CD172a+ cells producing IL-1β and TNF are increased in inflamed tissues in Crohn’s disease and can be targeted by CD47 fusion protein.
Abstract
In mice, the transfer of CD172a+ (SIRP-α) dendritic cells (DCs) elicits T cell–driven colitis, whereas treatment with CD47-Fc protein, a CD172a-binding agent, confers protection. The aim of this study was to elucidate the nature and functional properties of human CD172a+ DCs in chronic intestinal inflammation. Here, we show that CD172a+CD11c+ cells accumulate in the mesenteric lymph nodes (mLNs) and inflamed intestinal mucosa in patients with Crohn’s disease (CD). These cells are distinct from resident DCs and may coexpress markers typically associated with monocyte-derived inflammatory DCs such as CD14 and/or DC-SIGN, E-Cadherin, and/or CX3CR1. Spontaneous IL-1β and TNF production by HLA-DR+ cells in CD tissues is restricted to those expressing CD172a. An avidity-improved CD47 fusion protein (CD47-Var1) suppresses the release of a wide array of inflammatory cytokines by CD172a+ cells, which may include HLA-DR−CD172a+ neutrophils, in inflamed colonic explant cultures and impairs the ability of HLA-DR+CD172a+ cells to activate memory Th17 but not Th1 responses in mLNs. In conclusion, targeting CD172a+ cells may represent novel therapeutic perspectives for patients with CD.
The gastrointestinal tract is lined with a single layer of epithelial cells that separates the gut lumen from the connective tissue and the immune system (Kaser et al., 2010; MacDonald et al., 2011). Because it is constantly exposed to dietary and environmental antigens and to an estimated community of 1014 commensal bacteria, the immune system is confronted with the difficult task of enforcing tolerance to innocuous environmental antigens while also protecting against invading pathogens. An aberrant immune response to the intestinal microbiota contributes to the pathogenesis of Crohn’s disease (CD), a chronic inflammatory bowel disease (IBD) that affects genetically predisposed individuals (Chassaing and Darfeuille-Michaud, 2011; Maloy and Powrie, 2011).
Mononuclear phagocytes, which include a large population of macrophages (MΦ) and rare subsets of DCs, are critical for the establishment and maintenance of gut homeostasis (Coombes and Powrie, 2008; Varol et al., 2010). However, myeloid cell heterogeneity in phenotype, origin, and function has led to confusion over the classification between MΦ and DCs, especially in mucosal tissues (Gautier et al., 2012; Miller et al., 2012). In murine tissues, CD11c is not an adequate marker to identify DCs because it is also expressed in varying levels on F4/80+ MΦ (Medina-Contreras et al., 2011; Rivollier et al., 2012). This is in contrast to resident MΦ in human lamina propria (LP), which do not express CD11c (Smith et al., 2011). In mice, macrophage–dendritic cell progenitors (MDPs) give rise to dedicated common DC precursors (precDCs) and monocytes via developmental pathways that are governed by Flt3L and M-CSF, respectively (Liu et al., 2009). Both the CD103+CD11b+ and CD103+CD11b− DC subsets originate from precDCs. Tissue-resident CD103−CX3CR1+ mononuclear phagocytes, which are the dominant population in the murine gut LP, derive from Ly6Chigh circulating monocytes. Murine intestinal homeostasis has been demonstrated to critically depend on a delicate equilibrium between tolerogenic migratory CD103+CX3CR1− DCs and pathogenic CD103−CX3CR1+ mononuclear phagocytes (Jaensson et al., 2008; Bogunovic et al., 2009; Varol et al., 2009). In fact, mice genetically depleted of CD103+ DCs and CX3CR1+ MΦ do not develop spontaneous inflammation (Birnberg et al., 2008). Animals that have a predominance of CX3CR1+ cells in the LP develop exacerbated colitis (Varol et al., 2009). However, both CX3CR1+F4/80+CD103− LP MΦ and CD103+ DCs can induce gut tolerance through the generation and/or maintenance of the suppressive activity of Foxp3+ regulatory T cells, and CX3CR1 deficiency leads to exacerbated DSS-induced colitis (Denning et al., 2007; Sun et al., 2007; Medina-Contreras et al., 2011).
Recent studies have independently demonstrated that CD103−E-Cadherin+ and CD103−SIRP-α+ (CD172a) cells induce experimental colitis in mice (Fortin et al., 2009; Siddiqui et al., 2010). These pathogenic cells accumulate in the inflamed colons and/or LNs. The CD103−E-Cadherin+ cells originate from Ly6Chigh circulating monocytes that migrate in a CCR7-independent manner to the mesenteric LNs (mLNs), whereas the CD103−CD172a+ DCs accumulate in the inflamed colons and mLNs via a CD47-dependent process. These cell populations promote T cell driven anti-CD40–mediated colitis and drive Th17-associated TNBS colitis in mice; the latter can be ameliorated by the administration of a CD47-Fc fusion protein that putatively targets the CD172a+ cells. Whether human equivalents of the colitogenic CD103−CD172a+ cells exist and whether they can be targeted by CD47-Fc in the mLNs (inductive site) and/or intestinal tissues (effector site) of CD patients remains unknown.
Previous studies have reported the presence of CD14+ MΦ in situ in the colons of CD patients (Grimm et al., 1995a). Imaging analyses of intestinal mucosal tissues of CD patients have also revealed the existence of several distinct DC populations including DC-SIGN (CD209)+CD11c+ DCs, CD83+ DCs, CD103+ DCs, plasmacytoid DCs (pDCs) and Slan+ monocytes/DCs (de Baey et al., 2003; Jaensson et al., 2008; te Velde et al., 2003; Verstege et al., 2008). In addition, a CD33+CD14+ intermediate MΦ/DC subset has been detected at similar frequencies throughout the nonlesional and lesional gut mucosa in CD patients (Kamada et al., 2008).
In this study, we provide compelling evidence for the accumulation of proinflammatory cytokine-producing HLA-DR+CD172a+ cells that coexpress or not E-Cadherin and CX3CR1 in the mLNs and inflamed mucosa of CD patients. These cells are a major source of IL-1β, IL-6, and TNF and can be targeted by an avidity-improved CD47 fusion protein (CD47-Var1) in inflamed CD tissues.
RESULTS
HLA-DR+CD172a+ cells accumulate in the mLNs and inflamed intestinal mucosa of CD patients
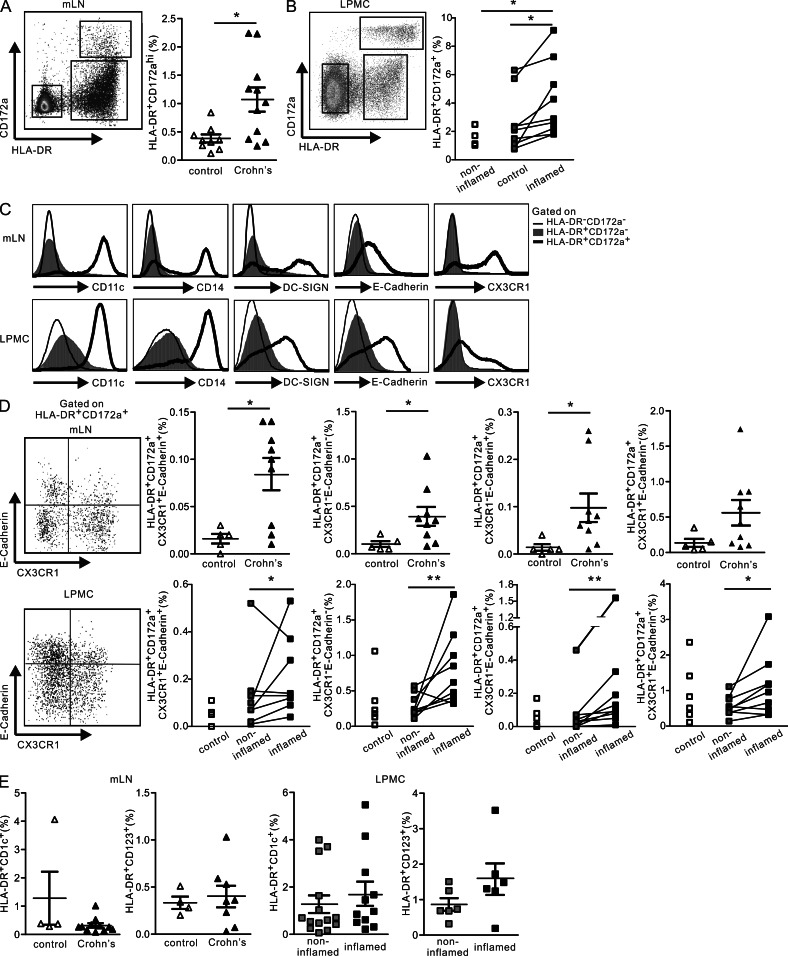
We first analyzed the mLNs and inflamed or noninflamed intestinal tissues of patients with CD or unrelated bowel disease (control/non-IBD) and searched for the human counterparts of the murine pathogenic CD103−CD172a+ DCs. HLA-DR+CD172a+ cells were detected in the mLNs (Fig. 1 A) and inflamed intestinal mucosa of CD patients (Fig. 1 B). The frequency of HLA-DR+CD172a+ cells was significantly increased in the mLN and LP mononuclear cell (LPMC) suspensions isolated from inflamed mucosal sites versus those samples isolated either from symptomless regions of CD patients or from control (non-IBD) specimens. The great majority of HLA-DR+CD172a+ cells from the mLNs were CD103−, whereas CD172a+CD103+ cells were detected in the inflamed gut tissues (unpublished data), corroborating previous observations in mice (Jaensson et al., 2008). In human skin–derived LNs from healthy donors, CD172a appears to identify most HLA-DR+CD11c+ DCs, including CD14+ MΦ-like cells and resident DCs (Segura et al., 2012). In this study, we found that CD11c expression in mLNs and gut tissues was limited to the HLA-DR+CD172a+ cell subset; HLA-DR+CD172a− and HLA-DR−CD172a− cells were CD11c− (Fig. 1 C).
Figure 1.
HLA-DR+CD172a+ cells are detected in increased proportions in the mLNs and intestinal tissues of CD patients. Mesenteric LN cellular suspensions (mLNs) and LPMCs were prepared from control (non-IBD, diverticulosis) and CD patients (noninflamed and inflamed intestinal tissues). (A and B) Flow cytometry plots of HLA-DR and CD172a expression and the percentages of HLA-DR+CD172a+ cells. n = 9 controls and n = 11 CD patients (mLNs; A) and n = 5 controls and n = 9 CD patients (LPMC; B). Data are presented as the mean ± SEM. Unpaired, two-tailed Student’s t test with Welch correction (mLNs and LPMC, controls versus CD patients) and paired, two-tailed Student’s t test after Log10 transformation (LPMC, noninflamed versus inflamed) were used to assess significance. (C) Flow cytometry histograms of CD11c, CD14, DC-SIGN, E-Cadherin, and CX3CR1 expression in the HLA-DR−CD172a− (thin lines), HLA-DR+CD172a− (shaded lines), and HLA-DR+CD172a+ gated cells (bold lines). Representative of at least five CD patients. (D) Representative dot plots for E-Cadherin and CX3CR1 expression by HLA-DR+CD172a+ gated cells, and the percentage of the cell population in each quadrant. n = 5 controls and n = 9 CD patients (mLNs); n = 5 controls and n = 8 CD patients (LPMC). Data are presented as the mean ± SEM. Unpaired, two-tailed Student’s t test with Welch correction (mLNs and LPMC, controls versus CD patients) and paired, two-tailed Student’s t test after Log10 transformation (LPMC, noninflamed versus inflamed) were used to assess significance. (E) The percentages of HLA-DR+CD1c+ and HLA-DR+CD123+ cells. n = 4 controls and n = 8 CD patients (mLNs); n = 6 CD patients (LPMC). Data are presented as the mean ± SEM. *, P < 0.05; **, P < 0.01.
We next determined which of the resident DC subsets, CD14+ MΦ-like cells or the recently described monocyte-derived DC-SIGN (CD209)+ DCs (Mo-DCs; Cheong et al., 2010), accumulated in the mLNs and intestinal mucosa of CD patients. CD14 and DC-SIGN were expressed by ∼50% of the HLA-DR+CD172a+ cells in the mLNs (Fig. 1 C). Notably, a significant fraction of HLA-DR+CD172a+CD11c+ cells coexpressed CD14 and DC-SIGN in the mLNs (unpublished data). In the LP, CD14 marked the majority of HLA-DR+CD172a+ cells but not the HLA-DR+CD172a− cells (Fig. 1 C). In situ analysis by IHC further revealed that CD172a expression was scattered throughout the mucosa in the inflamed colons (unpublished data), a region populated by recently recruited CD14+ MΦ in CD patients (Grimm et al., 1995a). In this regard, the HLA-DR+CD172a+CD11c+CD14+ cells observed in the gut mucosa appeared to be distinct from the human resident LP MΦ because the latter are characterized as CD11c−CD14−CD11b−CD13+HLA-DR+ cells (Kamada et al., 2008; Smythies et al., 2005). Of note, the HLA-DR+CD172a+ LP cell population also included regulatory MΦ (CD206+ cells) and, in agreement with recent studies, the CD206+/HLA-DR+ cell ratio was decreased in the inflamed versus noninflamed portions of the mucosa of CD patients (unpublished data; Vos et al., 2012).
In mice, E-Cadherin marks a murine colitogenic DC subset that can be directly recruited from the blood to the mLNs (Siddiqui et al., 2010), whereas the CD103−CX3CR1+ mononuclear gut phagocytes originate from Ly6Chigh monocytes that are recruited to the inflamed mucosal tissues and differentiate into CX3CRlow CD11b+ cells (Bar-On et al., 2011; Rivollier et al., 2012; Bain et al., 2013). Further phenotypic analysis revealed that a significant proportion of the HLA-DR+CD172a+ cells expressed E-Cadherin or CX3CR1 in lymphoid and nonlymphoid CD tissues (Fig. 1 C). When the HLA-DR+CD172a+ cells were further subdivided according to E-Cadherin and CX3CR1 expression, it appeared that the CX3CR1−E-Cadherin− cells predominated over the CX3CR1+E-Cadherin+ cells in both the mLNs and LP (Fig. 1 D). Importantly, these two cell populations, together with the CX3CR1−E-Cadherin+ cells, significantly accumulated in the mLNs and inflamed gut mucosa of CD patients. The frequency of CX3CR1+E-Cadherin− cells was augmented in the LP but not in the mLNs of CD donors. In fact, the CX3CR1+ cells in the mLNs coexpressed CD1c but not CD123 and are thus considered to be conventional DCs. The percentage of HLA-DR+CD172a+ CD1c−CD123− cells significantly increased in the samples from CD patients, further indicating that the HLA-DR+CD172a+ cells that accumulated in the mLNs are distinct from resident DCs (unpublished data). Notably, similar proportions of CD1c and CD123 DC subsets were detected in the mLNs and gut tissues of control and CD patients (Fig. 1 E).
Collectively, these findings provide the first evidence for the detection and accumulation of HLA-DR+CD172a+ cells in the lymphoid and mucosal tissues of CD patients, regardless of their expression of CX3CR1 and/or E-Cadherin.
TNF and IL-1β are selectively produced by CD172a+ cells in the peripheral tissues of CD patients
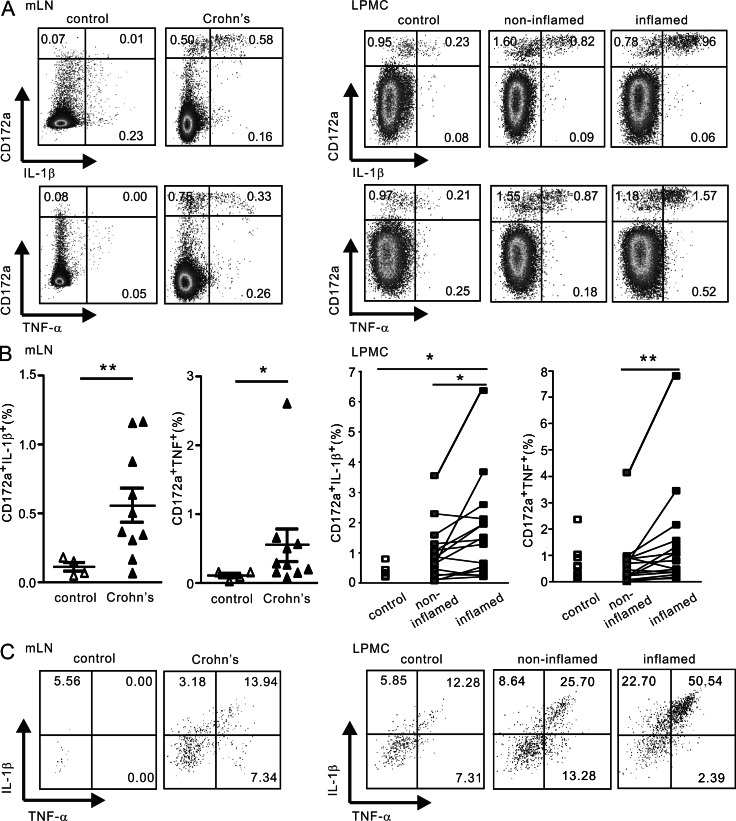
We next evaluated the proinflammatory profile of HLA-DR+CD172a+ cells in peripheral tissues. As depicted in Fig. 2, mLN cells and ex vivo–isolated LPMCs from CD patients spontaneously expressed IL-1β and TNF without the addition of exogenous stimuli. Notably, the CD172a+ but not CD172a− cells represented the major source of spontaneous IL-1β and TNF production (Fig. 2 A). The frequency of CD172a+ IL-1β+ or TNF+ cells in the mLNs and inflamed intestinal mucosa from CD patients was significantly higher compared with in noninflamed or control specimens (Fig. 2 B). In fact, TNF and IL-1β were coproduced by ∼15–50% of the HLA-DR+CD172a+ cells, which mainly comprised the CX3CR1−E-Cadherin− and CX3CR1+E-Cadherin− subsets in mLNs and LPMC, respectively (Fig. 2 C and not depicted). Thus, these data indicate that proinflammatory cytokine production is restricted to CD172a+ cells in CD patients.
Figure 2.
Proinflammatory cytokine production is restricted to CD172a+ cells in the mLNs and LPMC from CD patients. Freshly isolated mLN cells and ex vivo isolated LPMC were stained for cell surface CD172a and HLA-DR followed by intracytoplasmic staining for IL-1β and TNF in the absence of brefeldin. (A) Representative flow cytometry plots of CD172a and IL-1β or TNF expression among total CD45+ cells. (B) Percentages of CD172a+IL-1β+ cells and CD172a+TNF+ cells. n = 4 controls and n = 10 CD patients (mLNs) and n = 6 controls and n = 15 CD patients (LPMC). Data are presented as the mean ± SEM. Unpaired, two-tailed Student’s t test after Log10 transformation (mLN and LPMC, controls versus CD patients), and paired, two-tailed Student’s t test after Log10 transformation (LPMC, noninflamed versus inflamed) were used to assess significance. *, P < 0.05; **, P < 0.01. (C) Representative flow cytometry plots of coexpression of IL-1β and TNF on HLA-DR+CD172a+ gated cells.
A CD47 fusion protein specifically identifies HLA-DR+CD172a+ cells in the blood, mLNs, and mucosa of CD patients
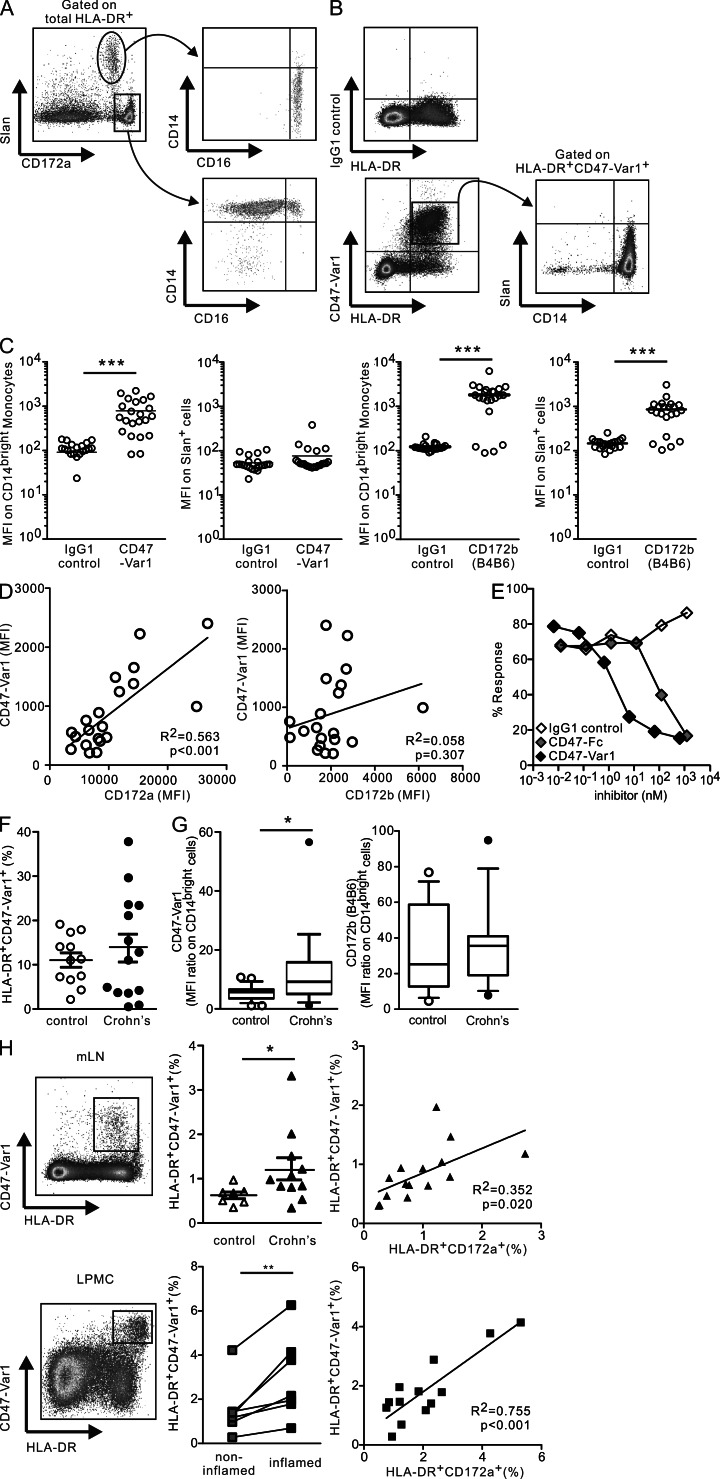
We therefore examined the possibility of selectively targeting these proinflammatory cytokine-producing cells in peripheral tissues of CD patients through CD172a. However, the most commonly used anti-CD172a mAb, clone SE5A5, which was used in the present study, was initially reported to also recognize an epitope common to both human CD172a and CD172b (Seiffert et al., 2001), and none of the commercially available mAbs specifically recognize CD172a (Zhao et al., 2011). Because CD47 is a specific receptor for CD172a (SIRPα) but not for CD172b (Brown and Frazier, 2001; Latour et al., 2001), and CD47-Fc prevents the development and relapse of colitis when administered to mice (Fortin et al., 2009), we first compared the binding characteristics of a newly developed avidity-improved human CD47 fusion protein (CD47-Var1) to CD172a mAb in the blood of control donors (Fig. 3). Human blood monocytes can be subdivided into two major subsets: CD14brightCD16+or− and CD14dimCD16+Slan+or− cells (Schäkel et al., 2006; Cros et al., 2010). Accordingly, we examined CD172a in relation to Slan expression within the circulating HLA-DR+ cells. CD172a mAb stained CD14bright cells, which are reported to be the circulating precursors of intestinal CD14+ Mϕ in CD patients (Grimm et al., 1995b). The CD14dimCD16+Slan+ cells that infiltrated CD ileum (de Baey et al., 2003) appeared as CD172adim (Fig. 3 A). In contrast, CD47-Var1 specifically marked CD14bright cells but not Slan+ cells, despite both cell subsets expressing similar levels of CD172b. This indicates that CD47-Var1 does not bind to CD172b and that Slan+ cells express low or no CD172a (Fig. 3, B and C). In fact, CD47-Var1 binding significantly correlated with the expression of CD172a but not CD172b on CD14bright monocytes (Fig. 3 D). Notably, the IC50 of the bond between HLA-DR+ cells and CD47-Var1 (1.4 nM) was 70-fold higher than that previously reported for CD47-Fc (100 nM; Braun et al., 2006) (Fig. 3 E).
Figure 3.
CD47-Var1 selectively detects HLA-DR+CD172a+ cells in blood and peripheral tissues. HLA-DR+CD172a+ cells were analyzed in PBMC (A, B, F, and G) or whole blood (hemolyzed; C–E). (A and B) Representative flow cytometry plots of CD172a mAb (A) or CD47-Var1 or IgG1 control fusion protein (B) staining together with MHC class II (HLA-DR), Slan, CD14, and CD16 mAbs. Data were representative of five experiments. (C) MFI of CD47-Var1 binding and CD172b staining on CD14bright monocytes or CD14dimSlan+ cells. n = 22. The bars in the graphs represent the means. Unpaired, two-tailed Student’s t test (IgG1 control versus CD47-Var1 or CD172b) was used to assess significance. (D) Correlation between the MFI of CD47-Var1 and CD172a or CD172b expression on CD14bright monocytes. n = 20. Two-tailed Pearson’s correlation test (between CD172a or CD172b and CD47-Var1) was used to assess significance. (E) Inhibition of CD47-Var1 binding on CD14bright monocytes by unlabeled CD47 fusion protein (CD47-Fc), CD47-Var1, or IgG1 control. Data are representative of six experiments. (F) Percentages of HLA-DR+CD47-Var1+ cells in PBMCs from controls and CD patients. n = 12 controls and n = 14 CD patients. Data are presented as the mean ± SEM. Unpaired, two-tailed Student’s t test (controls versus CD patients) was used to assess significance. (G) MFI ratio of CD47-Var1 or CD172b by CD14bright monocytes from controls and CD patients. n = 13 controls and n = 12 CD patients. Data are presented as box-and-whiskers plots with 10–90%iles. Unpaired, two-tailed Student’s t test with Welch correction (controls versus CD patients) was used to assess significance. (H) Representative flow cytometry plots of HLA-DR and CD47-Var1 expression, the percentages of HLA-DR+CD47-Var1+ cells, and correlation between the percentage of HLA-DR+CD172a+ and the percentage of HLA-DR+CD47-Var1+ cells in mLNs and LPMCs. n = 7 controls and n = 11 CD patients (mLNs) and n = 7 CD patients (LPMCs). Data are presented as the mean ± SEM. Unpaired, two-tailed Student’s t test with Welch correction (mLNs, controls versus CD patients), paired, two-tailed Student’s t test (LPMC, noninflamed versus inflamed), and two-tailed Pearson’s correlation test (between CD172a and CD47-Var1) were used to assess significance. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
CD47-Var1 identified ∼10–15% of HLA-DR+ cells in the blood of control and CD patients (Fig. 3 F). However, CD172a (CD47-Var1 binding) but not CD172b expression was significantly increased on the CD14brightSlan− cells in CD patients when compared with non-IBD patients, indicating that CD172a is highly expressed on circulating CD14bright HLA-DR+ cells in CD patients (Fig. 3 G). Finally, we demonstrated that HLA-DR+CD47-Var1+ cells could also be detected in the mLNs and intestinal mucosa (Fig. 3 H). The percentages of HLA-DR+CD47-Var1+ cells significantly increased in the mLNs of CD patients as well as in LPMC from the inflamed tissue when compared with mLNs of control patients or noninflamed tissues from CD patients and correlated with the percentage of HLA-DR+CD172a+ cells. The frequency of HLA-DR+ cells stained with CD172a mAb tended to be higher than that identified by CD47-Var1 in mLNs, suggesting the presence of HLA-DR+CD172b+ cells with low CD172a expression in lymphoid organs.
CD47-Var1 therefore selectively identifies CD172a but not CD172b on HLA-DR+ cells that accumulate in the mLNs and intestinal mucosa of CD patients, and thus can be used to target proinflammatory cytokine-producing cells in CD tissues.
CD172a+ cells can be targeted by CD47-Fc in the peripheral tissues of CD patients
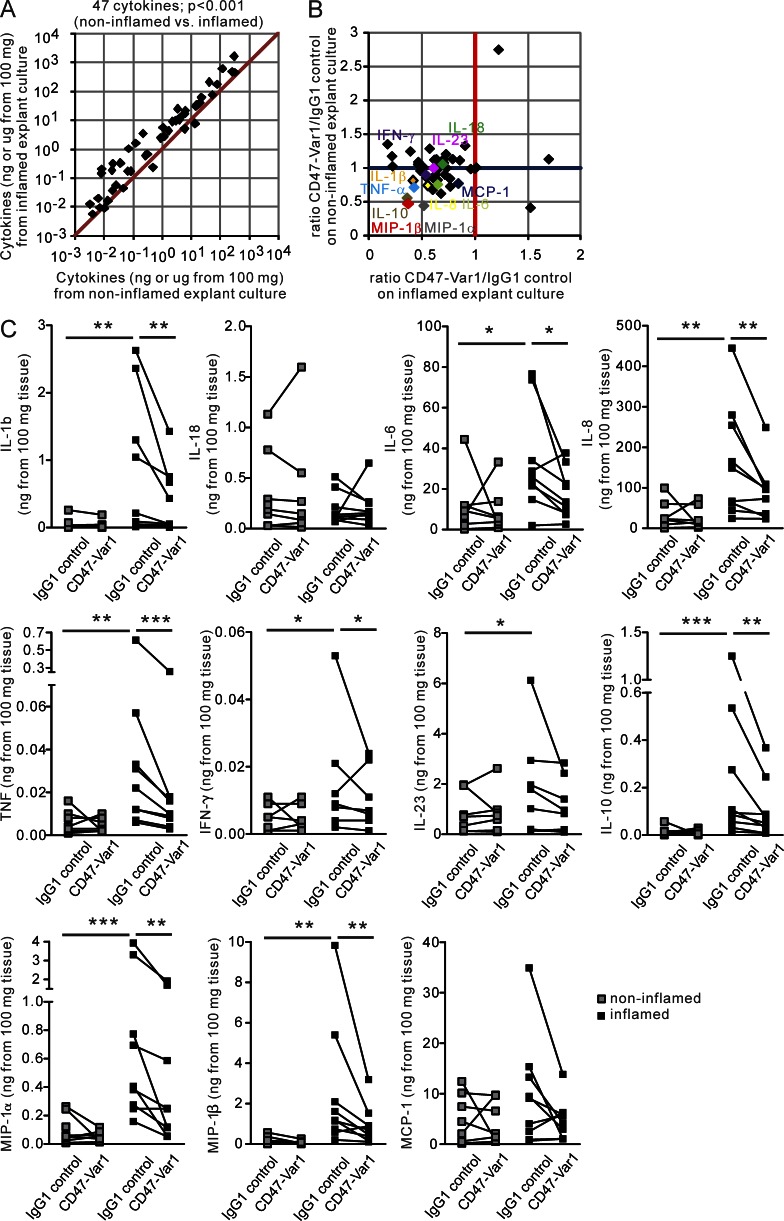
We have previously reported that CD47-Fc alters the migration and function of in vitro–generated human Mo-DCs (Latour et al., 2001; Braun et al., 2006), and in this study we have provided evidence that HLA-DR+CD172a+ cells are detected by CD47-Var1 in lymphoid and nonlymphoid CD tissues. We first added CD47-Var1 to colonic tissue explant cultures and examined the proinflammatory cytokine profile by Multi-Analyte Profiling (MAP) technology. The inflamed CD tissues (mean of 5 CD patients) displayed a pronounced proinflammatory profile when compared with noninflamed tissues, as indicated by a panel of 47 cytokines (Fig. 4 A). We demonstrated that exposure of inflamed mucosal explants to CD47-Var1 but not IgG1 control strongly suppressed the release of a large panel of cytokines from the inflamed tissue without significantly affecting the secretion of cytokines by the noninflamed tissues in CD patients (Fig. 4 B). Noteworthy, CD47-Var1 significantly dampened the production of proinflammatory cytokines, including IL-1β, IL-6, IL-8, TNF, IFN-γ, and, to a lesser extent, IL-23, as well as the monocyte chemoattractants MIP-1α and MIP-1β. More precisely, the inhibition of proinflammatory cytokine production by CD47-Var1 protein was observed in surgical specimens prepared from six patients who failed to respond to anti-TNF therapy (Fig. 4 C). In contrast, CD47-Var1 did not suppress IL-18 and MCP-1 release, whereas it decreased the production of IL-10.
Figure 4.
CD47-Var1 inhibits proinflammatory cytokine release by inflamed intestinal tissue from CD patients. Colonic tissue explants were cultured in the presence of CD47-Var1 or IgG1 control. The cytokine production profile was examined in the culture supernatants. The production of 47 cytokines was normalized to the absolute amount (ng or μg) of cytokine secreted by 100 mg of colonic tissue. (A) Scatter plot of cytokine production in noninflamed (x axis) versus inflamed (y axis) tissues. Data are presented as the mean of 5 independent experiments (n = 5 CD patients). Two-tailed Wilcoxon matched pairs signed rank test (noninflamed versus inflamed) was used to assess significance. (B) Scatter plot of cytokine production in explant cultures expressed as the ratio of CD47-Var1/IgG1 control for inflamed (x axis) versus noninflamed (y axis) tissues. Values <1 on the x axis reflect the inhibition by CD47-Var1 in inflamed tissue. Data are the mean from five independent experiments (n = 5 CD patients) (C) Cytokine release in noninflamed and inflamed tissues in CD47-Var1– or IgG1 control-treated explant cultures. n = 8 CD patients. Paired, two-tailed Student’s t test after Log10 transformation (noninflamed versus inflamed and IgG1 control versus CD47-Var1) was used to assess significance. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
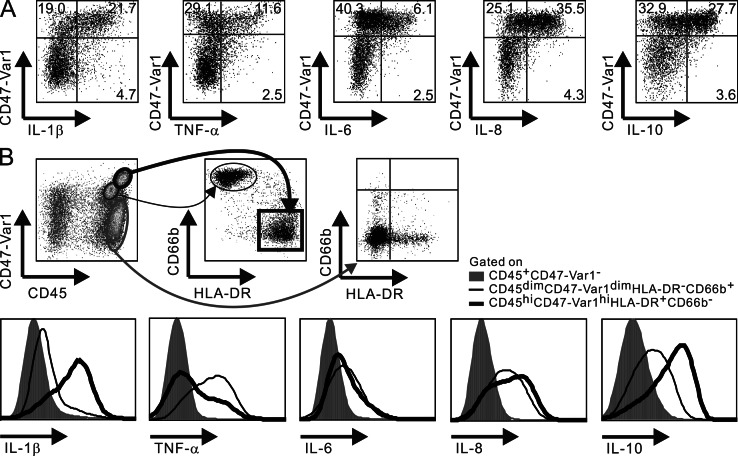
We next confirmed that among HLA-DR+ cells, CD47-Var1+ cells represented the main producers of IL-1β and TNF in the inflamed LPMC and extended these observations to IL-6, IL-8, and IL-10 (Fig. 5 A). Accordingly, the HLA-DR+CD47-Var1− cells were found to be negative for intracellular cytokines in the intestinal mucosa from CD patients. However, neutrophils (CD45dimCD66b+HLA-DR− cells) were observed in the inflamed LP tissues of a subgroup of CD patients and were found to be CD47-Var1dim (Fig. 5 B). In this patient subgroup, neutrophils but not CD45+CD47-Var1− cells expressed TNF, IL-6, IL-8, and IL-10 but low levels of IL-1β. These results indicate that neutrophils may represent an additional target for CD47-Var1 in inflamed colons and further demonstrate that cytokine expression is restricted to CD172a+ cells in hematopoietic cells, whether or not they express HLA-DR.
Figure 5.
CD47-Var1 identifies cytokine-producing HLA-DR+ and HLA-DR− CD172a+ cells in inflamed colons from CD patients. Ex vivo–isolated LP cells from inflamed colons of CD patients were stained for intracellular cytokines (IL-1β, TNF, IL-6, IL-8, and IL-10). (A) Representative flow cytometry plots of CD47-Var1 and cytokine expression among HLA-DR+ cells. (B) CD45 and CD47-Var1 expression was analyzed on total hematopoietic (CD45+) and nonhematopoietic (CD45−) cells. CD45+ cells were further subdivided according to HLA-DR and CD66b expressions. Intracellular expression of cytokines (IL-1β, TNF, IL-6, IL-8, and IL-10) was examined on CD45+CD47-Var1− (shaded lines), CD45dimCD47-Var1dimHLA-DR−CD66b+ (thin lines), and CD45hiCD47-Var1hiHLA-DR+CD66b− (bold lines) gated cells. Data are representative of at least three independent experiments.
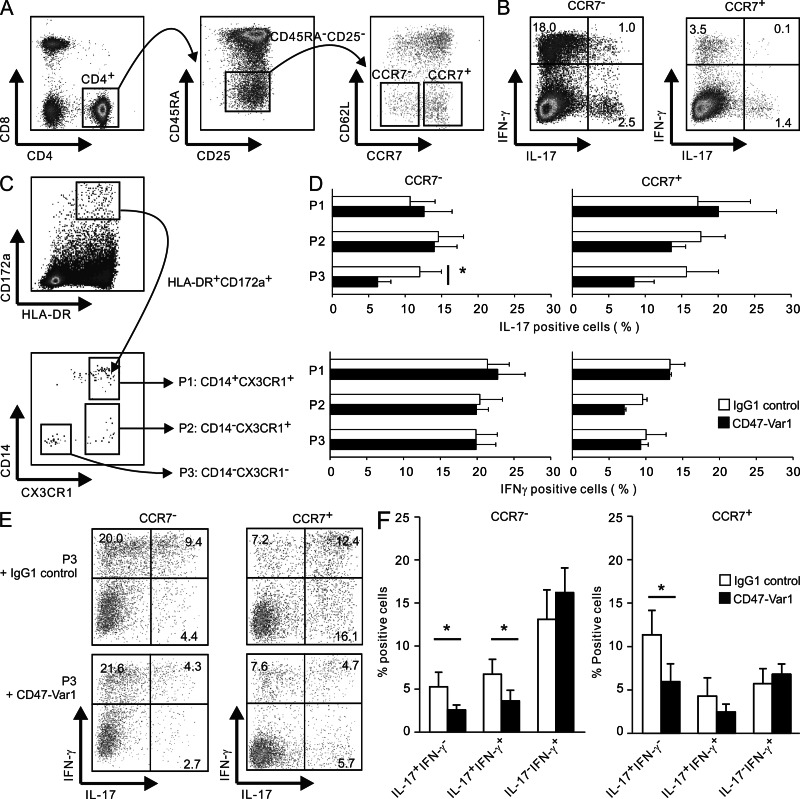
Finally, we addressed whether CX3CR1+ or CX3CR1−HLA-DR+CD172a+ cell subsets purified from the mLNs of CD patients could activate effector memory CD4 T cells isolated from the same mLN donor, and examined how CD47-Var1 interfered with Th cell fate (Fig. 6). Effector memory CD45RA−CD25−CD62Llow CD4+ T cells were purified as high (CCR7−) and low (CCR7+) IL-17 plus IFN-γ–producing cells (Kryczek et al., 2011) and co-cultured with CD14+CX3CR1+ (P1), CD14−CX3CR1+ (P2; CD1c+DCs), or CD14−CX3CR1− (P3, which may include E-Cadherin+ cells) HLA-DR+CD172a+ cells (Fig. 6, A to C). CD47-Var1 significantly impaired Th17 but not Th1 responses selectively in P3/CD62LlowCCR7− T cell co-cultures (Fig. 6 D). More specifically, CD47-Var1 suppressed the emergence of IL-17+IFN-γ− and IL-17+IFN-γ+, but not IL-17-IFN-γ+ cells in CD62LlowCCR7− and CD62LlowCCR7+ effector memory CD4 T cells co-cultured with P3 cells (Fig. 6, E and F).
Figure 6.
CD47-Var1 impairs memory Th17 and Th17/Th1, but not Th1, responses by CD62LloCD4 effector T cells co-cultured with HLA-DR+CD172a+ cells isolated from mLNs of CD patients. (A) Two subsets of effector memory CD62LloCD4 T cells (CCR7− and CCR7+) gated on CD8−CD4+CD45RA−CD25−T cells were purified from mLN cellular suspension of CD patients. (B) CCR7− and CCR7+ T cells were cultured in the presence of IL-2 for 24 h, and intracytoplasmic staining for IL-17 and IFN-γ was performed after PMA/ionomycin restimulation. (C) Three subpopulations (CD14+CX3CR1+, P1; CD14−CX3CR1+, P2; and CD14−CX3CR1−, P3) of HLA-DR+CD172a+ cells were purified from mLNs of CD patients. (A–C) Representative flow cytometry plots (n = 5 CD donors). (D to F) CCR7− and CCR7+ T cells were co-cultured with autologous P1 (n = 4), P2 (n = 3), or P3 (n = 5) subsets at a 5:1 ratio for 5 d in the presence of Staphylococcus aureus cowan 1 and CD47-Var1 or IgG1 control. Intracytoplasmic expression of IL-17 and IFN-γ was analyzed on CD2+ cells after PMA/ionomycin restimulation (mean ± SEM; D). Representative dot plots of IL-17 and IFN-γ staining in T cell/P3 co-culture (E) Data are the mean ± SEM from five independent experiments (P3) (F). Paired, two-tailed Student’s t test after Log10 transformation was used to assess significance. *, P < 0.05.
In summary, we conclude that the human counterparts of the murine colitogenic CD172a+ DCs, identified as proinflammatory cytokine producing HLA-DR+CD172a+ cells, accumulate in the mLNs and intestinal mucosa of CD patients and can be targeted by CD47 fusion proteins.
DISCUSSION
Several studies in animal models support a role for DCs in the pathogenesis of IBD (Coombes and Powrie, 2008), yet the nature and functional properties of the human counterparts of these colitogenic CD172a+ DCs has not been reported. Here, we show that HLA-DR+CD172a+ cells accumulate in the mLNs and the inflamed intestinal mucosa of CD patients, and demonstrate that IL-1β, TNF, IL-6, IL-8, or IL-10 production is selectively produced by CD172a+ but not CD172a− cells. In inflamed intestinal tissue explants, CD47-Var1, which specifically binds CD172a, profoundly inhibits the production of a wide array of proinflammatory cytokines, and in mLNs it impairs the ability of HLA-DR+CD172a+ cells to stimulate memory Th17 but not Th1 responses. We conclude that HLA-DR+CD172a+ cells may contribute to CD pathogenesis at effector and inductive sites and can be targeted therapeutically by a CD47 fusion protein.
The delineation between DCs, MΦ, and the more recently described inflammatory Mo-DCs, in terms of phenotype, molecular signature, and function remains an open and highly debated issue in both murine and human tissues, especially in mucosal sites (Varol et al., 2009; Gautier et al., 2012; Miller et al., 2012; Rivollier et al., 2012; Plantinga et al., 2013; Segura et al., 2013). In agreement with a previous study (Verstege et al., 2008), we found that the classical resident DCs (CD14−CX3CR1+CD1c+) and pDCs (CD14−CX3CR1−CD123+) are found in similar proportions in noninflamed and inflamed mLNs and colons, and are thus unlikely to represent a recruited cell population. In human LPMC, we showed that >90% of the HLA-DR+CD172a+ cells displayed CD14, whereas it was expressed by ∼50% of these cells in the mLNs. In this regard, the HLA-DR+CD172a+CD14+ colonic cells resemble the CD14+ MΦ subpopulation detected in situ in intestinal CD tissues (Grimm et al., 1995a) and the so-called intermediate CD14+CD33+CD16−CD68+CX3CR1+CD70−CD123+CD1c− MΦ/DCs. However, the latter are detected throughout the gut mucosa of IBD patients (Kamada et al., 2008). The intestinal intermediate CD14+ MΦ/DC subset drives naive T cell differentiation into Th1 and Th17 cells in vitro (Kamada et al., 2009), and thus they might fulfill the DC criteria. Furthermore, half of the HLA-DR+CD172a+ cells identified in the mLNs and LPMC also displayed DC-SIGN, in agreement with a previous report that showed high levels of DC-SIGN expression by IHC in the mucosa of CD patients (te Velde et al., 2003). This phenotype is reminiscent of in vitro–generated Mo-DCs (Baba et al., 2008) and the Mo-DCs detected in the lymphoid organs of infected mice(Cheong et al., 2010), but contrasts with recently described CD14+DC-SIGN−CD1c+ inflammatory DCs detected in the synovial fluid of rheumatoid arthritis patients (Segura et al., 2013). Definitive classification of HLA-DR+CD172a+ cells into MΦ versus inflammatory Mo-DCs awaits further morphological and molecular studies.
In contrast to murine CD103−CD11b+ MΦ, which all express CD172a and CX3CR1 in LP, whether they are inflammatory or not, CD172a+ cells comprised only ∼15% of the HLA-DR+ cells in inflamed gut mucosa, with about ∼40% displaying CX3CR1. In mice, CD172a marks all myeloid cells, including lung and intestinal MΦ, as well as granulocytes and mucosal CD103−CD11b+ DCs in the skin, airway, and gut (Fortin et al., 2009; Ginhoux et al., 2009; Raymond et al., 2009). Murine CD11b+CX3CR1+ cells are considered as resident mucosal tissue MΦ because these cells cannot be retraced as a homogenous population in the LNs (Schulz et al., 2009). The CD11chighCD11b+CX3CR1low DCs (also called CD11c+ MΦ) mediate pro-Th17 cell function, as opposed to CD11c−CD11b+CX3CR1high MΦ (bona fide MΦ; Medina-Contreras et al., 2011). Subdivisions according to the levels of CX3CR1 expression in CX3CR1-GFP mice provide further evidence for a functional dichotomy. The CX3CR1high cells retain a noninflammatory profile during intestinal inflammation, whereas CX3CR1low cells accumulate in the inflamed mucosa (Weber et al., 2011). The latter may develop into the former at steady state (Bain et al., 2013). However, the human CD172a+CX3CR1+ cells described in this study appear distinct from the resident CD11c−CD11b−CD14−HLA-DR+CD13+ MΦ that represent the largest population of mononuclear phagocytes in the human body (Smythies et al., 2005; Smith et al., 2011). The killing and elimination of invading pathogens are the primary functions of human intestinal MΦ. As such, the intestinal MΦ are essentially protective (Smith et al., 2011). Because the ubiquitously expressed CD47 Ag delivers a “do not eat me” signal to CD172a+ APC, the lack of CD172a expression on resident human intestinal MΦ may facilitate their phagocytic function (van den Berg and van der Schoot, 2008).
The challenge with human studies is to define which of the circulating monocyte or DC populations may represent the precursors of the HLA-DR+CD172a+ cells detected in the lymphoid and nonlymphoid tissues. The inflammatory CD14bright monocytes, which are the human counterparts of murine Ly6Chigh cells, may represent candidate precursors of the proinflammatory CD14+ cells in the gut or Mo-DCs in the mLNs (Tamoutounour et al., 2012). Of interest, autologous CD14+ monocytes injected into CD patients are retraced as intestinal CD14+ cells, and the transfer of Ly6Chigh cells into mice generates CD103−CX3CR1+ mononuclear phagocytes (Grimm et al., 1995b; Varol et al., 2009). Here, we showed that the proportion of circulating HLA-DR+CD172abrightCD14brightCD16− monocytes is similar in PBL from control and CD donors. However, HLA-DR+CD172a+CD14+ cells accumulated in the inflamed colons, in agreement with the detection of CD14brightCD103− cells in the ileum of CD donors (Bain et al., 2013). We further postulate that intestinal HLA-DR+CD172a+ cells that may coexpress E-Cadherin and/or CX3CR1 have the capacity to migrate into mLNs because they were also found in increased proportions in the mLNs of CD patients. Recent studies indicate that CD103−CX3CR1+CD172a+ mononuclear phagocytes, previously considered as a nonmigratory intestinal cell population, traffic from gut to mLNs in mice (Cerovic et al., 2013; Diehl et al., 2013). However, the HLA-DR+CD172a+CD1c−CX3CR1+cells, which coexpress CD14 and E-Cadherin, may originate from circulating CD14+ monocytes that are recruited directly to mLNs from the bloodstream (Siddiqui et al., 2010; Tamoutounour et al., 2012).
Human CD14+ MΦ produce IL12p40, TNF, IL-6, and IL-1β in response to in vitro culture with commensal bacteria (Kamada et al., 2008). Our data demonstrated that, among the ex vivo–isolated HLA-DR+ cells, only those that coexpressed CD172a and were recognized by CD47-Var1 produced IL-1β in the absence of external stimuli in the gut mucosa and mLNs. The inflammasome is a crucial molecular platform that regulates the activation of caspase-1 and the processing of IL-1β. However, how the main effectors of the inflammasome, IL-1 and IL-18, contribute to the development and perpetuation of IBD is rather complex (Maeda et al., 2005; Mills and Dunne, 2009). In brief, the protective function of these mediators, which involves epithelial cell repair, contrasts with their pathogenic role in maintaining intestinal inflammation. The increased production of IL-1β by HLA-DR+CD172a+ cells at inflamed sites in CD patients reinforces the idea that the overproduction of IL-1β correlates with overt inflammation and enhanced disease susceptibility in CD patients with Nod2 mutations (Villani et al., 2009). Here, we further demonstrated that CD47 fusion protein profoundly alters the in vitro proinflammatory cytokine profile released from inflamed mucosal explants, including those extracted from patients who were refractory to anti-TNF therapy. More specifically, exposure to CD47-Var1 disabled HLA-DR+CD172a+ cells by reducing IL-1β, IL-6, IL-8, TNF, and, to a lesser extent, IL-23 release. CD47-Var1 also reduced IL-10 production. Although intestinal epithelial cells represent a major source of IL-10 in noninflamed gut tissues, the release of IL-10 was augmented in inflamed colons, corroborating earlier studies (Autschbach et al., 1998). These data suggest that local IL-10 is insufficient to control overt inflammation in CD colons. In that regard, treatment with IL-10 does not ameliorate human CD nor established murine experimental colitis (Herfarth and Schölmerich, 2002). Furthermore, TNF, IL-6, and IL-8 secretion was restricted to CD172a+ cells, that include HLA-DR+ (inflammatory DCs) and HLA-DR− cells (neutrophils) in the LP of CD patients.
The frequency of IL-23+CD68+ MΦ is augmented in situ in inflamed tissues (Schenk et al., 2007). Together with IL-23, IL-1β can promote the generation of Th17 cells (Acosta-Rodriguez et al., 2007). Recent studies have supported the concept that CD is a Th1/Th17-associated autoinflammatory disease (Kobayashi et al., 2008). Our data indicate that CD47-Var1 impaired the ability of HLA-DR+CD172a+ CX3CR1− cells isolated from mLNs of CD donors, to stimulate in vitro memory Th17, Th17/Th1 but not Th1 responses. The CD47 fusion protein, when administered in vivo, could also reduce the recruitment of colitogenic CD172a+ cells to tissues and mLNs (Fortin et al., 2009). In that regard, our results indicate that CD47-Var1 decreased the production of MIP-1α, which is reported to attract inflammatory monocytes to inflamed gut (Schulthess et al., 2012).
Collectively, we have identified functional HLA-DR+CD172a+ cells, which are increased in the mLNs and intestines of CD patients, and conclude that these cells represent the human counterparts of murine colitogenic DCs. Confusion still remains regarding the classification of these mononuclear phagocytes as Mo-DCs or MΦ in peripheral tissues. HLA-DR+CD172a+ cells are an important source of proinflammatory cytokines, and therefore, we propose to refer to these cells as monocyte-derived effector cells (MDECs) or inflammatory DCs, as they appear to be distinct from classical DCs, monocytes, and MΦ in LP. In addition, MDECs are distinct from the anergic resident intestinal MΦ because they represent an important source of TNF and IL-1β in the mucosal tissues and mLNs of CD patients. The binding of the CD47 fusion protein to MDECs reflects the expression of CD172a, which becomes a suitable target for CD. Indeed, the suppression of multiple cytokines through a rather selective target, i.e., the proinflammatory CD172a+ MDECs and perhaps CD172a+ neutrophils, while sparing the protective CD172a− cells (tolerogenic CD103+ DCs, cCLE9A/CD141+ cells, and resident LP MΦ) might offer a therapeutic advantage over single-agent therapies. Indeed, only 50% of CD patients achieved remission under single anti-TNF or anti-IL12p40 treatments, the best currently available treatments for this debilitating chronic IBD. In conclusion, the administration of the CD47 fusion protein opens a novel therapeutic avenue for IBD patients.
MATERIALS AND METHODS
Clinical tissue samples.
CD patients and non-IBD control donors (largely medical check-ups, colon cancer, or diverticulitis cases) were recruited from the gastroenterology and digestive tract surgery departments at Centre Hospitalier de L’Université de Montréal (CHUM) in compliance with the Institutional CHUM Ethic Research Committee, and written informed consent was obtained from all patients. Peripheral blood samples were collected from all donors (non-IBD, n = 30; IBD, n = 67). Intestinal tissue samples were obtained from endoscopic biopsies (colons) or surgically resected specimens (colon, n = 29; ileum, n = 7). Specimens were obtained from unaffected areas of control donors or noninflamed and inflamed regions of CD patients on the basis of clinical, endoscopic and histological findings according to established criteria. The mLNs were obtained from surgical specimens. Peripheral blood samples were also obtained from healthy volunteers in compliance with the Swiss Red Cross Center, Basel.
Intestinal tissue explant cultures.
Dissected mucosal tissues (∼100 mg/piece) were cultured in RPMI 1640 medium (Wisent) supplemented with 10% FCS (Wisent) and an antibiotic cocktail (10 µg/ml of Vancomycin; Hospira), 50 µg/ml of Meropenem (AstraZeneca Canada), 50 µg/ml of Gentamicin (Invitrogen), 2.5 µg/ml of Fungizone (Invitrogen), and 12.5 µg/ml of polymyxin B (Invitrogen) in a 70% O2 and 5% CO2 saturated 37°C culture incubator for 24 h. In some experiments, CD47-Var1 fusion protein or IgG1 control fusion protein (Novartis Institute, Basel, Switzerland) was added at a concentration of 10 µg/ml. The culture supernatants were collected, and the proinflammatory cytokine profile was assessed using MAP technology (Inflammation MAP v1.0; Myriad RBM). The data were normalized by the weight of the tissue and culture volume. Data are presented as the absolute amount of cytokine released from 100 mg of tissue.
Cellular suspension preparation.
PBMC were prepared by density gradient centrifugation of heparinized peripheral blood. In some experiments, WBCs were obtained by hemolyzing the whole blood with ACK lysing buffer (Lonza, Basel, Switzerland). LPMC were isolated from intestinal specimens using a modification of the protocol described by Bull and Bookman (1977). In brief, the dissected mucosal tissue was cut into small pieces, incubated in HBSS (Sigma-Aldrich) containing 1 mM DTT and 1 mM EDTA (Sigma-Aldrich) for 45 min at 37°C and enzymatically digested with 0.25 mg/ml of collagenase D (Roche) and 0.01 mg/ml of DNase I (Roche) for 45–60 min at 37°C along with mechanical dissociation using a gentleMACS Dissociator (Miltenyi Biotec). Mesenteric LNs were harvested and passed through a 70-µm pore mesh to obtain the cellular suspension.
Flow cytometry analysis.
Whole blood cells (hemolyzed), PBMCs, mLN cells, and LPMCs were stained for surface antigens and fixed and stained for intracellular cytokine expression using fluoresceinated monoclonal antibodies to CD1c, CD4, CD8, CD11c, CD14, CD16, CD62L, CD66b, CD123, CD172a (clone SE5A5), CD172b (clone B4B6), CD197 (CCR7), CX3CR1, HLA-DR, IL-1β, IL-6, IL-8, IL-10, and TNF (BioLegend), CD45, CD209 (DC-SIGN) and CD324 (E-Cadherin; BD), Slan (Miltenyi Biotec), MHC class II (ID Laboratories), Alexa Fluor 647–conjugated CD47-Var1, and IgG1 control fusion proteins (Novartis Institute). Data were acquired and analyzed using the BD FACS Aria II system. FACS analysis was performed on CD45+ gated hematopoietic cells in LPMCs. The mean fluorescent intensity (MFI) ratio of positive and negative populations was used to normalize the signals to the background.
HLA-DR+CD172a+/memory CD4 T cell co-cultures.
Two subsets of effector memory CD45RA−CD25−CD62LloCD4 T cells (CCR7− and CCR7+; Kryczek et al., 2011) were isolated from mLNs. T cells were either cultured with 100 U/ml of IL-2 (R&D Systems) for 24 h in 96-well round-bottom plates in RPMI 1640 medium supplemented with 10% FCS penicillin and streptomycin or co-cultures with autologous HLA-DR+CD172a+ cells (CD14+CX3CR1+, CD14−CX3CR1+, and CD14−CX3CR1−) at a 5:1 ratio for 5 d in the presence of Staphylococcus aureus cowan 1 (at a dilution of 1/20,000) and CD47-Var1 or IgG1 control fusion proteins (10 µg/ml; Zielinski et al., 2012). T cells were restimulated by PMA (Sigma-Aldrich) and ionomycin (EMD Millipore) for the last 6 h of culture. Brefeldin A (EMD Millipore) was further added in the last 3 h of restimulation culture. Cell surface CD2 (BioLegend) staining followed by intracytoplasmic staining for IL-17 (R&D Systems) and IFN-γ (BioLegend) was performed and then analyzed by flow cytometry.
Statistical analysis.
The statistical analyses were performed on untransformed data unless otherwise indicated. Paired Student’s t tests or Wilcoxon paired tests were performed where applicable. For nonpaired data, Mann-Whitney tests or unpaired Student’s t tests with Welch correction were used. The Pearson test was used for correlation significance analysis.
Acknowledgments
We thank Dr. Michel Lemoyne, Dr. Eric DeBroux, and Dr. Richard Ratelle (CHUM) for their support to provide clinical samples for the research project. We also thank Dr. Guy Delespesse (CRCHUM) for critical comments and reading the manuscript.
This work was supported by the Canadian Institute for Health and Research, Crohn’s and Colitis Foundation of Canada, and grant in aid from Novartis Institute.
G. Weckbecker, F. Kolbinger, C. Heusser, T. Huber, and K. Welzenbach are working for the Novartis Institute of Biomedical Research, which is engaged in the development of medicine to treat autoimmune diseases, among other things. The other authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- CD
- Crohn’s disease
- CD47-Var1
- avidity-improved CD47 fusion protein
- IBD
- inflammatory bowel disease
- LP
- lamina propria
- LPMC
- LP mononuclear cell
- MDP
- macrophage-dendritic cell progenitor
- mLN
- mesenteric LN
- Mo-DC
- monocyte-derived DC
- MΦ
- macrophage
- precDC
- common DC precursor
References
- Acosta-Rodriguez E.V., Napolitani G., Lanzavecchia A., Sallusto F. 2007. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat. Immunol. 8:942–949 10.1038/ni1496 [DOI] [PubMed] [Google Scholar]
- Autschbach F., Braunstein J., Helmke B., Zuna I., Schürmann G., Niemir Z.I., Wallich R., Otto H.F., Meuer S.C. 1998. In situ expression of interleukin-10 in noninflamed human gut and in inflammatory bowel disease. Am. J. Pathol. 153:121–130 10.1016/S0002-9440(10)65552-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba N., Samson S., Bourdet-Sicard R., Rubio M., Sarfati M. 2008. Commensal bacteria trigger a full dendritic cell maturation program that promotes the expansion of non-Tr1 suppressor T cells. J. Leukoc. Biol. 84:468–476 10.1189/jlb.0108017 [DOI] [PubMed] [Google Scholar]
- Bain C.C., Scott C.L., Uronen-Hansson H., Gudjonsson S., Jansson O., Grip O., Guilliams M., Malissen B., Agace W.W., Mowat A.M. 2013. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6C(hi) monocyte precursors. Mucosal Immunol. 6:498–510 10.1038/mi.2012.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-On L., Zigmond E., Jung S. 2011. Management of gut inflammation through the manipulation of intestinal dendritic cells and macrophages? Semin. Immunol. 23:58–64 10.1016/j.smim.2011.01.002 [DOI] [PubMed] [Google Scholar]
- Birnberg T., Bar-On L., Sapoznikov A., Caton M.L., Cervantes-Barragán L., Makia D., Krauthgamer R., Brenner O., Ludewig B., Brockschnieder D., et al. 2008. Lack of conventional dendritic cells is compatible with normal development and T cell homeostasis, but causes myeloid proliferative syndrome. Immunity. 29:986–997 10.1016/j.immuni.2008.10.012 [DOI] [PubMed] [Google Scholar]
- Bogunovic M., Ginhoux F., Helft J., Shang L., Hashimoto D., Greter M., Liu K., Jakubzick C., Ingersoll M.A., Leboeuf M., et al. 2009. Origin of the lamina propria dendritic cell network. Immunity. 31:513–525 10.1016/j.immuni.2009.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun D., Galibert L., Nakajima T., Saito H., Quang V.V., Rubio M., Sarfati M. 2006. Semimature stage: a checkpoint in a dendritic cell maturation program that allows for functional reversion after signal-regulatory protein-alpha ligation and maturation signals. J. Immunol. 177:8550–8559 [DOI] [PubMed] [Google Scholar]
- Brown E.J., Frazier W.A. 2001. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 11:130–135 10.1016/S0962-8924(00)01906-1 [DOI] [PubMed] [Google Scholar]
- Bull D.M., Bookman M.A. 1977. Isolation and functional characterization of human intestinal mucosal lymphoid cells. J. Clin. Invest. 59:966–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerovic V., Houston S.A., Scott C.L., Aumeunier A., Yrlid U., Mowat A.M., Milling S.W. 2013. Intestinal CD103(-) dendritic cells migrate in lymph and prime effector T cells. Mucosal Immunol. 6:104–113 10.1038/mi.2012.53 [DOI] [PubMed] [Google Scholar]
- Chassaing B., Darfeuille-Michaud A. 2011. The commensal microbiota and enteropathogens in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 140:1720–1728 10.1053/j.gastro.2011.01.054 [DOI] [PubMed] [Google Scholar]
- Cheong C., Matos I., Choi J.H., Dandamudi D.B., Shrestha E., Longhi M.P., Jeffrey K.L., Anthony R.M., Kluger C., Nchinda G., et al. 2010. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell. 143:416–429 10.1016/j.cell.2010.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes J.L., Powrie F. 2008. Dendritic cells in intestinal immune regulation. Nat. Rev. Immunol. 8:435–446 10.1038/nri2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cros J., Cagnard N., Woollard K., Patey N., Zhang S.Y., Senechal B., Puel A., Biswas S.K., Moshous D., Picard C., et al. 2010. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 33:375–386 10.1016/j.immuni.2010.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Baey A., Mende I., Baretton G., Greiner A., Hartl W.H., Baeuerle P.A., Diepolder H.M. 2003. A subset of human dendritic cells in the T cell area of mucosa-associated lymphoid tissue with a high potential to produce TNF-alpha. J. Immunol. 170:5089–5094 [DOI] [PubMed] [Google Scholar]
- Denning T.L., Wang Y.C., Patel S.R., Williams I.R., Pulendran B. 2007. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat. Immunol. 8:1086–1094 10.1038/ni1511 [DOI] [PubMed] [Google Scholar]
- Diehl G.E., Longman R.S., Zhang J.X., Breart B., Galan C., Cuesta A., Schwab S.R., Littman D.R. 2013. Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX(3)CR1(hi) cells. Nature. 494:116–120 10.1038/nature11809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin G., Raymond M., Van V.Q., Rubio M., Gautier P., Sarfati M., Franchimont D. 2009. A role for CD47 in the development of experimental colitis mediated by SIRPalpha+CD103- dendritic cells. J. Exp. Med. 206:1995–2011 10.1084/jem.20082805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier E.L., Shay T., Miller J., Greter M., Jakubzick C., Ivanov S., Helft J., Chow A., Elpek K.G., Gordonov S., et al. ; Immunological Genome Consortium 2012. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat. Immunol. 13:1118–1128 10.1038/ni.2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F., Liu K., Helft J., Bogunovic M., Greter M., Hashimoto D., Price J., Yin N., Bromberg J., Lira S.A., et al. 2009. The origin and development of nonlymphoid tissue CD103+ DCs. J. Exp. Med. 206:3115–3130 10.1084/jem.20091756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm M.C., Pavli P., Van de Pol E., Doe W.F. 1995a. Evidence for a CD14+ population of monocytes in inflammatory bowel disease mucosa—implications for pathogenesis. Clin. Exp. Immunol. 100:291–297 10.1111/j.1365-2249.1995.tb03667.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm M.C., Pullman W.E., Bennett G.M., Sullivan P.J., Pavli P., Doe W.F. 1995b. Direct evidence of monocyte recruitment to inflammatory bowel disease mucosa. J. Gastroenterol. Hepatol. 10:387–395 10.1111/j.1440-1746.1995.tb01589.x [DOI] [PubMed] [Google Scholar]
- Herfarth H., Schölmerich J. 2002. IL-10 therapy in Crohn’s disease: at the crossroads. Treatment of Crohn’s disease with the anti-inflammatory cytokine interleukin 10. Gut. 50:146–147 10.1136/gut.50.2.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaensson E., Uronen-Hansson H., Pabst O., Eksteen B., Tian J., Coombes J.L., Berg P.L., Davidsson T., Powrie F., Johansson-Lindbom B., Agace W.W. 2008. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J. Exp. Med. 205:2139–2149 10.1084/jem.20080414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada N., Hisamatsu T., Okamoto S., Chinen H., Kobayashi T., Sato T., Sakuraba A., Kitazume M.T., Sugita A., Koganei K., et al. 2008. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J. Clin. Invest. 118:2269–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada N., Hisamatsu T., Honda H., Kobayashi T., Chinen H., Kitazume M.T., Takayama T., Okamoto S., Koganei K., Sugita A., et al. 2009. Human CD14+ macrophages in intestinal lamina propria exhibit potent antigen-presenting ability. J. Immunol. 183:1724–1731 10.4049/jimmunol.0804369 [DOI] [PubMed] [Google Scholar]
- Kaser A., Zeissig S., Blumberg R.S. 2010. Inflammatory bowel disease. Annu. Rev. Immunol. 28:573–621 10.1146/annurev-immunol-030409-101225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T., Okamoto S., Hisamatsu T., Kamada N., Chinen H., Saito R., Kitazume M.T., Nakazawa A., Sugita A., Koganei K., et al. 2008. IL23 differentially regulates the Th1/Th17 balance in ulcerative colitis and Crohn’s disease. Gut. 57:1682–1689 10.1136/gut.2007.135053 [DOI] [PubMed] [Google Scholar]
- Kryczek I., Zhao E., Liu Y., Wang Y., Vatan L., Szeliga W., Moyer J., Klimczak A., Lange A., Zou W. 2011. Human TH17 cells are long-lived effector memory cells. Sci. Transl. Med. 3:104ra100 10.1126/scitranslmed.3002949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latour S., Tanaka H., Demeure C., Mateo V., Rubio M., Brown E.J., Maliszewski C., Lindberg F.P., Oldenborg A., Ullrich A., et al. 2001. Bidirectional negative regulation of human T and dendritic cells by CD47 and its cognate receptor signal-regulator protein-alpha: down-regulation of IL-12 responsiveness and inhibition of dendritic cell activation. J. Immunol. 167:2547–2554 [DOI] [PubMed] [Google Scholar]
- Liu K., Victora G.D., Schwickert T.A., Guermonprez P., Meredith M.M., Yao K., Chu F.F., Randolph G.J., Rudensky A.Y., Nussenzweig M. 2009. In vivo analysis of dendritic cell development and homeostasis. Science. 324:392–397 10.1126/science.1171243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald T.T., Monteleone I., Fantini M.C., Monteleone G. 2011. Regulation of homeostasis and inflammation in the intestine. Gastroenterology. 140:1768–1775 10.1053/j.gastro.2011.02.047 [DOI] [PubMed] [Google Scholar]
- Maeda S., Hsu L.C., Liu H., Bankston L.A., Iimura M., Kagnoff M.F., Eckmann L., Karin M. 2005. Nod2 mutation in Crohn’s disease potentiates NF-kappaB activity and IL-1beta processing. Science. 307:734–738 10.1126/science.1103685 [DOI] [PubMed] [Google Scholar]
- Maloy K.J., Powrie F. 2011. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 474:298–306 10.1038/nature10208 [DOI] [PubMed] [Google Scholar]
- Medina-Contreras O., Geem D., Laur O., Williams I.R., Lira S.A., Nusrat A., Parkos C.A., Denning T.L. 2011. CX3CR1 regulates intestinal macrophage homeostasis, bacterial translocation, and colitogenic Th17 responses in mice. J. Clin. Invest. 121:4787–4795 10.1172/JCI59150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.C., Brown B.D., Shay T., Gautier E.L., Jojic V., Cohain A., Pandey G., Leboeuf M., Elpek K.G., Helft J., et al. ; Immunological Genome Consortium 2012. Deciphering the transcriptional network of the dendritic cell lineage. Nat. Immunol. 13:888–899 10.1038/ni.2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills K.H., Dunne A. 2009. Immune modulation: IL-1, master mediator or initiator of inflammation. Nat. Med. 15:1363–1364 10.1038/nm1209-1363 [DOI] [PubMed] [Google Scholar]
- Plantinga M., Guilliams M., Vanheerswynghels M., Deswarte K., Branco-Madeira F., Toussaint W., Vanhoutte L., Neyt K., Killeen N., Malissen B., et al. 2013. Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity. 38:322–335 10.1016/j.immuni.2012.10.016 [DOI] [PubMed] [Google Scholar]
- Raymond M., Rubio M., Fortin G., Shalaby K.H., Hammad H., Lambrecht B.N., Sarfati M. 2009. Selective control of SIRP-alpha-positive airway dendritic cell trafficking through CD47 is critical for the development of T(H)2-mediated allergic inflammation. J. Allergy Clin. Immunol. 124:1333–1342: e1 10.1016/j.jaci.2009.07.021 [DOI] [PubMed] [Google Scholar]
- Rivollier A., He J., Kole A., Valatas V., Kelsall B.L. 2012. Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J. Exp. Med. 209:139–155 10.1084/jem.20101387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäkel K., von Kietzell M., Hänsel A., Ebling A., Schulze L., Haase M., Semmler C., Sarfati M., Barclay A.N., Randolph G.J., et al. 2006. Human 6-sulfo LacNAc-expressing dendritic cells are principal producers of early interleukin-12 and are controlled by erythrocytes. Immunity. 24:767–777 10.1016/j.immuni.2006.03.020 [DOI] [PubMed] [Google Scholar]
- Schenk M., Bouchon A., Seibold F., Mueller C. 2007. TREM-1—expressing intestinal macrophages crucially amplify chronic inflammation in experimental colitis and inflammatory bowel diseases. J. Clin. Invest. 117:3097–3106 10.1172/JCI30602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulthess J., Meresse B., Ramiro-Puig E., Montcuquet N., Darche S., Bègue B., Ruemmele F., Combadière C., Di Santo J.P., Buzoni-Gatel D., Cerf-Bensussan N. 2012. Interleukin-15-dependent NKp46+ innate lymphoid cells control intestinal inflammation by recruiting inflammatory monocytes. Immunity. 37:108–121 10.1016/j.immuni.2012.05.013 [DOI] [PubMed] [Google Scholar]
- Schulz O., Jaensson E., Persson E.K., Liu X., Worbs T., Agace W.W., Pabst O. 2009. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J. Exp. Med. 206:3101–3114 10.1084/jem.20091925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura E., Valladeau-Guilemond J., Donnadieu M.H., Sastre-Garau X., Soumelis V., Amigorena S. 2012. Characterization of resident and migratory dendritic cells in human lymph nodes. J. Exp. Med. 209:653–660 10.1084/jem.20111457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura E., Touzot M., Bohineust A., Cappuccio A., Chiocchia G., Hosmalin A., Dalod M., Soumelis V., Amigorena S. 2013. Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity. 38:336–348 10.1016/j.immuni.2012.10.018 [DOI] [PubMed] [Google Scholar]
- Seiffert M., Brossart P., Cant C., Cella M., Colonna M., Brugger W., Kanz L., Ullrich A., Bühring H.J. 2001. Signal-regulatory protein alpha (SIRPalpha) but not SIRPbeta is involved in T-cell activation, binds to CD47 with high affinity, and is expressed on immature CD34(+)CD38(-) hematopoietic cells. Blood. 97:2741–2749 10.1182/blood.V97.9.2741 [DOI] [PubMed] [Google Scholar]
- Siddiqui K.R., Laffont S., Powrie F. 2010. E-cadherin marks a subset of inflammatory dendritic cells that promote T cell-mediated colitis. Immunity. 32:557–567 10.1016/j.immuni.2010.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P.D., Smythies L.E., Shen R., Greenwell-Wild T., Gliozzi M., Wahl S.M. 2011. Intestinal macrophages and response to microbial encroachment. Mucosal Immunol. 4:31–42 10.1038/mi.2010.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythies L.E., Sellers M., Clements R.H., Mosteller-Barnum M., Meng G., Benjamin W.H., Orenstein J.M., Smith P.D. 2005. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J. Clin. Invest. 115:66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C.M., Hall J.A., Blank R.B., Bouladoux N., Oukka M., Mora J.R., Belkaid Y. 2007. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 204:1775–1785 10.1084/jem.20070602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamoutounour S., Henri S., Lelouard H., de Bovis B., de Haar C., van der Woude C.J., Woltman A.M., Reyal Y., Bonnet D., Sichien D., et al. 2012. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur. J. Immunol. 42:3150–3166 10.1002/eji.201242847 [DOI] [PubMed] [Google Scholar]
- te Velde A.A., van Kooyk Y., Braat H., Hommes D.W., Dellemijn T.A., Slors J.F., van Deventer S.J., Vyth-Dreese F.A. 2003. Increased expression of DC-SIGN+IL-12+IL-18+ and CD83+IL-12-IL-18- dendritic cell populations in the colonic mucosa of patients with Crohn’s disease. Eur. J. Immunol. 33:143–151 10.1002/immu.200390017 [DOI] [PubMed] [Google Scholar]
- van den Berg T.K., van der Schoot C.E. 2008. Innate immune ‘self’ recognition: a role for CD47-SIRPalpha interactions in hematopoietic stem cell transplantation. Trends Immunol. 29:203–206 10.1016/j.it.2008.02.004 [DOI] [PubMed] [Google Scholar]
- Varol C., Vallon-Eberhard A., Elinav E., Aychek T., Shapira Y., Luche H., Fehling H.J., Hardt W.D., Shakhar G., Jung S. 2009. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 31:502–512 10.1016/j.immuni.2009.06.025 [DOI] [PubMed] [Google Scholar]
- Varol C., Zigmond E., Jung S. 2010. Securing the immune tightrope: mononuclear phagocytes in the intestinal lamina propria. Nat. Rev. Immunol. 10:415–426 10.1038/nri2778 [DOI] [PubMed] [Google Scholar]
- Verstege M.I., ten Kate F.J., Reinartz S.M., van Drunen C.M., Slors F.J., Bemelman W.A., Vyth-Dreese F.A., te Velde A.A. 2008. Dendritic cell populations in colon and mesenteric lymph nodes of patients with Crohn’s disease. J. Histochem. Cytochem. 56:233–241 10.1369/jhc.7A7308.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villani A.C., Lemire M., Fortin G., Louis E., Silverberg M.S., Collette C., Baba N., Libioulle C., Belaiche J., Bitton A., et al. 2009. Common variants in the NLRP3 region contribute to Crohn’s disease susceptibility. Nat. Genet. 41:71–76 10.1038/ng.285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos A.C., Wildenberg M.E., Arijs I., Duijvestein M., Verhaar A.P., de Hertogh G., Vermeire S., Rutgeerts P., van den Brink G.R., Hommes D.W. 2012. Regulatory macrophages induced by infliximab are involved in healing in vivo and in vitro. Inflamm. Bowel Dis. 18:401–408 10.1002/ibd.21818 [DOI] [PubMed] [Google Scholar]
- Weber B., Saurer L., Schenk M., Dickgreber N., Mueller C. 2011. CX3CR1 defines functionally distinct intestinal mononuclear phagocyte subsets which maintain their respective functions during homeostatic and inflammatory conditions. Eur. J. Immunol. 41:773–779 10.1002/eji.201040965 [DOI] [PubMed] [Google Scholar]
- Zhao X.W., van Beek E.M., Schornagel K., Van der Maaden H., Van Houdt M., Otten M.A., Finetti P., Van Egmond M., Matozaki T., Kraal G., et al. 2011. CD47-signal regulatory protein-α (SIRPα) interactions form a barrier for antibody-mediated tumor cell destruction. Proc. Natl. Acad. Sci. USA. 108:18342–18347 10.1073/pnas.1106550108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski C.E., Mele F., Aschenbrenner D., Jarrossay D., Ronchi F., Gattorno M., Monticelli S., Lanzavecchia A., Sallusto F. 2012. Pathogen-induced human TH17 cells produce IFN-γ or IL-10 and are regulated by IL-1β. Nature. 484:514–518 10.1038/nature10957 [DOI] [PubMed] [Google Scholar]