Retinoic acid attenuates colitis and is associated with increased IL-22 production from γδ T cells and innate lymphoid cells and enhanced antimicrobial peptide expression.
Abstract
Retinoic acid (RA), a vitamin A metabolite, modulates mucosal T helper cell responses. Here we examined the role of RA in regulating IL-22 production by γδ T cells and innate lymphoid cells in intestinal inflammation. RA significantly enhanced IL-22 production by γδ T cells stimulated in vitro with IL-1β or IL-18 and IL-23. In vivo RA attenuated colon inflammation induced by dextran sodium sulfate treatment or Citrobacter rodentium infection. This was associated with a significant increase in IL-22 secretion by γδ T cells and innate lymphoid cells. In addition, RA treatment enhanced production of the IL-22–responsive antimicrobial peptides Reg3β and Reg3γ in the colon. The attenuating effects of RA on colitis were reversed by treatment with an anti–IL-22 neutralizing antibody, demonstrating that RA mediates protection by enhancing IL-22 production. To define the molecular events involved, we used chromatin immunoprecipitation assays and found that RA promoted binding of RA receptor to the IL-22 promoter in γδ T cells. Our findings provide novel insights into the molecular events controlling IL-22 transcription and suggest that one key outcome of RA signaling may be to shape early intestinal immune responses by promoting IL-22 synthesis by γδ T cells and innate lymphoid cells.
The vitamin A metabolite retinoic acid (RA) is produced predominantly by DCs in the gut, skin, lungs, and their associated draining LNs (Guilliams et al., 2010). RA production by DCs is enhanced by inflammatory stimuli, and RA signaling is increased at sites of inflammation (Yokota et al., 2009; Pino-Lagos et al., 2011). The effect of RA is mediated by two classes of receptors, the RA receptors (RARs) and the retinoid X receptors, which act as transcription factors to regulate gene expression. These receptors are expressed by lymphoid cells, and recent studies have highlighted the importance of RA in regulating the homing capacity, activation, and differentiation of T cells (Iwata et al., 2004; Mora et al., 2006; Hall et al., 2011b). RA promotes induction of CD4+Foxp3+ T regulatory cells (Coombes et al., 2007; Denning et al., 2007; Sun et al., 2007) and inhibits the differentiation of IL-17–producing CD4+ T helper cells (Th17; Mucida et al., 2007; Elias et al., 2008). Although RA plays a role in immune homeostasis and the maintenance of intestinal tolerance in the steady-state, it has the reciprocal role of promoting effector T cell responses during infection or autoimmune inflammation (DePaolo et al., 2011; Hall et al., 2011a).
IL-22 in the intestine induces epithelial cell repair and secretion of antimicrobial peptides that limit bacterial dissemination and intestinal inflammation (Zheng et al., 2008; Sonnenberg et al., 2012). IL-22–deficient mice are more susceptible to colitis (Zenewicz et al., 2008), and IL-22 production is increased in the intestine of patients with Crohn’s disease or ulcerative colitis (Geremia et al., 2011); however, little is known about the regulatory pathways controlling IL-22 production. The IL-23R signaling pathway and the nuclear factors aryl hydrocarbon receptor (AhR) and RAR-related orphan receptor gamma (RORγt) have been implicated in promoting IL-22 (Simonian et al., 2010; Qiu et al., 2012), although how these pathways interact with the IL-22 locus and the requirement for additional factors have not been investigated. γδ T cells and RORγt-expressing lamina propria innate lymphoid cells (ILC3; Spits et al., 2013) are two key sources of innate IL-22 (Chen et al., 2002; Sutton et al., 2009; Simonian et al., 2010; Li et al., 2011; Sawa et al., 2011; Spits and Di Santo, 2011), although IL-22 expression is not limited to these cell types (Zenewicz et al., 2007).
In the present study, we show that RA protects against colitis by promoting innate IL-22 production. RA enhanced IL-22 production by γδ T cells and ILC3, and this corresponded with attenuated dextran sodium sulfate (DSS)– and Citrobacter rodentium infection–induced colon inflammation.
RESULTS AND DISCUSSION
RA enhances IL-22 production by LN γδ T cells and intestinal ILC3
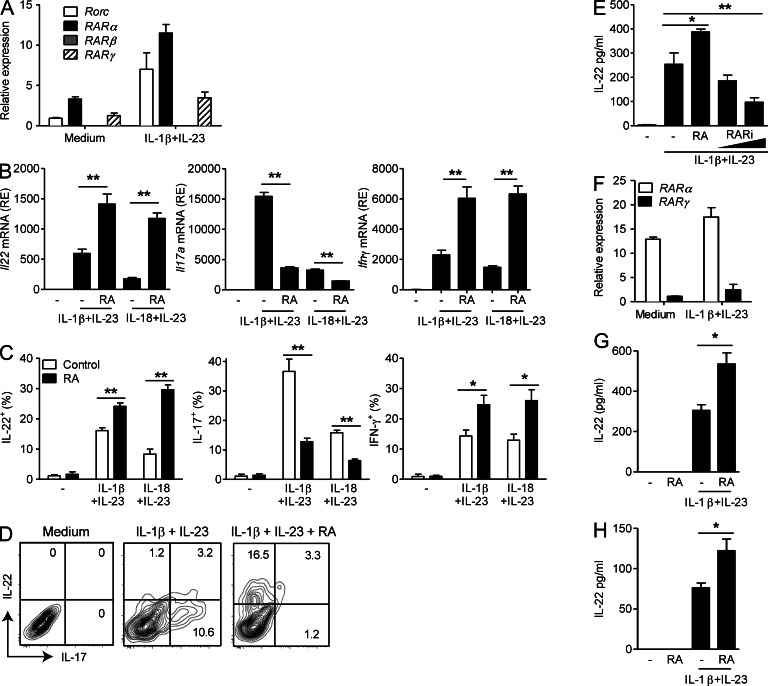
We have previously shown that γδ T cells in LNs of mice can produce IL-22 in response to IL-1β or IL-18 with IL-23 independent of TCR stimulation (Sutton et al., 2009; Lalor et al., 2011), but the effects of RA on IL-22 production have not been investigated. Purified LN γδ T cells expressed both Rarα and Rarγ, and their expression increased upon stimulation with IL-1β and IL-23, as did Rorc expression (Fig. 1 A).
Figure 1.
RA enhances IL-22 production by γδ T cells and ILC3. (A) Relative mRNA expression of Rorc, Rarα, -β, and -γ in purified γδ T cells from LNs, ±IL-1β and IL-23 stimulation for 48 h. (B) Relative mRNA expression (RE) of Il22, Il17a, and Ifnγ in LN γδ T cells stimulated with IL-1β and IL-23 or IL-18 and IL-23 with or without RA. (C) IL-22, IL-17, and IFN-γ production by ICS on purified LN γδ T cells stimulated with IL-1β and IL-23 or IL-18 and IL-23 ± RA or vehicle control for 72 h (mean ± SE). (D) ICS on purified LN γδ T cells stimulated with IL-1β and IL-23 ± RA. (E) IL-22 production by ELISA on purified LN γδ T cells stimulated with IL-1β and IL-23 for 72 h ± 100 nM RA or 0.5 or 5.0 µM RARi (mean ± SD). (F) Relative mRNA expression of Rarα and -γ in FACS-sorted lamina propria NCR+ ILC3 (CD3−CD19−CD11c−NK1.1−NKp46+) with and without stimulation with IL-1β and IL-23 for 48 h. (A, B, and F) Results are mean and SD values for triplicate samples. (G and H) IL-22 production detected by ELISA on lamina propria NCR+ ILC3 (G) or γδ T cells (H) stimulated with IL-1β and IL-23 ± RA (mean ± SD). Results are representative of two to four independent experiments (n = 3 for A, B, E, and F; n = 4 for C, G, and H; D is representative of four samples). *, P < 0.05; and **, P < 0.01 versus DMSO control.
We examined the effect of RA on IL-22 production by LN γδ T cells. Addition of RA to purified γδ T cells significantly enhanced Il22 mRNA production induced by IL-1β and IL-23 or IL-18 and IL-23 (Fig. 1 B). RA also enhanced IFN-γ but suppressed IL-17 production by γδ T cells (Fig. 1 B). We observed similar results when cytokine production was analyzed by flow cytometry (Fig. 1 C). CD27− γδ T cells produced IL-17 and IL-22 after stimulation with IL-1β and IL-23 (Fig. 1 D and not depicted), and RA appears to act as a molecular switch to inhibit IL-17 and promote IL-22 production. Treatment with an RAR inhibitor (RARi) hindered IL-22 production induced by purified γδ T cells stimulated with IL-1β and IL-23 (Fig. 1 E).
NKp46+ ILC3 (NCR+ ILC3) purified from the intestinal lamina propria also expressed Rarα and Rarγ (Fig. 1 F). Furthermore, RA enhanced IL-22 production by NCR+ ILC3 (Fig. 1 G) and γδ T cells from the lamina propria (Fig. 1 H). In contrast, RA did not enhance IL-22 production by CD4+ T cells, although it did suppress IL-17 (not depicted). These results suggest that RA plays an important role in enhancing innate lymphocyte production of IL-22.
DSS treatment induces RA production in the colon, and RA is protective against colon inflammation
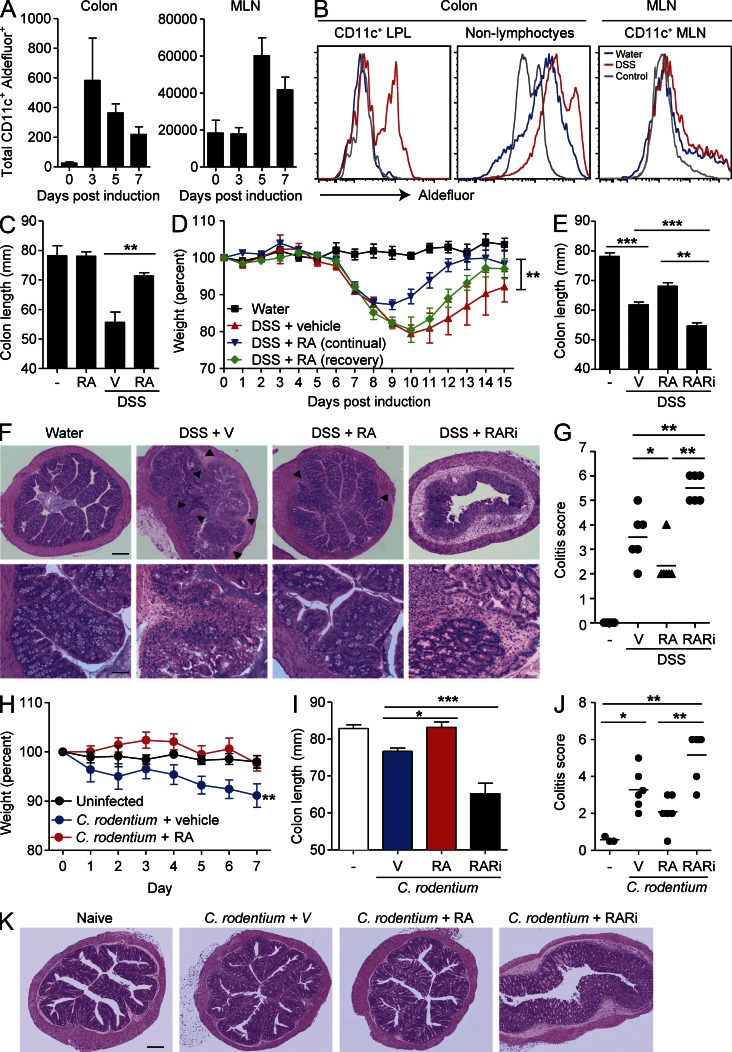
We tested the hypothesis that RA acts to enhance IL-22 production in vivo leading to protection against colitis. First we examined whether endogenous RA is produced during development of DSS-induced colitis. We examined expression of active aldehyde dehydrogenases, enzymes involved in RA production, by cleavage of a fluorescent synthetic substrate, ALDEFLUOR, which accumulates within the cell after cleavage. The total number of ALDEFLUOR+CD11c+ DCs dramatically increased in the colon of mice treated with DSS, peaking on day 3, and remained increased at day 7 (Fig. 2 A). ALDEFLUOR+CD11c+ DCs were also enhanced in mesenteric LNs (MLNs) and peaked 5 d after DSS treatment (Fig. 2, A and B). We also observed an increase in ALDEFLUOR staining of non-lymphocytes in the colon (Fig. 2 B). These results show that RA production by DCs and non-lymphocytes (possibly epithelial or stromal cells) increases during intestinal inflammation and may play a role in controlling DSS-induced inflammation.
Figure 2.
RA protects against DSS-induced colitis. (A and B) Colon LPLs and MLNs were prepared from naive mice or mice treated with 2% DSS in their drinking water for 3–7 d. Cells were stained with ALDEFLUOR, CD45, and anti-CD11c. (A) Mean ± SEM (n = 5) ALDEFLUOR+CD11c+ cells. (B) Sample FACS plots of CD11c+ cells and non-lymphocytes (CD45− cells) from naive mice (blue) or DSS-treated mice (red) isolated from colon or MLNs. ALDEFLUOR-negative control is shown in gray. (C) Mice were given normal water or 2% DSS in their drinking water for 7 d, and every second day mice were treated i.p. with 200 µg RA or vehicle (V) only. Colon lengths were recorded on day 7 (n = 6). (D) Mice were given water or 2% DSS in their drinking water for 7 d and were then allowed to recover with normal drinking water for a further 7 d. Mice were treated every second day i.p. with 200 µg RA or vehicle from days 1–7 or only in the recovery period (days 7–14). (E) Mice were treated with normal water or 2% DSS in their drinking water for 7 d, and every second day mice were treated i.p. with 200 µg RA, 400 µg RARi, or vehicle. Colon lengths were recorded on day 7 (mean ± SE; n = 6). (F) Sections from the ascending colons were stained with H&E. Areas of inflammatory cell infiltration are shown as arrowheads. (G) Histological scores for inflammatory cell infiltration and tissue disruption. (H–K) Mice were infected with 2 × 109 CFUs of C. rodentium and treated with RA or vehicle, and weight loss was monitored. Weights (H), colon lengths (I), colitis scores (J), and histology on day 7 (K) in mice infected with C. rodentium and treated with RA or RARi. (G and J) Horizontal lines are means. Bars: (F [top] and K) 160 µm; (F, bottom) 45 µm. Results in all panels are mean ± SE (n = 5–6 mice) from two to three independent experiments or representative sections from one of six mice per group. *, P < 0.05; **, P < 0.01; and ***, P < 0.001 versus vehicle.
Previous studies showed that mice reared on a vitamin A–deficient diet or mice lacking RARα have altered gut homeostasis resulting from defects in T helper cell activation, goblet cell hyperplasia, and alterations in the gut microbiome (Cha et al., 2010; Hall et al., 2011a). Treatment of mice with DSS results in damage to epithelial cells in the colon, thereby compromising barrier function and leading to inflammation, characterized by loss of crypt structure and gross shortening of the colon. Treatment of mice with RA for 7 d significantly reduced the DSS-induced colon shortening when compared with mice treated with DSS alone (Fig. 2 C). RA treatment also promoted recovery from colitis, even if administered after colitis was established (Fig. 2 D). To examine the role of endogenous RA without the possible conditioning effects of rearing mice on a vitamin A–deficient diet, we treated mice with an RARi. Blocking RA signaling enhanced colon shortening induced by DSS (Fig. 2 E). Histopathological analysis revealed that mice treated with DSS had morphological changes in their ascending colon, with crypt damage and inflammatory cell infiltrate characteristic of acute colitis, and that this intestinal inflammation was reversed by treatment with RA and exacerbated by treatment with RARi (Fig. 2, F and G). RA also reversed early weight loss (Fig. 2 H), colon shortening (Fig. 2 I), and intestinal inflammation (Fig. 2, J and K) induced by infection of mice with C. rodentium. RARi treatment exacerbated intestinal damage, as determined by colon shortening and histology, induced by C. rodentium infection (Fig. 2, I–K). These findings demonstrate that treatment with RA protects against intestinal damage in two models of colitis and suggest that endogenous RA plays a role in controlling intestinal inflammation.
RA increases IL-22 production in the colon
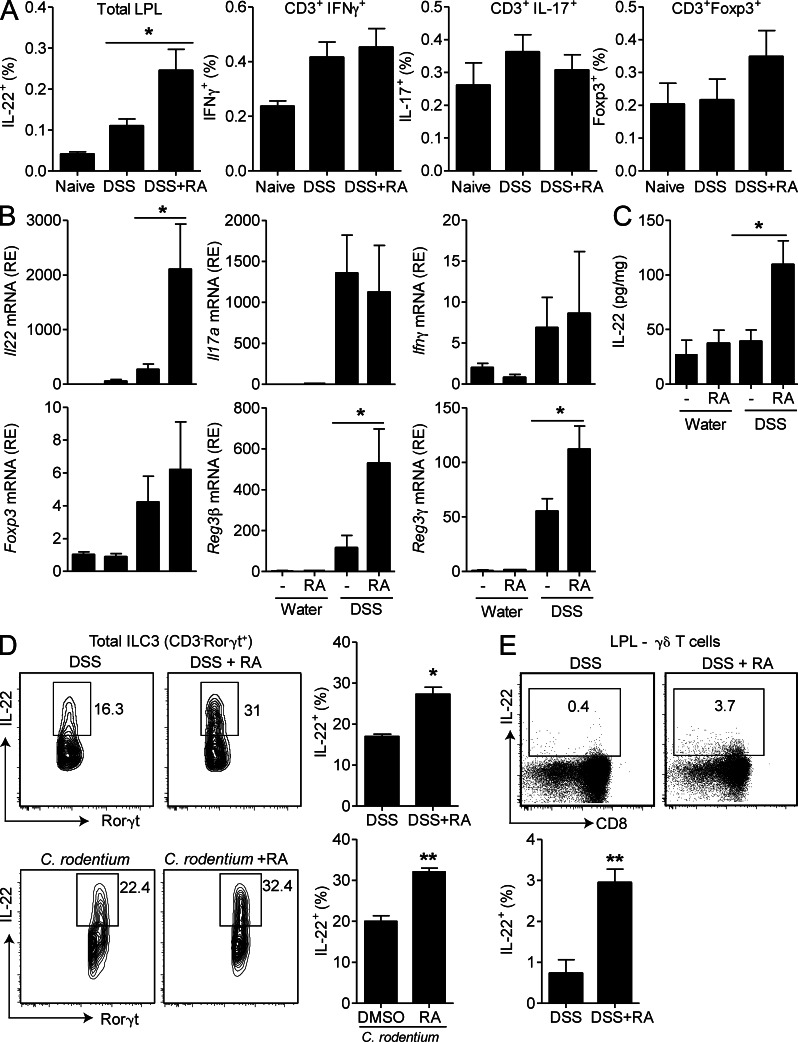
It has been reported that RA modulates production of IL-17 and IFN-γ by CD4+ T cells, cytokines thought to promote intestinal inflammation (Mucida et al., 2007; Elias et al., 2008; DePaolo et al., 2011). We have demonstrated that RA promotes IL-22 secretion by NCR+ ILC3 and γδ T cells ex vivo and that administration of RA attenuates intestinal inflammation. Here we found that development of DSS-induced colitis was associated with an increase in IL-22–, IFN-γ–, IL-17–expressing cells (Fig. 3 A) and/or mRNA (Fig. 3 B). Treatment with RA did not significantly affect Il17, Ifnγ, or Foxp3 but significantly enhanced expression of Il22 mRNA (Fig. 3 B) and IL-22 protein (Fig. 3, A and C) in colons. Furthermore, expression of the antimicrobial peptides Reg3β and Reg3γ, which are produced by intestinal epithelial cells in response to IL-22, were also increased in the colons of mice treated with RA (Fig. 3 B). RA enhanced IL-22 production by ILC3 during colitis induced by DSS treatment or C. rodentium infection (Fig. 3 D). Furthermore, RA treatment enhanced the frequency of IL-22–secreting γδ T cells (Fig. 3 E). These results suggest that treatment with RA stimulates IL-22 production by ILC3 and γδ T cells during intestinal inflammation.
Figure 3.
RA enhances IL-22 and antimicrobial peptide expression in the intestine. (A–D) Mice were treated with DSS ± RA, as described in Fig. 2. (A) LPLs were purified and ICS performed for IL-22, IL-17, IFN-γ, and Foxp3; gated on total LPLs or CD3+ LPLs as indicated and pooled data (n = 6). (B and C) Colons were removed on day 7, and relative mRNA expression (RE) of Il22, Il17a, Ifnγ, Foxp3, Reg3β, and Reg3γ was quantified by RT-PCR (B), and IL-22 concentrations in colon homogenates were quantified by ELISA (C; mean ± SEM; n = 6). (D and E) LPLs were purified from the small intestine of RA-treated mice after DSS treatment or C. rodentium infection, and ICS was performed for IL-22 and quantified by flow cytometry. (D) Representative plots of CD3−Rorγt+ ILC3; pooled data are shown in the right panel (n = 6). (E) Representative plots of γδ T cells (CD3+γδTCR+), and pooled data are shown in the right panel (n = 6). *, P < 0.05; and **, P < 0.01 versus control. Results are representative of two or three independent experiments.
IL-22 and γδ T cells mediate the protective effect of RA in intestinal inflammation
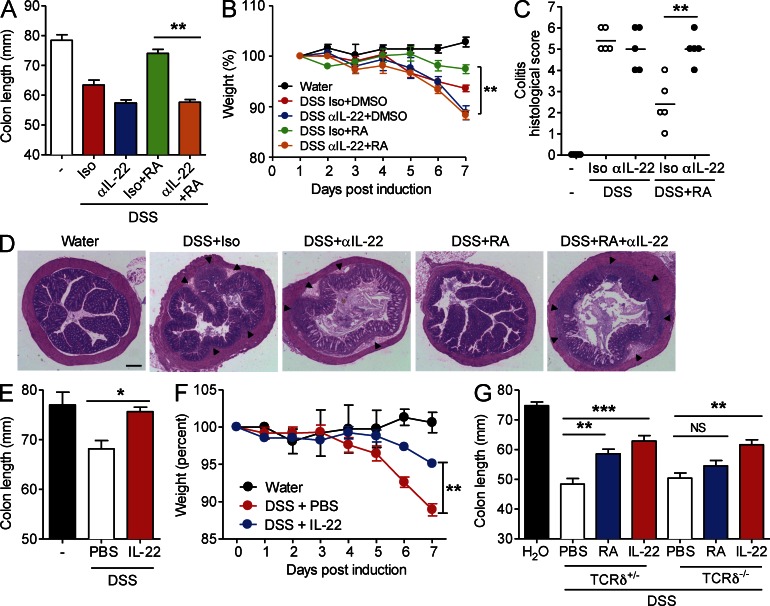
We have shown that RA enhances IL-22 production by γδ T cells and that RA protects against colon inflammation. To test whether the protective effect of RA is mediated through IL-22, we neutralized IL-22 in vivo. Mice treated with DSS and isotype control antibody developed colitis, and disease symptoms were reduced by treatment with RA (Fig. 4, A and B). In contrast, administration of neutralizing antibodies to IL-22 reversed the protective effect of RA on colon length (Fig. 4 A) and weight loss (Fig. 4 B). Histological analysis revealed that anti–IL-22 reversed the protective effect of RA on colon inflammation and no longer prevented crypt damage in the colons of mice with DSS-induced colitis (Fig. 4, C and D). To confirm that IL-22 plays an antiinflammatory role in DSS-induced colitis, we examined the effect of direct administration of rIL-22. Treatment of wild-type mice with rIL-22 reversed colon shortening (Fig. 4 E) to a similar degree as treatment with RA (Fig. 2 B) and protected against weight loss (Fig. 4 F). We next used TCRδ−/− mice to examine the role of γδ T cells in mediating the protective effect of RA in colitis. We did not observe protection against colon shortening in TCRδ−/− mice treated with RA, whereas RA did protect TCRδ+/− littermate controls (Fig. 4 G). In contrast, treatment with rIL-22 significantly reduced colon shortening in TCRδ−/− mice (Fig. 4 G). Collectively, our study demonstrates that endogenous IL-22 is protective against DSS-induced colon inflammation and that administration of IL-22 can enhance protection. In addition, RA attenuates intestinal inflammation at least in part by enhancing IL-22 production by γδ T cells, ILC3, and possibly other cell types.
Figure 4.
IL-22 mediates the protective effect of RA against DSS-induced colitis. (A–C) Mice were treated with DSS ± RA as described in Fig. 2 and given anti–IL-22 or 500 µg of an isotype control antibody i.p. once on day 0. (A) Colon lengths were recorded on day 7 (mean ± SD; n = 5). (B) Weights of mice were recorded daily (mean ± SD; n = 5). **, P < 0.01. (C) Histological scores for inflammatory cell infiltration and tissue disruption after H&E staining of sections from the ascending colons of mice (n = 5). Horizontal lines are means. **, P < 0.01 for anti–IL-22 + RA versus isotype + RA. (D) Representative sections with areas of inflammatory cell infiltration shown as arrowheads. Bar, 160 µm. (E and F) WT mice were treated with normal water or 2% DSS in their drinking water for 7 d and were treated i.p. with 500 ng rIL-22 or PBS as a control (mice were treated every day for 7 d). (E) Colon lengths were recorded on day 7; mean ± SEM (n = 6); *, P < 0.05. (F) Mice were weighed daily; mean ± SD (n = 6); **, P < 0.01 for rIL-22 versus PBS. (G) TCRδ−/− or litter mate control TCRδ+/− mice were treated with 2% DSS in their drinking water for 7 d and were treated i.p. with RA every second day or treated with rIL-22 daily, and colon lengths were recorded (mean ± SD; n = 5). **, P < 0.01; and ***, P < 0.001.
RAR binds the Il22 promoter
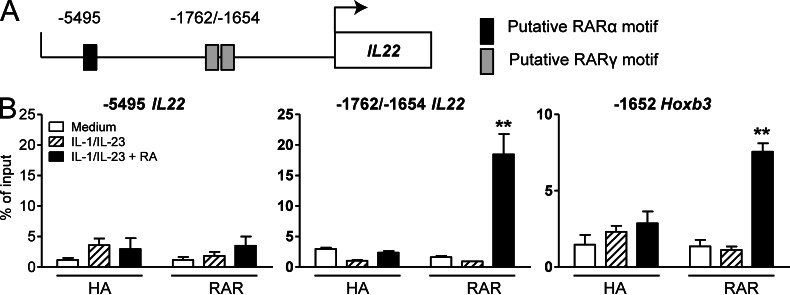
The molecular events controlling Il22 transcription are not well defined. We determined the effect of RA on transcription of factors known to regulate IL-22 production and found that treatment of γδ T cells with IL-1β and IL-23 enhanced Il1r, Il23r, and Rorγt expression, but addition of RA did not influence their expression or the expression of Arnt, Ahr, or Cyp1a1, a known target gene of AhR (not depicted). In addition, RA did not enhance expression of the γδ TCR (not depicted). Our in vitro studies with γδ T cells were performed in the absence of a TCR stimulus, suggesting that RA acts independently of TCR signaling to enhance IL-22 production. Because RARs act as transcription factors, we examined the possibility that RARα and RARγ act directly on the Il22 locus. We performed promoter analysis and found putative binding sites for RARα and RARγ in the promoter region of Il22 (Fig. 5 A). Using chromatin immunoprecipitation with a pan-RAR antibody, we observed binding of RAR to a site containing two predicted RARγ-binding motifs (−1762 bp/−1654 bp) in the Il22 promoter in γδ T cells. Importantly, binding of RAR to this site was detected in cells stimulated with IL-1β, IL-23, and RA but not with IL-1β and IL-23 alone (Fig. 5 B). We did not observe any enrichment of the RARα-binding site (−5495 bp) in the promoter region of Il22, indicating that RARs were not bound to this site under these conditions. Binding of RAR to the promoter of Hoxb3 was included as a positive control, as it is a known RAR target gene (Fig. 5 B, right). Our findings show that RAR transcription factors bind to the Il22 promoter and provide a plausible mechanism whereby RA directly promotes Il22 transcription.
Figure 5.
RAR binds the Il22 promoter to enhance Il22 transcription. (A) Putative RARα and RARγ response elements in the mouse Il22 promoter region. The transcription initiation site was designated as 1. (B) A pan-RAR antibody (or control HA antibody) was used to detect binding of RAR to the promoter region of Il22 in LN γδ T cells after stimulation with IL-1β, IL-23, and RA. The predicted RARα-binding site is located −5,495 bp upstream, whereas the predicted adjacent RARγ-binding sites are −1,762/−1,654 bp upstream of the transcription initiation site. RAR binding to the Hoxb3 promoter region is shown as a positive control. Data shown are mean ± SEM (n = 4). **, P < 0.01.
In this study, we have identified RAR as novel transcriptional regulators of the Il22 promoter. We have also demonstrated a previously unidentified function for RA in enhancing IL-22 production by γδ T cells and ILC3 and protecting against colon inflammation by initiating the repair process in the intestine. Together these findings suggest that RA can enhance innate lymphocyte function, integrating and enhancing signals from the environment whether they are pro- or antiinflammatory to promote CD4+ effector responses. Our study also demonstrates that RA initiates tissue repair by increasing innate IL-22 production by γδ T cells and innate lymphoid cells.
MATERIALS AND METHODS
Mice.
C57BL/6 mice were obtained from Harlan UK and maintained at Trinity College Dublin in a specific pathogen–free facility. TCRδ-deficient mice were obtained from the Jackson Laboratory. Animal protocols were reviewed and approved by the Trinity College Dublin animal ethics committee.
Induction and assessment of DSS colitis.
Mice were given 2% DSS (molecular weight: 36,000-50,000; MP Biomedicals) in their drinking water for 7 d, and mice were weighed every 24 h. Mice were treated i.p. with 200 µg all-trans RA (Enzo Life Sciences), 400 µg RARi (BMS 493; Tocris Bioscience), or DMSO as a control every second day. Where indicated, mice were given one i.p. dose of 500 µg anti–IL-22 (clone IL22JOP; eBioscience) or 500 µg rat IgG2a κ isotype control (eBioscience). In some experiments, mice were treated every day i.p. with 500 ng mouse rIL-22 (R&D Systems). Sections from the ascending colon of each mouse were analyzed using hematoxylin and eosin (H&E) staining. Colitis severity was assessed by a combined score of colon cellular infiltration (0–3, according to the number and localization of the inflammatory cells) and tissue disruption (0–3, according to the severity of mucosal and crypts damages) as described previously (Smith et al., 2007). The histological scoring was performed in a blinded fashion.
Intestinal inflammation induced by C. rodentium infection.
Mice were inoculated with 2 × 109 CFUs of C. rodentium by oral gavage. Mice were treated i.p. with 400 µg RARi (BMS 493) or 200 µg RA every second day. Mice were weighed daily and analyzed between 6 and 8 d after infection.
Cell preparation and stimulation.
LN γδ T cells were sorted using a mouse γδ T cell isolation kit (MACS; Miltenyi Biotech). Cells were cultured in cRPMI (RPMI containing 10% fetal calf serum, 100 U/ml penicillin, 100 µg/ml streptomycin, 2 mM l-glutamine [Invitrogen], and 50 µM 2-ME [Sigma-Aldrich]) with 10 ng/ml IL-1β, 10 ng/ml IL-23, or 10 ng/ml IL-18 and 100 nM all-trans RA or 0.5 or 5.0 µM RARi. IL-22 concentrations in cell supernatants were determined by ELISA (R&D Systems). Intestinal lamina propria lymphocytes (LPLs) were extracted from the small intestine. In brief, the small intestine was collected and the Peyer’s patches removed. Intestines were opened longitudinally and cut into small pieces (<5 mm). Intraepithelial cells were removed by washing with HBSS and incubating with 5 mM EDTA for 20 min at 37°C. The intestinal pieces were washed with cRPMI, and LPLs were isolated by digestion with 1 µg/ml DNase (Sigma-Aldrich) and 500 µg/ml Collagenase D (Roche) for 40 min at 37°C. The LPL fractions were purified by 40/70% Percoll (GE Healthcare) gradient. In some experiments, NCR+ ILC3 (CD3−CD19−CD11c−NK1.1−NKp46+ cells) were sorted by FACS (MoFlo; Dako). Lamina propria γδ T cells were identified by sorting CD3+γδTCR+ LPLs.
Flow cytometry.
Purified γδ T cells were stimulated for 48–72 h, and brefeldin A (Sigma-Aldrich) was added for the last 4 h of culture. LPLs were restimulated with IL-1β and IL-23 for 12 h in the presence of brefeldin A. Cells were stained for surface markers CD3 (clone 500A2; BD), CD8 (clone 53-6.7; eBioscience), γδ TCR (clone eBioGL3; eBioscience), NKp46 (clone 29A1.4; eBioscience), or CD11c (clone N418; eBioscience). Intracellular cytokine staining (ICS) was performed with an IntraStain kit (Dako) or with Foxp3 fixation/permeabilization concentrate and diluent buffers (eBioscience) when staining for RORγt. Antibodies for ICS include IL-22 (clone 1H8PWSR; eBioscience), IL-17A (clone eBio17B7; eBioscience), IFN-γ (clone XMG1.2; eBioscience), and RORγt (clone B2D; eBioscience). Samples were analyzed with a FACSCanto (BD) with FlowJo software (Tree Star), with isotype or unstained controls to determine gating. The presence of cells displaying aldehyde dehydrogenase activity was determined using an ALDEFLUOR staining kit (STEMCELL Technologies) as per the manufacturer’s instructions.
Real-time PCR.
After cell stimulation or homogenization of colon sections, RNA was extracted using an RNeasy kit (QIAGEN) per the manufacturer’s instructions. For samples from mice treated with DSS, mRNA was further purified using the Dynabeads mRNA purification kit (Invitrogen). RT was performed using high-capacity cDNA RT kit (Applied Biosystems) followed by real-time PCR using an ABI PRISM7500 Sequence Detection System (Applied Biosystems). Analysis of Il22, Il17a, Ifnγ, Foxp3, Reg3β, Reg3γ, Il1r, Il23r, Rorγt, Arnt, Ahr, or Cyp1a1 mRNA levels was performed using commercially available primer/probe sets (Applied Biosystems). Relative levels of expression were determined by normalization to Gapdh or 18S rRNA.
Chromatin immunoprecipitation.
Purified LN γδ T cells were stimulated for 48 h with IL-1β and IL-23 (both 10 ng/ml) in the presence or absence or RA and fixed with 1% formaldehyde. Cell lysates were sheared and immunoprecipitated with pan-RAR antibody (clone M-545; Santa Cruz Biotechnology, Inc.) or a control HA antibody (Santa Cruz Biotechnology, Inc.). Bound DNA was purified and analyzed by quantitative PCR for enrichment of predicted RAR-binding sites. Primer sequences are available on request.
Statistical analysis.
Data were compared by two-tailed Student’s t test, one-way ANOVA, or Mann–Whitney U test. Where significant differences were found, the Tukey-Kramer multiple comparisons test was used for identifying differences between individual groups.
Acknowledgments
We thank B. Moran for technical help with FACS.
This work was supported by a Science Foundation Ireland Strategic Research Cluster grant and a Principal Investigator Award to K.H.G. Mills.
K.H.G. Mills is Co-Founder and shareholder of Opsona Therapeutics Ltd. and TriMod Therapeutics Ltd., University start-up companies involved in the development of immunotherapeutics.
Footnotes
Abbreviations used:
- AhR
- aryl hydrocarbon receptor
- DSS
- dextran sodium sulfate
- ICS
- intracellular cytokine staining
- LPL
- lamina propria lymphocyte
- MLN
- mesenteric LN
- RA
- retinoic acid
- RAR
- RA receptor
- RARi
- RAR inhibitor
References
- Cha H.R., Chang S.Y., Chang J.H., Kim J.O., Yang J.Y., Kim C.H., Kweon M.N. 2010. Downregulation of Th17 cells in the small intestine by disruption of gut flora in the absence of retinoic acid. J. Immunol. 184:6799–6806 10.4049/jimmunol.0902944 [DOI] [PubMed] [Google Scholar]
- Chen Y., Chou K., Fuchs E., Havran W.L., Boismenu R. 2002. Protection of the intestinal mucosa by intraepithelial gamma delta T cells. Proc. Natl. Acad. Sci. USA. 99:14338–14343 10.1073/pnas.212290499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes J.L., Siddiqui K.R., Arancibia-Cárcamo C.V., Hall J., Sun C.M., Belkaid Y., Powrie F. 2007. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β– and retinoic acid–dependent mechanism. J. Exp. Med. 204:1757–1764 10.1084/jem.20070590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning T.L., Wang Y.C., Patel S.R., Williams I.R., Pulendran B. 2007. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat. Immunol. 8:1086–1094 10.1038/ni1511 [DOI] [PubMed] [Google Scholar]
- DePaolo R.W., Abadie V., Tang F., Fehlner-Peach H., Hall J.A., Wang W., Marietta E.V., Kasarda D.D., Waldmann T.A., Murray J.A., et al. 2011. Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens. Nature. 471:220–224 10.1038/nature09849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias K.M., Laurence A., Davidson T.S., Stephens G., Kanno Y., Shevach E.M., O’Shea J.J. 2008. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood. 111:1013–1020 10.1182/blood-2007-06-096438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geremia A., Arancibia-Cárcamo C.V., Fleming M.P., Rust N., Singh B., Mortensen N.J., Travis S.P., Powrie F. 2011. IL-23–responsive innate lymphoid cells are increased in inflammatory bowel disease. J. Exp. Med. 208:1127–1133 10.1084/jem.20101712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilliams M., Crozat K., Henri S., Tamoutounour S., Grenot P., Devilard E., de Bovis B., Alexopoulou L., Dalod M., Malissen B. 2010. Skin-draining lymph nodes contain dermis-derived CD103(-) dendritic cells that constitutively produce retinoic acid and induce Foxp3(+) regulatory T cells. Blood. 115:1958–1968 10.1182/blood-2009-09-245274 [DOI] [PubMed] [Google Scholar]
- Hall J.A., Cannons J.L., Grainger J.R., Dos Santos L.M., Hand T.W., Naik S., Wohlfert E.A., Chou D.B., Oldenhove G., Robinson M., et al. 2011a. Essential role for retinoic acid in the promotion of CD4(+) T cell effector responses via retinoic acid receptor alpha. Immunity. 34:435–447 10.1016/j.immuni.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J.A., Grainger J.R., Spencer S.P., Belkaid Y. 2011b. The role of retinoic acid in tolerance and immunity. Immunity. 35:13–22 10.1016/j.immuni.2011.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata M., Hirakiyama A., Eshima Y., Kagechika H., Kato C., Song S.Y. 2004. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 21:527–538 10.1016/j.immuni.2004.08.011 [DOI] [PubMed] [Google Scholar]
- Lalor S.J., Dungan L.S., Sutton C.E., Basdeo S.A., Fletcher J.M., Mills K.H. 2011. Caspase-1-processed cytokines IL-1beta and IL-18 promote IL-17 production by gammadelta and CD4 T cells that mediate autoimmunity. J. Immunol. 186:5738–5748 10.4049/jimmunol.1003597 [DOI] [PubMed] [Google Scholar]
- Li Y., Innocentin S., Withers D.R., Roberts N.A., Gallagher A.R., Grigorieva E.F., Wilhelm C., Veldhoen M. 2011. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 147:629–640 10.1016/j.cell.2011.09.025 [DOI] [PubMed] [Google Scholar]
- Mora J.R., Iwata M., Eksteen B., Song S.Y., Junt T., Senman B., Otipoby K.L., Yokota A., Takeuchi H., Ricciardi-Castagnoli P., et al. 2006. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 314:1157–1160 10.1126/science.1132742 [DOI] [PubMed] [Google Scholar]
- Mucida D., Park Y., Kim G., Turovskaya O., Scott I., Kronenberg M., Cheroutre H. 2007. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 317:256–260 10.1126/science.1145697 [DOI] [PubMed] [Google Scholar]
- Pino-Lagos K., Guo Y., Brown C., Alexander M.P., Elgueta R., Bennett K.A., De Vries V., Nowak E., Blomhoff R., Sockanathan S., et al. 2011. A retinoic acid–dependent checkpoint in the development of CD4+ T cell–mediated immunity. J. Exp. Med. 208:1767–1775 10.1084/jem.20102358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J., Heller J.J., Guo X., Chen Z.M., Fish K., Fu Y.X., Zhou L. 2012. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 36:92–104 10.1016/j.immuni.2011.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa S., Lochner M., Satoh-Takayama N., Dulauroy S., Bérard M., Kleinschek M., Cua D., Di Santo J.P., Eberl G. 2011. RORγt+ innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nat. Immunol. 12:320–326 10.1038/ni.2002 [DOI] [PubMed] [Google Scholar]
- Simonian P.L., Wehrmann F., Roark C.L., Born W.K., O’Brien R.L., Fontenot A.P. 2010. γδ T cells protect against lung fibrosis via IL-22. J. Exp. Med. 207:2239–2253 10.1084/jem.20100061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P., Mangan N.E., Walsh C.M., Fallon R.E., McKenzie A.N., van Rooijen N., Fallon P.G. 2007. Infection with a helminth parasite prevents experimental colitis via a macrophage-mediated mechanism. J. Immunol. 178:4557–4566 [DOI] [PubMed] [Google Scholar]
- Sonnenberg G.F., Monticelli L.A., Alenghat T., Fung T.C., Hutnick N.A., Kunisawa J., Shibata N., Grunberg S., Sinha R., Zahm A.M., et al. 2012. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science. 336:1321–1325 10.1126/science.1222551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits H., Di Santo J.P. 2011. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat. Immunol. 12:21–27 10.1038/ni.1962 [DOI] [PubMed] [Google Scholar]
- Spits H., Artis D., Colonna M., Diefenbach A., Di Santo J.P., Eberl G., Koyasu S., Locksley R.M., McKenzie A.N., Mebius R.E., et al. 2013. Innate lymphoid cells—a proposal for uniform nomenclature. Nat. Rev. Immunol. 13:145–149 10.1038/nri3365 [DOI] [PubMed] [Google Scholar]
- Sun C.M., Hall J.A., Blank R.B., Bouladoux N., Oukka M., Mora J.R., Belkaid Y. 2007. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 204:1775–1785 10.1084/jem.20070602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton C.E., Lalor S.J., Sweeney C.M., Brereton C.F., Lavelle E.C., Mills K.H. 2009. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 31:331–341 10.1016/j.immuni.2009.08.001 [DOI] [PubMed] [Google Scholar]
- Yokota A., Takeuchi H., Maeda N., Ohoka Y., Kato C., Song S.Y., Iwata M. 2009. GM-CSF and IL-4 synergistically trigger dendritic cells to acquire retinoic acid-producing capacity. Int. Immunol. 21:361–377 10.1093/intimm/dxp003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenewicz L.A., Yancopoulos G.D., Valenzuela D.M., Murphy A.J., Karow M., Flavell R.A. 2007. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 27:647–659 10.1016/j.immuni.2007.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenewicz L.A., Yancopoulos G.D., Valenzuela D.M., Murphy A.J., Stevens S., Flavell R.A. 2008. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 29:947–957 10.1016/j.immuni.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Valdez P.A., Danilenko D.M., Hu Y., Sa S.M., Gong Q., Abbas A.R., Modrusan Z., Ghilardi N., de Sauvage F.J., Ouyang W. 2008. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 14:282–289 10.1038/nm1720 [DOI] [PubMed] [Google Scholar]