PLA2G2D ameliorates skin inflammation through mobilizing pro-resolving lipid mediators.
Abstract
Resolution of inflammation is an active process that is mediated in part by antiinflammatory lipid mediators. Although phospholipase A2 (PLA2) enzymes have been implicated in the promotion of inflammation through mobilizing lipid mediators, the molecular entity of PLA2 subtypes acting upstream of antiinflammatory lipid mediators remains unknown. Herein, we show that secreted PLA2 group IID (PLA2G2D) is preferentially expressed in CD11c+ dendritic cells (DCs) and macrophages and displays a pro-resolving function. In hapten-induced contact dermatitis, resolution, not propagation, of inflammation was compromised in skin and LNs of PLA2G2D-deficient mice (Pla2g2d−/−), in which the immune balance was shifted toward a proinflammatory state over an antiinflammatory state. Bone marrow-derived DCs from Pla2g2d−/− mice were hyperactivated and elicited skin inflammation after intravenous transfer into mice. Lipidomics analysis revealed that PLA2G2D in the LNs contributed to mobilization of a pool of polyunsaturated fatty acids that could serve as precursors for antiinflammatory/pro-resolving lipid mediators such as resolvin D1 and 15-deoxy-Δ12,14-prostaglandin J2, which reduced Th1 cytokine production and surface MHC class II expression in LN cells or DCs. Altogether, our results highlight PLA2G2D as a “resolving sPLA2” that ameliorates inflammation through mobilizing pro-resolving lipid mediators and points to a potential use of this enzyme for treatment of inflammatory disorders.
In many acute and chronic disorders, unresolved inflammation is a major mechanism of disease pathogenesis (Libby, 2002). Inflammation is a protective host response to foreign challenge or tissue injury that, if unopposed, could lead to loss of tissue structure and function. Many inflammatory processes have self-limiting and self-resolving systems, which are regulated by antiinflammatory or pro-resolving mediators during the course of inflammation (Serhan et al., 2008). Uncontrolled balance between pro- and antiinflammatory mediators is now considered to be a central feature in many cases of acute and chronic inflammation.
Lipid mediators derived from polyunsaturated fatty acids (PUFAs) have recently attracted much attention as critical regulators of inflammatory responses (Serhan, 2007). Eicosanoids, such as prostaglandins and leukotrienes derived from ω-6 arachidonic acid (AA; C20:4), are generally known as proinflammatory lipid mediators (Samuelsson et al., 1987). However, some AA metabolites such as 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) and lipoxins have antiinflammatory capacities (Rossi et al., 2000; Straus et al., 2000). Furthermore, depending on tissue-specific contexts and receptor subtype occupancy, prostaglandin E2 (PGE2), and D2 (PGD2) often suppress the recruitment or activation of neutrophils, macrophages, or DCs (Gomez et al., 2005; Trivedi et al., 2006; Rajakariar et al., 2007; Honda et al., 2009). Recently, novel families of lipid mediators derived from ω-3 eicosapentaenoic acid (EPA; C20:5) and docosahexaenoic acid (DHA; C22:6), such as resolvins and protectins, have been shown to possess potent pro-resolving activities and thereby participate in the resolution of inflammation (Serhan et al., 2002; Mukherjee et al., 2004). PUFAs can also drive alternative antiinflammatory programs by acting on PUFA-sensing plasma membrane (e.g., GPR120) or nuclear (e.g., PPARγ) receptors (Diep et al., 2000; Oh et al., 2010), or by attenuating ER stress (Ariyama et al., 2010).
Biosynthesis of PUFA-derived lipid mediators is initiated by hydrolysis of phospholipids by phospholipase A2 (PLA2; Murakami et al., 2011). Of more than 30 members of the PLA2 family, group IVA cytosolic PLA2α (cPLA2α; Bonventre et al., 1997; Uozumi et al., 1997) and, in certain cases, group VIA Ca2+-independent PLA2 (iPLA2β; Moon et al., 2008) play major roles in stimulus-coupled AA release for eicosanoid generation. However, the molecular entity of PLA2 enzymes that can trigger antiinflammatory lipid mediator programs still remains obscure. The secreted PLA2 (sPLA2) family, which consists of 11 isoforms, has long been thought to play proinflammatory roles (Lambeau and Gelb, 2008; Murakami et al., 2010). Indeed, studies using transgenic or knockout mice for sPLA2s have revealed their redundant or nonredundant proinflammatory roles in arthritis, asthma, or cardiovascular disorders through lipid mediator-dependent or -independent mechanisms (Ohtsuki et al., 2006; Bostrom et al., 2007; Henderson et al., 2007; Muñoz et al., 2007; Fujioka et al., 2008; Boilard et al., 2010). However, the simple idea that sPLA2s always serve as offensive players of inflammation needs careful evaluation, as PLA2G2A protects from infection by degrading bacterial membranes (Weinrauch et al., 1996), PLA2G5 facilitates phagocytotic clearance of harmful materials by macrophages (Balestrieri et al., 2009; Boilard et al., 2010), and PLA2G10, when overexpressed in macrophages, can prime antiinflammatory, rather than proinflammatory, responses through production of PGE2 and 15d-PGJ2 (Curfs et al., 2008). Although sPLA2s can liberate various fatty acids, including ω-3 PUFAs, in vitro, it remains unknown whether they could participate in some inflammation resolution processes by driving antiinflammatory lipid mediators in vivo.
Group IID sPLA2 (PLA2G2D) was initially identified as an isoform structurally related to PLA2G2A and is abundantly expressed in lymphoid organs (Ishizaki et al., 1999; Shakhov et al., 2000; Valentin et al., 1999). Although PLA2G2D-transfected cells exhibited augmented AA release (Murakami et al., 2001) and infusion of an artificial PLA2G2D-Fc fusion protein into mice attenuated autoimmune diseases (von Allmen et al., 2009), its precise functions and mechanistic actions in vivo remain obscure. Herein, using mice lacking PLA2G2D (Pla2g2d−/−), we show that PLA2G2D is a lymphoid tissue-associated “resolving sPLA2” that participates in the resolution of contact hypersensitivity (CHS). This is the first demonstration of a particular sPLA2 subtype that lies upstream of the pro-resolving lipid mediators in a tissue-specific context.
RESULTS
PLA2G2D is preferentially expressed in lymphoid tissue-resident DCs and macrophages
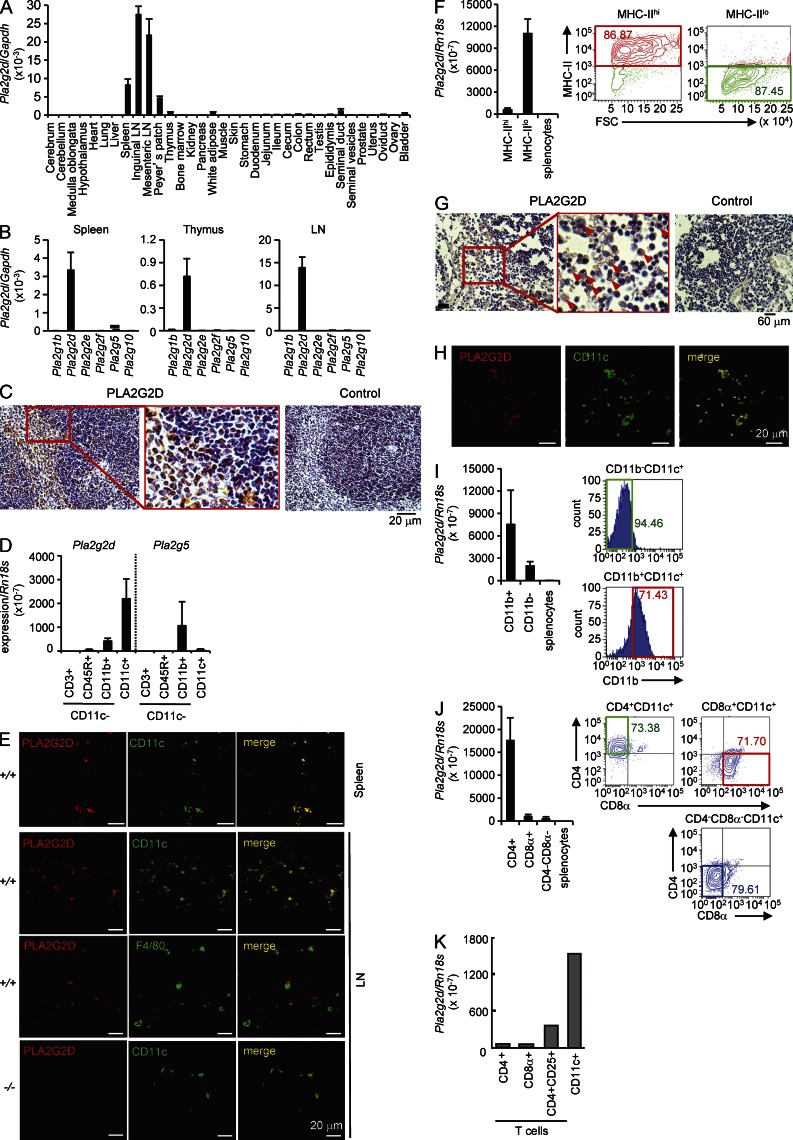
We first aimed to determine the sites where PLA2G2D is intrinsically expressed. Previous studies have reported that PLA2G2D is expressed in secondary lymphoid organs (Ishizaki et al., 1999; Valentin et al., 1999; Shakhov et al., 2000). Consistently, quantitative RT-PCR for Pla2g2d in various tissues of C57BL/6 mice revealed its preferential expression in secondary lymphoid organs, including inguinal and mesenteric LNs, Peyer’s patch, and spleen (Fig. 1 A). Among the sPLA2s, PLA2G2D is a major isoform expressed in cells from lymphoid organs (Fig. 1 B). In mouse spleen, cells with adhesive morphology and large cytoplasm in the red pulp and marginal zone, as well as focally in the white pulp, were stained with anti-PLA2G2D but not control antibody, whereas PLA2G2D staining was scarcely seen in lymphocytes (judging from round cell shape and small cytoplasm; Fig. 1 C).
Figure 1.
Expression of PLA2G2D in CD11c+ DCs. (A) Quantitative RT-PCR of Pla2g2d relative to Gapdh in various tissues of C57BL/6 mice (mean ± SEM from four mice, pooled from two experiments). (B) Quantitative RT-PCR of sPLA2 mRNAs relative to Gapdh in cells from mouse spleen, thymus, and LNs (mean ± SEM from five mice, pooled from two experiments). (C) Immunohistochemistry of mouse spleen with anti–mouse PLA2G2D or control antibody. A boxed area is magnified. (D) Quantitative RT-PCR of Pla2g2d and Pla2g5 relative to Rn18s in several splenic cell populations (mean ± SEM from three mice, a representative of three experiments). The separation procedure of individual cells is illustrated in Fig. S1. (E) Confocal immunofluorescence microscopy of PLA2G2D, CD11c, or F4/80 in spleen and LNs from Pla2g2d+/+ (+/+) and Pla2g2d−/− (−/−) mice. (F) The AutoMACS-sorted splenic CD11c+ cells were sorted for MHC class II by FACS (right), and then analyzed for expression of Pla2g2d relative to Rn18s (mean ± SEM from four mice, a representative of two experiments; left). (G) Immunohistochemistry of human LNs with anti–human PLA2G2D or control antibody. A boxed area is magnified. Arrows indicate PLA2G2D+ cells. (H) Confocal immunofluorescence microscopy of PLA2G2D and CD11c in human LNs. (I and J) AutoMACS-sorted CD11c+ cells from mouse splenocytes were further FACS-sorted for CD11b (I) or CD4 and CD8 (J; right), and then examined for expression of Pla2g2d relative to Rn18s (mean ± SEM from four mice, representative results from three experiments; left). (K) Expression of Pla2g2d relative to Rn18s in CD4+, CD8+, or CD4+CD25+ T cells and CD11c+ cells (a representative of two experiments). FACS profiles are representative of two (F) or three (I and J) experiments. Images are representative of two (H) or three (C, E, and G) experiments.
To accurately determine the cellular type(s) in which PLA2G2D is expressed, mouse splenocytes were sorted into CD11c+ and CD11c− cells, and the CD11c− population was further sorted for CD3ε (a T cell marker), CD45R (a B cell marker), and CD11b (a macrophage marker; Fig. S1). Quantitative RT-PCR revealed that Pla2g2d was enriched in the CD11c+ fraction (Fig. 1 D), suggesting that PLA2G2D is expressed in lymphoid tissue-resident CD11c+ cells of the mononuclear phagocyte lineage, including DCs and a subset of macrophages. In comparison, Pla2g5, which was detected in mouse spleen at a lower level, was distributed in CD11b+CD11c− macrophages, whereas Pla2g1b, Pla2g2a (absent in C57BL/6 mice because of a natural mutation), Pla2g2e, Pla2g2f, and Pla2g10 were hardly detected in these cells (Fig. 1, B and D). When mouse spleen and LNs were subjected to confocal immunofluorescence microscopy, PLA2G2D was largely colocalized with CD11c and only partially with F4/80, a genuine macrophage marker (Fig. 1 E), indicating that it is expressed in CD11c+ DCs and a subpopulation of CD11c+F4/80+ macrophages. Further sorting of the AutoMACS-sorted CD11c+ cells revealed the distribution of Pla2g2d in MHC class IIlo cells in preference to MHC class IIhi cells (Fig. 1 F), suggesting that PLA2G2D is mainly expressed in DCs of low activation state and is down-regulated in DCs undergoing activation (also see below). Staining of PLA2G2D was also found in human LNs, where it was distributed in cells within the paracortical area and was colocalized with CD11c (Fig. 1 G, H).
In mouse spleen, lymphoid-resident conventional DCs are subdivided into three major subsets, including CD11b+CD4+CD8α− DCs (CD4+ DCs), CD11b−CD8α+ DCs (CD8α+ DCs), and CD11b+CD4−CD8α− DCs (CD4−CD8α− DCs; Vremec et al., 2000; Tamura et al., 2005). When the AutoMACS-sorted CD11c+ cells were further FACS-sorted for CD11b, Pla2g2d mRNA was enriched in the CD11b+ population (Fig. 1 I). FACS sorting of another pool of CD11c+ cells for CD4 and CD8α showed more abundant expression of Pla2g2d in CD4+ DCs than in CD8α+ or CD4−CD8− DCs (Fig. 1 J). Thus, among the conventional DCs, PLA2G2D is preferentially expressed in the CD11b+CD4+CD8α− DC subset. Because PLA2G2D is expressed in T reg cells (von Allmen et al., 2009), we compared the expression of Pla2g2d in CD11c+ cells and CD4+CD25+ T reg cells in mouse spleen. Among T cells, Pla2g2d was enriched in CD4+CD25+ T reg cells relative to total CD4+ and CD8α+ T cells, as previously reported (von Allmen et al., 2009). However, its expression was markedly higher in CD11c+ cells than in T reg cells (Fig. 1 K).
Generation of Pla2g2d−/− mice
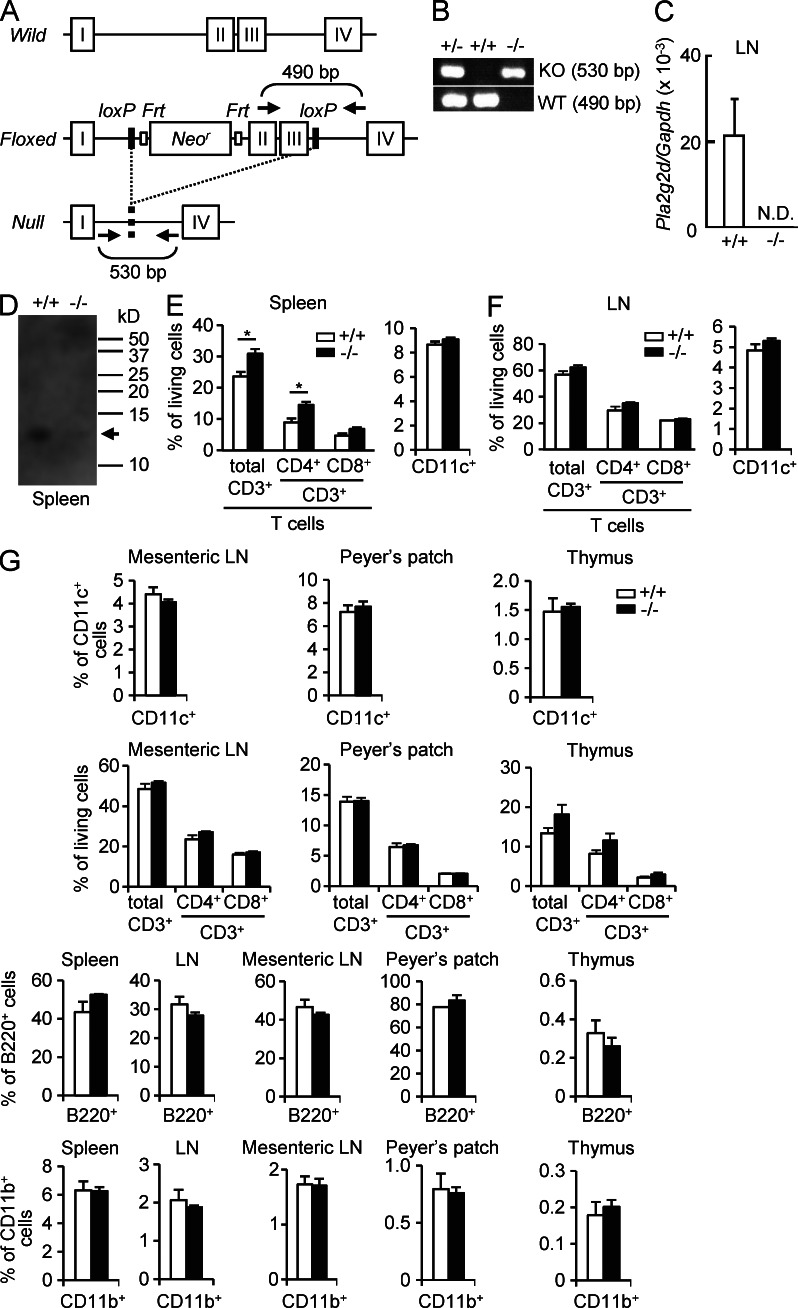
To investigate the roles of PLA2G2D in vivo, we generated Pla2g2d−/− mice by gene targeting. Construction of the Pla2g2d targeting vector is illustrated in Fig. 2 A. Appropriately targeted ES cell clones were used to obtain chimeric mice that transmitted the targeted locus through the germ line. Male mice carrying a targeted Pla2g2d gene were crossed with female CAG-Cre transgenic mice, which allowed deletion of exons 2 and 3 from the Pla2g2d gene in all tissues. Heterozygous mice carrying a mutated Pla2g2d allele (Pla2g2d+/−) were backcrossed onto the C57BL/6 background (n > 12). Successful ablation of the Pla2g2d gene was confirmed by PCR genotyping from tail biopsy (Fig. 2 B) and by the absence of its mRNA (Fig. 2 C) and protein (Fig. 2 D) in the LNs and spleen of Pla2g2d−/− mice as demonstrated by quantitative RT-PCR and immunoblotting, respectively. Also, PLA2G2D staining was absent in the LNs of Pla2g2d−/− mice (Fig. 1 E), confirming the specificity of the anti-PLA2G2D antibody used here.
Figure 2.
Gene targeting of Pla2g2d. (A) Strategy for gene targeting of the Pla2g2d gene. Positions of PCR primers for genotyping are indicated. (B) An example of PCR genotyping of Pla2g2d+/+, Pla2g2d+/−, and Pla2g2d−/− mice. (C) Quantitative RT-PCR of Pla2g2d expression in the inguinal LNs of Pla2g2d+/+ and Pla2g2d−/− mice (mean ± SEM from four mice per group). (D) Western blotting of PLA2G2D protein in the spleen of Pla2g2d+/+ and Pla2g2d−/− mice. Arrow indicates the position of 14-kD PLA2G2D protein. A representative blot of three experiments is shown. (E and F) Proportions of T cells and DCs in spleen (E) and inguinal LNs (F) were determined by FACS (mean ± SEM from three mice per group; *, P < 0.05). (G) Immune cells from the indicated tissues from Pla2g2d+/+ and Pla2g2d−/− mice were subjected to FACS analysis to examine the populations of CD11c+ DCs, CD3ε+ T cells (both CD4+ and CD8+ populations), CD45R+ (B220+) B cells, and CD11b+ monocytes/macrophages (mean ± SEM from three mice per group). Representative results of two experiments are shown in (B, C, and E–G). In C–G, 7-wk-old male mice were used.
The ratio of the genotypes of heterozygous male and female offspring exhibited Mendelian proportions, and homozygous null mice were indistinguishable from WT littermates in terms of survival rates, appearance, fertility, and gross behavior, suggesting that the lack of PLA2G2D did not affect embryonic and postnatal development in normal states. The serum levels of biomedical markers and the proportions of blood cells were similar between the genotypes (Tables S1 andS2). DC, T cell, B cell, and macrophage populations in spleen, inguinal, and mesenteric LNs, Peyer’s patch, and thymus were indistinguishable between Pla2g2d−/− and Pla2g2d+/+ mice, except that the splenic proportion of CD4+ T cells was slightly greater in Pla2g2d−/− mice (Fig. 2, E–G). Thus, PLA2G2D ablation did not profoundly affect hematopoiesis or lymphopoiesis under normal conditions. LN expression of other PLA2s was similar between the genotypes (unpublished data), confirming that the lack of PLA2G2D was not compensated by other PLA2s.
Impaired resolution of hapten-induced CHS in Pla2g2d−/− mice
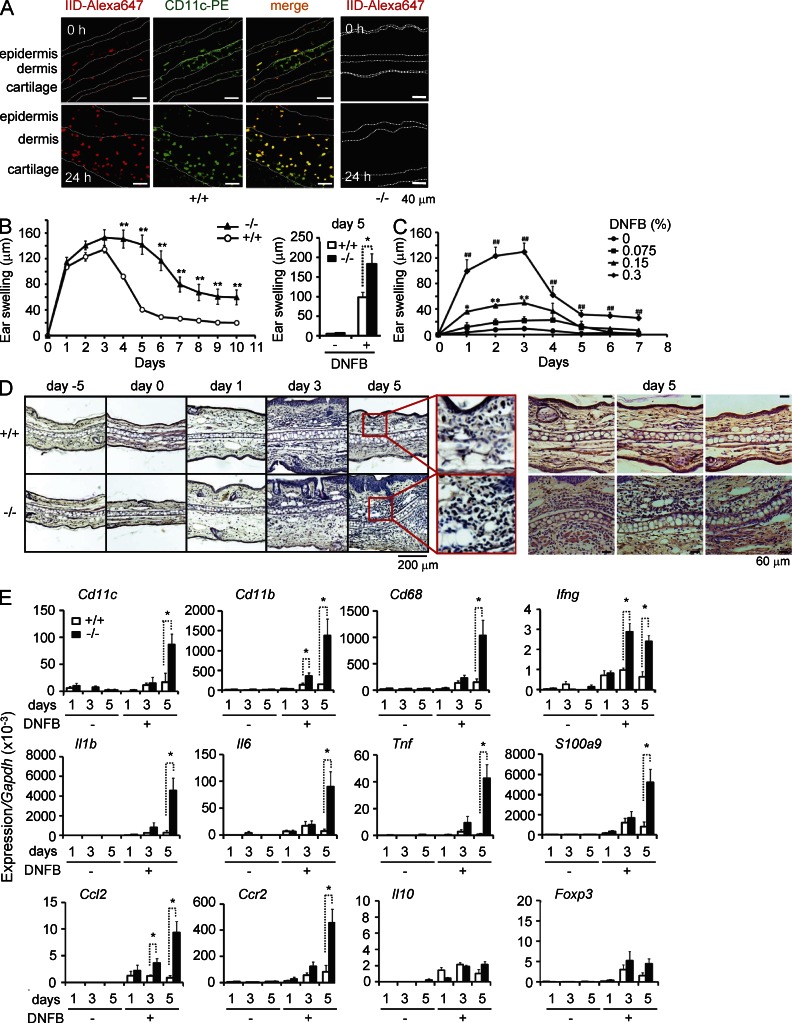
To assess the immunoregulatory role of PLA2G2D in vivo, Pla2g2d−/− mice and WT littermates were subjected to hapten-induced CHS. In this well-known model for Th1 response, application of the hapten antigen dinitrofluorobenzene (DNFB) onto abdominal skin (sensitization), followed by the second application of the same antigen onto ear skin (elicitation) induced ear swelling. In normal skin, there are resident CD11c+ populations including epidermal Langerhans cells and dermal DCs, which capture antigen and migrate into draining LNs (Heath and Carbone, 2009). Although Pla2g2d expression was low in nonlymphoid tissues (Fig. 1 A), confocal microscopy of the ear revealed that scattered PLA2G2D signal was colocalized in dermal and to a lesser extent in epidermal CD11c+ cells in WT mice, while it was absent in Pla2g2d−/− skin (Fig. 3 A). Inflammation recruits monocytes that are differentiated into CD11c+ inflammatory DCs or macrophages, which act as a peripheral stimulator of T cells (Heath and Carbone, 2009). Remarkably, cells expressing both PLA2G2D and CD11c were increased in the dermis and even epidermis after DNFB challenge for 24 h (Fig. 3 A), indicating that PLA2G2D is also expressed in many, if not all, inflammatory DCs or macrophages migrating into the inflamed skin.
Figure 3.
Impaired resolution of skin inflammation in Pla2g2d−/− mice in a DNFB-induced CHS model. (A) Confocal immunofluorescence microscopy of PLA2G2D (IID-Alexa647; red) and CD11c (CD11c-PE; green) in ear skin from Pla2g2d+/+ (+/+) and Pla2g2d−/− (−/−) mice before and 24 h after elicitation of DNFB-induced CHS. Green signal in the cartilage is nonspecific. Images are representative of two experiments. (B) Monitoring of ear thickness over the elicitation period of CHS in Pla2g2d+/+ and Pla2g2d−/− mice (left). Result is a representative of three experiments (mean ± SEM from 7 Pla2g2d+/+ and 8 Pla2g2d−/− mice per group; **, P < 0.01). (right) Pooled data on day 5 from three experiments (mean ± SEM from 12 mice per group without DNFB or 21 (+/+) and 23 (−/−) mice with DNFB; *, P < 0.05). (C) Monitoring of ear thickness after treatment of WT mice with various doses of DNFB (mean ± SEM from 5 mice per group; *, P < 0.05 and **, P < 0.01, 0 versus 0.15% DNFB; #, P < 0.01, 0% versus 0.3% DNFB; representative of three experiments). (D) Hematoxylin/eosin staining of ear skin sections obtained at the indicated times from Pla2g2d+/+ and Pla2g2d−/− mice. Images are representative of four experiments. The ears on day 5 are magnified (red boxes). Other examples of ear histology on day 5 are also shown (right). (E) Quantitative RT-PCR of various pro- and antiinflammatory marker genes relative to Gapdh in the ear skins of Pla2g2d+/+ and Pla2g2d−/− mice at the indicated time points. Data are representative of three experiments (mean ± SEM from 3 mice without DNFB and four mice with DNFB per group; *P < 0.05).
We then compared the process of CHS between Pla2g2d+/+ and Pla2g2d−/− mice. In WT mice, ear swelling peaked by day 3 and declined to nearly the basal level by day 6 after DNFB challenge (Fig. 3 B). At lower doses of DNFB, milder edema peaked on days 2–3, and then shifted to the declining phase (Fig. 3 C), confirming that the onset and resolution were not affected by the intensity of the stimulus. The initial process of ear swelling by day 3 occurred normally in Pla2g2d−/− mice, with no significant difference from Pla2g2d+/+ mice. After reaching the peak, ear edema in Pla2g2d−/− mice continued to maintain the maximally exaggerated state by day 5, and then decayed at a slower rate by day 10, at which time the ear was still thicker than in WT mice (Fig. 3 B), implying a delayed resolution in the null mice. In accordance, histological examination of the ears showed more severe ear swelling, epidermal hyperplasia, and inflammatory cell infiltration in Pla2g2d−/− mice than in Pla2g2d+/+ mice on day 5 (Fig. 3 D).
Quantitative RT-PCR of ear skin revealed that the expression levels of inflammatory markers, including Cd11c, Cd11b, and Cd68 (surface markers of inflammatory DCs or macrophages), Ifng (IFN-γ; a Th1 cytokine), Il1b, Il6, and Tnf (IL-1β, IL-6, and TNF; proinflammatory cytokines), Ccl2, and Ccr2 (a monocyte-attracting chemokine and its receptor), and S100a9 (a marker of epidermal hyperplasia) peaked by day 3, and then declined by day 5 in Pla2g2d+/+ mice, whereas they were dramatically increased on day 5 in Pla2g2d−/− mice (Fig. 3 E). A robust increase of Ifng in Pla2g2d−/− mice over Pla2g2d+/+ mice became obvious on day 3 before that of other proinflammatory markers, implying that the exacerbated inflammation in the null mice stemmed from the augmented Th1 response (Lu et al., 1998). In contrast, expression levels of Il10 (IL-10), an antiinflammatory cytokine (Fickenscher et al., 2002), and of Foxp3, a marker of T reg cells (Shevach, 2009), in Pla2g2d−/− skin did not differ significantly from those in Pla2g2d+/+ skin throughout the experimental periods (Fig. 3 E), suggesting that T reg cell migration or proliferation in the inflamed skin was unaffected by PLA2G2D deficiency. Thus, during the resolution process of CHS, a cytokine milieu in the affected skin is skewed toward a proinflammatory profile rather than an antiinflammatory profile in Pla2g2d−/− mice.
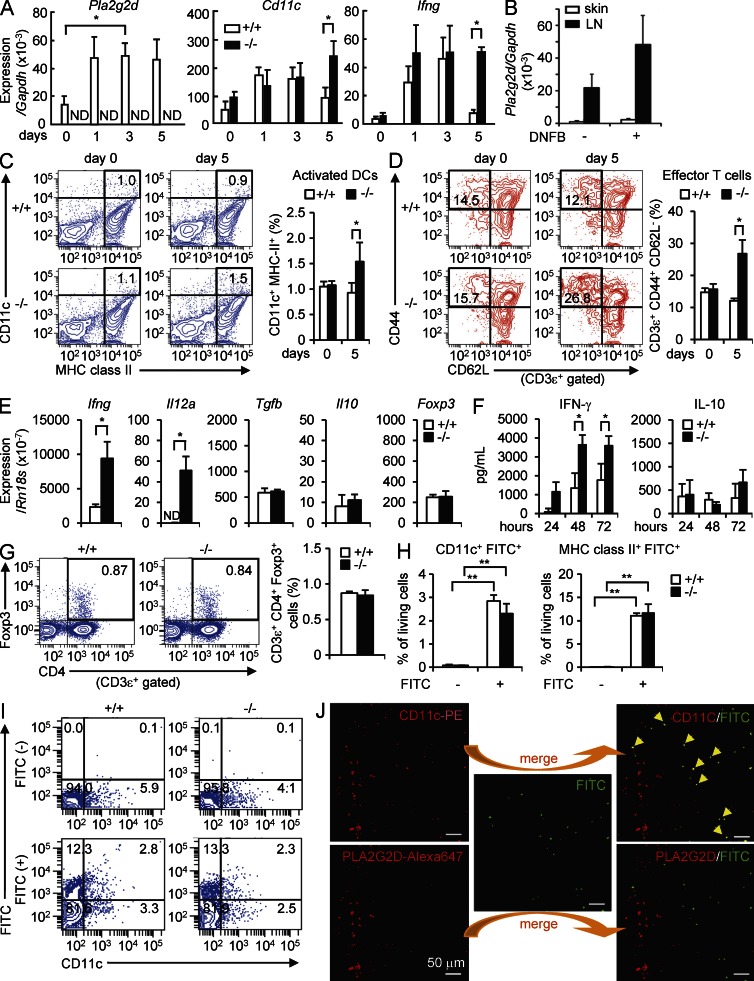
We also examined the inflammatory status in the draining LNs during the elicitation phase of CHS. After DNFB challenge to the ears, Pla2g2d expression in the LNs of WT mice was increased on days 1 to 5 (Fig. 4 A), reaching a much higher level than in the skin (Fig. 4 B). The modest increase of Pla2g2d was accompanied by a concomitant increase of Cd11c and a robust increase of Ifng on days 1–3 (Fig. 4 A). Subsequently, the expressions of Cd11c and Ifng were declined to their basal levels by day 5 in Pla2g2d+/+ mice, underscoring the resolution of LN inflammation. In Pla2g2d−/− LNs, however, the expression levels of Cd11c and Ifng still remained at their highest levels on day 5 (Fig. 4 A). On day 5, CD11c+MHC class II+ activated DCs (Fig. 4 C) and CD3ε+CD44+CD62L− effector T cells (Fig. 4 D) were greater in Pla2g2d−/− LNs than in Pla2g2d+/+ LNs. Thus, as in the affected skin, inflammation failed to resolve in the draining LNs of DNFB-challenged Pla2g2d−/− mice relative to Pla2g2d+/+ mice.
Figure 4.
Impaired resolution of LN inflammation in Pla2g2d−/− mice in a DNFB-induced CHS model. (A) Quantitative RT-PCR of Pla2g2d, Cd11c, and Ifng relative to Gapdh in the LNs of Pla2g2d+/+ (+/+) and Pla2g2d−/− (−/−) mice during the elicitation phase of CHS (mean ± SEM from four mice per group). (B) Quantitative RT-PCR of Pla2g2d relative to Gapdh in the ear skin and LNs of Pla2g2d+/+ and Pla2g2d−/− mice on day 5 with or without DNFB challenge (mean ± SEM from four mice per group). (C and D) FACS for activated DCs (CD11c+MHC class II+; C) and effector T cells (CD3ε+CD44+CD62L−; D) in the LNs of Pla2g2d+/+ and Pla2g2d−/− mice. Representative FACS profiles and average scores (mean ± SEM from three mice per group) are shown. (E and F) LN cells harvested from DNFB-sensitized Pla2g2d+/+ and Pla2g2d−/− mice on day 0 were cultured with 100 µg/ml DNBS ex vivo to assess the expression of pro- and antiinflammatory markers at 24 h by quantitative RT-PCR (E) or the secretion of cytokines over 72 h by ELISA (F; mean ± SEM from four mice per group). (G) FACS for CD4 and FoxP3 on CD3ε+-gated T cells from Pla2g2d+/+ and Pla2g2d−/− LNs on day 5. Representative FACS profiles and average scores (mean ± SEM from four mice per group) are shown. (H and I) FACS for FITC in combination with CD11c or MHC class II in skin-draining LNs 24 h after treatment of the abdominal skins of Pla2g2d+/+ and Pla2g2d−/− mice with 200 µl of 1% FITC in acetone/dibutyl phthalate (1:1). Average scores of FITC+CD11c+ or FITC+MHC class II+ cells (mean ± SEM from four mice per group; H) and representative FACS profiles (I) are shown. (J) A representative images of confocal microscopy of PLA2G2D, CD11c, and FITC in the LNs of WT mice used in (H and I). Arrowheads indicate the cells in which CD11c and FITC signals were colocalized. Results are representative of two (C–J) or three (A and B) experiments. *, P < 0.05; **, P < 0.01.
LN cells were harvested from DNFB-sensitized Pla2g2d−/− and Pla2g2d+/+ mice and cultured for 24 h ex vivo in the presence of dinitrobenzene sulfonic acid (DNBS; a water-soluble form of DNFB). Expression levels of the Th1 cytokines Ifng and Il12a were markedly higher in Pla2g2d−/− LN cells than in WT cells, whereas those of the antiinflammatory cytokines Tgfb and Il10, as well as the T reg cell marker Foxp3, were unaffected by the absence of PLA2G2D (Fig. 4 E). In agreement, the elevated secretion of IFN-γ, but not IL-10, in the culture of Pla2g2d−/− LN cells relative to Pla2g2d+/+ LN cells over time was confirmed by ELISA (Fig. 4 F), and the unaltered CD4+FoxP3+ T reg cell population in Pla2g2d−/− LNs was confirmed by flow cytometry (Fig. 4 G). These results indicate again that the proinflammatory signal exceeds the antiinflammatory signal in Pla2g2d−/− LNs compared with Pla2g2d+/+ LNs. However, our results do not support the proposal that PLA2G2D facilitates the expansion and immunosuppressive function of T reg cells (von Allmen et al., 2009), at least in the CHS model used here.
To verify whether PLA2G2D deficiency did not affect the initial process of CHS, we examined the migration of skin-resident DCs into the draining LNs during the sensitization phase. After capture of a foreign antigen, skin-resident DCs are activated and migrate into the regional LNs, where they present the antigen to T cells (Heath and Carbone, 2009). The fluorescent hapten antigen FITC was topically applied onto the skin of Pla2g2d+/+ and Pla2g2d−/− mice, and then the migration of FITC-captured skin DCs into the draining LNs was monitored by flow cytometry. The proportion of FITC+CD11c+ or FITC+MHC class II+ DCs was similar between Pla2g2d+/+ and Pla2g2d−/− LNs (Fig. 4, H and I), implying that the lack of PLA2G2D does not affect antigen uptake, recruitment, and activation of skin DCs migrating into the LNs. Notably, many of the FITC+CD11c+ cells in the LNs did not display PLA2G2D signal (Fig. 4 J), suggesting that PLA2G2D expression was decreased after activation and LN recruitment of skin DCs. Thus, the apparent effect of PLA2G2D deficiency is restricted to the late period of CHS.
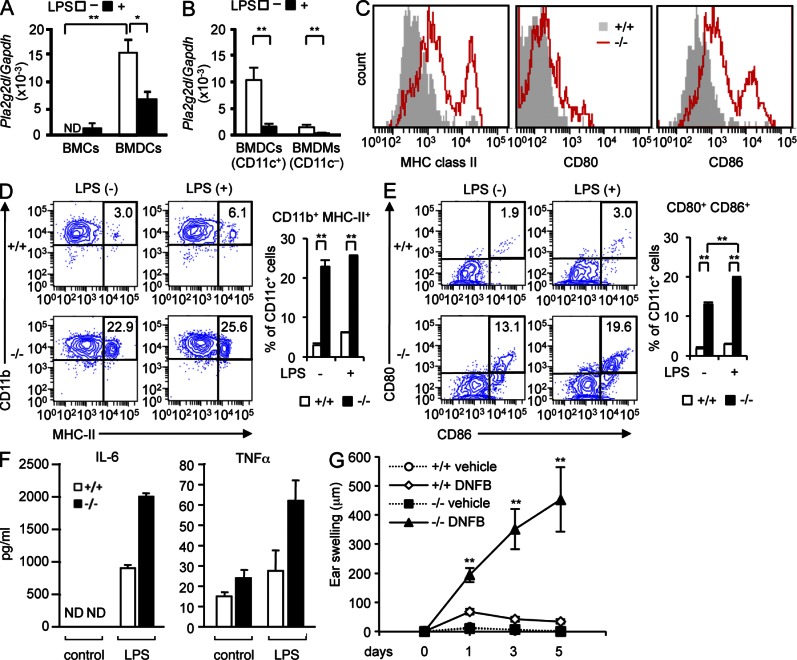
Pla2g2d−/− DCs are hyperactivated
To assess whether the increased immune responses in Pla2g2d−/− mice is attributable to some abnormalities in DCs, we analyzed GM-CSF–driven BM-derived DCs (CD11b+CD11c+ BMDCs; McGeachy, 2011). Although the expression of Pla2g2d was minimal in BM cells, it was robustly induced in GM-CSF-driven BMDCs (Fig. 5 A). Compared with BMDCs, Pla2g2d expression in M-CSF–driven BM-derived macrophages (CD11b+CD11c− BMDMs) was low (Fig. 5 B), agreeing with the preferential expression of PLA2G2D in CD11c+ DCs in vivo. When BMDCs or even BMDMs were treated for 24 h with LPS, Pla2g2d expression was reduced markedly (Fig. 5, A and B), a finding reminiscent of its down-regulation by LPS in mouse tissues (Suzuki et al., 2000; von Allmen et al., 2009) and human macrophages (Lindbom et al., 2005). Thus, corroborating with the data shown in Figs. 1 F and 4 J, PLA2G2D expression inversely correlates with DC activation.
Figure 5.
Absence of PLA2G2D hyperactivates BMDCs. (A and B) Quantitative RT-PCR of Pla2g2d relative to Gapdh in BM cells (BMCs), BMDCs, and BMDMs from WT mice, with or without stimulation with 1 µg/ml LPS (mean ± SEM from 3 (A) and 5 (B) mice per group). (C) FACS profiles of CD11c-gated BMDCs from Pla2g2d+/+ (+/+) and Pla2g2d−/− (−/−) mice for surface expression of MHC class II, CD80, and CD86. (D and E) Representative FACS profiles and average scores (mean ± SEM from three mice per group) for surface expression of CD11b and MHC class II (D) or CD80 and CD86 (E) in Pla2g2d+/+ and Pla2g2d−/− BMDCs with or without stimulation with 10 ng/ml LPS for 24 h. (F) Secretion of cytokines from Pla2g2d+/+ and Pla2g2d−/− BMDCs treated for 24 h with or without 10 ng/ml LPS (mean ± SEM from three mice per group). (G) BMDCs from Pla2g2d+/+ and Pla2g2d−/− mice were sensitized with DNBS and then intravenously transferred into C57BL/6 mice. After 5 d, DNFB was painted on the ear skin to monitor ear thickness over time (mean ± SEM from four mice per group). Data are representative of two (B and F) or three (A, C–E, and G) experiments. ND, not detected. *, P < 0.05; **, P < 0.01.
Even without stimulation, Pla2g2d−/− BMDCs had greater surface expression of MHC class II, CD80, and CD86 than Pla2g2d+/+ BMDCs (Fig. 5 C). Further incubation with a suboptimal concentration of LPS increased more CD11b+MHC class II+ (Fig. 5 D) and CD80+CD86+ (Fig. 5 E) BMDCs from Pla2g2d−/− mice than from Pla2g2d+/+ mice. There were substantial increases in IL-6 and TNF secretion from Pla2g2d−/− BMDCs over Pla2g2d+/+ BMDCs in response to suboptimal LPS (Fig. 5 F). Moreover, when C57BL/6 mice were intravenously transferred with Pla2g2d+/+ or Pla2g2d−/− BMDCs that had been sensitized with DNBS, and then subjected to DNFB challenge, mice transferred with Pla2g2d−/− BMDCs exhibited greater edema than those transferred with Pla2g2d+/+ BMDCs (Fig. 5 G). Thus, PLA2G2D deficiency allows BMDCs to be hyperactivated, leading to more severe inflammation.
Altered lipid profiles in the LNs of Pla2g2d−/− mice
Because PLA2G2D is a lipolytic enzyme, we speculated that the impaired resolution of CHS in Pla2g2d−/− mice might result from some perturbed lipid metabolisms. We therefore performed electrospray ionization mass spectrometry (ESI-MS) to comprehensively monitor the levels of free PUFAs and their oxygenated metabolites in the draining LNs and skin of Pla2g2d−/− mice in comparison with Pla2g2d+/+ mice on day 5 after DNFB challenge, a time point when Pla2g2d−/− mice exhibited an impaired resolution of inflammation.
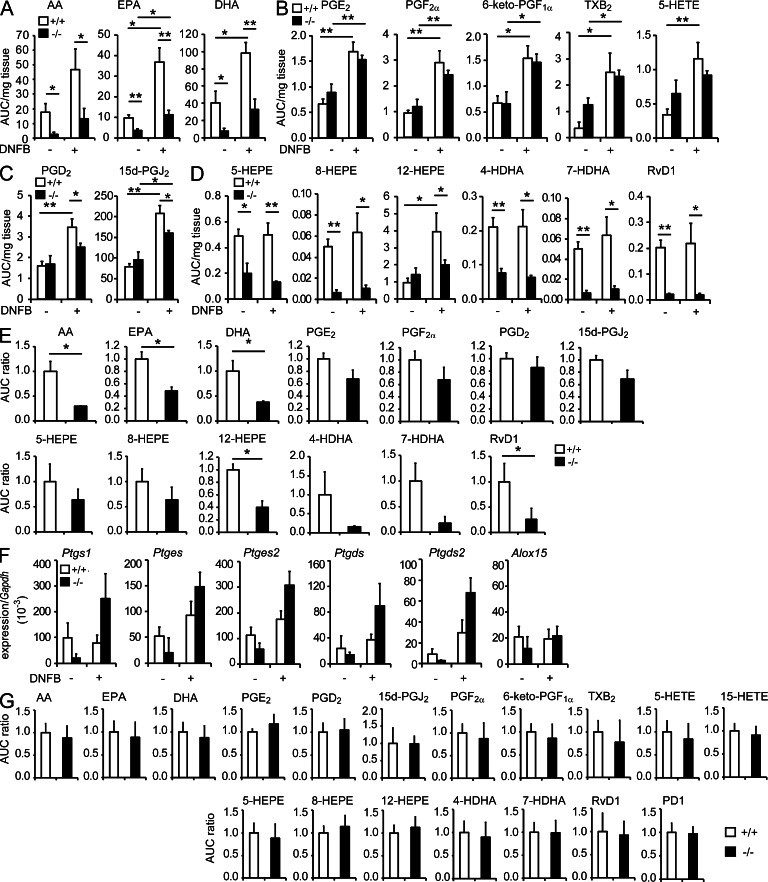
Bioactive metabolomes for AA-, EPA-, and DHA-derived products revealed that DNFB challenge led to substantial increases in free AA, EPA, and DHA, with concomitant increases in many if not all of their bioactive metabolites, in the LNs of Pla2g2d+/+ mice. Remarkably, the levels of AA, EPA, and DHA were significantly lower in Pla2g2d−/− LNs than in Pla2g2d+/+ LNs in the presence or even in the absence of DNFB challenge (Fig. 6 A), suggesting that PLA2G2D plays a role in the constitutive and inducible supply of a large portion of these PUFAs in the LNs. However, the levels of many AA metabolites, including the cyclooxygenase products PGE2, PGF2α, 6-keto-PGF1α (an end-product of PGI2), and thromboxane (TX) B2 (an end-product of TXA2), as well as the 5-lipoxygenase product 5-hydroxyeicosatetraenoic acid (HETE), some of which promote Th1-dominated inflammation (Byrum et al., 1999; Kabashima et al., 2003; Nagamachi et al., 2007; Yao et al., 2009; Nakajima et al., 2010), did not differ significantly between the genotypes in DNFB-treated and -untreated settings (Fig. 6 B). In contrast, the DNFB-induced increases in the AA-derived antiinflammatory prostanoid 15d-PGJ2 and its precursor PGD2 occurred only partially in Pla2g2d−/− mice relative to Pla2g2d+/+ mice (Fig. 6 C). These results suggest that the PLA2G2D-sensitive AA pool is preferentially coupled with the antiinflammatory PGD2/15d-PGJ2 axis, whereas another AA pool remaining in Pla2g2d−/− mice, which may be supplied by cPLA2α (Bonventre et al., 1997; Uozumi et al., 1997), is sufficient for full mobilization of the proinflammatory AA metabolites.
Figure 6.
LC-ESI/MS profiling of PUFAs and their bioactive mediators. (A-D) LC-ESI/MS of free PUFAs (A), proinflammatory AA metabolites (B), antiinflammatory AA metabolites (C), and EPA or DHA metabolites (D) in the LNs of Pla2g2d+/+ (+/+) and Pla2g2d−/− (−/−) mice on day 5 of CHS, with or without DNFB treatment (mean ± SEM from five mice per group). Values are AUC (area under the curve) per mg tissue, as evaluated by comparison with an internal standard (1 AUC = ∼50 pg). (E) LC-ESI/MS of free PUFAs and their metabolites in the LNs of Pla2g2d+/+ and Pla2g2d−/− mice on day 1 after DNFB challenge (mean ± SEM from three mice per group). (F) Quantitative RT-PCR of lipid mediator-biosynthetic enzymes relative to Gapdh in the LNs on day 5 after DNFB challenge (mean ± SEM from three mice per group). (G) LC-ESI/MS of free PUFAs and their metabolites in the ear skin of DNFB-challenged Pla2g2d+/+ and Pla2g2d−/− mice on day 5 (mean ± SEM from 10 mice per group). In E and G, values are expressed as AUC ratio, with the level of each product in Pla2g2d+/+ mice being regarded as 1. HEPE, hydroxyeicosapentaenoic acid; HDHA, hydroxydocosahexaenoic acid; PD1, protectin D1. Representative results of two (E and F) or three (A–D) experiments and pooled results of two experiments (G) are shown. *, P < 0.05; **, P < 0.01.
In addition, the EPA and DHA bioactive metabolomes revealed that the levels of ω-3 EPA- and DHA-derived metabolites, which included the potent pro-resolving lipid mediator resolvin D1 (RvD1), were considerably lower in Pla2g2d−/− LNs than in Pla2g2d+/+ LNs regardless of DNFB challenge (Fig. 6 D). Thus, along with the reduction in the inducible production of ω-6 AA-derived 15d-PGJ2 (Fig. 6 C), the absence of PLA2G2D causes a marked reduction in the steady-state levels of ω-3 PUFAs and their enzymatically oxygenated products. The lipidomic profile on day 1, an early peak point of CHS, was similar to that on day 5 (Fig. 6 E). Hence, the constitutive supply of pro-resolving lipid mediators by PLA2G2D may be important for the early entry into resolution, rather than for limiting the maximal inflammatory response. To obtain a possible explanation for these changes in lipid profiles in Pla2g2d−/− LNs, we examined the expression of downstream enzymes responsible for the conversion of PUFAs to bioactive metabolites. Notably, the expression levels of prostanoid-biosynthetic enzymes tended to be higher in Pla2g2d−/− LNs than in Pla2g2d+/+ LNs, whereas that of 12/15-lipoxygenase, an enzyme crucial for the conversion of ω-3 PUFAs to antiinflammatory lipid mediators, was similar in both genotypes (Fig. 6 F). Thus, in Pla2g2d−/− LNs, the diminished supply of AA might be counter-balanced by the elevated expression of downstream enzymes for proinflammatory lipid mediators, while the reduction of ω-3 PUFAs might be linked to the reduction of pro-resolving lipid mediators because of the lack of compensatory up-regulation of 12/15-lipoxygenase.
Lipidomics analysis of the affected skin on day 5 of CHS revealed that none of the free PUFAs and their metabolites were significantly altered in Pla2g2d−/− mice relative to Pla2g2d+/+ mice (Fig. 6 G). Thus, despite the increased skin inflammation, overall lipid mediator profiles in the skin were unaffected by PLA2G2D deficiency. Altogether, it is likely that PLA2G2D acts mainly in lymphoid tissues, where it supplies a particular pool of PUFAs that is selectively coupled with antiinflammatory/pro-resolving lipid mediator programs and thereby contributes to the resolution of CHS primarily in the LNs and secondarily in the skin.
PLA2G2D releases PUFAs from phosphatidylethanolamine in LN membranes
Having established that the deficiency of PLA2G2D reduces the LN levels of PUFAs such as AA and DHA, we next sought to gain insights into phospholipid subclasses from which PLA2G2D could release these PUFAs. To this end, we extracted total lipids from LNs and incubated them with or without recombinant mouse PLA2G2D protein. Then, the lipids were subjected to ESI-MS to analyze the reduction of phospholipid species and the increase of lysophospholipids and free fatty acids.
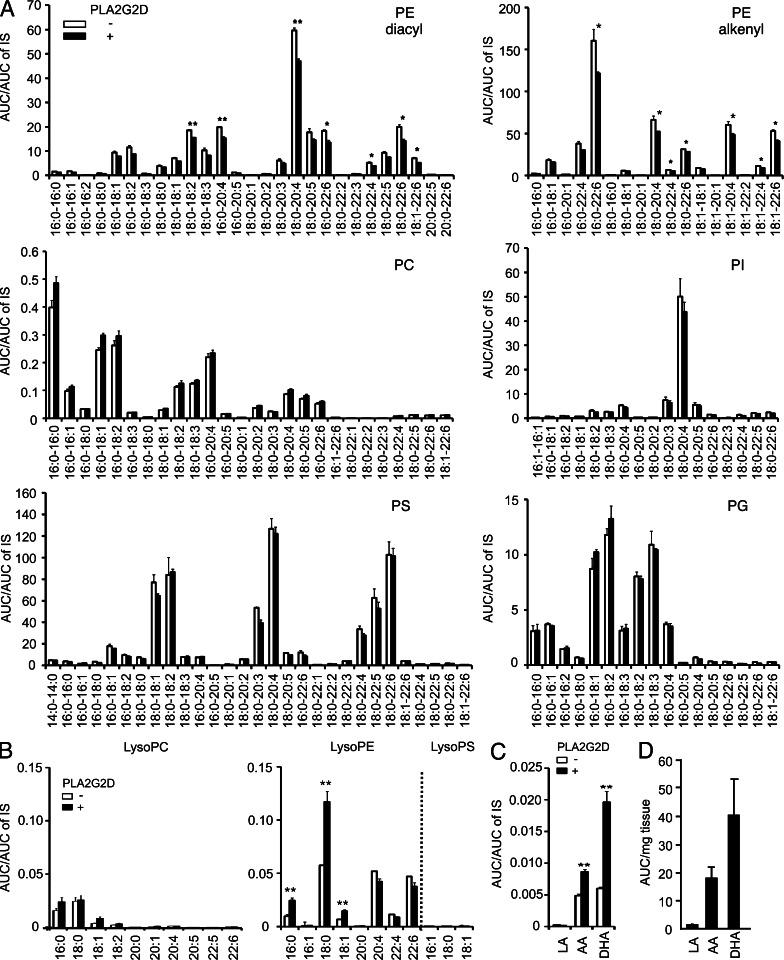
We found that PLA2G2D selectively decreased several diacyl and alkenyl forms of phosphatidylethanolamine (PE) molecular species containing unsaturated fatty acids, particularly those containing AA and DHA, whereas phosphatidylcholine (PC), phosphatidylinositol (PI), phosphatidylserine (PS), and phosphatidylglycerol (PG) species were barely affected by the enzyme (Fig. 7 A). The decreases of particular PE species by PLA2G2D were accompanied by concomitant increases in lysophosphatidylethanolamine (LysoPE) species with saturated or monounsaturated fatty acid (which are likely to be sn-1 bound), supporting the PLA2 reaction (Fig. 7 B). In agreement with the unaltered PC and PS levels (Fig. 7 A), there were no increases in lysophosphatidylcholine (LysoPC) and lysophosphatidylserine (LysoPS) species (Fig. 7 B). Among the PUFAs, DHA and AA were increased in marked preference to linoleic acid (LA; C18:2) after the treatment with PLA2G2D (Fig. 7 C), an observation that agreed with the selective reduction of PE species bearing DHA or AA (Fig. 7 A). Moreover, this PUFA preference in vitro was recapitulated by marked abundance of free DHA and AA relative to LA in the LNs in vivo (Fig. 7 D). These results collectively suggest that PLA2G2D selectively hydrolyzes PUFA-containing PE in LN membranes to provide DHA and AA.
Figure 7.
Hydrolysis of LN-derived phospholipids by recombinant PLA2G2D. (A–C) ESI-MS of phospholipids (A), lysophospholipids (B), and fatty acids (C) in total lipids extracted from the LNs and incubated with (+) or without (−) 200 nM recombinant mouse PLA2G2D (mean ± SEM, n = 3 per group). (D) ESI-MS of LA, AA, and DHA in the LNs of WT mice (mean ± SEM from five mice). Results in A–D are representative of three independent experiments. *, P < 0.05; **, P < 0.01.
The lipid products altered in Pla2g2d−/− LNs dampen the DC-mediated immune responses
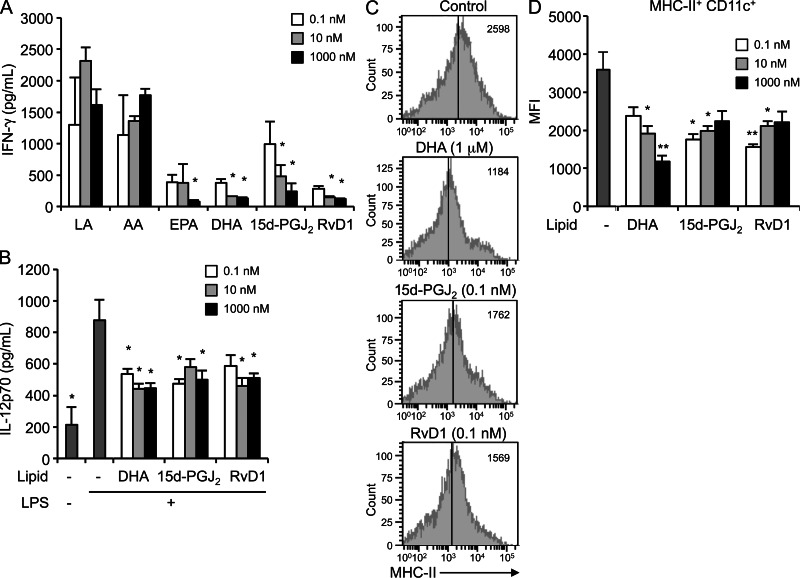
Finally, to assess whether the lipid products altered in Pla2g2d−/− LNs could indeed modulate the immune response, LN cells from DNFB-sensitized mice were stimulated with DNBS in the presence of the lipids. EPA, DHA, 15d-PGJ2 and RvD1 dampened IFN-γ secretion, whereas LA and AA were ineffective (Fig. 8 A), confirming the immune regulation by the ω-3 PUFA products and the ω-6 cyclopentenone prostanoid. These antiinflammatory lipids also efficiently blocked IFN-γ production by Pla2g2d−/− LN cells (unpublished data). The levels of these lipids in WT LNs were estimated to be within a range of nanomolar to micromolar orders (Fig. 6 A), which were sufficient to block IFN-γ production by LN cells (Fig. 8 A). Furthermore, DHA, 15d-PGJ2, and RvD1 reduced IL-12 secretion (Fig. 8 B) and surface expression of MHC class II (Fig. 8 C, D) in LPS-stimulated BMDCs. Together with previous studies reporting the antiinflammatory effects of ω-3 PUFAs on CHS models (Danno et al., 1993; Tomobe et al., 2000) and on DCs or macrophages (Groeger et al., 2010; Draper et al., 2011), our present results indicate that the lipid mediator balance is shifted toward a proinflammatory status in favor of an antiinflammatory status in Pla2g2d−/− LNs during the resolution phase of CHS.
Figure 8.
Effects of PUFAs and their metabolites on activation of LN cells and DCs. (A) LN cells obtained from DNFB-sensitized WT mice were stimulated with DNBS for 24 h in the presence of the indicated lipids to assess IFN-γ secretion (mean ± SEM, n = 3 per group; *, P < 0.05 versus the same concentration of LA). (B–D) BMDCs were stimulated with 10 ng/ml LPS in the presence or absence of the indicated concentrations of lipids for 24 h to assess the release of IL-12p70 by ELISA (mean ± SEM, n = 4 per group; *, P < 0.05 versus plus LPS without lipids; B) and the surface expression of MHC class II by flow cytometry (C and D). Representative FACS profiles with median fluorescence intensity (MFI) are indicated (C). Average scores of the MFI are evaluated (mean ± SEM, n = 4 per group; *, P < 0.05 and **, P < 0.01 versus without lipids; D). Results are representative of two (A) or three (B–D) independent experiments.
DISCUSSION
Although the last decade has seen progress in deciphering the pathways that activates innate and acquired immunity, the mechanisms that limit these responses to secure homeostasis have not yet been fully defined. Antiinflammatory or pro-resolving lipid mediators can explain some of the beneficial actions of ω-3 PUFA-rich fish oils in various diseases (Yokoyama et al., 2007). Although the understanding of PUFA-oxygenated products with pro-resolving activities has recently been expanding (Serhan, 2007; Serhan et al., 2008), an interest in exploring the role of PLA2s that can supply PUFA precursors upstream of their biosynthetic conversion to pro-resolving lipid mediators has emerged. Here, we have shown that PLA2G2D is an immunosuppressive sPLA2 that is abundantly expressed in CD11c+ cells in lymphoid organs and that its absence delays the resolution of CHS. Importantly, pro-resolving lipid mediators and their precursors were reduced in Pla2g2d−/− LNs, implying that PLA2G2D resolves inflammation by driving antiinflammatory lipid mediators in this tissue. Because of its pro-resolving role in CHS, along with a recent study showing an immunosuppressive effect of PLA2G2D-Fc fusion protein (von Allmen et al., 2009), we propose that PLA2G2D represents a “resolving sPLA2” that mobilizes antiinflammatory or pro-resolving lipid mediators in lymphoid tissues.
PLA2G2D is mainly located in a cell population expressing CD11c, a molecular signature of DCs. DCs are antigen-presenting cells specialized to capture and process antigens for presentation on MHC products and then to control differentiation of T cells (Villadangos and Schnorrer, 2007), and they are highly diverged in terms of their locations, surface marker expressions, and functions (Heath and Carbone, 2009). Lymphoid-resident DCs present antigen that drains to LNs and stimulate the initial steps of T cell activation; migratory DCs transport antigen from peripheral tissues to regional LNs and stimulate the next steps of T cell activation; as immune stimulation continues, blood monocytes recruited to peripheral sites develop into inflammatory DCs, which migrate into LNs to provide a continuing stimulus for T cell activation; and TipDCs, another unique DC subset, mediate T cell–independent innate immune defense (Itano et al., 2003; Serbina et al., 2003; Allenspach et al., 2008; Nakano et al., 2009). Among the lymphoid-resident DC subsets, CD8α+ DCs are responsible for cell-mediated immune responses, whereas CD4+ DCs regulate humoral responses (Maldonado-López et al., 1999; Pulendran et al., 1999; den Haan et al., 2000; Pooley et al., 2001). PLA2G2D is preferentially expressed in lymphoid tissue-resident CD4+CD11b+ DCs as well as in dermal DCs, monocyte-derived inflammatory DCs/macrophages, and ex vivo-derived, GM-CSF-driven BMDCs.
Although PLA2G2D is largely dispensable for normal development of DCs as well as lymphocytes and other myeloid cells, its deficiency impairs resolution, rather than progression, of CHS with over-production of proinflammatory markers, while holding antiinflammatory markers unchanged, in both skin and regional LNs. Robust elevation of DC/macrophage surface markers and proinflammatory cytokines/chemokines in hapten-challenged Pla2g2d−/− mice over WT mice in the late phase of CHS indicates that PLA2G2D deficiency perturbs the termination program for the recruitment and activation of monocyte-derived inflammatory DCs/macrophages. In support of this, GM-CSF-driven BMDCs from Pla2g2d−/− mice have elevated expression of cell activation markers and can elicit skin inflammation after transferred into mice. The decrease of PLA2G2D expression in activated DCs also accords with the antiinflammatory property of this enzyme. In addition, a modestly increased splenic CD4+ T cells in Pla2g2d−/− mice under normal condition might result from the increased activation of CD4+ DCs.
During the process of inflammation, lipid mediator profiles switch from proinflammatory to antiinflammatory products, which in turn contribute to sequestration of the recruitment of inflammatory cells to the site (Levy et al., 2001; Serhan et al., 2008). Of importance, lipidomics profiling has delineated a critical role of PLA2G2D in the supply of a pool of free PUFAs that can be selectively linked to an array of pro-resolving lipid products in the skin-draining LNs. Thus, several DHA- and EPA-oxygenated products and AA-derived 15d-PGJ2 were significantly reduced in the LNs by PLA2G2D deletion. The LN levels of free AA, EPA, and DHA were lower in Pla2g2d−/− mice even under noninflamed conditions, suggesting that PLA2G2D participates in the steady-state supply of a particular PUFA pool within the LNs. In contrast, Th1-promoting AA-derived mediators appear to be mostly derived from the PLA2G2D-independent AA pool, thus revealing an unexplored compartmentalization of distinct PUFA pools toward pro- versus antiinflammatory pathways. Although the molecular basis for the distinct compartmentalization of the pro- and antiinflammatory lipid pathways in the LNs is unclear at present, it may rely on when and where PLA2G2D secreted from DCs could encounter its target phospholipids during the process of immune response. The differential expression of downstream biosynthetic enzymes, each of which may be localized in distinct cell populations, could provide partly an explanation for the different coupling of PLA2G2D with pro- versus antiinflammatory lipid mediators. The preferred target phospholipids of PLA2G2D are particular PE molecular species with PUFAs such as DHA and AA. Considering that PE usually resides in the inner leaflet of the plasma membrane in resting cells, PLA2G2D might act on these PE species exposed on activated cells by a process of flip-flop or those released extracellularly from the cells (e.g., exosomes or microvesicles). Supporting the latter possibility, sPLA2 acts on microvesicles shed from cells at inflamed sites (Fourcade et al., 1995) and leukocyte-derived microvesicles, which are temporally generated in inflammatory exudates during resolution, contain esterified biosynthetic precursors of pro-resolving mediators (Norling et al., 2011).
Collectively, we conclude that PLA2G2D is preferentially coupled with the biosynthesis of pro-resolving lipid mediators that could resolve LN inflammation. Our results are compatible with a recent report showing that mouse lymphoid tissues produce RvD1, which can regulate antibody production (Ramon et al., 2012). Also, free PUFAs by themselves might be protective against inflammation through acting on PUFA-sensing receptors (Diep et al., 2000; Oh et al., 2010) or through reducing the stress response (Ariyama et al., 2010). As such, the lack of PLA2G2D, and accordingly the decrease of the pro-resolving lipid mediators driven by this enzyme, may tip the balance toward a more aggressive inflammatory response in the LNs and thereby in the skin. The lipid-mobilizing role of PLA2G2D in local skin microenvironments remains to be elucidated.
PLA2G5 is expressed in macrophages and DCs (Giannattasio et al., 2010; Satake et al., 2004), and we have shown here that it is preferentially localized in CD11b+CD11c− splenic macrophages. The expression of PLA2G2D in lymphoid organs and in CD11c+ cells is much higher than that of PLA2G5 and other sPLA2 isoforms. Beyond their distinct expression patterns, these two sPLA2s afford opposite effects on DC functions and immunity; PLA2G5 facilitates antigen capture and presentation by DCs toward airway inflammation (Giannattasio et al., 2010), while PLA2G2D has pro-resolving roles in CHS (as shown in this study by gene targeting) and in experimental multiple sclerosis and colitis (as shown by injection of PLA2G2D-Fc fusion protein; von Allmen et al., 2009). Although the mechanism underlying the functional difference between these two sPLA2s is unclear, it might rely at least partly on their distinct expression levels and on their different substrate selectivity in that PLA2G2D prefers PE bearing PUFAs (this study), whereas PLA2G5 prefers PE and PC bearing fatty acids with low degree of unsaturation (Chen and Dennis, 1998; Mitsuishi et al., 2007). Non-redundant roles of multiple sPLA2s in a given process have also been documented for arthritis (offensive PLA2G2A versus defensive PLA2G5; Boilard et al., 2010) and for male reproduction (PLA2G3 for sperm maturation versus PLA2G10 for sperm activation; Escoffier et al., 2010; Sato et al., 2010). The contrasting roles of PLA2G2D versus PLA2G5 in the DC-related immunity represent another example of these occasions. Thus, individual sPLA2s, which exhibit distinct substrate preferences or cellular locations in a given tissue or cell type, act spatiotemporally on different membrane targets, thereby linking to distinct biological outcomes. Although pan-sPLA2 inhibitors, which block classical sPLA2s broadly, attenuate several disease models (Rosenson et al., 2009), dampening offensive and defensive sPLA2s altogether might limit their therapeutic use (Bowton et al., 2005; Bradley et al., 2005). Our results point to an alternative strategy to use PLA2G2D, a resolving sPLA2, for treatment of human disorders.
MATERIALS AND METHODS
Targeted disruption of the Pla2g2d gene.
The Pla2g2d-targeting vector was constructed with a neomycin resistance (Neor) gene that was inserted between exons 1 and 2 of the Pla2g2d gene. ES cell transfection and embryo injections were performed by the Transgenic Resources Program (Department of Comparative Medicine, University of Washington, Seattle, WA). ES clones with homologous recombination were screened by PCR and Southern blotting. After germline transmission, Pla2g2d+/− mice were bred with C57BL/6NCrSLc (Japan SLC) mice (n > 12). Genotyping was performed on genomic DNA from tail biopsies by PCR using the primer pairs 5′-CCATCTGAACTTGAGTGGGAAGTGCCA-3′ and 5′-GCCTATGGGTCCCTCATGAG-3′ (Sigma Genosys), which amplified a 490-bp fragment specific for the WT allele, and 5′-GTGCTGTCAGAAGCATGATTGTT-3′ and 5′-GCCTATGGGTCCCTCATGAG-3′, which amplified a 530-bp fragment specific for the mutant allele. The reaction was 95°C for 10 s and then 35 cycles of 95°C for 0 s and 65°C for 1 min on Applied Biosystems 9800 Fast Thermal Cycler (Applied Biosystems). The PCR products were analyzed by 1.5% (wt/vol) agarose gel electrophoresis with ethidium bromide. All mice were housed in climate-controlled (23°C) specific pathogen–free facilities with a 12-h light-dark cycle, with free access to standard laboratory food (CE2; Laboratory Diet; CLEA Japan) and water. All procedures involving animals were performed in accordance with protocols approved by the Institutional Animal Care and Use Committees of the Tokyo Metropolitan Institute of Medical Science, Showa University, and the University of Washington.
Hapten-induced CHS.
On day −5, mice (7–12-wk-old, male) were sensitized with 50 µl of 0.5% (vol/vol) DNFB in acetone/olive oil (4/1, vol/vol) on shaved abdominal skin. On day 0, ears were challenged by 20 µl of 0.3% DNFB to their dorsal and ventral surfaces. Ear thickness was measured for each mouse before and after elicitation for various time periods at a predetermined site with a micrometer, and the difference is expressed as ear swelling.
Quantitative RT-PCR.
Total RNA was extracted from tissues and cells using TRIzol reagent (Invitrogen). First-strand cDNA synthesis was performed using a High Capacity cDNA Reverse transcription kit (Applied Biosystems). PCR reactions were performed using Power SYBR Green PCR system (Applied Biosystems) or TaqMan Gene Expression System (Applied Biosystems) on the ABI7700 Quantitative PCR system (Applied Biosystems). The probe/primer sets used were listed in Table S3.
Immunohistochemistry.
Paraffin-embedded tissue sections (∼4–7 µm thick) were incubated with Target Retrieval Solution (Dako), and then with rabbit anti–mouse or human PLA2G2D antibody, which did not cross-react with other sPLA2s (Degousee et al., 2002), or control antibody at 1:2,000 dilution in 10 mM Tris-HCl (pH 7.4) containing 0.15 M NaCl and 0.1% BSA (TBS-BSA) overnight at 4°C. The sections were then treated with an EnVision+ System Staining kit (Dako) with diaminobenzidine substrate, followed by counterstaining with hematoxylin (Murakami et al., 2005; Sato et al., 2010). Human LN sections were obtained by surgery at Toho University Ohmori Hospital (Tokyo, Japan) after approval by the ethical committee of the faculty and informed consents from patients.
Confocal laser microscopy.
Mouse frozen tissues mounted in OCT compound (Sakura Finetek) were cut into 20-µm thick sections using a cryomicrotome (CM3050S; Leica). The sections were incubated with 1.5 x Block Ace (DS Pharma Biomedical) in TBS for 30 min, and then with rabbit anti–mouse PLA2G2D antibody, phycoerythrin-conjugated hamster anti–mouse CD11c antibody (N418; eBioscience), or FITC-labeled rat anti–mouse F4/80 antibody (BM8; BioLegend) in TBS-BSA overnight at 4°C. For human tissues, rabbit anti–human PLA2G2D antibody and Alexa Fluor 488–labeled mouse anti–human CD11c antibody (3.9; BioLegend) were used. The sections were then treated with 1 µg/ml Alexa Fluor 647–labeled goat anti–rabbit IgG (Invitrogen) in TBS-BSA for 1 h, mounted in a VectaShield mounting medium (Vector laboratories), and analyzed with a confocal laser scanning microscope (LSM510 META; Carl Zeiss).
Flow cytometry.
Mouse spleens or inguinal LNs were excised, minced in Hanks’ solution (Nissui Pharmaceutical) with 2% (vol/vol) heat-inactivated FBS (Invitrogen) and 0.05% (wt/vol) sodium azide (Nacalai Tesque), and incubated with 400 U/ml collagenase type II (Worthington) with shaking for 30 min at 37°C. After adding 10 mM EDTA, the suspensions were passed through Cell Strainer 70-µm Nylon (Falcon; BD), and then centrifuged at 300 g for 5 min at 4°C. Splenocytes were treated for 2 min on ice with 10 mM Tris-HCl (pH 8.0) containing 0.84% (wt/vol) ammonium chloride to lyse red blood cells, centrifuged, and suspended in Hanks’ solution. For AutoMACS analysis, the cells were resuspended at 107 cells/ml in AutoMACS buffer (PBS, 0.5% [wt/vol] BSA, and 2 mM EDTA), blocked with 0.5 µg/ml rat anti–mouse CD16/CD32 antibody (mouse BD Tc Block; BD) for 5 min at 4°C, and were added with 1/4 volume of appropriate MicroBeads (Miltenyi Biotec). After incubation for 15 min at 4°C, cells (∼108 cells/500 µl) were resuspended in AutoMACS buffer and applied to AutoMACS Pro Separator (Miltenyi Biotec). For flow cytometry, the cells were blocked with mouse BD Tc Block, incubated with marker antibodies (listed in Table S4), and analyzed with a FACSAria III (BD) and FlowJo (Tree Star) software.
Culture of LN cells.
LN cells (106 cells/ml) were isolated from mice that had been sensitized with DNFB for 5 d and cultured with or without 100 µg/ml DNBS (Alfa Aesar) in culture medium (RPMI 1640 containing 10% heat-inactivated FBS, 50 µM 2-ME, 2 mM l-glutamine, 25 mM Hepes, 1 mM nonessential amino acids, 1 mM sodium pyruvate, 100 U/ml penicillin, and 100 µg/ml streptomycin). After appropriate periods, RNA was extracted from the cells and subjected to quantitative RT-PCR, and the supernatants were subjected to ELISA for cytokines (eBioscience). As required for experiments, PUFAs, 15d-PGJ2, and RvD1 (Cayman Chemicals) were added to the culture.
Culture of BMDCs and BMDMs.
BM cells were cultured with mouse GM-CSF (10 ng/ml; PeproTech) for 9 d and with mouse M-CSF (100 ng/ml; Kyowa Kirin) for 3 d to obtain BMDCs and BMDMs, respectively (Lutz et al., 1999). The cells (2 × 106 cells/ml) were stimulated with LPS (Sigma-Aldrich) for 24 h to assess Pla2g2d expression by quantitative RT-PCR, cytokine release by ELISA, or surface expression of DC activation markers by FACS. For transfer of BMDCs into mice, the cells (2 × 106 cells/ml) were treated with 100 µg/ml DNBS for 24 h, resuspended in PBS at 106 cells/200 µl, and injected intravenously into recipient mice. After 5 d, the ears were challenged with 40 µl of 0.3% DNFB to monitor ear swelling.
Immunoblotting.
Spleen (10 mg) was homogenized in 100 µl of 20 mM Tris-HCl (pH7.5) with a Polytron homogenizer. Protein concentrations were determined with a BCA protein assay kit (Thermo Fisher Scientific). The homogenates (10 µg protein) were subjected to SDS-PAGE on 15% (wt/vol) gels under nonreducing conditions and were electroblotted onto PVDF membranes (Bio-Rad Laboratories) with a semi-dry blotter (Transblot SD; Bio-Rad Laboratories). The membranes were blocked with 5% (wt/vol) skim milk in PBS containing 0.05% (vol/vol) Tween 20 (PBS-T), probed with goat anti-PLA2G2D antibody (sc-104297; Santa Cruz Biotechnology, Inc.) at 1:5,000 dilution in PBS-T for 2 h, incubated with horseradish peroxidase–conjugated anti-goat IgG (Zymed) at 1:5,000 dilution in PBS-T for 2 h, and then visualized by ECL Prime Western blotting detection reagent (GE Healthcare) with LAS-4000 (Fuji Film).
ESI-MS.
Tissues were soaked in 10 vol of methanol and homogenized with a Polytron homogenizer. After overnight incubation at −20°C, water was added to the mixture to give a final methanol concentration of 10% (vol/vol). As internal standards, 1 ng of d4-labeled leukotriene B4, d4-labeled PGD2, and d8-labeled 15-HETE (Cayman Chemicals) were added to the samples. The samples in 10% methanol were applied to Sep-Pak C18 cartridges (Waters), washed with 10 ml of hexane, eluted with 3 ml of methyl formate, dried up under N2 gas, and solved in 60% methanol. The analysis of PUFAs and their metabolites was performed using a 4000Q-TRAP quadrupole-linear ion trap hybrid mass spectrometer (AB Sciex) with liquid chromatography (LC; LC-20AP; Shimazu) combined with an HTC PAL autosampler (CTC Analytics). The sample was applied to the Develosil C30-UG column (1 × 150 mm i.d., 3 µm particle; Nomura Chemical) coupled for ESI-MS/MS. The samples injected by an autosampler (10 µl) were separated by a step gradient with mobile phase A (water containing 0.1% acetic acid) and mobile phase B (acetonitrile: methanol = 4: 1; vol/vol) at a flow rate of 50 µl/min at 45°C. The detection of each lipid was performed by multiple reaction monitoring (Yamamoto et al., 2011).
PLA2 reaction.
Lipids were extracted from mouse LNs by the method of Bligh and Dyer (Bligh and Dyer, 1959). The LN membrane mimic composed of the tissue-extracted lipids (250 µg) was sonicated for 5 min in 100 mM Tris-HCl (pH 7.4) containing 4 mM CaCl2 and then incubated for 1 h at 37°C with 200 nM recombinant mouse PLA2G2D (Rouault et al., 2007). After incubation, the lipids were mixed with internal standards (250 pmol of PE or PC with 14:0-14:0), extracted, and subjected to ESI-MS for detections of fatty acids, phospholipids, and lysophospholipids (Sato et al., 2010; Yamamoto et al., 2011).
Statistical analysis.
Data are presented as the mean ± SEM. Statistical significance was evaluated by two-tailed Student’s t test and Dunnett test at a significance level of P < 0.05.
Online supplemental material.
Fig. S1 shows flow cytometry of splenocytes used in Fig. 1 D. Tables S1 and S2 show serum parameters and circulating blood cells in Pla2g2d−/− and Pla2g2d+/+ mice under normal conditions. Table S3 is a list of primers used in quantitative RT-PCR. Table S4 is a list of antibodies used in flow cytometry. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20121887/DC1.
Supplementary Material
Acknowledgments
We would like to thank Dr. Takehiko Yokomizo (Juntendo University, Tokyo, Japan) for technical assistance.
This work was supported by grants in aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan 22116005, 24390021 (to M. Murakami), 24890296 (to Y. Miki), 23591665 (to K. Yamamoto), 23790119 (to Y. Taketomi), and 23790120 (to H. Sato), Promoting Individual Research to Nurture the Seeds of Future Innovation and Organizing Unique Innovative Network (PRESTO) of Japan Science and Technology Agency (JST; to M. Murakami), and the Uehara, Mitsubishi, Terumo, and Toray Science Foundations (to M. Murakami).
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- 15d-PGJ2
- 15-deoxy-Δ12,14-prostaglandin J2
- AA
- arachidonic acid
- BMDC
- BM-derived DC
- CHS
- contact hypersensitivity
- cPLA2
- Ca2+-dependent cytosolic PLA2
- DHA
- docosahexaenoic acid
- DNBS
- dinitrobenzene sulfonic acid
- DNFB
- dinitrofluorobenzene
- EPA
- eicosapentaenoic acid
- ESI-MS
- electrospray ionization mass spectrometry
- HETE
- hydroxyeicosatetraenoic acid
- iPLA2
- Ca2+-independent PLA2
- LA
- linoleic acid
- LC
- liquid chromatography
- LysoPC
- lysophosphatidylcholine
- LysoPE
- lysophosphatidylethanolamine
- LysoPS
- lysophosphatidylserine
- PC
- phosphatidylcholine
- PD1
- protectin D1 (10(S),17(S)-dihydroxy-4Z,7Z,11E,13Z,15E,19Z-DHA)
- PE
- phosphatidylethanolamine
- PG
- phosphatidylglycerol
- PGD2
- prostaglandin D2
- PGE2
- prostaglandin E2
- PLA2
- phospholipase A2
- PLA2G2D
- group IID sPLA2
- PUFA
- polyunsaturated fatty acid
- RvD1
- resolvin D1 (7S,8R,17S-trihydroxy-4Z,9E,11E,13Z,15E,19Z-DHA)
- sPLA2
- secreted PLA2
- T reg cell
- regulatory T cell
References
- Allenspach E.J., Lemos M.P., Porrett P.M., Turka L.A., Laufer T.M. 2008. Migratory and lymphoid-resident dendritic cells cooperate to efficiently prime naive CD4 T cells. Immunity. 29:795–806 10.1016/j.immuni.2008.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariyama H., Kono N., Matsuda S., Inoue T., Arai H. 2010. Decrease in membrane phospholipid unsaturation induces unfolded protein response. J. Biol. Chem. 285:22027–22035 10.1074/jbc.M110.126870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balestrieri B., Maekawa A., Xing W., Gelb M.H., Katz H.R., Arm J.P. 2009. Group V secretory phospholipase A2 modulates phagosome maturation and regulates the innate immune response against Candida albicans. J. Immunol. 182:4891–4898 10.4049/jimmunol.0803776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligh E.G., Dyer W.J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911–917 10.1139/o59-099 [DOI] [PubMed] [Google Scholar]
- Boilard E., Lai Y., Larabee K., Balestrieri B., Ghomashchi F., Fujioka D., Gobezie R., Coblyn J.S., Weinblatt M.E., Massarotti E.M., et al. 2010. A novel anti-inflammatory role for secretory phospholipase A2 in immune complex-mediated arthritis. EMBO Mol. Med. 2:172–187 10.1002/emmm.201000072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonventre J.V., Huang Z., Taheri M.R., O’Leary E., Li E., Moskowitz M.A., Sapirstein A. 1997. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature. 390:622–625 10.1038/37635 [DOI] [PubMed] [Google Scholar]
- Bostrom M.A., Boyanovsky B.B., Jordan C.T., Wadsworth M.P., Taatjes D.J., de Beer F.C., Webb N.R. 2007. Group V secretory phospholipase A2 promotes atherosclerosis: evidence from genetically altered mice. Arterioscler. Thromb. Vasc. Biol. 27:600–606 10.1161/01.ATV.0000257133.60884.44 [DOI] [PubMed] [Google Scholar]
- Bowton D.L., Dmitrienko A.A., Israel E., Zeiher B.G., Sides G.D. 2005. Impact of a soluble phospholipase A2 inhibitor on inhaled allergen challenge in subjects with asthma. J. Asthma. 42:65–71 10.1081/JAS-200044748 [DOI] [PubMed] [Google Scholar]
- Bradley J.D., Dmitrienko A.A., Kivitz A.J., Gluck O.S., Weaver A.L., Wiesenhutter C., Myers S.L., Sides G.D. 2005. A randomized, double-blinded, placebo-controlled clinical trial of LY333013, a selective inhibitor of group II secretory phospholipase A2, in the treatment of rheumatoid arthritis. J. Rheumatol. 32:417–423 [PubMed] [Google Scholar]
- Byrum R.S., Goulet J.L., Snouwaert J.N., Griffiths R.J., Koller B.H. 1999. Determination of the contribution of cysteinyl leukotrienes and leukotriene B4 in acute inflammatory responses using 5-lipoxygenase- and leukotriene A4 hydrolase-deficient mice. J. Immunol. 163:6810–6819 http://www.jimmunol.org/content/163/12/6810 [PubMed] [Google Scholar]
- Chen Y., Dennis E.A. 1998. Expression and characterization of human group V phospholipase A2. Biochim. Biophys. Acta. 1394:57–64 10.1016/S0005-2760(98)00098-8 [DOI] [PubMed] [Google Scholar]
- Curfs D.M., Ghesquiere S.A., Vergouwe M.N., van der Made I., Gijbels M.J., Greaves D.R., Verbeek J.S., Hofker M.H., de Winther M.P. 2008. Macrophage secretory phospholipase A2 group X enhances anti-inflammatory responses, promotes lipid accumulation, and contributes to aberrant lung pathology. J. Biol. Chem. 283:21640–21648 10.1074/jbc.M710584200 [DOI] [PubMed] [Google Scholar]
- Danno K., Ikai K., Imamura S. 1993. Anti-inflammatory effects of eicosapentaenoic acid on experimental skin inflammation models. Arch. Dermatol. Res. 285:432–435 10.1007/BF00372139 [DOI] [PubMed] [Google Scholar]
- Degousee N., Ghomashchi F., Stefanski E., Singer A., Smart B.P., Borregaard N., Reithmeier R., Lindsay T.F., Lichtenberger C., Reinisch W., et al. 2002. Groups IV, V, and X phospholipases A2s in human neutrophils: role in eicosanoid production and gram-negative bacterial phospholipid hydrolysis. J. Biol. Chem. 277:5061–5073 10.1074/jbc.M109083200 [DOI] [PubMed] [Google Scholar]
- den Haan J.M., Lehar S.M., Bevan M.J. 2000. CD8(+) but not CD8(-) dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med. 192:1685–1696 10.1084/jem.192.12.1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diep Q.N., Touyz R.M., Schiffrin E.L. 2000. Docosahexaenoic acid, a peroxisome proliferator-activated receptor-α ligand, induces apoptosis in vascular smooth muscle cells by stimulation of p38 mitogen-activated protein kinase. Hypertension. 36:851–855 10.1161/01.HYP.36.5.851 [DOI] [PubMed] [Google Scholar]
- Draper E., Reynolds C.M., Canavan M., Mills K.H., Loscher C.E., Roche H.M. 2011. Omega-3 fatty acids attenuate dendritic cell function via NF-κB independent of PPARγ. J. Nutr. Biochem. 22:784–790 10.1016/j.jnutbio.2010.06.009 [DOI] [PubMed] [Google Scholar]
- Escoffier J., Jemel I., Tanemoto A., Taketomi Y., Payre C., Coatrieux C., Sato H., Yamamoto K., Masuda S., Pernet-Gallay K., et al. 2010. Group X phospholipase A2 is released during sperm acrosome reaction and controls fertility outcome in mice. J. Clin. Invest. 120:1415–1428 10.1172/JCI40494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fickenscher H., Hör S., Küpers H., Knappe A., Wittmann S., Sticht H. 2002. The interleukin-10 family of cytokines. Trends Immunol. 23:89–96 10.1016/S1471-4906(01)02149-4 [DOI] [PubMed] [Google Scholar]
- Fourcade O., Simon M.F., Viodé C., Rugani N., Leballe F., Ragab A., Fournié B., Sarda L., Chap H. 1995. Secretory phospholipase A2 generates the novel lipid mediator lysophosphatidic acid in membrane microvesicles shed from activated cells. Cell. 80:919–927 10.1016/0092-8674(95)90295-3 [DOI] [PubMed] [Google Scholar]
- Fujioka D., Saito Y., Kobayashi T., Yano T., Tezuka H., Ishimoto Y., Suzuki N., Yokota Y., Nakamura T., Obata J.E., et al. 2008. Reduction in myocardial ischemia/reperfusion injury in group X secretory phospholipase A2-deficient mice. Circulation. 117:2977–2985 10.1161/CIRCULATIONAHA.107.743997 [DOI] [PubMed] [Google Scholar]
- Giannattasio G., Fujioka D., Xing W., Katz H.R., Boyce J.A., Balestrieri B. 2010. Group V secretory phospholipase A2 reveals its role in house dust mite-induced allergic pulmonary inflammation by regulation of dendritic cell function. J. Immunol. 185:4430–4438 10.4049/jimmunol.1001384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez P.F., Pillinger M.H., Attur M., Marjanovic N., Dave M., Park J., Bingham C.O., III, Al-Mussawir H., Abramson S.B. 2005. Resolution of inflammation: prostaglandin E2 dissociates nuclear trafficking of individual NF-κB subunits (p65, p50) in stimulated rheumatoid synovial fibroblasts. J. Immunol. 175:6924–6930 http://www.jimmunol.org/content/175/10/6924 [DOI] [PubMed] [Google Scholar]
- Groeger A.L., Cipollina C., Cole M.P., Woodcock S.R., Bonacci G., Rudolph T.K., Rudolph V., Freeman B.A., Schopfer F.J. 2010. Cyclooxygenase-2 generates anti-inflammatory mediators from ω-3 fatty acids. Nat. Chem. Biol. 6:433–441 10.1038/nchembio.367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath W.R., Carbone F.R. 2009. Dendritic cell subsets in primary and secondary T cell responses at body surfaces. Nat. Immunol. 10:1237–1244 10.1038/ni.1822 [DOI] [PubMed] [Google Scholar]
- Henderson W.R., Jr, Chi E.Y., Bollinger J.G., Tien Y.T., Ye X., Castelli L., Rubtsov Y.P., Singer A.G., Chiang G.K., Nevalainen T., et al. 2007. Importance of group X-secreted phospholipase A2 in allergen-induced airway inflammation and remodeling in a mouse asthma model. J. Exp. Med. 204:865–877 10.1084/jem.20070029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T., Matsuoka T., Ueta M., Kabashima K., Miyachi Y., Narumiya S. 2009. Prostaglandin E(2)-EP(3) signaling suppresses skin inflammation in murine contact hypersensitivity. J. Allergy Clin. Immunol. 124:809–818: e2 10.1016/j.jaci.2009.04.029 [DOI] [PubMed] [Google Scholar]
- Ishizaki J., Suzuki N., Higashino K., Yokota Y., Ono T., Kawamoto K., Fujii N., Arita H., Hanasaki K. 1999. Cloning and characterization of novel mouse and human secretory phospholipase A(2)s. J. Biol. Chem. 274:24973–24979 10.1074/jbc.274.35.24973 [DOI] [PubMed] [Google Scholar]
- Itano A.A., McSorley S.J., Reinhardt R.L., Ehst B.D., Ingulli E., Rudensky A.Y., Jenkins M.K. 2003. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 19:47–57 10.1016/S1074-7613(03)00175-4 [DOI] [PubMed] [Google Scholar]
- Kabashima K., Sakata D., Nagamachi M., Miyachi Y., Inaba K., Narumiya S. 2003. Prostaglandin E2-EP4 signaling initiates skin immune responses by promoting migration and maturation of Langerhans cells. Nat. Med. 9:744–749 10.1038/nm872 [DOI] [PubMed] [Google Scholar]
- Lambeau G., Gelb M.H. 2008. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu. Rev. Biochem. 77:495–520 10.1146/annurev.biochem.76.062405.154007 [DOI] [PubMed] [Google Scholar]
- Levy B.D., Clish C.B., Schmidt B., Gronert K., Serhan C.N. 2001. Lipid mediator class switching during acute inflammation: signals in resolution. Nat. Immunol. 2:612–619 10.1038/89759 [DOI] [PubMed] [Google Scholar]
- Libby P. 2002. Inflammation in atherosclerosis. Nature. 420:868–874 10.1038/nature01323 [DOI] [PubMed] [Google Scholar]
- Lindbom J., Ljungman A.G., Tagesson C. 2005. Interferon γ-induced gene expression of the novel secretory phospholipase A2 type IID in human monocyte-derived macrophages is inhibited by lipopolysaccharide. Inflammation. 29:108–117 10.1007/s10753-006-9007-x [DOI] [PubMed] [Google Scholar]
- Lu B., Ebensperger C., Dembic Z., Wang Y., Kvatyuk M., Lu T., Coffman R.L., Pestka S., Rothman P.B. 1998. Targeted disruption of the interferon-γ receptor 2 gene results in severe immune defects in mice. Proc. Natl. Acad. Sci. USA. 95:8233–8238 10.1073/pnas.95.14.8233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz M.B., Kukutsch N., Ogilvie A.L., Rössner S., Koch F., Romani N., Schuler G. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods. 223:77–92 10.1016/S0022-1759(98)00204-X [DOI] [PubMed] [Google Scholar]
- Maldonado-López R., De Smedt T., Michel P., Godfroid J., Pajak B., Heirman C., Thielemans K., Leo O., Urbain J., Moser M. 1999. CD8α+ and CD8α− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J. Exp. Med. 189:587–592 10.1084/jem.189.3.587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeachy M.J. 2011. GM-CSF: the secret weapon in the T(H)17 arsenal. Nat. Immunol. 12:521–522 10.1038/ni.2044 [DOI] [PubMed] [Google Scholar]
- Mitsuishi M., Masuda S., Kudo I., Murakami M. 2007. Human group III phospholipase A2 suppresses adenovirus infection into host cells. Evidence that group III, V and X phospholipase A2s act on distinct cellular phospholipid molecular species. Biochim. Biophys. Acta. 1771:1389–1396 10.1016/j.bbalip.2007.09.006 [DOI] [PubMed] [Google Scholar]
- Moon S.H., Jenkins C.M., Mancuso D.J., Turk J., Gross R.W. 2008. Smooth muscle cell arachidonic acid release, migration, and proliferation are markedly attenuated in mice null for calcium-independent phospholipase A2β. J. Biol. Chem. 283:33975–33987 10.1074/jbc.M805817200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee P.K., Marcheselli V.L., Serhan C.N., Bazan N.G. 2004. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc. Natl. Acad. Sci. USA. 101:8491–8496 10.1073/pnas.0402531101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz N.M., Meliton A.Y., Arm J.P., Bonventre J.V., Cho W., Leff A.R. 2007. Deletion of secretory group V phospholipase A2 attenuates cell migration and airway hyperresponsiveness in immunosensitized mice. J. Immunol. 179:4800–4807 http://www.jimmunol.org/content/179/7/4800 [DOI] [PubMed] [Google Scholar]
- Murakami M., Koduri R.S., Enomoto A., Shimbara S., Seki M., Yoshihara K., Singer A., Valentin E., Ghomashchi F., Lambeau G., et al. 2001. Distinct arachidonate-releasing functions of mammalian secreted phospholipase A2s in human embryonic kidney 293 and rat mastocytoma RBL-2H3 cells through heparan sulfate shuttling and external plasma membrane mechanisms. J. Biol. Chem. 276:10083–10096 10.1074/jbc.M007877200 [DOI] [PubMed] [Google Scholar]
- Murakami M., Masuda S., Ueda-Semmyo K., Yoda E., Kuwata H., Takanezawa Y., Aoki J., Arai H., Sumimoto H., Ishikawa Y., et al. 2005. Group VIB Ca2+-independent phospholipase A2γ promotes cellular membrane hydrolysis and prostaglandin production in a manner distinct from other intracellular phospholipases A2. J. Biol. Chem. 280:14028–14041 10.1074/jbc.M413766200 [DOI] [PubMed] [Google Scholar]
- Murakami M., Taketomi Y., Girard C., Yamamoto K., Lambeau G. 2010. Emerging roles of secreted phospholipase A2 enzymes: Lessons from transgenic and knockout mice. Biochimie. 92:561–582 10.1016/j.biochi.2010.03.015 [DOI] [PubMed] [Google Scholar]
- Murakami M., Taketomi Y., Miki Y., Sato H., Hirabayashi T., Yamamoto K. 2011. Recent progress in phospholipase A₂ research: from cells to animals to humans. Prog. Lipid Res. 50:152–192 10.1016/j.plipres.2010.12.001 [DOI] [PubMed] [Google Scholar]
- Nagamachi M., Sakata D., Kabashima K., Furuyashiki T., Murata T., Segi-Nishida E., Soontrapa K., Matsuoka T., Miyachi Y., Narumiya S. 2007. Facilitation of Th1-mediated immune response by prostaglandin E receptor EP1. J. Exp. Med. 204:2865–2874 10.1084/jem.20070773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S., Honda T., Sakata D., Egawa G., Tanizaki H., Otsuka A., Moniaga C.S., Watanabe T., Miyachi Y., Narumiya S., Kabashima K. 2010. Prostaglandin I2-IP signaling promotes Th1 differentiation in a mouse model of contact hypersensitivity. J. Immunol. 184:5595–5603 10.4049/jimmunol.0903260 [DOI] [PubMed] [Google Scholar]
- Nakano H., Lin K.L., Yanagita M., Charbonneau C., Cook D.N., Kakiuchi T., Gunn M.D. 2009. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nat. Immunol. 10:394–402 10.1038/ni.1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norling L.V., Spite M., Yang R., Flower R.J., Perretti M., Serhan C.N. 2011. Cutting edge: Humanized nano-proresolving medicines mimic inflammation-resolution and enhance wound healing. J. Immunol. 186:5543–5547 10.4049/jimmunol.1003865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh D.Y., Talukdar S., Bae E.J., Imamura T., Morinaga H., Fan W., Li P., Lu W.J., Watkins S.M., Olefsky J.M. 2010. GPR120 is an ω-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 142:687–698 10.1016/j.cell.2010.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuki M., Taketomi Y., Arata S., Masuda S., Ishikawa Y., Ishii T., Takanezawa Y., Aoki J., Arai H., Yamamoto K., et al. 2006. Transgenic expression of group V, but not group X, secreted phospholipase A2 in mice leads to neonatal lethality because of lung dysfunction. J. Biol. Chem. 281:36420–36433 10.1074/jbc.M607975200 [DOI] [PubMed] [Google Scholar]
- Pooley J.L., Heath W.R., Shortman K. 2001. Cutting edge: intravenous soluble antigen is presented to CD4 T cells by CD8− dendritic cells, but cross-presented to CD8 T cells by CD8+ dendritic cells. J. Immunol. 166:5327–5330 http://www.jimmunol.org/content/166/9/5327 [DOI] [PubMed] [Google Scholar]
- Pulendran B., Smith J.L., Caspary G., Brasel K., Pettit D., Maraskovsky E., Maliszewski C.R. 1999. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc. Natl. Acad. Sci. USA. 96:1036–1041 10.1073/pnas.96.3.1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajakariar R., Hilliard M., Lawrence T., Trivedi S., Colville-Nash P., Bellingan G., Fitzgerald D., Yaqoob M.M., Gilroy D.W. 2007. Hematopoietic prostaglandin D2 synthase controls the onset and resolution of acute inflammation through PGD2 and 15-deoxyDelta12 14 PGJ2. Proc. Natl. Acad. Sci. USA. 104:20979–20984 10.1073/pnas.0707394104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon S., Gao F., Serhan C.N., Phipps R.P. 2012. Specialized proresolving mediators enhance human B cell differentiation to antibody-secreting cells. J. Immunol. 189:1036–1042 10.4049/jimmunol.1103483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenson R.S., Hislop C., McConnell D., Elliott M., Stasiv Y., Wang N., Waters D.D.; PLASMA Investigators 2009. Effects of 1-H-indole-3-glyoxamide (A-002) on concentration of secretory phospholipase A2 (PLASMA study): a phase II double-blind, randomised, placebo-controlled trial. Lancet. 373:649–658 10.1016/S0140-6736(09)60403-7 [DOI] [PubMed] [Google Scholar]
- Rossi A., Kapahi P., Natoli G., Takahashi T., Chen Y., Karin M., Santoro M.G. 2000. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase. Nature. 403:103–108 10.1038/47520 [DOI] [PubMed] [Google Scholar]
- Rouault M., Le Calvez C., Boilard E., Surrel F., Singer A., Ghomashchi F., Bezzine S., Scarzello S., Bollinger J., Gelb M.H., Lambeau G. 2007. Recombinant production and properties of binding of the full set of mouse secreted phospholipases A2 to the mouse M-type receptor. Biochemistry. 46:1647–1662 10.1021/bi062119b [DOI] [PubMed] [Google Scholar]
- Samuelsson B., Dahlén S.E., Lindgren J.A., Rouzer C.A., Serhan C.N. 1987. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 237:1171–1176 10.1126/science.2820055 [DOI] [PubMed] [Google Scholar]
- Satake Y., Diaz B.L., Balestrieri B., Lam B.K., Kanaoka Y., Grusby M.J., Arm J.P. 2004. Role of group V phospholipase A2 in zymosan-induced eicosanoid generation and vascular permeability revealed by targeted gene disruption. J. Biol. Chem. 279:16488–16494 10.1074/jbc.M313748200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Taketomi Y., Isogai Y., Miki Y., Yamamoto K., Masuda S., Hosono T., Arata S., Ishikawa Y., Ishii T., et al. 2010. Group III secreted phospholipase A2 regulates epididymal sperm maturation and fertility in mice. J. Clin. Invest. 120:1400–1414 10.1172/JCI40493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbina N.V., Salazar-Mather T.P., Biron C.A., Kuziel W.A., Pamer E.G. 2003. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 19:59–70 10.1016/S1074-7613(03)00171-7 [DOI] [PubMed] [Google Scholar]
- Serhan C.N. 2007. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu. Rev. Immunol. 25:101–137 10.1146/annurev.immunol.25.022106.141647 [DOI] [PubMed] [Google Scholar]
- Serhan C.N., Hong S., Gronert K., Colgan S.P., Devchand P.R., Mirick G., Moussignac R.L. 2002. Resolvins: a family of bioactive products of ω-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. 196:1025–1037 10.1084/jem.20020760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C.N., Chiang N., Van Dyke T.E. 2008. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 8:349–361 10.1038/nri2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakhov A.N., Rubtsov A.V., Lyakhov I.G., Tumanov A.V., Nedospasov S.A. 2000. SPLASH (PLA2IID), a novel member of phospholipase A2 family, is associated with lymphotoxin deficiency. Genes Immun. 1:191–199 10.1038/sj.gene.6363659 [DOI] [PubMed] [Google Scholar]
- Shevach E.M. 2009. Mechanisms of Foxp3+ T regulatory cell-mediated suppression. Immunity. 30:636–645 10.1016/j.immuni.2009.04.010 [DOI] [PubMed] [Google Scholar]
- Straus D.S., Pascual G., Li M., Welch J.S., Ricote M., Hsiang C.H., Sengchanthalangsy L.L., Ghosh G., Glass C.K. 2000. 15-deoxy-δ 12,14-prostaglandin J2 inhibits multiple steps in the NF-κ B signaling pathway. Proc. Natl. Acad. Sci. USA. 97:4844–4849 10.1073/pnas.97.9.4844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N., Ishizaki J., Yokota Y., Higashino K., Ono T., Ikeda M., Fujii N., Kawamoto K., Hanasaki K. 2000. Structures, enzymatic properties, and expression of novel human and mouse secretory phospholipase A(2)s. J. Biol. Chem. 275:5785–5793 10.1074/jbc.275.8.5785 [DOI] [PubMed] [Google Scholar]
- Tamura T., Tailor P., Yamaoka K., Kong H.J., Tsujimura H., O’Shea J.J., Singh H., Ozato K. 2005. IFN regulatory factor-4 and -8 govern dendritic cell subset development and their functional diversity. J. Immunol. 174:2573–2581 http://www.jimmunol.org/content/174/5/2573 [DOI] [PubMed] [Google Scholar]
- Tomobe Y.I., Morizawa K., Tsuchida M., Hibino H., Nakano Y., Tanaka Y. 2000. Dietary docosahexaenoic acid suppresses inflammation and immunoresponses in contact hypersensitivity reaction in mice. Lipids. 35:61–69 10.1007/s11745-000-0495-0 [DOI] [PubMed] [Google Scholar]
- Trivedi S.G., Newson J., Rajakariar R., Jacques T.S., Hannon R., Kanaoka Y., Eguchi N., Colville-Nash P., Gilroy D.W. 2006. Essential role for hematopoietic prostaglandin D2 synthase in the control of delayed type hypersensitivity. Proc. Natl. Acad. Sci. USA. 103:5179–5184 10.1073/pnas.0507175103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uozumi N., Kume K., Nagase T., Nakatani N., Ishii S., Tashiro F., Komagata Y., Maki K., Ikuta K., Ouchi Y., et al. 1997. Role of cytosolic phospholipase A2 in allergic response and parturition. Nature. 390:618–622 10.1038/37622 [DOI] [PubMed] [Google Scholar]
- Valentin E., Koduri R.S., Scimeca J.C., Carle G., Gelb M.H., Lazdunski M., Lambeau G. 1999. Cloning and recombinant expression of a novel mouse-secreted phospholipase A2. J. Biol. Chem. 274:19152–19160 10.1074/jbc.274.27.19152 [DOI] [PubMed] [Google Scholar]
- Villadangos J.A., Schnorrer P. 2007. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat. Rev. Immunol. 7:543–555 10.1038/nri2103 [DOI] [PubMed] [Google Scholar]
- von Allmen C.E., Schmitz N., Bauer M., Hinton H.J., Kurrer M.O., Buser R.B., Gwerder M., Muntwiler S., Sparwasser T., Beerli R.R., Bachmann M.F. 2009. Secretory phospholipase A2-IID is an effector molecule of CD4+CD25+ regulatory T cells. Proc. Natl. Acad. Sci. USA. 106:11673–11678 10.1073/pnas.0812569106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vremec D., Pooley J., Hochrein H., Wu L., Shortman K. 2000. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J. Immunol. 164:2978–2986 http://www.jimmunol.org/content/164/6/2978 [DOI] [PubMed] [Google Scholar]
- Weinrauch Y., Elsbach P., Madsen L.M., Foreman A., Weiss J. 1996. The potent anti-Staphylococcus aureus activity of a sterile rabbit inflammatory fluid is due to a 14-kD phospholipase A2. J. Clin. Invest. 97:250–257 10.1172/JCI118399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Taketomi Y., Isogai Y., Miki Y., Sato H., Masuda S., Nishito Y., Morioka K., Ishimoto Y., Suzuki N., et al. 2011. Hair follicular expression and function of group X secreted phospholipase A2 in mouse skin. J. Biol. Chem. 286:11616–11631 10.1074/jbc.M110.206714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C., Sakata D., Esaki Y., Li Y., Matsuoka T., Kuroiwa K., Sugimoto Y., Narumiya S. 2009. Prostaglandin E2-EP4 signaling promotes immune inflammation through Th1 cell differentiation and Th17 cell expansion. Nat. Med. 15:633–640 10.1038/nm.1968 [DOI] [PubMed] [Google Scholar]
- Yokoyama M., Origasa H., Matsuzaki M., Matsuzawa Y., Saito Y., Ishikawa Y., Oikawa S., Sasaki J., Hishida H., Itakura H., et al. ; Japan EPA lipid intervention study (JELIS) Investigators 2007. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 369:1090–1098 10.1016/S0140-6736(07)60527-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.