B., Fuhrer A., Herren S., Jakob S., Ayuni E., Mueller C., Brunner T. 2004. Intestinal epithelial cells synthesize glucocorticoids and regulate T cell activation. J. Exp. Med. 200:1635–1646 10.1084/jem.20031958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste A., Dubuquoy L., Barnouin R., Annicotte J.S., Magnier B., Notti M., Corazza N., Antal M.C., Metzger D., Desreumaux P., et al. 2007. LRH-1-mediated glucocorticoid synthesis in enterocytes protects against inflammatory bowel disease. Proc. Natl. Acad. Sci. USA. 104:13098–13103 10.1073/pnas.0702440104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross B.C., Bond P.J., Sadowski P.G., Jha B.K., Zak J., Goodman J.M., Silverman R.H., Neubert T.A., Baxendale I.R., Ron D., Harding H.P. 2012. The molecular basis for selective inhibition of unconventional mRNA splicing by an IRE1-binding small molecule. Proc. Natl. Acad. Sci. USA. 109:E869–E878 10.1073/pnas.1115623109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De P., Roy S.G., Kar D., Bandyopadhyay A. 2011. Excess of glucocorticoid induces myocardial remodeling and alteration of calcium signaling in cardiomyocytes. J. Endocrinol. 209:105–114 10.1530/JOE-10-0431 [DOI] [PubMed] [Google Scholar]
- Deng L., Adachi T., Kitayama K., Bungyoku Y., Kitazawa S., Ishido S., Shoji I., Hotta H. 2008. Hepatitis C virus infection induces apoptosis through a Bax-triggered, mitochondrion-mediated, caspase 3-dependent pathway. J. Virol. 82:10375–10385 10.1128/JVI.00395-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak A.M., Osage J.E., Monahan R.A., Dickersin G.R. 1980. Crohn’s disease: transmission electron microscopic studies. III. Target tissues. Proliferation of and injury to smooth muscle and the autonomic nervous system. Hum. Pathol. 11:620–634 10.1016/S0046-8177(80)80073-6 [DOI] [PubMed] [Google Scholar]
- Eri R.D., Adams R.J., Tran T.V., Tong H., Das I., Roche D.K., Oancea I., Png C.W., Jeffery P.L., Radford-Smith G.L., et al. 2011. An intestinal epithelial defect conferring ER stress results in inflammation involving both innate and adaptive immunity. Mucosal Immunol. 4:354–364 10.1038/mi.2010.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii Y., Khoshnoodi J., Takenaka H., Hosoyamada M., Nakajo A., Bessho F., Kudo A., Takahashi S., Arimura Y., Yamada A., et al. 2006. The effect of dexamethasone on defective nephrin transport caused by ER stress: a potential mechanism for the therapeutic action of glucocorticoids in the acquired glomerular diseases. Kidney Int. 69:1350–1359 [DOI] [PubMed] [Google Scholar]
- Gartner H., Shukla P., Markesich D.C., Solomon N.S., Oesterreicher T.J., Henning S.J. 2002. Developmental expression of trehalase: role of transcriptional activation. Biochim. Biophys. Acta. 1574:329–336 10.1016/S0167-4781(02)00231-2 [DOI] [PubMed] [Google Scholar]
- Gregorieff A., Stange D.E., Kujala P., Begthel H., van den Born M., Korving J., Peters P.J., Clevers H. 2009. The ets-domain transcription factor Spdef promotes maturation of goblet and paneth cells in the intestinal epithelium. Gastroenterology. 137:1333–1345 [DOI] [PubMed] [Google Scholar]
- Hao X., Yao A., Gong J., Zhu W., Li N., Li J. 2012. Berberine ameliorates pro-inflammatory cytokine-induced endoplasmic reticulum stress in human intestinal epithelial cells in vitro. Inflammation. 35:841–849 10.1007/s10753-011-9385-6 [DOI] [PubMed] [Google Scholar]
- Hasnain S.Z., Lourie R., Das I., Chen A.C., McGuckin M.A. 2012. The interplay between endoplasmic reticulum stress and inflammation. Immunol. Cell Biol. 90:260–270 10.1038/icb.2011.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasnain S.Z., Tauro S., Das I., Tong H., Chen A.C., Jeffery P.L., McDonald V., Florin T.H., McGuckin M.A. 2013. IL-10 promotes production of intestinal mucus by suppressing protein misfolding and endoplasmic reticulum stress in goblet cells. Gastroenterology. 144:357–368 10.1053/j.gastro.2012.10.043 [DOI] [PubMed] [Google Scholar]
- Heazlewood C.K., Cook M.C., Eri R., Price G.R., Tauro S.B., Taupin D., Thornton D.J., Png C.W., Crockford T.L., Cornall R.J., et al. 2008. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 5:e54 10.1371/journal.pmed.0050054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert D.N., Foellmer B., Helenius A. 1995. Glucose trimming and reglucosylation determine glycoprotein association with calnexin in the endoplasmic reticulum. Cell. 81:425–433 10.1016/0092-8674(95)90395-X [DOI] [PubMed] [Google Scholar]
- Hosoi T., Ozawa K. 2010. Endoplasmic reticulum stress in disease: mechanisms and therapeutic opportunities. Clin. Sci. 118:19–29 10.1042/CS20080680 [DOI] [PubMed] [Google Scholar]
- Jung P., Sato T., Merlos-Suárez A., Barriga F.M., Iglesias M., Rossell D., Auer H., Gallardo M., Blasco M.A., Sancho E., et al. 2011. Isolation and in vitro expansion of human colonic stem cells. Nat. Med. 17:1225–1227 10.1038/nm.2470 [DOI] [PubMed] [Google Scholar]
- Kamimoto T., Shoji S., Hidvegi T., Mizushima N., Umebayashi K., Perlmutter D.H., Yoshimori T. 2006. Intracellular inclusions containing mutant alpha1-antitrypsin Z are propagated in the absence of autophagic activity. J. Biol. Chem. 281:4467–4476 10.1074/jbc.M509409200 [DOI] [PubMed] [Google Scholar]
- Kaser A., Lee A.-H., Franke A., Glickman J.N., Zeissig S., Tilg H., Nieuwenhuis E.E., Higgins D.E., Schreiber S., Glimcher L.H., Blumberg R.S. 2008. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 134:743–756 10.1016/j.cell.2008.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman R.J. 2002. Orchestrating the unfolded protein response in health and disease. J. Clin. Invest. 110:1389–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasa M., Abraham S.M., Boucheron C., Saklatvala J., Clark A.R. 2002. Dexamethasone causes sustained expression of mitogen-activated protein kinase (MAPK) phosphatase 1 and phosphatase-mediated inhibition of MAPK p38. Mol. Cell. Biol. 22:7802–7811 10.1128/MCB.22.22.7802-7811.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H.F., Kong N. 1990. Perturbation of cellular calcium blocks exit of secretory proteins from the rough endoplasmic reticulum. J. Biol. Chem. 265:10893–10899 [PubMed] [Google Scholar]
- Maeda K., Fujihara M., Harasawa R. 2009. Bovine viral diarrhea virus 2 infection activates the unfolded protein response in MDBK cells, leading to apoptosis. J. Vet. Med. Sci. 71:801–805 10.1292/jvms.71.801 [DOI] [PubMed] [Google Scholar]
- Malhotra J.D., Kaufman R.J. 2007. The endoplasmic reticulum and the unfolded protein response. Semin. Cell Dev. Biol. 18:716–731 10.1016/j.semcdb.2007.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manguso F., Balzano A. 2007. Meta-analysis: the efficacy of rectal beclomethasone dipropionate vs. 5-aminosalicylic acid in mild to moderate distal ulcerative colitis. Aliment. Pharmacol. Ther. 26:21–29 10.1111/j.1365-2036.2007.03349.x [DOI] [PubMed] [Google Scholar]
- Marciniak S.J., Ron D. 2006. Endoplasmic reticulum stress signaling in disease. Physiol. Rev. 86:1133–1149 10.1152/physrev.00015.2006 [DOI] [PubMed] [Google Scholar]
- Martens C., Bilodeau S., Maira M., Gauthier Y., Drouin J. 2005. Protein-protein interactions and transcriptional antagonism between the subfamily of NGFI-B/Nur77 orphan nuclear receptors and glucocorticoid receptor. Mol. Endocrinol. 19:885–897 10.1210/me.2004-0333 [DOI] [PubMed] [Google Scholar]
- McGuckin M.A., Eri R., Simms L.A., Florin T.H., Radford-Smith G. 2009. Intestinal barrier dysfunction in inflammatory bowel diseases. Inflamm. Bowel Dis. 15:100–113 10.1002/ibd.20539 [DOI] [PubMed] [Google Scholar]
- McGuckin M.A., Eri R.D., Das I., Lourie R., Florin T.H. 2010. ER stress and the unfolded protein response in intestinal inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 298:G820–G832 10.1152/ajpgi.00063.2010 [DOI] [PubMed] [Google Scholar]
- Medigeshi G.R., Lancaster A.M., Hirsch A.J., Briese T., Lipkin W.I., Defilippis V., Früh K., Mason P.W., Nikolich-Zugich J., Nelson J.A. 2007. West Nile virus infection activates the unfolded protein response, leading to CHOP induction and apoptosis. J. Virol. 81:10849–10860 10.1128/JVI.01151-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modica S., Gofflot F., Murzilli S., D’Orazio A., Salvatore L., Pellegrini F., Nicolucci A., Tognoni G., Copetti M., Valanzano R., et al. 2010. The intestinal nuclear receptor signature with epithelial localization patterns and expression modulation in tumors. Gastroenterology. 138:636–648: 648: e1–e12 10.1053/j.gastro.2009.09.060 [DOI] [PubMed] [Google Scholar]
- Mueller M., Cima I., Noti M., Fuhrer A., Jakob S., Dubuquoy L., Schoonjans K., Brunner T. 2006. The nuclear receptor LRH-1 critically regulates extra-adrenal glucocorticoid synthesis in the intestine. J. Exp. Med. 203:2057–2062 10.1084/jem.20060357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanua S., Yoshimura F.K. 2004. Mink epithelial cell killing by pathogenic murine leukemia viruses involves endoplasmic reticulum stress. J. Virol. 78:12071–12074 10.1128/JVI.78.21.12071-12074.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noah T.K., Kazanjian A., Whitsett J., Shroyer N.F. 2010. SAM pointed domain ETS factor (SPDEF) regulates terminal differentiation and maturation of intestinal goblet cells. Exp. Cell Res. 316:452–465 10.1016/j.yexcr.2009.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivari S., Molinari M. 2007. Glycoprotein folding and the role of EDEM1, EDEM2 and EDEM3 in degradation of folding-defective glycoproteins. FEBS Lett. 581:3658–3664 10.1016/j.febslet.2007.04.070 [DOI] [PubMed] [Google Scholar]
- Park K.S., Korfhagen T.R., Bruno M.D., Kitzmiller J.A., Wan H., Wert S.E., Khurana Hershey G.K., Chen G., Whitsett J.A. 2007. SPDEF regulates goblet cell hyperplasia in the airway epithelium. J. Clin. Invest. 117:978–988 10.1172/JCI29176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.-W., Zhen G., Verhaeghe C., Nakagami Y., Nguyenvu L.T., Barczak A.J., Killeen N., Erle D.J. 2009. The protein disulfide isomerase AGR2 is essential for production of intestinal mucus. Proc. Natl. Acad. Sci. USA. 106:6950–6955 10.1073/pnas.0808722106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder M., Kaufman R.J. 2005. ER stress and the unfolded protein response. Mutat. Res. 569:29–63 10.1016/j.mrfmmm.2004.06.056 [DOI] [PubMed] [Google Scholar]
- Shang J., Lehrman M.A. 2004. Discordance of UPR signaling by ATF6 and Ire1p-XBP1 with levels of target transcripts. Biochem. Biophys. Res. Commun. 317:390–396 10.1016/j.bbrc.2004.03.058 [DOI] [PubMed] [Google Scholar]
- Shang Y., Wang F., Bai C., Huang Y., Zhao L.J., Yao X.P., Li Q., Sun S.H. 2011. Dexamethasone protects airway epithelial cell line NCI-H292 against lipopolysaccharide induced endoplasmic reticulum stress and apoptosis. Chin. Med. J. (Engl.). 124:38–44 [PubMed] [Google Scholar]
- Shkoda A., Ruiz P.A., Daniel H., Kim S.C., Rogler G., Sartor R.B., Haller D. 2007. Interleukin-10 blocked endoplasmic reticulum stress in intestinal epithelial cells: impact on chronic inflammation. Gastroenterology. 132:190–207 10.1053/j.gastro.2006.10.030 [DOI] [PubMed] [Google Scholar]
- Sidler D., Renzulli P., Schnoz C., Berger B., Schneider-Jakob S., Flück C., Inderbitzin D., Corazza N., Candinas D., Brunner T. 2011. Colon cancer cells produce immunoregulatory glucocorticoids. Oncogene. 30:2411–2419 10.1038/onc.2010.629 [DOI] [PubMed] [Google Scholar]
- Solomon N.S., Gartner H., Oesterreicher T.J., Henning S.J. 2001. Development of glucocorticoid-responsiveness in mouse intestine. Pediatr. Res. 49:782–788 10.1203/00006450-200106000-00012 [DOI] [PubMed] [Google Scholar]
- Trabucchi E., Mukenge S., Baratti C., Colombo R., Fregoni F., Montorsi W. 1986. Differential diagnosis of Crohn’s disease of the colon from ulcerative colitis: ultrastructure study with the scanning electron microscope. Int. J. Tissue React. 8:79–84 [PubMed] [Google Scholar]
- Vembar S.S., Brodsky J.L. 2008. One step at a time: endoplasmic reticulum-associated degradation. Nat. Rev. Mol. Cell Biol. 9:944–957 10.1038/nrm2546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X., Yang Z., Rey F.E., Ridaura V.K., Davidson N.O., Gordon J.I., Semenkovich C.F. 2012. Fatty acid synthase modulates intestinal barrier function through palmitoylation of mucin 2. Cell Host Microbe. 11:140–152 10.1016/j.chom.2011.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Wu M., Song R., Ma J., Shi Y., Qin W., Jin Y. 2009. A glucocorticoid amplifies IL-2-induced selective expansion of CD4(+)CD25(+)FOXP3(+) regulatory T cells in vivo and suppresses graft-versus-host disease after allogeneic lymphocyte transplantation. Acta Biochim. Biophys. Sin. (Shanghai). 41:781–791 10.1093/abbs/gmp067 [DOI] [PubMed] [Google Scholar]
- Xu Z.X., Viviano C.J., Rooney S.A. 1995. Glucocorticoid stimulation of fatty-acid synthase gene transcription in fetal lung: antagonism by retinoic acid. Am. J. Physiol. 268:L683–L690 [DOI] [PubMed] [Google Scholar]
- Xue X., Piao J.H., Nakajima A., Sakon-Komazawa S., Kojima Y., Mori K., Yagita H., Okumura K., Harding H., Nakano H. 2005a. Tumor necrosis factor alpha (TNFalpha) induces the unfolded protein response (UPR) in a reactive oxygen species (ROS)-dependent fashion, and the UPR counteracts ROS accumulation by TNFalpha. J. Biol. Chem. 280:33917–33925 10.1074/jbc.M505818200 [DOI] [PubMed] [Google Scholar]
- Xue X., Piao J.H., Nakajima A., Sakon-Komazawa S., Kojima Y., Mori K., Yagita H., Okumura K., Harding H., Nakano H. 2005b. Tumor necrosis factor alpha (TNFalpha) induces the unfolded protein response (UPR) in a reactive oxygen species (ROS)-dependent fashion, and the UPR counteracts ROS accumulation by TNFalpha. J. Biol. Chem. 280:33917–33925 10.1074/jbc.M505818200 [DOI] [PubMed] [Google Scholar]