Loss of TRAF3 results in reduced TCR signaling and defective up-regulation of T-bet and CD122 in iNKT cells that impairs their proliferation and survival.
Abstract
TCR signaling is a prerequisite for early stage development of invariant natural killer T (iNKT) cells, whereas IL-15 signaling is required for expansion and maturation at later stages. In this study, we show that TNF receptor associated factor 3 (TRAF3) plays a critical role in the transition between these two distinct signaling pathways and developmental stages. TRAF3-deficient iNKT cells in CD4CreTRAF3flox/flox (T-TRAF3−/−) mice exhibit defective up-regulation of T-bet and CD122, two critical molecules for IL-15 signaling, and as a consequence, IL-15–mediated iNKT cell proliferation and survival are impaired. Consistently, development of iNKT cells in T-TRAF3−/− mice shows a major defect at developmental stages 2 and 3, but not stages 0 and 1. We further demonstrated that defective T-bet up-regulation occurring during the stage 1 to stage 2 transition results from reduced TCR signaling in TRAF3−/− iNKT cells. In addition, mature TRAF3−/− iNKT cells displayed defective cytokine responses upon TCR stimulation. Collectively, our results reveal that by modulating the relative strength of TCR signaling, TRAF3 is an important regulator of iNKT cell development and functions.
TNF receptor associated factor 3 (TRAF3), a member of the TRAF family of intracellular signaling proteins, has multiple effects on signal transduction by the TNF receptor superfamily as well as other receptor families (Häcker, et al., 2011; Hildebrand et al., 2011). The importance of TRAF3 is highlighted by the finding that general deletion of the traf3 gene results in mouse death within 2 wk of birth (Xu et al., 1996). Accumulating data indicate that the role of TRAF3 is highly receptor and cell type dependent. TRAF3 promotes production of type I interferon and IL-10 in myeloid cells after TLR stimulation (Häcker et al., 2006; Oganesyan et al., 2006), whereas it negatively regulates B cell survival and CD40 signaling, as well as TLR signals to B cells (Bishop and Xie, 2007; Xie et al., 2011b). TRAF3 also negatively regulates IL-17 signaling in myeloid cells by interfering with the formation of an IL-17R–Act1–TRAF6 complex (Zhu et al., 2010). Newly identified roles for TRAF3 in T cell biology were revealed recently by our group. Producing and analyzing CD4CreTraf3flox/flox (T-TRAF3−/−) mice, we found that deficiency of TRAF3 in mature CD4+ and CD8+ T cells causes defective T cell–dependent antibody production, T cell cytotoxic function, and proximal TCR-mediated kinase activation. Interestingly, TRAF3 was recruited to the TCR complex upon TCR plus CD28 stimulation, demonstrating the association of TRAF3 with the TCR complex (Xie et al., 2011a). Notably, the potential roles of TRAF3 in other aspects of T cell biology have not been characterized.
Invariant NK T (iNKT) cells are important regulators in a variety of immune diseases (Vincent et al., 2003; Taniguchi et al., 2003; Bendelac et al., 2007, Scanlon et al., 2011). They possess several unique features that distinguish them from other immune cells, including expression of an invariant TCR-α chain, both T cell and NK cell markers, and production of copious amounts of cytokines very rapidly upon stimulation. Their four developmental stages (stages 0–3) are also distinctive, with different receptor-mediated signals and sets of transcription factors required for progression through each stage (Borowski and Bendelac, 2005; Das et al., 2010; D’Cruz et al., 2010). TCR signaling in conjunction with the SLAM (signaling lymphocytic-activation molecule) is essential for early stage iNKT cell development, whereas IL-15 signaling is absolutely required for terminal expansion and maturation (Godfrey and Berzins, 2007; Godfrey et al., 2010; Engel and Kronenberg, 2012). However, how the transition between signals from early to later stages is finely regulated to promote iNKT cell development remains ill defined. In this study, we identify TRAF3 as an essential regulator of iNKT cell development and function by modulating events dependent on the strength of TCR signaling.
RESULTS AND DISCUSSION
TRAF3 is required for iNKT cell development
Studies of the T-TRAF3−/− mouse demonstrate that TRAF3 plays an essential role in T cell function and enhances TCR/CD28 signaling. Interestingly, although mature conventional T cells show striking functional defects in the absence of TRAF3, they develop normally and are present in normal numbers (Xie et al., 2011a). In contrast, we observed that T-TRAF3−/− mice exhibited an ∼10-fold decrease in iNKT cells in the thymus and liver, and a twofold decrease in spleen in both percentage and total number (Fig. 1, A and B). Thus, in the current study, we investigated the role of TRAF3 in the development and function of iNKT cells. Together with reduced iNKT cell numbers, there was remarkably less IFN-γ and IL-4 produced by TRAF3-deficient iNKT cells upon in vivo stimulation with α-galactosylceramide (α-GalCer; Fig. 1 C). These observations led us to hypothesize that TRAF3 plays a distinct role in the development of iNKT cells, compared with conventional αβ T cells (Xie et al., 2011a).
Figure 1.
TRAF3 is required for iNKT cell development. (A, B, and E) Thymus, spleen, and liver mononuclear cells were stained for TCR-β and PBS57-CD1d tetramer. iNKT cells defined as double-positive cells are shown. (A and B) Percentage (thymus [LMC vs. T-TRAF3−/−]: P < 0.001; spleen: P = 0.182; liver: P < 0.001; A) and total number (thymus: P = 0.011, spleen: P = 0.522, liver: P = 0.007; B). Horizontal bars represent mean values. (C) 5 µg α-GalCer was injected into LMC or T-TRAF3−/− mice. Serum IFN-γ and IL-4 were measured by ELISA. (D) DN1 to DN3 cells in thymi of LckCreTRAF3flox/flox mice were sorted, and TRAF3 and actin were detected by Western blot. Data are representative of three independent experiments. (E) Frequency of iNKT cells in LMC and LckCreTRAF3flox/flox mice. (F and G) Cells from BM chimeric mice were stained and percentage of iNKT cells, CD4+, or CD8+ T cells in WT (CD45.1+) or T-TRAF3−/− (CD45.2+) BM-derived cells is shown. (H) Lineage-negative BM cells from T-TRAF3−/− mice were transduced with retrovirus (GFP positive) and transferred into sublethally irradiated Rag1−/− mice. Percentages of iNKT cells in GFP-negative (T-TRAF3−/−) and GFP-positive (TRAF3 virus, or empty virus) cells are shown. Error bars in A, C, E, G, and H are mean values ± SD of four to six mice.
Lck-Cre mediates depletion of floxed genes from as early as the DN2 T cell developmental stage in the thymus (Lee et al., 2001), earlier than that mediated by CD4-Cre. To examine the possibility that the presence of a few iNKT cells in T-TRAF3−/− mice might result from residual TRAF3 protein when iNKT cells start developing, we crossed TRAF3flox/flox mice with Lck-Cre mice. TRAF3 was completely depleted from the DN3 stage in LckCreTraf3flox/flox mice, demonstrated by Western blot (Fig. 1 D). With this new mouse model, we found that iNKT cells were reduced to similar levels as in the CD4-Cre T-TRAF3−/− mouse (Fig. 1 E). This result implies that TRAF3’s role is important at very specific stages during iNKT cell development.
TRAF3 affects iNKT development in a cell-intrinsic manner
To explore whether defective iNKT cell development in T-TRAF3−/− mice is a cell-autonomous effect, we generated BM chimeric mice by transferring a 1:1 ratio of BM from C57BL/6 mice (WT, CD45.1+) and T-TRAF3−/− mice (CD45.2+), into lethally irradiated WT mice (CD45.1+CD45.2+). 8 wk later, analysis of recipients showed that the percentage of iNKT cells developing from T-TRAF3−/− BM was ∼10-fold less than from WT BM in all lymphoid organs examined, whereas the percentage of CD4+ and CD8+ T cells was not different (Fig. 1, F and G). Similar results were obtained with 1:20 or 20:1 ratios of mixed BM (unpublished data). In addition, the expression levels of CD1d, CD150, and Ly108 on double-positive thymocytes were not different between T-TRAF3−/− and littermate control (LMC) mice (unpublished data). To determine whether this is a TRAF3-specific cell-autonomous effect, T-TRAF3−/− BM was transduced with a virus expressing the traf3 gene and transplanted into sublethally irradiated Rag1 deficient (Rag1−/−) mice. Analysis of these mice 8 wk after reconstitution showed that forced expression of TRAF3 significantly increased the percentage of iNKT cells, but empty viral vector had no effect (Fig. 1 H). These results demonstrate that TRAF3 affects iNKT cell development in a cell-intrinsic manner.
TRAF3 affects iNKT cell development after positive selection
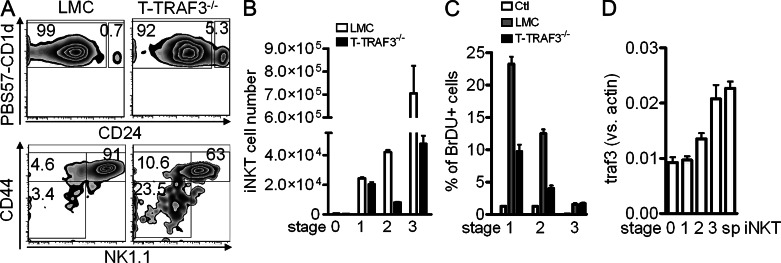
It is well established that iNKT cell development in the thymus undergoes four distinct stages (Godfrey, et al., 2010). The presence of a few thymic iNKT cells in T-TRAF3−/− mice allowed us to estimate at which stages TRAF3 exerts its effect. With enriched thymic iNKT cells, we observed 10-fold fewer absolute numbers of iNKT cells in T-TRAF3−/− than in LMC mice. However, the percentage of stage 0 and stage 1 iNKT cells was more than sevenfold higher, and for stage 2 was twofold higher; in contrast, the percentage of INKT cells in stage 3 was lower in T-TRAF3−/− mice than in LMC mice (Fig. 2 A). Therefore, the absolute number of iNKT cells in stage 2 and, in particular, stage 3 was drastically reduced, whereas the numbers in stage 0 and 1 were relatively normal or just slightly reduced in T-TRAF3−/− mice (Fig. 2 B). This result indicates that TRAF3 is not essential for precursor selection (stage 0) and transition from stage 0 to 1 but is critical for the burst proliferation from stage 1 to stages 2 and 3. Consistent with this observation, there was reduced proliferation of iNKT cells at stages 1 and 2 in T-TRAF3−/− mice (Fig. 2 C). These results, in conjunction with findings in LckCreTRAF3flox/flox mice that TRAF3 deficiency did not completely block iNKT cell development, indicate that the involvement of TRAF3 in iNKT cell development is stage specific. We next explored whether the expression level of traf3 differs in distinct stages. Results indicate that traf3 gene expression increased during iNKT cell development, being highest in stage 3 and lowest in stages 0 and 1 (Fig. 2 D). The expression level of traf3 suggests that TRAF3 might play a more important role in mature iNKT cells.
Figure 2.
TRAF3 deficiency affects iNKT cell development in a stage-specific manner. Enriched thymic iNKT cells were stained for CD24, CD44, and NK1.1. The CD24Low population was gated to the lower panel (A) for further analysis of different developmental stages of iNKT cells. (A and B) Percentage (A) and absolute number (B) of iNKT cells in stages 0–3 (stage 0: CD24hi; P = 0.2067 [LMC vs. T-TRAF3−/−]; stage 1: CD24lowCD44−NK1.1low, P = 0.3886; stage 2: CD24low CD44+NK1.1low, P < 0.0001; stage 3: CD24lowCD44+NK1.1hi, P < 0.0001). Error bars are mean values ± SD of six mice. (C) Thymic iNKT cells were enriched and surface-stained after BrDU in vivo incorporation. BrDU staining was performed and analyzed in different stages of iNKT cells. (D) traf3 mRNA expression in different stages of iNKT cells and splenic iNKT cells was measured by real-time PCR (stage 1 vs. stage 3: P = 0.014). Error bars in C and D are mean values ± SD of three separate experiments.
Defective response of TRAF3-deficient iNKT cells to IL-15 stimulation
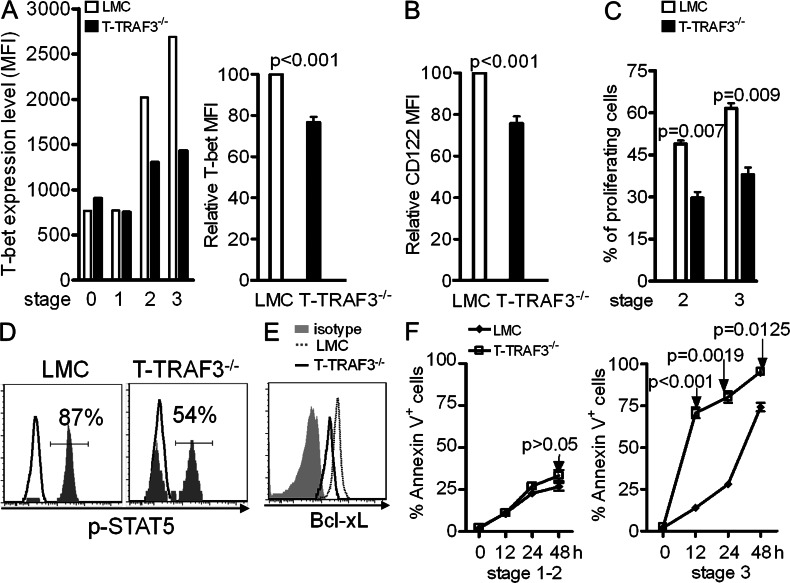
IL-15 is important in terminal stage expansion, maturation, and homeostasis of iNKT cells (Matsuda et al., 2002; Castillo et al., 2010; Gordy et al., 2011). Our observations with T-TRAF3−/− mice share similarities with the phenotypes reported for IL-15–deficient mice, which encouraged us to explore whether TRAF3 deficiency affects IL-15–mediated iNKT cell expansion and homeostasis. Because the expression of CD122 on iNKT cells can be regulated by T-bet (Townsend et al., 2004; Matsuda et al., 2007), we first examined the expression level of T-bet and CD122. Flow cytometry data showed that expression of T-bet is remarkably reduced (Fig. 3 A), as is CD122 (Fig. 3 B) in TRAF3−/− thymic iNKT cells. Notably, T-bet or CD122 exhibited only basal level expression at stages 0 and 1 but were drastically up-regulated in stages 2 and 3 in LMC mice, consistent with the distinct roles of TCR and IL-15 signaling in different stages of iNKT development and maturation. To investigate whether the reduction of T-bet and CD122 affected IL-15–mediated proliferation of iNTK cells, thymocytes depleted of CD8+ T cells were CFSE labeled and incubated with IL-15 for 4 d. Assessment of CFSE dilution by flow cytometry showed much lower cell proliferation in TRAF3−/− than in LMC stages 2 and 3 iNKT cells (Fig. 3 C). IL-15–induced phosphorylation of STAT5 was also impaired in T-TRAF3−/− iNKT cells (Fig. 3 D). These results indicate that TRAF3 deficiency impairs IL-15–mediated proliferation of iNKT cells by reducing T-bet and CD122 expression.
Figure 3.
Defective response of TRAF3-deficient iNKT cells to IL-15 stimulation. Enriched thymic iNKT cells were surface stained as in Fig 2 and further stained for T-bet (A) or Bcl-xL (E). (A, left) Mean fluorescence intensity (MFI) of T-bet in a single representative mouse from each group. (A, right) Bar graph representing a summary of relative T bet expression in TRAF3−/− versus LMC stage 2 iNKT cells (the MFI for the LMC was set at 100%). (B) CD122 expression on stage 2 iNKT cells. Error bars in A and B represent mean values ± SD of four mice. (C) Proliferation of stage 2 and 3 thymic iNKT cells was evaluated by the percentage of CFSE dilution. Error bars are mean values ± SD of three separate experiments. (D) IL-15–induced phosphorylation of STAT5 in thymic iNKT cells (data represent three independent experiments, LMC = 85 ± 4.7%, T-TRAF3−/− = 51 ± 5.1%, P < 0.01). (E) Bcl-xL expression in stage 3 iNKT cells. Data are from one of three mice with similar results (LMC vs. T-TRAF3−/− MFI = 3,636 ± 606 vs. 1,809 ± 23, P = 0.027). (F) Tetramer-enriched thymic iNKT cells were incubated for indicated time. Surface staining to distinguish different developmental stages was performed and followed by staining for Annexin V and PI. Error bars are mean values ± SD of three separate experiments.
Bcl-xL is a major target of IL-15 signaling and essential for maintaining iNKT cell survival (Gordy et al., 2011). Examination of Bcl-xL expression in T-TRAF3−/− mice shows that stage 3 thymic iNKT cells expressed much less than LMC (Fig. 3 E). To explore whether this results in impaired survival, we estimated survival status of iNKT cells in vitro. Upon tetramer stimulation, stage 3 T-TRAF3−/− iNKT cells underwent much faster cell death at all time points examined, but this was confined to stage 3 of maturation (Fig. 3 F). Similarly, more cell death was found in TRAF3−/− iNKT cells without stimulation (unpublished data). These results suggest that reduced expression of Bcl-xL could contribute to decreased iNKT cell number in T-TRAF3−/− mice. Collectively, TRAF3 deficiency led to a failure to up-regulate T-bet and CD122 and further impaired both IL-15 mediated terminal expansion and survival of iNKT cells.
TRAF3 is required for T-bet up-regulation by impacting TCR signaling during iNKT cell development
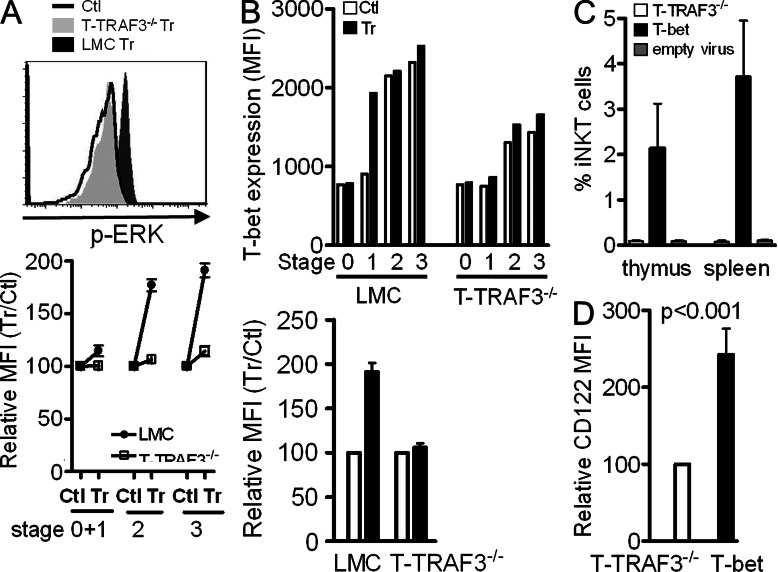
Up-regulation of T-bet can be initiated and maintained by TCR signaling (Bajénoff, et al., 2002; Matsuoka, et al., 2004) in synergy with IFN-γ/STAT-1 signaling (Lighvani, et al., 2001; Afkarian, et al., 2002). Our previous report that TRAF3 is required for normal levels of TCR signaling in conventional T cells (Xie et al., 2011a) prompted us to hypothesize that defective up-regulation of T-bet during the transition from stage 1 to stage 2 results from reduced strength of TCR signaling. With optimized stimulation by PBS57-CD1d tetramer and staining conditions, we found that phosphorylation of the MAP kinases ERK and p38 was detectable in LMC iNKT cells within 2–5 min of tetramer stimulation. However, ERK phosphorylation at all stages of T-TRAF3−/− iNKT cell development was defective compared with that seen in LMC iNKT cells (Fig. 4 A). Similar results were obtained for p-p38 (unpublished data). To verify that weakened TCR signaling was responsible for reduced T-bet expression, mice were treated with anti-CD3 Ab. 18 h later, thymic iNKT cells were enriched and stained for T-bet. Results show that T-bet expression was markedly increased in stage 1 of LMC iNKT cells, which was equal to the level in stages 2 and 3 in untreated control iNKT cells. However, there was only minimal up-regulation of T-bet in T-TRAF3−/− iNKT cells after anti-CD3 Ab treatment (Fig. 4 B). Notably, T-bet expression in stages 2 and 3 was not further up-regulated upon stimulation in LMC iNKT cells, indicating that it reaches its peak levels at these stages. These results support our hypothesis that TRAF3 is required for optimal TCR signaling to up-regulate T-bet expression during the transition from stages 1 to 2. To assess whether defective T-bet expression is responsible for impaired iNKT cell development, T-bet overexpression by retroviral transduction in BM chimeric mice showed that the percentage of iNKT cells was dramatically increased in T-bet–overexpressing cells, compared with virus-negative cells or those transduced with viral vector only. Consistently, CD122 expression was also increased in T-bet–overexpressing cells (Fig. 4, C and D). This result indicates that T-bet overexpression can rescue defective iNKT cell development induced by TRAF3 deficiency.
Figure 4.
TRAF3 is required for optimal TCR signaling and T-bet up-regulation. (A) Tetramer-enriched thymic iNKT cells were incubated at 37°C for 2 min and fixed immediately. Surface staining (as in Fig 2) and intracellular staining for p-ERK after permeabilization were performed. Top: histogram shows p-ERK staining of stage 3 iNKT cells. Bottom: percentage of treated (Tr) versus control (Ctl) cell MFI (set for 100%) is shown. (B) Thymic iNKT cells from anti-CD3 Ab-treated mice were stained for T-bet. Top: MFI of T-bet in cells of a single mouse from each group. Bottom: percentage of Tr versus Ctl MFI (set for 100%) of stage 1 iNKT cells (Tr vs. Ctl for LMC, P = 0.001; for T-TRAF3−/−, P = 0.46). Error bars in A and B are mean values ± SD of three independent experiments. (C) Percentage of iNKT cells derived from BM overexpressing T-bet (T-bet) versus without T-bet overexpression (T-TRAF3−/−) or expressing viral vector only (P < 0.001 for both thymic and splenic iNKT cells). (D) CD122 expression in thymic iNKT cells with or without T-bet overexpression. Error bars in C and D are mean values ± SD of four mice.
TRAF3-deficient iNKT cells are functionally impaired
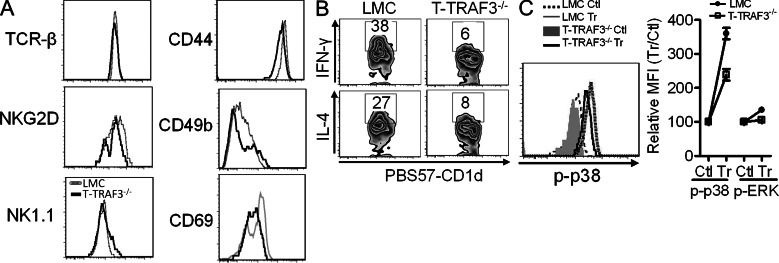
During iNKT cell development, stage 2 immature cells migrate into the periphery, further mature into functional iNKT cells, and acquire NK cell surface markers. Analysis of cell surface markers of splenic iNKT cells showed that they are either slightly to moderately decreased or unchanged (Fig. 5 A), indicating that a few iNKT cells can still mature in the absence of TRAF3. To investigate whether the function of these mature iNKT cells is impacted by TRAF3 deficiency, cytokine production by peripheral iNKT cells stimulated with α-GalCer was examined. Intracellular staining revealed that the percentage of TRAF3−/− iNKT cells producing IFN-γ and IL-4 was markedly reduced compared with LMC iNKT cells (Fig. 5 B). Results from BM chimera mice showed that defective IFN-γ and IL-4 production by TRAF3−/− iNKT cells was a cell-intrinsic defect (unpublished data). This result is comparable with the phenotype of TRAF3−/− conventional CD4+ and CD8+ T cells, which show defective cytokine production in response to TCR stimulation (Xie, et al., 2011a). Examination of TCR signaling showed that TRAF3−/− splenic iNKT cells exhibited much less p-ERK and p-p38 after tetramer stimulation than LMC iNKT cells (Fig. 5 C). These results indicate that TRAF3 is also required for TCR signaling during mature iNKT cell function.
Figure 5.
TRAF3-deficient iNKT cells display defective response to stimulation. (A) Splenocytes from LMC or T-TRAF3−/− mice were stained for indicated surface markers. iNKT cells were gated and shown in histograms. Data shown are representative of one of six mice (for MFI of TCR-β, NKG2D, and NK1.1, P > 0.05; for MFI of CD44, CD49b, and CD69, P < 0.05). (B) Intracellular IFN-γ and IL-4 in splenic iNKT cells were evaluated by flow cytometry after α-GalCer stimulation. Data shown are representative of one of four mice (for IFN-γ, P = 0.002 [LMC vs. T-TRAF3−/−]; for IL-4, P = 0.027). (C) Splenocytes were stained with PE-labeled PBS57-CD1d tetramer at 4°C for 45 min and then incubated at 37°C for 2 min. Surface staining for TCR-β and intracellular staining for p-ERK and p-p38 were performed after fixation and permeabilization. Right: p-p38 staining. Left: relative MFI comparing Tr versus Ctl, Ctl set at 100%. Error bars are mean values ± SD of three independent experiments.
The stage-specific effect of TRAF3 on iNKT cell development suggests that TRAF3 is a key connector between TCR and IL-15 signaling. TRAF3 deficiency didn’t completely block the development of stages 2 and 3, indicating the involvement of additional regulators in the transition stages. Defective iNKT cell development is consistent with our findings that the percentage of memory (CD44HiCD122Hi) CD8+ T cells is decreased in T-TRAF3−/− mice (unpublished data). These defects in both iNKT cells and CD8+ T cells indicate that they might share similar mechanisms. One possibility is that when they become mature T cells with down-regulation of CD24, TRAF3 plays more important roles in optimizing TCR signaling. Our findings also raise future questions: why is TRAF3 required for iNKT cell but not conventional T cell development? Does TRAF3 play distinct roles in different T cell subsets? Precisely how does TRAF3 participate in proximal TCR signaling? All are the focus of our ongoing studies. The findings of this study reveal TRAF3 to have rheostat function in modulating TCR signal strength. Our results highlight the diverse roles played by TRAF3 in different immune cells and the importance of TRAF3 in multiple aspects of T cell biology.
MATERIALS AND METHODS
Animals.
TRAF3flox/flox mice were previously described (Xie et al., 2007) and backcrossed with C57BL/6 mice for 10 generations. TRAF3flox/flox mice were bred with CD4Cre mice (Taconic) and LckCre mice (The Jackson Laboratory). CD45.2+ C57BL/6 and congenic CD45.1+ C57BL/6 mice (The Jackson Laboratory) were bred to generate CD45.2 and CD45.1 double-positive mice. Rag1−/− mice were provided by F. Sutterwala (University of Iowa, Iowa City, IA). Mice of 6–12 wk of age were used for all experiments. All mice were maintained in facilities under specific pathogen-free conditions at The University of Iowa and were used in accordance with National Institutes of Health guidelines under an animal protocol approved by the Animal Care and Use Committee of the University of Iowa.
Cytokine detection.
For serum cytokine detection, 5 µg α-GalCer (Avanti Polar Lipids, Alabaster, AL) in 100 µl PBS was injected intraperitoneally into mice. Serum was collected at 2 and 5 h after injection. IFN-γ and IL-4 were measured by ELISA according to the manufacturer’s instructions (ELISA Ready-SET-Go; eBioscience). For secondary activation of B cells, splenocytes were collected 5 h after injection and stained with antibodies against CD69 and B220. To detect cytokine production by single cells, splenocytes were isolated at 1.5 h after α-GalCer injection and incubated with Brefeldin A in vitro for another 2 h. Intracellular staining for IL-4 and IFN-γ was performed using Cytofix/Cytoperm reagents (BD).
Retrovirus transduction and bone marrow chimeras.
Recipient CD45.1+CD45.2+ congenic C57BL/6 mice were given 1,100 rad γ-irradiation 16 h before transfer. BM cells harvested from the tibiae and femurs of T-TRAF3−/− (CD45.2+) and WT (CD45.1+) mice were depleted of B220+ and CD3+ cells by magnetic bead separation (Milteyni Biotec). 5 × 106 cells from each mouse strain were mixed and injected intravenously into recipient mice.
For virus packaging, the mouse traf3 or Tbx21 gene was cloned and inserted into the retrovirus backbone pMIG. pMIG,pMIG-traf3 or pMIG-Tbx21 and helper vector pCLECO were cotransfected into 293T epithelial cells using lipofectamine (Invitrogen). Supernatant was harvested after 48 h. Lineage-negative BM cells were purified using a kit (Milteyni Biotec), stimulated overnight with a cytokine combination (IL-6, IL-3, and SCF [PeproTech]), and transduced with viral supernatant. Rag1−/− mice were sublethally irradiated with 500 rad γ-irradiation and rested overnight. 0.5 × 106 transduced BM cells were transferred by i.v. injection. The resulting chimeras were analyzed 8 wk later.
Flow cytometry.
Single-cell suspensions were prepared from thymi and spleens, and erythrocytes were lysed. Liver mononuclear cells were isolated (Xie et al., 2011a). For flow cytometry staining, nonspecific Ab binding was blocked with anti–mouse CD16/CD32 mAb and cells stained with fluorescently labeled antibodies against TCR-β (H57-597), CD4 (L3T4), CD8α (53–6.7), B220 (RA3-6B2), CD24 (M1/69), CD44 (IM7), NK1.1 (PK136), CD69 (H1.2F3), CD25 (eBio7D4), NKG2D (A10), CD49b (DX5), CD1d (1B1), CD45.2 (104), CD45.1 (A20), CD122 (TM-b1), IL-4 (BVD6-24G2), IFN-γ (XMG1.2), T-bet (eBio4B10), SLAM6 (13G3-19D), and CD150 (9D1). For Bcl-xL staining, cells were stained with anti–Bcl-xL Ab (54H6) followed by anti–mouse–APC secondary Ab. All antibodies were purchased from eBioscience, BD, or Cell Signaling Technology. For intracellular staining of T-bet and Bcl-xL, cells were first stained for surface markers, fixed and permeabilized with the Foxp3 Staining Buffer Set (eBioscience), and stained with relevant Abs. For thymic iNKT cell purification, thymocytes were stained with PE-labeled PBS57-CD1d tetramer, followed by anti-PE beads and purified with a magnetic column (Milteyni Biotec). Alternatively, thymic iNKT cells were enriched by depleting CD8+ cells, using PE-labeled anti-CD8 Ab and anti-PE beads. BrDU in vivo incorporation was measured according to the manufacturer’s instructions (BrDU Flow kit; BD). Flow cytometric analysis and cell sorting were performed using a FACS LSRII or Aria (BD) at The University of Iowa Flow Cytometry Facility. Results were analyzed with FlowJo software (Tree Star).
In vitro survival assay.
Enriched thymic iNKT cells were stained with or without PBS57-CD1d tetramer and incubated in complete RPMI medium. Samples were taken at various time points and stained for cell surface markers and then stained with FITC-labeled Annexin V (eBioscience) in Annexin V binding buffer for 15 min. Finally, propidium iodide (PI) was added and cells were analyzed by flow cytometry without fixation.
In vitro proliferation assay.
Thymocytes depleted of CD8+ cells were labeled for 10 min with 10 µM CFSE in accordance with manufacturer’s instructions (Invitrogen). IL-15 was added at indicated concentrations and cells were incubated in complete medium for 4 d. CFSE dilution was analyzed by flow cytometry.
TCR signaling induced by PBS57-CD1d tetramer.
Thymic iNKT cells enriched at 4°C with PE-labeled PBS57-CD1d tetramer or splenocytes stained with tetramer were incubated in a 37°C water bath for 2 or 5 min. Cells were fixed immediately with 4% paraformaldehyde for 10 min at room temperature, washed, and permeabilized with 0.1% Triton X-100 for 5 min. Surface staining was performed after washing, followed by intracellular staining for phospho-ERK or phospho-p38 (Cell Signaling Technology). Cells were analyzed by flow cytometry.
In vivo stimulation with anti-CD3 Ab.
For T-bet up-regulation experiments, LMC or T-TRAF3−/− mice were injected intravenously with 25 µg anti-CD3 Ab (2C11; eBioscience). Thymic iNKT cells were enriched after 18 h and surface-stained for CD24, NK1.1, and CD44, followed by intracellular staining for T-bet. Cells were analyzed by flow cytometry.
Cell sorting and Western blots.
Thymocytes from LckCreTRAF3flox/flox mice were stained and sorted for DN1, DN2, and DN3. Cell lysates were separated by 10% SDS-PAGE and transferred to PVDF membranes. Anti-TRAF3 (M20) and anti-actin (C-2; Santa Cruz Biotechnology, Inc.) Abs were used for TRAF3 and actin detection.
Real-time PCR.
Different stages of thymic iNKT cells were sorted. RNA was extracted with RNeasy Protocol (QIAGEN) and cDNA synthesized using SuperScript II (Invitrogen). RT-PCR was performed on an ABI PRISM 7900 Sequence Detection System (Applied Biosystems) using SYBR Green (Applied Biosystems). cDNA levels were determined with a standard curve and normalized to β-actin. TRAF3 primers were: forward 5′-GAGCAAGGAGGCTACAAGGAG-3′ and reverse 5′-CATGCAGCTCTCGCAGAAC-3′; β-actin primers were: forward 5′-TGGCACCCAGCACAATGAA-3′ and reverse 5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′.
IL-15 signaling.
Thymocytes depleted of CD8+ T cells or splenocytes were incubated with IL-15 for 20 min. They were fixed immediately with 4% paraformaldehyde for 10 min at room temperature, washed, and permeabilized with 0.1% Triton X-100 for 5 min. Surface staining was performed with anti-NK1.1 and TCR Abs after washing. Phosphorylation of STAT5 was detected with anti-p-STAT5 Ab (D47E7; Cell Signaling Technology). Cells were analyzed by flow cytometry.
Statistics.
Data represent mean ± SEM. Statistical comparisons of differences between sample means used Student’s t test.
Acknowledgments
We thank Dr. Jon Houtman for critical review of the manuscript and Dr. John Colgan for advising for retrogenic BM chimera mice. Unloaded and PBS-57–loaded CD1d tetramers conjugated with Phycoerythrin or Allophycocyanin were supplied by the National Institutes of Health tetramer facility.
The work of the authors was supported by a grant from the National Institutes of Health (R01 AI28847 to G.A. Bishop) and a Research Career Scientist Award from the Dept. of Veterans Affairs (to G.A. Bishop). This material is based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development.
The authors declare no financial or commercial conflict of interest.
Footnotes
Abbreviations used:
- α-galactosylceramide
- α-GalCer
- iNKT
- invariant NK T
- LMC
- littermate control
- MFI
- mean fluorescence intensity
- TRAF3
- TNF receptor associated factor 3
References
- Afkarian M., Sedy J.R., Yang J., Jacobson N.G., Cereb N., Yang S.Y., Murphy T.L., Murphy K.M. 2002. T-bet is a STAT1-induced regulator of IL-12R expression in naïve CD4+ T cells. Nat. Immunol. 3:549–557 10.1038/ni794 [DOI] [PubMed] [Google Scholar]
- Bajénoff M., Wurtz O., Guerder S. 2002. Repeated antigen exposure is necessary for the differentiation, but not the initial proliferation, of naive CD4(+) T cells. J. Immunol. 168:1723–1729 [DOI] [PubMed] [Google Scholar]
- Bendelac A., Savage P.B., Teyton L. 2007. The biology of NKT cells. Annu. Rev. Immunol. 25:297–336 10.1146/annurev.immunol.25.022106.141711 [DOI] [PubMed] [Google Scholar]
- Bishop G.A., Xie P. 2007. Multiple roles of TRAF3 signaling in lymphocyte function. Immunol. Res. 39:22–32 10.1007/s12026-007-0068-1 [DOI] [PubMed] [Google Scholar]
- Borowski C., Bendelac A. 2005. Signaling for NKT cell development: the SAP-FynT connection. J. Exp. Med. 201:833–836 10.1084/jem.20050339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo E.F., Acero L.F., Stonier S.W., Zhou D., Schluns K.S. 2010. Thymic and peripheral microenvironments differentially mediate development and maturation of iNKT cells by IL-15 transpresentation. Blood. 116:2494–2503 10.1182/blood-2010-03-277103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das R., Sant’Angelo D.B., Nichols K.E. 2010. Transcriptional control of invariant NKT cell development. Immunol. Rev. 238:195–215 10.1111/j.1600-065X.2010.00962.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Cruz L.M., Yang C.Y., Goldrath A.W. 2010. Transcriptional regulation of NKT cell development and homeostasis. Curr. Opin. Immunol. 22:199–205 10.1016/j.coi.2010.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel I., Kronenberg M. 2012. Making memory at birth: understanding the differentiation of natural killer T cells. Curr. Opin. Immunol. 24:184–190 10.1016/j.coi.2012.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey D.I., Berzins S.P. 2007. Control points in NKT-cell development. Nat. Rev. Immunol. 7:505–518 10.1038/nri2116 [DOI] [PubMed] [Google Scholar]
- Godfrey D.I., Stankovic S., Baxter A.G. 2010. Raising the NKT cell family. Nat. Immunol. 11:197–206 10.1038/ni.1841 [DOI] [PubMed] [Google Scholar]
- Gordy L.E., Bezbradica J.S., Flyak A.I., Spencer C.T., Dunkle A., Sun J., Stanic A.K., Boothby M.R., He Y.W., Zhao Z., et al. 2011. IL-15 regulates homeostasis and terminal maturation of NKT cells. J. Immunol. 187:6335–6345 10.4049/jimmunol.1003965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häcker H., Redecke V., Blagoev B., Kratchmarova I., Hsu L.-C., Wang G.G., Kamps M.P., Raz E., Wagner H., Häcker G., et al. 2006. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature. 439:204–207 10.1038/nature04369 [DOI] [PubMed] [Google Scholar]
- Häcker H., Tseng P.H., Karin M. 2011. Expanding TRAF function: TRAF3 as a tri-faced immune regulator. Nat. Rev. Immunol. 11:457–468 10.1038/nri2998 [DOI] [PubMed] [Google Scholar]
- Hildebrand J.M., Yi Z., Buchta C.M., Poovassery J., Stunz L.L., Bishop G.A. 2011. Roles of tumor necrosis factor receptor associated factor 3 (TRAF3) and TRAF5 in immune cell functions. Immunol. Rev. 244:55–74 10.1111/j.1600-065X.2011.01055.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P.P., Fitzpatrick D.R., Beard C., Jessup H.K., Lehar S., Makar K.W., Pérez-Melgosa M., Sweetser M.T., Schlissel M.S., Nguyen S., et al. 2001. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 15:763–774 10.1016/S1074-7613(01)00227-8 [DOI] [PubMed] [Google Scholar]
- Lighvani A.A., Frucht D.M., Jankovic D., Yamane H., Aliberti J., Hissong B.D., Nguyen B.V., Gadina M., Sher A., Paul W.E., O’Shea J.J. 2001. T-bet is rapidly induced by interferon-γ in lymphoid and myeloid cells. Proc. Natl. Acad. Sci. USA. 98:15137–15142 10.1073/pnas.261570598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda J.L., Gapin L., Sidobre S., Kieper W.C., Tan J.T., Ceredig R., Surh C.D., Kronenberg M. 2002. Homeostasis of V α 14i NKT cells. Nat. Immunol. 3:966–974 10.1038/ni837 [DOI] [PubMed] [Google Scholar]
- Matsuda J.L., George T.C., Hagman J., Gapin L. 2007. Temporal dissection of T-bet functions. J. Immunol. 178:3457–3465 [DOI] [PubMed] [Google Scholar]
- Matsuoka K., Inoue N., Sato T., Okamoto S., Hisamatsu T., Kishi Y., Sakuraba A., Hitotsumatsu O., Ogata H., Koganei K., et al. 2004. T-bet upregulation and subsequent interleukin 12 stimulation are essential for induction of Th1 mediated immunopathology in Crohn’s disease. Gut. 53:1303–1308 10.1136/gut.2003.024190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oganesyan G., Saha S.K., Guo B., He J.Q., Shahangian A., Zarnegar B., Perry A., Cheng G. 2006. Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response. Nature. 439:208–211 10.1038/nature04374 [DOI] [PubMed] [Google Scholar]
- Scanlon S.T., Thomas S.Y., Ferreira C.M., Bai L., Krausz T., Savage P.B., Bendelac A. 2011. Airborne lipid antigens mobilize resident intravascular NKT cells to induce allergic airway inflammation. J. Exp. Med. 208:2113–2124 10.1084/jem.20110522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi M., Harada M., Kojo S., Nakayama T., Wakao H. 2003. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annu. Rev. Immunol. 21:483–513 10.1146/annurev.immunol.21.120601.141057 [DOI] [PubMed] [Google Scholar]
- Townsend M.J., Weinmann A.S., Matsuda J.L., Salomon R., Farnham P.J., Biron C.A., Gapin L., Glimcher L.H. 2004. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity. 20:477–494 10.1016/S1074-7613(04)00076-7 [DOI] [PubMed] [Google Scholar]
- Vincent M.S., Gumperz J.E., Brenner M.B. 2003. Understanding the function of CD1-restricted T cells. Nat. Immunol. 4:517–523 10.1038/ni0603-517 [DOI] [PubMed] [Google Scholar]
- Xie P., Stunz L.L., Larison K.D., Yang B., Bishop G.A. 2007. Tumor necrosis factor receptor-associated factor 3 is a critical regulator of B cell homeostasis in secondary lymphoid organs. Immunity. 27:253–267 10.1016/j.immuni.2007.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie P., Kraus Z.J., Stunz L.L., Liu Y., Bishop G.A. 2011a. TNF receptor-associated factor 3 is required for T cell-mediated immunity and TCR/CD28 signaling. J. Immunol. 186:143–155 10.4049/jimmunol.1000290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie P., Poovassery J., Stunz L.L., Smith S.M., Schultz M.L., Carlin L.E., Bishop G.A. 2011b. Enhanced Toll-like receptor (TLR) responses of TNFR-associated factor 3 (TRAF3)-deficient B lymphocytes. J. Leukoc. Biol. 90:1149–1157 10.1189/jlb.0111044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Cheng G., Baltimore D. 1996. Targeted disruption of TRAF3 leads to postnatal lethality and defective T-dependent immune responses. Immunity. 5:407–415 10.1016/S1074-7613(00)80497-5 [DOI] [PubMed] [Google Scholar]
- Zhu S., Pan W., Shi P., Gao H., Zhao F., Song X., Liu Y., Zhao L., Li X., Shi Y., Qian Y. 2010. Modulation of experimental autoimmune encephalomyelitis through TRAF3-mediated suppression of interleukin 17 receptor signaling. J. Exp. Med. 207:2647–2662 10.1084/jem.20100703 [DOI] [PMC free article] [PubMed] [Google Scholar]