Abstract
Background.
Gender-specific trajectories of lower extremity function (LEF) and the potential for bias in LEF estimation due to differences in survival have been understudied.
Methods.
We evaluated longitudinal data from 690 initially nondisabled adults age 70 or older from the Precipitating Events Project. LEF was assessed every 18 months for 12 years using a modified Short Physical Performance Battery (mSPPB). Hierarchical linear models with adjustments for length-of-survival estimated the intraindividual trajectory of LEF and differences in trajectory intercept and slope between men and women.
Results.
LEF declined following a nonlinear trajectory. In the full sample, and among participants with high (mSPPB 10–12) and intermediate (mSPPB 7–9) baseline LEF, the rate-of-decline in mSPPB was slower in women than in men, with no gender differences in baseline mSPPB scores. Among participants with low baseline LEF (mSPPB ≤6), men had a higher starting mSPPB score, whereas women experienced a deceleration in the rate-of-decline over time. In all groups, participants who survived longer had higher starting mSPPB scores and slower rates-of-decline compared with those who died sooner.
Conclusions.
Over the course of 12 years, older women preserve LEF better than men. Nonadjustment for differences in survival results in overestimating the level and underestimating the rate-of-decline in LEF over time.
Keywords: Lower extremity function, Trajectories, Gender differences, Survival bias
Introduction
Maintaining independent function among community-dwelling older adults is a central goal of clinical medicine and public health. Achieving this goal requires a thorough understanding of the antecedents to mobility impairment. In nondisabled and even high-functioning older adults, lower extremity function (LEF) captures subtle changes in functioning and is a strong predictor of incident disability (1,2), hospitalization and nursing home admission (3,4), and mortality (4,5).
Because impairments in physical performance develop over time, possibly nonmonotonically (6), trajectories of physical performance indicators, such as LEF, are useful outcome measures. Yet, most data come from cross-sectional and short-term transition studies; thus, there is little information regarding the long-term LEF trajectory (ie, starting level, rate, and magnitude of change) in older adults. Cross-sectional studies, indicating poorer LEF with increasing age (7–9), provide valuable information about the distribution of LEF at various ages, but do not differentiate between intra- and interindividual variation in performance, may confound age and cohort differences, and have been shown to underestimate the true change in performance over time, by favoring highly functioning individuals who survive longer in older cohorts (10). Studies of changes in LEF between two time-points, despite confirming the decline in performance over time, have been generally restricted to short follow-up periods and ethnic- or sex-specific samples (7,10–12). Furthermore, the estimation of average (eg, annualized) rates of change in LEF using the observed actual change between two time-points relies on an assumption of linearity, which may not be appropriate (13).
The apparent paradox in the relationship between health and survival of men and women—“women are sicker, but men die quicker” (14)—has been extensively documented for a multitude of health outcomes, including physical performance. Thus, despite surviving longer than men, women tend to perform worse on objective assessments of physical capabilities (6,9,15). However, gender differences in the trajectories of LEF over longer follow-up periods have been understudied and uncertainty remains as to whether the observed cross-sectional differences in physical performance are due to differences in the level and/or rate of change over time in these functional measures.
To address these gaps, this study aims to determine the long-term average trajectory of LEF in a large cohort of older persons and to compare the LEF trajectories between older men and women, while accounting for the potential bias due to gender differences in health status and survival.
Methods
Analytic Sample and Data Collection
Data came from the Precipitating Events Project, an ongoing longitudinal study of 754 initially nondisabled community-dwelling adults aged 70 or older, who underwent comprehensive assessments at 18-month intervals for 12 years (1999–2011). The study design, inclusion and exclusion criteria, and enrollment response rates have been described in detail elsewhere (16). Briefly, participants were deemed eligible for enrollment in the study if they were 70 years or older and reported no need for assistance with four essential activities of daily living (bathing, dressing, walking across a room, and transferring from a chair). Clinically significant cognitive impairment with no available proxy, life expectancy of less than 12 months, inability to speak English, and plans to leave the area were exclusion criteria. Of those eligible, 75.2% agreed to participate and were enrolled between March 1998 and October 1999. Physically frail individuals, defined on the basis of slow gait speed (ie, required >10 seconds to walk along a 10-foot course and back as quickly as possible), were oversampled. Comprehensive assessments were completed at baseline and repeated at 18-month intervals, with the exception of 126 months, for a total of 144 months (8 assessments).
For these analyses, we excluded participants with only one assessment because change in LEF could not be evaluated. Of the 754 Precipitating Events Project participants, 57 (7.6%) died and 7 (0.9%) dropped out prior to the 18-month assessment and hence were excluded, leaving an analytic sample of 690 participants (descriptive characteristics of excluded participants are provided in the Supplementary Appendix). Deaths were ascertained by reviewing the local obituaries and/or from the next-of-kin or another knowledgeable person during a follow-up interview. Over the follow-up period, 470 (68.1%) participants in the analytic sample died (median length-of-survival 7.6 years, interquartile range 4.6–10.3 years); attrition among survivors was very low (11 participants; median length-of-stay in the study 4.5 years, interquartile range 1.5–9.0 years).
Measures
LEF was assessed at each wave using a modified version of the Short Physical Performance Battery (mSPPB) (4) that included 3 chair stands instead of 5, a 20-foot walk with a turn instead of a 4-meter walk, and balance assessment using the same three maneuvers (side-by-side, semi-tandem, and tandem stands) and scoring as the standard SPPB (17). The SPPB score is a highly reliable and responsive summary performance measure (18), comprised of three hierarchical timed tests of balance, short-distance walking speed, and repeated chair stands. For each test, a five-level summary scale from 0 (unable to perform the task) to 4 (1–4 representing quartiles calculated separately for each test) was created according to established procedure (4). An overall mSPPB score (range: 0–12) was calculated for each subject by summing the scores on the three tests; higher scores indicate better LEF. In a sample of 26 older persons, mSPPB showed strong concordance with the standard SPPB (intraclass correlation coefficient = 0.88, p < .001). The baseline mSPPB score was categorized per convention into high (range: 10–12, inclusive), intermediate (range: 7–9, inclusive), and low (range: 0–6) for stratified analyses (19).
Sociodemographic characteristics included gender (0 = male, 1 = female), age-at-baseline (years), education (years of education completed), race (0 = white, 1 = non-white), and living arrangement (0 = lives with someone else, 1 = lives alone).
Modifiable risk factors (MRFs) were included as potential confounders (2). Body mass index was calculated at each wave using the following formula: [weight (in kilograms)/(height)2(in meters)]; physical activity level was assessed at each wave using a modified Physical Activity Score for the Elderly (range: 0–360) (20); smoking status (0 = nonsmoker, 1 = current smoker) and alcohol use (0 = nondrinker, 1 = drinker) were recorded at baseline.
Health status indicators assessed at each wave were included to account for the well-known gender differentials in morbidity (21). The sum of 9 self-reported, physician-diagnosed chronic diseases (hypertension, myocardial infarction, congestive health failure, stroke, cancer, diabetes, arthritis, hip fracture, and lung disease) was calculated from individual-disease questions (0 = absent, 1 = present). The short-form 11-item CES-D score (22) was used to calculate a full CES-D equivalent-score (range: 0–60), according to a previously described procedure (23). Cognitive functioning was assessed using the Mini Mental State Examination score (range: 0–30) (24).
For participants who died during the study, data were censored at the last assessment prior to death and the length-of-survival in the study was calculated (in years) by subtracting the date-of-enrollment from the verified date-of-death. For participants alive for the 144-month evaluation, a length-of-survival of 12.0 years was assigned.
Statistical Analysis
Hierarchical (multilevel) generalized linear models were used to calculate the LEF trajectory, defined by a starting level (intercept), change over time (slope), and acceleration or deceleration (curvature), as a function of time, while accounting for intra-individual and inter-individual variability across repeated measurements and cross-level interactions between time and each of the covariates (25). We tested a series of models sequentially adjusted to control for differences in survival, sociodemographic characteristics, MRFs, and health status. For all the models, linear and nonlinear (quadratic and cubic) patterns of change in LEF over time were considered. Detailed model specification and additional statistical considerations are provided in the Supplementary Appendix. To control for potential bias associated with differences in mortality (26), length-of-survival in the study (in years, calculated from baseline) was included as an individual-level (ie, varying from participant to participant) covariate in the appropriate models. Similar approaches have been used in recent analyses of trajectories of health in older adults (27,28). The statistical significance level was set at p < .05 (two tailed). All analyses were performed using HLM 6.6 software (Scientific Software International, Lincolnwood, IL).
Results
The descriptive characteristics of the analytic sample are shown in Table 1.
Table 1.
Descriptive Characteristics of Analytic Sample
| All Baseline mSPPB | Baseline mSPPB Subgroups | |||||
|---|---|---|---|---|---|---|
| Full Sample | Women | Men | High (10–12) | Intermediate (7–9) | Low (0–6) | |
| (N = 690) | (N = 451) | (N = 239) | (N = 160) | (N = 225) | (N = 305) | |
| (n = 4111 observations) | (n = 2913 observations) | (n = 1198 observations) | (n = 1074 observations) | (n = 1406 observations) | (n = 1631 observations) | |
| Mean (SD) or % | Mean (SD) or % | Mean (SD) or % | Mean (SD) or % | Mean (SD) or % | Mean (SD) or % | |
| Baseline | ||||||
| mSPPB score (0–12) | 6.9 (2.9) | 6.5 (2.9) | 7.7 (2.8) | 10.9 (0.9) | 7.8 (0.8) | 4.2 (1.4) |
| Age (y) | 78.3 (5.1) | 78.2 (5.2) | 78.4 (5.0) | 75.7 (3.9) | 77.6 (4.8) | 80.1 (5.3) |
| Women (%) | 65.4 | — | — | 53.1 | 65.3 | 71.8 |
| White (%) | 90.1 | 90.0 | 90.4 | 93.8 | 92.4 | 86.6 |
| Education (y) | 11.9 (2.9) | 11.8 (2.8) | 12.3 (3.0) | 12.9 (2.7) | 12.2 (2.7) | 11.3 (2.9) |
| Live alone (%) | 39.7 | 48.6 | 23.0 | 29.4 | 35.1 | 48.5 |
| Body mass index | 26.9 (5.1) | 27.2 (5.6) | 26.4 (4.3) | 26.4 (4.5) | 26.6 (4.5) | 27.4 (5.8) |
| Smoker (%) | 8.3 | 8.2 | 8.4 | 6.9 | 8.9 | 8.5 |
| Alcohol user (%) | 22.2 | 15.7 | 34.3 | 31.8 | 27.6 | 13.1 |
| PASE (1–360) | 91.7 (57.1) | 81.9 (52.2) | 110.2 (61.2) | 131.9 (56.3) | 100.3 (55.7) | 64.2 (42.3) |
| Chronic diseases (1–9) | 1.7 (1.2) | 1.7 (1.2) | 1.7 (1.2) | 1.3 (1.0) | 1.6 (1.2) | 2.0 (1.2) |
| Cognitive score (0–30) | 26.8 (2.4) | 26.9 (2.4) | 26.6 (2.5) | 27.4 (2.2) | 27.0 (2.3) | 26.3 (2.6) |
| Depressive symptoms (1–60) | 8.8 (8.5) | 9.8 (9.1) | 6.9 (6.8) | 6.2 (7.0) | 7.5 (7.6) | 11.2 (9.1) |
| Attrition | ||||||
| Died (%) | 68.1 | 66.1 | 71.9 | 47.5 | 67.6 | 79.3 |
| Survival (y) | 8.9 (3.4) | 9.1 (3.3) | 8.4 (3.5) | 10.1 (3.0) | 9.4 (3.1) | 7.8 (3.4) |
Note: mSPPB = modified Short Physical Performance Battery score; PASE = Physical Activity Score for the Elderly; SD = standard deviation.
Gender Differences in mSPPB Trajectories
The model with gender but unadjusted for all other covariates (M0; Table 2) showed an average mSPPB trajectory with an intercept of 5.27 (p < .001), and a declining trajectory best fit by a cubic function, with a negative linear slope (b = −0.67, p < .001), a negative (accelerating) quadratic slope (b = −0.01, p < .01), and a positive cubic slope (b = 0.01, p < .001).
Table 2.
Lower Extremity Function (mSPPB) Trajectory Estimates for the Full Sample†
| Unadjusted‡ | Survival adjusted‡ | Health Adjusted‡ | MRFs Adjusted‡ | Fully Adjusted Except Survival | Fully Adjusted‡ | |
|---|---|---|---|---|---|---|
| (M0) | (M1) | (M2.1) | (M2.2) | (M3.1) | (M3.2) | |
| Fixed effects | ||||||
| Intercept§ | ||||||
| Intercept|| | 5.27* | 4.86* | 4.75* | 4.33* | 5.20* | 4.75* |
| Women | −0.73** | −1.08* | −0.76* | −0.40 | −0.18 | −0.41 |
| Survival | — | 0.73* | 0.44* | 0.53* | — | 0.39* |
| Linear slope | ||||||
| Intercept | −0.67* | −0.92* | −0.74* | −0.82* | −0.51* | −0.66* |
| Women | 0.21* | 0.18* | 0.17* | 0.16** | 0.16** | 0.15** |
| Survival | — | 0.13* | 0.07* | 0.10* | — | 0.06* |
| Quadratic slope | ||||||
| Intercept | −0.01** | −0.04*** | −0.02 | −0.04*** | −0.03*** | −0.02 |
| Women | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Survival | — | 0.00 | −0.01 | 0.00 | — | −0.01 |
| Cubic slope | ||||||
| Intercept | 0.01* | 0.00* | 0.01* | 0.01** | 0.01** | 0.01** |
| Women | −0.00 | −0.00 | −0.00 | −0.00 | −0.00 | −0.00 |
| Survival | — | −0.00 | −0.00*** | −0.00 | — | −0.00*** |
| Random effects (variance) | ||||||
| Intercept | 8.67* | 5.93* | 4.36* | 4.05* | 4.24* | 3.76* |
| Linear slope | 0.11* | 0.09* | 0.07* | 0.08* | 0.07* | 0.06* |
| Quadratic slope | 0.00* | 0.00* | 0.00* | 0.00* | 0.00* | 0.00* |
| Cubic slope | 0.00*** | 0.00*** | 0.00*** | 0.00 | 0.00*** | 0.00*** |
| Level-1, E¶ | 1.94 | 1.91 | 1.83 | 1.88 | 1.83 | 1.81 |
| Deviance | 17354.4 | 17104.1 | 16937.1 | 17001.1 | 16991.6 | 16938.4 |
| Estimated parameters (nr.) | 11 | 11 | 11 | 11 | 11 | 11 |
Notes: mSPPB = modified Short Physical Performance Battery score.
† N = 690 participants/4111 observations.
‡Unadjusted M0 is the time-only model; M1,adjusted for length-of-survival; M2.1, adjusted for length-of-survival and health status; M2.2, adjusted for length-of-survival and modifiable risk factors, but not for health status; M3.1, adjusted for health status and MRFs, but not for length-of-survival; M3.2, fully adjusted for length-of-survival, health status and MRFs. M1, M2.1, M2.2, M3.1, and M3.2 tested with and without adjustment for age-at-baseline, education, race, and living arrangement; results from adjusted models are presented in the table.
§Time(t) centered at its grand mean in all models; consequently, mSPPB intercept should be interpreted as mSPPB score at mean follow-up time (6 years).
||Intercept for each fixed effect represents the estimate when all other variables are held constant as appropriate at 0 (binary variables) or at sample mean (continuous variables).
¶Level 1, E represents the residual intra-individual (ie, wave-to-wave) variation in mSPPB.
*p value < .001, **p value < .01, ***p value < .05.
The unadjusted model indicated significant gender differences in the mSPPB intercept and linear slope. Compared with men, women had a lower mSPPB intercept (b = −0.73, p < .01), but a slower rate-of-decline (b = 0.21, p < .001 for linear slope; nonsignificant quadratic and cubic slope gender coefficients). These results were robust to the inclusion of length-of-survival (M1) and health status (M2.1) indicators. After adjustment for baseline differences and intra-individual changes in MRFs (M2.2), the intercept for gender became nonsignificant (b = −0.40, p > .05), indicating that the better initial LEF observed in men was likely explained by differences in health risk factors. Nevertheless, the significant linear slope for women (b = 0.16, p < .01 in M2.2), denoting a slower decline in LEF in women, was preserved.
Finally, the fully adjusted model (M3.2) showed that men and women followed mSPPB trajectories characterized by nonsignificant differences in intercepts (b = −0.41, p > .05) and by a significant difference favorable to women in the linear slope (b = 0.15, p < .01).
Effect of Adjustment for Length-of-Survival
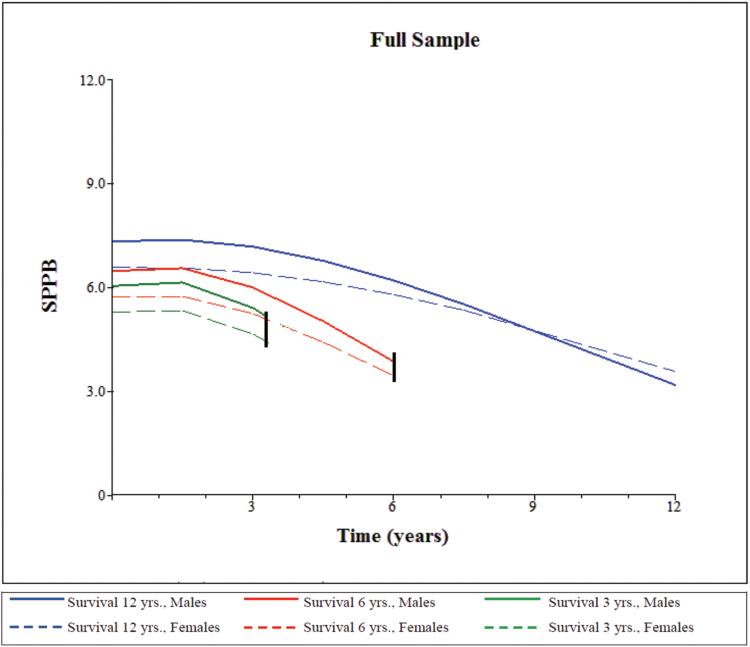
The length-of-survival was significantly associated with a higher mSPPB intercept and slower rate-of-decline in all the models (b = 0.39, p < .001 for intercept and b = 0.06, p < .001 for linear slope in M3.2, Table 2), indicating that not adjusting for differential survival would have resulted in an overestimation of the intercept (b = 5.27 in M0 compared with b = 4.86 in M1) and an underestimation of the rate-of-decline over time (b = −0.67 in M0 compared with b = −0.92 in M1). We also compared the mSPPB intercept and slopes from a model that excluded length-of-survival but included all other covariates (M3.1, Table 2) with those from the fully adjusted model (M3.2). These results confirmed the overestimation of mSPPB intercept (b = 5.20 M3.1 vs b = 4.75, p < .001 in M3.2) and the underestimation of linear slope (b = −0.51, p < .001 in M3.1 vs b = −0.66, p < .001 in M3.2) associated with nonadjustment for survival, thus indicating that the potential for survival bias remains even after adequate control for interindividual differences in health status, risk factors, and sociodemographic characteristics. Figure 1 illustrates differences (by sex) in LEF trajectories for participants who died after 3 years, those who died after 6 years, and survivors.
Figure 1.
Trajectories of lower extremity function (mSPPB) by sex and length-of-survival for the full sample.
Notes: mSPPB denotes the modified Short Physical Performance Battery score; trajectories were estimated using model M3.2 in Table 2; 3- and 6-year-length-of-survival models chosen for illustrative purposes; trajectories for length-of-survival of 12 years are descriptive of estimated lower extremity function course for survivors.
Baseline mSPPB Subgroup Analysis
Table 3 provides the stratified results according to baseline mSPPB subgroups. For simplicity purposes, only the results from models M1 (adjusted for length-of-survival) and M3.2 (fully adjusted) are shown.
Table 3.
Lower Extremity Function (mSPPB) Trajectory Estimates for High, Intermediate, and Low Baseline mSPPB Subgroups
| High mSPPB (10–12) (N = 160) | Intermediate mSPPB (7–9) (N = 225) | Low mSPPB (1–6) (N = 305) | ||||
|---|---|---|---|---|---|---|
| Survival Adjusted† | Fully Adjusted† | Survival Adjusted† | Fully Adjusted† | Survival Adjusted† | Fully Adjusted† | |
| M1 | M3.2 | M1 | M3.2 | M1 | M3.2 | |
| Fixed effects | ||||||
| Intercept‡ | ||||||
| Intercept§ | 7.44* | 8.60* | 5.15* | 5.94* | 2.71* | 2.71* |
| Women | −0.13 | 0.15 | −0.40 | −0.40 | −0.98** | −0.75** |
| Survival | 0.58* | 0.31*** | 0.62* | 0.41* | 0.55* | 0.37* |
| Linear slope | ||||||
| Intercept | −0.87* | −0.67* | −0.87* | −0.66* | −0.89* | −0.60* |
| Women | 0.32** | 0.28** | 0.16*** | 0.17*** | 0.12 | 0.05 |
| Survival | 0.19** | 0.07 | 0.13* | 0.07** | 0.08* | 0.05** |
| Quadratic slope | ||||||
| Intercept | −0.12** | −0.09** | −0.05*** | −0.02 | 0.01 | 0.02 |
| Women | 0.01 | 0.00 | 0.01 | 0.01 | 0.02*** | 0.02*** |
| Survival | 0.05** | 0.03*** | 0.01 | 0.00 | −0.01*** | −0.02** |
| Cubic slope | ||||||
| Intercept | −0.01** | 0.00 | 0.00 | 0.01 | 0.02* | 0.01** |
| Women | −0.01** | −0.01** | 0.00 | 0.00 | 0.00 | 0.00 |
| Survival | 0.01** | 0.01*** | 0.00 | 0.00 | −0.01** | −0.01* |
| Random effects (variance) | ||||||
| Intercept | 4.63* | 3.49* | 4.70* | 3.58* | 2.85* | 2.38* |
| Linear slope | 0.15* | 0.11* | 0.13* | 0.09* | 0.08* | 0.06* |
| Quadratic slope | 0.01* | 0.01* | 0.01* | 0.01* | 0.01*** | 0.01*** |
| Cubic slope | 0.00*** | 0.00 | 0.00 | 0.00*** | 0.00 | 0.00 |
| Level-1, E|| | 1.77 | 1.66 | 1.64 | 1.61 | 1.65 | 1.57 |
| Deviance | 4353.54 | 4571.16 | 5483.59 | 5755.94 | 6242.43 | 6514.75 |
| Estimated parameters (nr.) | 11 | 11 | 11 | 11 | 11 | 11 |
Notes: mSPPB = modified Short Physical Performance Battery score.
†M1, adjusted for length-of-survival; M3.2, fully adjusted for length-of-survival, health status and modifiable risk factors. M1 and M3.2 tested with and without adjustment for age-at-baseline, education, race, and living arrangement; results from adjusted models are presented in the table.
‡Time(t) centered at its grand mean in all models; consequently, mSPPB intercept should be interpreted as mSPPB score at mean follow-up time (6 years).
§Intercept for each fixed effect represents the estimate when all other variables are held constant as appropriate at 0 (binary variables) or at sample mean (continuous variables).
||Level 1, E represents the residual intra-individual (ie, wave-to-wave) variation in mSPPB.
*p value < .001, **p value < .01, ***p value < .05.
Despite different baseline mSPPB scores, the three subgroups experienced similar average declines in LEF over time (b = −0.67, p < .001; b = −0.66, p < .001; and b = −0.60, p < .001, for linear slope for high, intermediate and low subgroups, respectively), but only the high baseline performers experienced a significant acceleration in decline (b = −0.09, p < .01 for quadratic slope).
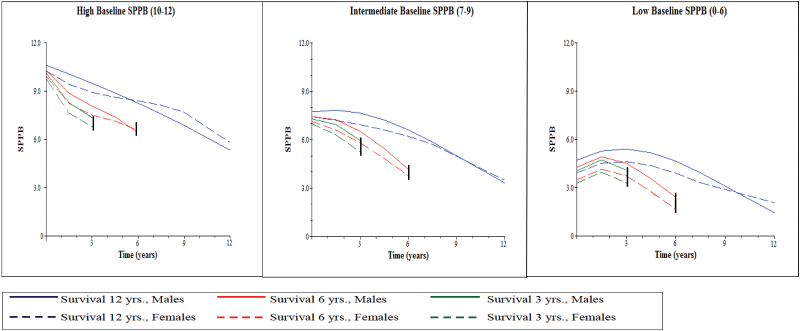
In the high and intermediate mSPPB subgroups, the gender coefficients for intercept were nonsignificant, but women experienced a slower loss of LEF compared with men (b = 0.28, p < .01 and b = 0.17, p < .05, linear slope in M3.2 for high and intermediate subgroups, respectively). In the low mSPPB subgroup, women had a worse mSPPB intercept (b = −0.75, p < .01 in M3.2) but a slowing in LEF decline over time (b = 0.02, p < .05 for quadratic slope, nonsignificant linear and cubic slope coefficients for gender in M3.2) compared with men. Gender-specific trajectories according to baseline mSPPB subgroup and length-of- survival are illustrated in Figure 2.
Figure 2.
Trajectories of lower extremity function (mSPPB) by gender and length-of-survival for high-, intermediate, and low baseline performers.
Notes: mSPPB denotes the modified Short Physical Performance Battery score; trajectories estimated using model M3.2 in Table 3 for each subgroup; 3- and 6-year-length-of-survival models were chosen for illustrative purposes; trajectories for length-of-survival of 12 years are descriptive of estimated lower extremity function course for survivors.
In all three subgroups, the intercept and slopes coefficients for length-of-survival showed that participants who survived longer in the study had a higher initial mSPPB score (b = 0.31, p < .05; b = 0.41, p < .001; and b = 0.37, p < .001, in M3.2 for the high, intermediate, and low mSPPB subgroups, respectively) and a slower decline in LEF (nonsignificant linear slope; b = 0.03, p < .05 quadratic slope; b = 0.01, p < .05 cubic slope in M3.2 for the high mSPPB group; b = 0.07, p < .01 linear slope; nonsignificant quadratic and cubic slopes for the intermediate subgroup; and b = 0.05, p < .01 for linear slope, b = −0.02, p < .01 quadratic slope, and b = −0.01, p < .001 cubic slope in M3.2). These results indicate that nonadjustment for length-of-survival would have led to biased intercept and slope estimates in all three subgroups.
Discussion
In this study, we estimated gender-based long-term trajectories of LEF, in terms of their level, magnitude, and rate of change, while accounting for potential bias due to survival and health status differences. Our results show that LEF tracked an average nonlinear declining trajectory and that women generally followed a trajectory with similar initial levels but substantially slower declines in function compared with men.
The SPPB has been used extensively to assess physical and functional health in community-dwelling older adults (1,4). Although previous cross-sectional and two time-points transition studies have suggested an age-related decrease in LEF (2,9), methodological limitations have precluded direct inferences to the “true” long-term trajectory of LEF in old age. Our results from a cohort of men and women observed for up to eight times for a period of 12 years indicate that on average LEF decreases from an initial estimated level of 6.9 by approximately 0.7 units per year following a marginally nonlinear trajectory (very small yet significant positive cubic slope term). Based on previously developed criteria, which estimated the size of meaningful changes in the standard SPPB score needed to anchor a self-reported functional loss at between 0.5 (small) and 1.0 (substantial), a decline of 0.7 mSPPB units corresponds to an intermediate meaningful change, equivalent to the loss of between one and two flights of stairs in climbing ability or between one and two blocks in walking ability (29,30).
Because physically frail participants were oversampled at baseline, and because other investigators have found that high baseline performers were more likely to decline than poor performers (7), we also estimated the LEF trajectories separately for participants with high, intermediate, and low baseline performance. The rate-of-decline was similar among the three subgroups; only the high-mSPPB subgroup experienced a slight acceleration in decline. One possible explanation for the discrepancy between our findings and the previous results showing variations in the rate-of-decline based on initial performance levels (7) is that higher baseline performers may have preclinical health conditions that become clinically apparent during the follow-up, thus triggering more precipitous declines in function, whereas low performers have worse but more stable health. Unlike the other studies, our analyses were adjusted for both baseline and time-varying health indicators, thus capturing transitions from preclinical to overt health conditions, and showed that negative health transitions (ie, increases in the index of chronic conditions and CES-D score, or declines in Mini Mental State Examination score) between follow-up assessments are indeed associated with concurrent declines in mSPPB (coefficients not in tables; available upon request).
After accounting for socioeconomic, behavioral, health, and survival differences, women had a substantial advantage in preserving LEF over time, regardless of the starting functional level; intercept differences favorable to men were observed only among initial low performers. As illustrated in Figures 1 and 2, these findings suggest that differences in performance levels between men and women may diminish and eventually reverse among longer surviving older adults. Other investigators have also found a faster rate-of-decline in isometric and isokinetic leg muscle strength, as well as in leg muscle mass among men compared with women (10,31). The annual decline in mSPPB score in our participants was about 23% slower in women (~0.15 mSPPB units less decline/year compared with ~0.66 units/year decline in men; M3.2 in Table 2); this difference is smaller than those previously reported, perhaps due to differences in participants’ age and health status (10,31). The potential mechanisms underlying gender differences in physical performance have been summarized elsewhere (9). Although our analyses controlled for several of these factors (eg, body weight, health behaviors, and risk of chronic and mental conditions), others, such as differences in lean vs fat mass, hormonal exposure, neurological and cardiovascular fitness, or inflammatory status, were not fully addressed. Future studies are needed to clarify how these factors affect the male–female differences in the trajectory of lower extremity performance.
To our knowledge, this is the first study to control for interindividual survival differences and to indirectly demonstrate that failure to account for length-of-survival would have resulted in an overestimation of the LEF intercept and an underestimation of the rate-of-decline over time. This is partially due to the fact that through selective attrition, sicker or frailer individuals, who likely have lower LEF levels and higher rates-of-decline prior to death, leave the cohort, whereas healthier individuals survive and contribute more observations. In addition, our results show that length-of-survival captures subtle differences in resilience not totally captured through health status or sociodemographic indicators, and that residual survival bias may occur despite careful and extensive adjustment for other interindividual differences. Thus, without adjustments for differences in survival, even results from longitudinal studies can underestimate the “true” expected decline in performance (32).
Several limitations in our study warrant comment. First, because data were not suitable for age-based analyses (28,33), the LEF trajectories were estimated as a function of time with adjustment for age-at-baseline, to minimize the potential for age-cohort confounding. Consequently, the results should not be extrapolated to represent the effect of age on LEF, but rather the evolution of LEF over time in this specific age group. Second, we evaluated LEF using a composite measure incorporating walking speed, balance, and repeated chair stands. A large cross-sectional study of gender differences in physical capabilities has shown dissimilarities in the associations between the three individual components of SPPB score and gender (9). It is possible that these individual measures change differentially over time or that changes in only one or two of these components drive the results. Future studies are needed to evaluate this possibility. Third, because our sample was drawn from persons in a single health plan from a defined urban area, the results may not be generalizable to the entire older population. However, the demographic characteristics of our sample are similar to those of the entire U.S. population, with the exception of racial/ethnic representation (34).
In summary, this study provides new information on the long-term trajectory of LEF in older men and women, and indicates that the potential for systematic bias due to nonadjustment for differences in survival exists in multiple-measurement studies of physical performance.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Acknowledgments
The work for this report was funded by grants from the National Institute on Aging—T32-AG1934 (to A.B.) and R37AG17560, R01AG022993 (to T.M.G). The study was conducted at the Yale Claude D. Pepper Older Americans Independence Center (P30AG21342). Dr. Gill is the recipient of a Midcareer Investigator Award in Patient-Oriented Research (K24AG021507) from the National Institute on Aging.
References
- 1. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stuck AE, Walthert JM, Nikolaus T, Büla CJ, Hohmann C, Beck JC. Risk factors for functional status decline in community-living elderly people: a systematic literature review. Soc Sci Med. 1999;48:445–469 [DOI] [PubMed] [Google Scholar]
- 3. Penninx BW, Ferrucci L, Leveille SG, Rantanen T, Pahor M, Guralnik JM. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. J Gerontol A Biol Sci Med Sci. 2000;55:M691–M697 [DOI] [PubMed] [Google Scholar]
- 4. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. A Biol Sci Med Sci. 1994;49:M85–M94 [DOI] [PubMed] [Google Scholar]
- 5. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Frederiksen H, Hjelmborg J, Mortensen J, McGue M, Vaupel JW, Christensen K. Age trajectories of grip strength: cross-sectional and longitudinal data among 8,342 Danes aged 46 to 102. Ann Epidemiol. 2006;16:554–562 [DOI] [PubMed] [Google Scholar]
- 7. Onder G, Penninx BW, Lapuerta P, et al. Change in physical performance over time in older women: the Women’s Health and Aging Study. J Gerontol A Biol Sci Med Sci. 2002;57:M289–M293 [DOI] [PubMed] [Google Scholar]
- 8. Frontera WR, Hughes VA, Lutz KJ, Evans WJ. A cross-sectional study of muscle strength and mass in 45- to 78-yr-old men and women. J Appl Physiol. 1991;71:644–650 [DOI] [PubMed] [Google Scholar]
- 9. Cooper R, Hardy R, Aihie Sayer A, et al. Age and gender differences in physical capability levels from mid-life onwards: the harmonisation and meta-analysis of data from eight UK cohort studies. PLoS ONE. 2011;6:e27899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hughes VA, Frontera WR, Wood M, et al. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci. 2001;56:B209–B217 [DOI] [PubMed] [Google Scholar]
- 11. Forrest KY, Zmuda JM, Cauley JA. Correlates of decline in lower extremity performance in older women: A 10-year follow-up study. J Gerontol A Biol Sci Med Sci. 2006;61:1194–1200 [DOI] [PubMed] [Google Scholar]
- 12. Wolinsky FD, Miller TR, Malmstrom TK, et al. Four-year lower extremity disability trajectories among African American men and women. J Gerontol A Biol Sci Med Sci. 2007;62:525–530 [DOI] [PubMed] [Google Scholar]
- 13. Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York, NY: Oxford University Press; 2003. [Google Scholar]
- 14. Lorber J, Moore LJ. Gender and the Social Construction of Illness. 2nd ed. Walnut Creek, CA: Altamira Press; 2002. [Google Scholar]
- 15. Bassey EJ, Harries UJ. Normal values for handgrip strength in 920 men and women aged over 65 years, and longitudinal changes over 4 years in 620 survivors. Clin Sci. 1993;84:331–337 [DOI] [PubMed] [Google Scholar]
- 16. Gill TM, Desai MM, Gahbauer EA, Holford TR, Williams CS. Restricted activity among community-living older persons: incidence, precipitants, and health care utilization. Ann Intern Med. 2001;135:313–321 [DOI] [PubMed] [Google Scholar]
- 17. Gill TM, Murphy TE, Barry LC, Allore HG. Risk factors for disability subtypes in older persons. J Am Geriatr Soc. 2009;57:1850–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ostir GV, Volpato S, Fried LP, Chaves P, Guralnik JM. Reliability and sensitivity to change assessed for a summary measure of lower body function: results from the Women’s Health and Aging Study. J Clin Epidemiol. 2002;55:916–921 [DOI] [PubMed] [Google Scholar]
- 19. Sayers SP, Guralnik JM, Newman AB, Brach JS, Fielding RA. Concordance and discordance between two measures of lower extremity function: 400 meter self-paced walk and SPPB. Aging Clin Exp Res. 2006;18:100–106 [DOI] [PubMed] [Google Scholar]
- 20. Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA. The physical activity scale for the elderly (PASE): evidence for validity. J Clin Epidemiol. 1999;52:643–651 [DOI] [PubMed] [Google Scholar]
- 21. Arber S, Cooper H. Gender differences in health in later life: the new paradox? Soc Sci Med. 1999;48:61–76 [DOI] [PubMed] [Google Scholar]
- 22. Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. J Aging Health. 1993;5:179–193 [DOI] [PubMed] [Google Scholar]
- 23. Penninx BW, Guralnik JM, Ferrucci L, Simonsick EM, Deeg DJ, Wallace RB. Depressive symptoms and physical decline in community-dwelling older persons. JAMA. 1998;279:1720–1726 [DOI] [PubMed] [Google Scholar]
- 24. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 25. Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. 2nd ed. Thousand Oaks, CA: Sage Publications; 2002. [Google Scholar]
- 26. Harel O, Hofer SM, Hoffman L, Pedersen NL, Johansson B. Population inference with mortality and attrition in longitudinal studies on aging: a two-stage multiple imputation method. Exp Aging Res. 2007;33:187–203 [DOI] [PubMed] [Google Scholar]
- 27. Quiñones AR, Liang J, Bennett JM, Xu X, Ye W. How does the trajectory of multimorbidity vary across Black, White, and Mexican Americans in middle and old age? J Gerontol B Psychol Sci Soc Sci. 2011;66:739–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liang J, Bennett JM, Shaw BA, et al. Gender differences in functional status in middle and older age: are there any age variations? J Gerontol B Psychol Sci Soc Sci. 2008;63:S282–S292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749 [DOI] [PubMed] [Google Scholar]
- 30. Kwon S, Perera S, Pahor M, et al. What is a meaningful change in physical performance? Findings from a clinical trial in older adults (the LIFE-P study). J Nutr Health Aging. 2009;13:538–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the Health, Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064 [DOI] [PubMed] [Google Scholar]
- 32. Stenholm S, Härkänen T, Sainio P, Heliövaara M, Koskinen S. Long-term changes in handgrip strength in men and women—accounting the effect of right censoring due to death. J Gerontol A Biol Sci Med Sci. 2012;67:1068–1074 [DOI] [PubMed] [Google Scholar]
- 33. Alwin DF, Hofer SM, McCammon RJ. Modeling the effects of time: Integrating demographic and developmental perspectives. In: Binstock RH, George LK, eds. Handbook of Aging and the Social Sciences. 6th ed. San Diego, CA: Elsevier; 2006:20–38 [Google Scholar]
- 34. Hardy SE, Gill TM. Recovery from disability among community-dwelling older persons. JAMA. 2004;291:1596–1602 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.