Abstract
Obesity in the elderly individuals is increasing at alarming rates and there is evidence suggesting that elderly individuals are more vulnerable to the deleterious cardiovascular effects of obesity than younger individuals. However, the specific mechanisms through which aging and obesity interact to promote the development of cardiovascular disease remain unclear. The present study was designed to test the hypothesis that aging exacerbates obesity-induced inflammation in perivascular adipose tissue, which contributes to increased vascular oxidative stress and inflammation in a paracrine manner. To test this hypothesis, we assessed changes in the secretome, reactive oxygen species production, and macrophage infiltration in periaortic adipose tissue of young (7 month old) and aged (24 month old) high-fat diet–fed obese C57BL/6 mice. High-fat diet–induced vascular reactive oxygen species generation significantly increased in aged mice, which was associated with exacerbation of endothelial dysfunction and vascular inflammation. In young animals, high-fat diet–induced obesity promoted oxidative stress in the perivascular adipose tissue, which was associated with a marked proinflammatory shift in the profile of secreted cytokines and chemokines. Aging exacerbated obesity-induced oxidative stress and inflammation and significantly increased macrophage infiltration in periaortic adipose tissue. Using cultured arteries isolated from young control mice, we found that inflammatory factors secreted from the perivascular fat tissue of obese aged mice promote significant prooxidative and proinflammatory phenotypic alterations in the vascular wall, mimicking the aging phenotype. Overall, our findings support an important role for localized perivascular adipose tissue inflammation in exacerbation of vascular oxidative stress and inflammation in aging, an effect that likely enhances the risk for development of cardiovascular diseases from obesity in the elderly individuals.
Key Words: Diabetes, Metabolic disease, Obesity, Adiposity, Fat
OVER the next several decades an increasing percentage of the population of the United States will reach retirement age and these changes will have a significant impact on the economy and health care system. Currently, more than 35% of these individuals are obese and if the current trend continues, nearly half of the elderly population will be obese by 2030 (1). It is highly likely that a dramatic rise in the incidence of obesity-related diseases will occur and significantly increases the risk of cardiovascular morbidity and mortality (2).
The mechanisms by which obesity promotes athero genesis are likely multifaceted and include induction of oxidative stress and inflammation in the vascular wall by circulating factors released into the bloodstream from the adipose tissue. In addition, atheroprone arteries, including the aorta, coronary arteries, carotid, and iliac arteries, are surrounded by significant amounts of perivascular adipose tissue. There is growing evidence that the perivascular adipose tissue is a highly important source of inflammatory cytokines and chemokines, which likely regulate the function and phenotype of the surrounding vascular cells in a paracrine manner (3–8). Recent studies suggest that in obesity the visceral adipose tissue exhibits increased inflammation, characterized by a marked proinflammatory shift in the secretome and increased macrophage infiltration (9–12). There is also evidence that aging promotes proinflammatory phenotypic alterations in the visceral adipose tissue (13,14). Furthermore, obesity has been shown to promote inflammation in perivascular adipose tissue (4,8,15). The available evidence suggests a key role for macrophage infiltration into perivascular adipose tissue and increased production of proinflammatory cytokines during initiation of vascular disease in obese individuals (4,5,8,15). Despite advances in our understanding of the role of perivascular adipose tissue in the vascular pathophysiology of obesity in young adults, there are virtually no studies addressing the synergistic effects of aging and obesity on perivascular adipose tissue inflammation and oxidative stress and the role of factors secreted from perivascular adipose tissue in regulation of vascular function in aging.
The present study was designed to test the hypothesis that aging exacerbates obesity-induced inflammation in perivascular adipose tissue, which contributes to increased vascular oxidative stress and inflammation in a paracrine manner. To test our hypotheses, we assessed changes in the secretome, reactive oxygen species (ROS) production, and macrophage infiltration in periaortic adipose tissue of young and aged high-fat diet (HFD)–fed obese C57BL/6 mice. Using cultured arteries isolated from young control mice, we also assessed whether inflammatory factors secreted from the perivascular fat tissue of obese aged mice promote prooxidative and proinflammatory phenotypic alterations in the vascular wall, mimicking the aging phenotype.
Methods
Animals and Diets
In the present study, we used young and aged male C57BL/6 mice (7 and 24 months old at the time of sacrifice, respectively) from the aging colony maintained at the National Institute on Aging. Five months prior to the planned sacrifice young and old animals were divided into four groups and placed on either a standard diet (SD) or HFD. The four groups were (i) young animals fed a SD, (ii) young animals fed a HFD, (iii) old animals fed a SD, and (iv) old animals fed a HFD. The high fat chow, commonly used to induce obesity, delivers 60% kcal from fat, whereas the SD provides only 10% kcal from fat (D12492, D12450B, respectively, Research Diets Inc., New Brunswick, NJ). The animals continued on the specified diets (with water and food ad libitum) for 5 months. Animals were housed in pairs in the Rodent Barrier Facility at the University of Oklahoma Health Sciences Center on a 12-hour light–dark cycle and weighed weekly. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Oklahoma Health Sciences Center.
Serum Biochemical Profile and Analysis of Circulating Levels of Metabolic Hormones and Inflammatory Cytokines
All experimental animals were fasted overnight 5 months after starting the HFD or SD. Tail vein blood samples were taken using a sterile lancet (Medipoint, Mineola, NY) and glucose was measured with a OneTouch UltraMini glucose meter (LifeScan, Milpitas, CA). Whole blood was collected and centrifuged at 2,500g for 20 minutes at 4°C; serum was collected, aliquoted, and stored at −80°C. The animals were then euthanized by rapid decapitation. Total IGF-1, leptin, and adiponectin levels in serum were determined by ELISA (R&D Systems, Minneapolis, MN) as previously described (16–20). Serum insulin levels were also assessed by ELISA (Millipore Corporation, Billerica, MA). Circulating triglyceride and total cholesterol levels were determined using commercially available enzymatic assays (Cayman Chemical, Ann Arbor, MI). Circulating levels of cytokines and chemokines, which are important biomarkers of aging (21–25) and obesity (20), were analyzed using a multiplex protein array system (Rules Based Medicine, Austin, TX) according to the manufacturer’s protocol, as described previously (20).
Vessel Isolation and Functional Studies
The aortas were isolated, cleaned, sectioned, and endothelial function was assessed by measuring relaxation of the aortic rings in response to acetylcholine, as previously described (26). Endothelium-independent vasorelaxation was assessed using S-nitroso-N-acetylpenicillamine, a nitric oxide donor. In brief, an aorta ring segment (2mm in length) was isolated from each animal and mounted on 40 µm stainless steel wires in myograph chambers (Danish Myo Technology A/S, Inc., Denmark) for measurement of isometric tension. The vessels were superfused with Krebs buffer solution (118mM NaCl, 4.7mM KCl, 1.5mM CaCl2, 25mM NaHCO3, 1.1mM MgSO4, 1.2mM KH2PO4, and 5.6mM glucose; at 37°C; gassed with 95% air and 5% CO2). Optimal passive tension (as determined from the vascular length–tension relationship) was applied for 1 hour (equilibration period) then relaxation of precontracted vessels (using 10–6 mol/L phenylephrine) to acetylcholine (from 10–9 to 10–6 mol/L) and S-nitroso-N-acetylpenicillamine (from 10–9 to 10–6 mol/L) was assessed.
Measurement of Vascular 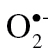 Production
Production
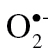 content in the aorta was determined using dihydroethidium (DHE), an oxidative fluorescent dye, as previously reported (27,28). Aortic rings complete with surrounding perivascular adipose tissue were incubated with DHE (3 × 10−6 mol/L; at 37oC for 30 minutes). After incubation, the vessels were washed three times with phosphate buffered saline, embedded in optimal cutting temperature medium, and cryosectioned. Images of
content in the aorta was determined using dihydroethidium (DHE), an oxidative fluorescent dye, as previously reported (27,28). Aortic rings complete with surrounding perivascular adipose tissue were incubated with DHE (3 × 10−6 mol/L; at 37oC for 30 minutes). After incubation, the vessels were washed three times with phosphate buffered saline, embedded in optimal cutting temperature medium, and cryosectioned. Images of  -specific red fluorescence were captured at 20× magnification and analyzed using Metamorph imaging software as previously reported (20,26,29). Three entire fields per specimen were analyzed with one image per field. The mean fluorescence intensities of DHE-stained nuclei in the endothelium and medial layer as well as in the perivascular adipose tissue were calculated for each specimen. Thereafter, the intensity values for each animal in the group were averaged.
-specific red fluorescence were captured at 20× magnification and analyzed using Metamorph imaging software as previously reported (20,26,29). Three entire fields per specimen were analyzed with one image per field. The mean fluorescence intensities of DHE-stained nuclei in the endothelium and medial layer as well as in the perivascular adipose tissue were calculated for each specimen. Thereafter, the intensity values for each animal in the group were averaged.
Quantitative Real-Time RT-PCR
Quantitative real-time RT-PCR was used to analyze mRNA expression in aorta samples as previously reported (30–33). Total RNA was isolated using a Mini RNA Isolation Kit (Zymo Research, Orange, CA). The mRNA was then reverse transcribed using Superscript III RT (Invitrogen) and mRNA expression was analyzed using a Strategen MX3000 platform (30,34–38). To determine the primer efficiencies, a dilution series of a standard vascular sample was quantified for each plate. Quantification was performed using an efficiency-corrected △△Cq method. Expression of the reference genes Hprt, Ywhaz, B2m, and Actb was quantified and a normalization factor was calculated based on the geometric mean for internal normalization. The oligonucleotide sequences for quantitative real-time RT-PCR are listed in Table 1. Fidelity of the PCR reaction was determined by melting temperature analysis and visualization of the product on a 2% agarose gel.
Table 1.
Oligonucleotides for Real-Time RT-PCR
| mRNA Targets | Description | Sense | Antisense |
|---|---|---|---|
| Cdkn2a | cyclin-dependent kinase inhibitor 2A; p16INK4a | AAGAGCAGAGCTAAATCC | TTTCTCATGCCATTCCTT |
| Nox2 | NADPH oxidase subunit GP91PHOX; cytochrome b-245, beta polypeptide | GAAGACAACTGGACAGGAACC | CCGACTCTGGCATTCACAC |
| Nox1 | NADPH oxidase 1 | TTCACAGTTATTCATATCATTGC | GAGATAGGCTGGAGAGAAC |
| Nos3 | nitric oxide synthase 3 (endothelial cell); eNOS | AGGTGACAAGCCGCATAC | CGAACGAAGTGACACAATCC |
| Tnfa | tumor necrosis factor alpha | GCACCACCATCAAGGACTC | AGGTCTGAAGGTAGGAAGGC |
| Il1b | interleukin 1, beta | TCTATACCTGTCCTGTGTAATG | GCTTGTGCTCTGCTTGTG |
| Il6 | interleukin 6 | TCCATCCAGTTGCCTTCTTG | TTTCTCATTTCCACGATTTCCC |
| Nlrp3 | NLR family, pyrin domain containing 3; nucleotide-binding oligomerization domain, leucine rich repeat and pyrin domaincontaining 3 | TGTTGTCCGATTCCTCTT | ACTTCAGTAGTTCCAGTCT |
| Icam1 | intercellular adhesion molecule 1 | TCATTCTCTATTGCCCCTG | CCTCCTCACTGATCTACTAT |
| Panx1 | pannexin 1 | CAATGACTTGAGCCTCTACA | CAGCACCTTCAGACACTT |
| P2rx7 | purinergic receptor P2X, ligand-gated ion channel, 7 | GTAGACTGTATTTGACTT | AAAGATAAAGGTGTAGAG |
| Hprt | hypoxanthine phosphoribosyltransferase 1 | TGCTGCGTCCCCAGACTTTTG | AGATAAGCGACAATCTACCAGAGG |
| B2m | Beta-2-microglobin | CGGTCGCTTCAGTCGTCAG | CAGTTCAGTATGTTCGGCTTCC |
| Ywhaz | tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide1 | ACTGTCTTGTCACCAACCATTC | GGGCTGTAGAGAGGATGAGG |
| Actb | beta actin | CCGTAAAGACCTCTATGCCAACAC | GGGGCCGGACTCATCG |
Cellular Antioxidant Capacity
To compare the capacity of cellular antioxidant systems to counterbalance the deleterious effects of oxidative stress in the vascular wall of SD- and HFD-fed young and aged mice, we assessed the Hydroxyl Radical Antioxidant Capacity using the OxiSelect Hydroxyl Radical Antioxidant Capacity Activity Assay (Cell Biolabs Inc., San Diego, CA) according to the manufacturer’s guidelines. The Hydroxyl Radical Antioxidant Capacity Activity Assay is based on the oxidation-mediated quenching of a fluorescent probe by hydroxyl radicals produced by a hydroxyl radical initiator and Fenton reagent. Antioxidants present in the tissues delay the quenching of the fluorescent probe until the antioxidant activity in the sample is depleted. The antioxidant capacity of the tissues was calculated on the basis of the area under the fluorescence decay curve, compared with an antioxidant standard curve obtained with gallic acid. Sample protein concentration was used for normalization purposes.
Apoptotic Cell Death
To compare cellular resistance to HFD-induced damage in the aorta of young and aged mice, increases in the rate of apoptosis were assessed. The vessels were homogenized in lysis buffer and caspase 3 activity, which is a useful measure of apoptosis, was determined as reported (39) using the Caspase-Glo 3/7 assay system (Promega). Luminescent intensity was measured using an Infinite M200 plate reader and was normalized to the sample protein concentration.
Protein Expression of MCP-1 and MIP-1α in the Aorta Arch
Previous studies reported that HFD-induced obesity modulates vascular expression of MCP-1 and MIP-1α (40). Thus, to characterize vascular inflammation in our cohorts, expression of MCP-1 and MIP-1α was analyzed in homogenates of the aortic arch using a magnetic bead array (Millipore). For normalization purposes, the sample protein content was determined by a spectrophotometric quantitation method using BCA reagent (Pierce Chemical Co., Rockford, IL).
Analysis of Macrophage Infiltration in Perivascular Adipose Tissue
Aortas with intact surrounding periaortic adipose tissue from HFD- and SD-fed young and aged mice were fixed overnight in 4% paraformaldehyde and embedded in paraffin for subsequent analyses. Five-micron tissue sections of the specimens were deparaffinized in xylene and immunohistochemistry was performed to quantify CD68+ macrophages in the perivascular adipose tissue. In brief, the sections were subjected to heat retrieval using Rodent Decloaker (Biocare Medical, Concord, CA) for 30 minutes at 85°C. To specifically detect macrophages, the sections were incubated with a mouse monoclonal antibody (ab31630, Abcam, Cambridge, MA) that recognizes CD68 (1:200, in phosphate buffered saline, overnight, at 4°C). A rat IgG secondary biotinylated antibody was used (Vector Labs, BA-4001, Burlingame, CA). A peroxidase-based ABC system and red chromogen AEC (both from Vector Laboratories) were used to identify the antigen–antibody reaction. An isotype-matched rat IgG and omission of primary antibody were used as controls. Nuclei were visualized by staining with hematoxylin. The number of CD68+ cells per field was counted by a blinded observer. Five fields per sample were analyzed.
Preparation of the Perivascular Adipose Tissue–Conditioned Media
We measured ~3mm above the diaphragm, and then removed the periaortic adipose tissue intact. Periaortic adipose tissue from each mouse was carefully cleaned, cut into small pieces, washed, and incubated in non–fetal serum containing Medium 199 at pH 7.4 for 24 hours (20mg of fat tissue per 100 µL) to obtain the corresponding secretomes (nonobese young, nonobese aged, obese young, obese aged). All procedures were conducted under sterile conditions. After 24 hours of incubation, the conditioned media from the explants were stored at −80oC until use.
Analysis of Secreted Cytokines in Conditioned Media
Profiling of cytokines and chemokines secreted by explanted perivascular adipose tissue was conducted using a multiplex protein array system (Rules Based Medicine). The concentration of a range of cytokines and growth factors involved in vascular physiology and pathophysiology (GM-CSF, IFNγ, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12/23 (p40), IL-13, IL-15, IL-17, MCP-1, MIP-1β, sCD40L, TNFα, and VEGF) was measured in the conditioned media. Adiponectin levels were determined by ELISA (R&D Systems). For normalization purposes, the sample protein content was determined by a spectrophotometric quantitation method using BCA reagent (Pierce Chemical Co.).
Bioassay Studies to Characterize the Prooxidative and Proinflammatory Vascular Effects of Perivascular Adipose Tissue Secretomes
We used an organoid culture-based bioassay approach utilizing aorta segments isolated from young (3 month old) control mice that were treated with perivascular adipose tissue–conditioned media (for 18 hours). The organoid culture system has been described previously (34). As endpoints, (i) ROS production and (ii) expression of inflammatory markers in the detector rings were assessed. To assess vascular oxidative stress induced by factors present in the conditioned media, H2O2 production in detector aorta segments was measured fluorometrically using the Amplex Red/horseradish peroxidase assay as described (26,41). The rate of H2O2 generation was assessed by measuring resorufin fluorescence for 60 minutes by a Tecan Infinite M200 plate reader. A calibration curve was constructed using H2O2 and the production of H2O2 in the samples was calculated as pmol H2O2 released per minute, normalized to tissue wet weight. To assess the proinflammatory effects of factors present in the conditioned media, mRNA expression of the following targets was assessed by real-time RT-PCR: Nox2, Nox1, Nos2 (endothelial nitric oxide synthase), Icam1, the inflammasome markers Nlrp3, Panx1, and P2rx7, and the inflammatory cytokines TNFα, IL-6, and IL-1β.
Data Analysis
Gene expression data were normalized to the respective control mean values. Statistical analyses of data were performed by one-way analysis of variance followed by Tukey’s Multiple Comparison Test. The strength of the linear relationship between values was assessed by calculating the coefficient of determination (r 2) as the square of Pearson’s product–moment correlation coefficient. p < .05 was considered statistically significant. Data are expressed as means ± SEM.
Results
Effects of Chronic HFD and Aging on Body Mass, Blood Glucose Levels, and Circulating Levels of Metabolic Hormones
In agreement with previous data (42,43), we show that both young and aged mice fed a HFD exhibit significant obesity (Table 2). The relative increase in body mass (calculated as a percentage of body mass at the beginning of treatment) of HFD-fed young mice was significantly greater as compared with HFD-fed aged animals (Table 2). Chronic feeding of a HFD resulted in a significant increase in fasting blood glucose levels in both young and aged mice (Table 3). Serum insulin levels were significantly elevated both in HFD-fed young and aged mice, as compared with the respective SD-fed age-matched controls (Table 3). The quantitative insulin sensitivity check index was calculated to assess insulin sensitivity using the inverse of the sum of the logarithms of the fasting insulin and fasting glucose: 1/(log[fasting insulin in µU/mL] + log[fasting glucose in mg/dL]). There was no significant difference in the quantitative insulin sensitivity check index between the SD-fed young and aged mice. The quantitative insulin sensitivity check index was significantly decreased both in HFD-fed young and aged mice as compared with that in the respective SD-fed age-matched controls (Table 3). SD-fed aged mice had low serum IGF-1 levels and they maintained low IGF-1 levels when fed a HFD (Table 3). Consumption of a HFD tended to decrease IGF-1 levels in young mice (Table 3). Both in young and aged mice fed a HFD there was a significant increase in serum levels of leptin (Table 3). Serum leptin is known to increase in proportion to body weight in animal models of obesity. Accordingly, we found that in HFD-fed young mice leptin levels were higher than in HFD-fed aged mice, which exhibited less body mass gain.
Table 2.
Changes in Body Mass of Young and Aged Mice Fed a High-Fat Diet (HFD) or Standard Diet (SD)
| Parameter | Young (SD) | Young (HFD) | Aged (SD) | Aged (HFD) |
|---|---|---|---|---|
| Body mass (at sacrifice, g) | 30.7±0.6 | 54.5±0.9* | 33.3±1.2 | 45.2±3.2*,†,‡ |
| Gain in body mass (%) | 11±1 | 91±4.3* | −4±1.3* | 32±8.3*,†,‡ |
Note: Data are means ± SEM.
*p < .05 vs SD-fed young control mice.
†p < .05 vs HFD-fed young mice.
‡ p < .05 vs SD-fed aged mice.
Table 3.
Effects of a High-Fat Diet (HFD) on Various Serum Biomarkers and Metabolic Parameters in Fasted Young and Aged C57BL/6 Mice
| Parameter | Young (SD) | Young (HFD) | Aged (SD) | Aged (HFD) |
|---|---|---|---|---|
| Total triglycerides (mg/dL) | 62.0±9.4 | 54.9±5.9 | 51.0±5.7 | 37.6±4.4 |
| Total cholesterol (mmol/L) | 5.4±0.5 | 6.7±0.6 | 3.7±0.1* | 3.6±0.3*,† |
| Glucose (mmol/L) | 6.6±0.4 | 11.0±0.7* | 6.7±0.3 | 9.5±0.9*,‡ |
| Leptin (ng/mL) | 1.2±0.2 | 50.8±7.0* | 0.4±0.1 | 15.4±0.6*,†,‡ |
| Adiponectin (µg/mL) | 7.4±0.6 | 8.0±0.8 | 6.0±0.6 | 4.0±0.5†,‡ |
| Insulin (ng/mL) | 0.5±0.3 | 1.1±0.1* | 0.3±0.1 | 0.7±0.1†,‡ |
| IGF-1 (ng/mL) | 465±78 | 233±30* | 201±22* | 251±34 |
| QUICKI | 0.358±0.006 | 0.272±0.003* | 0.350±0.006 | 0.290±0.010*,‡ |
Notes: Data are means ± SEM (n = 5–7 for each data point). QUICKI = quantitative insulin sensitivity check index.* p < .05 vs standard diet (SD)–fed young control mice.† p < .05 vs HFD-fed young mice.‡ p < .05 vs SD-fed aged mice.
Effects of Obesity and Aging on Endothelial Function, Vascular Oxidative Stress, and Vascular Inflammation
Representative fluorescent images of cross sections of DHE-stained aortas isolated from young and aged mice are shown in Figure 1A. Analysis of nuclear DHE fluorescent intensities indicated that aging is associated with significant increases in vascular ROS production (arbitrary units [AU]; young + SD: 100±3; aged + SD: 229±15; p < .05). HFD-induced obesity was also associated with significant increases in cellular  production in young and aged mice (young + HFD: 167±6; aged + HFD: 408±43; p < .05 vs young + HFD). Obesity-induced vascular oxidative stress was associated with significant endothelial dysfunction in aortas of young mice, as shown by the impaired relaxation responses to acetylcholine (Figure 1B). Obesity-induced endothelial dysfunction was exacerbated in aged mice, as shown by the significantly diminished acetylcholine-induced relaxations of aortas of HFD-fed aged animals (Figure 1B) compared with responses obtained in vessels from HFD-fed young mice. In contrast, aorta relaxations in response to the endothelium-independent vasodilator S-nitroso-N-acetylpenicillamine were unaffected by either aging or obesity (Figure 1C). We also found that aging exacerbated obesity-induced upregulation of the Nox2 subunit of the vascular nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (Figure 1D). Increased vascular oxidative stress in obese aged mice also associated with a significant decline in cellular antioxidant capacity, as indicated by the decreased Hydroxyl Radical Antioxidant Capacity (Figure 1E). This later finding accords with our recent observations that aging is associated with a homeostatic failure due to dysregulation of the Nrf2-mediated antioxidant response in the vasculature (35,36,44,45). Increased vascular oxidative stress in obese aged mice was also associated with increased apoptosis, as indicated by the increased caspase 3/7 activity (Figure 1F) in this cohort. Increased oxidative stress has been causally linked to vascular inflammation in aging (46). Here, we found that aging exacerbated obesity-induced vascular inflammation, as indicated by the significantly increased tissue levels of MCP-1 (Figure 1G) and MIP1α (Figure 1H) in HFD-fed aged mice. Because previous studies (47) suggested that obesity promotes endothelial cell senescence, we analyzed the vascular mRNA expression of p16INK4a, a biomarker of cell senescence. We found that aortas of HFD-fed aged mice exhibited a significant upregulation of mRNA expression of p16INK4a (Figure 1, Panel I).
production in young and aged mice (young + HFD: 167±6; aged + HFD: 408±43; p < .05 vs young + HFD). Obesity-induced vascular oxidative stress was associated with significant endothelial dysfunction in aortas of young mice, as shown by the impaired relaxation responses to acetylcholine (Figure 1B). Obesity-induced endothelial dysfunction was exacerbated in aged mice, as shown by the significantly diminished acetylcholine-induced relaxations of aortas of HFD-fed aged animals (Figure 1B) compared with responses obtained in vessels from HFD-fed young mice. In contrast, aorta relaxations in response to the endothelium-independent vasodilator S-nitroso-N-acetylpenicillamine were unaffected by either aging or obesity (Figure 1C). We also found that aging exacerbated obesity-induced upregulation of the Nox2 subunit of the vascular nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (Figure 1D). Increased vascular oxidative stress in obese aged mice also associated with a significant decline in cellular antioxidant capacity, as indicated by the decreased Hydroxyl Radical Antioxidant Capacity (Figure 1E). This later finding accords with our recent observations that aging is associated with a homeostatic failure due to dysregulation of the Nrf2-mediated antioxidant response in the vasculature (35,36,44,45). Increased vascular oxidative stress in obese aged mice was also associated with increased apoptosis, as indicated by the increased caspase 3/7 activity (Figure 1F) in this cohort. Increased oxidative stress has been causally linked to vascular inflammation in aging (46). Here, we found that aging exacerbated obesity-induced vascular inflammation, as indicated by the significantly increased tissue levels of MCP-1 (Figure 1G) and MIP1α (Figure 1H) in HFD-fed aged mice. Because previous studies (47) suggested that obesity promotes endothelial cell senescence, we analyzed the vascular mRNA expression of p16INK4a, a biomarker of cell senescence. We found that aortas of HFD-fed aged mice exhibited a significant upregulation of mRNA expression of p16INK4a (Figure 1, Panel I).
Figure 1.
Aging exacerbates high-fat diet (HFD)–induced vascular impairment. (Panel A) Representative micrographs showing nuclear dihydroethidium (DHE) fluorescence (white), representing cellular 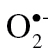 production, in sections of aortas isolated from young and aged mice fed a HFD or standard diet (SD). For orientation purposes, overlay of the nuclear DHE signal (excitation: 490nm, emission: 525nm) and autofluorescence of elastic laminae (excitation: 490nm, emission: 525nm) is shown. Original magnification: 20×. (Panels B and C) Relaxation of aorta rings isolated from young and aged SD- or HFD-fed mice. Vasomotor responses were induced by the endothelium-dependent agent acetylcholine (B) and S-nitroso-N-acetylpenicillamine (SNAP, Panel C), an endothelium-independent vasodilator. Data are means ± SEM; *p < .05 vs respective SD-fed control (n = 5–7). (Panel D) Quantitative real-time RT-PCR data showing mRNA expression of Nox2 in the aortas of HFD-fed and SD-fed young and aged mice. Data are mean ± SEM (n = 5–7); *p < .05 vs SD. (Panel E) Hydroxyl Radical Antioxidant Capacity (HORAC) in the aorta of young and aged SD- and HFD-fed mice. Data are mean ± SEM (n = 5–7); *p < .05 vs SD. (Panel F) HFD-induced increases in caspase 3/7 activity in aortas of young and aged mice. Data are mean ± SEM (n = 5–7); *p < . 05. (Panels G and H) Protein expression of MCP-1 (G) and MIP-1α (H) in aortas of HFD-fed and SD-fed young and aged mice. Data are means ± SEM (n = 5–7); *p < . 05 vs SD, #p < .05 vs young. (Panel I) mRNA expression of the senescence marker p16INK4a in aortas of HFD-fed and SD-fed young and aged mice. Data are mean ± SEM (n = 5–7); *p < . 05 vs SD, #p < .05 vs young.
production, in sections of aortas isolated from young and aged mice fed a HFD or standard diet (SD). For orientation purposes, overlay of the nuclear DHE signal (excitation: 490nm, emission: 525nm) and autofluorescence of elastic laminae (excitation: 490nm, emission: 525nm) is shown. Original magnification: 20×. (Panels B and C) Relaxation of aorta rings isolated from young and aged SD- or HFD-fed mice. Vasomotor responses were induced by the endothelium-dependent agent acetylcholine (B) and S-nitroso-N-acetylpenicillamine (SNAP, Panel C), an endothelium-independent vasodilator. Data are means ± SEM; *p < .05 vs respective SD-fed control (n = 5–7). (Panel D) Quantitative real-time RT-PCR data showing mRNA expression of Nox2 in the aortas of HFD-fed and SD-fed young and aged mice. Data are mean ± SEM (n = 5–7); *p < .05 vs SD. (Panel E) Hydroxyl Radical Antioxidant Capacity (HORAC) in the aorta of young and aged SD- and HFD-fed mice. Data are mean ± SEM (n = 5–7); *p < .05 vs SD. (Panel F) HFD-induced increases in caspase 3/7 activity in aortas of young and aged mice. Data are mean ± SEM (n = 5–7); *p < . 05. (Panels G and H) Protein expression of MCP-1 (G) and MIP-1α (H) in aortas of HFD-fed and SD-fed young and aged mice. Data are means ± SEM (n = 5–7); *p < . 05 vs SD, #p < .05 vs young. (Panel I) mRNA expression of the senescence marker p16INK4a in aortas of HFD-fed and SD-fed young and aged mice. Data are mean ± SEM (n = 5–7); *p < . 05 vs SD, #p < .05 vs young.
Effects of Obesity and Aging on Oxidative Stress and Macrophage Infiltration in the Perivascular Adipose Tissue
Because there are studies demonstrating that aging is associated with increased oxidative stress in the visceral adipose tissue (48), we assessed ROS production in perivascular adipose tissue in the present study. Analysis of nuclear DHE fluorescent intensities (Figure 2A) indicated that aging was associated with significant increases in cellular ROS production in the perivascular adipose tissue (AU; young + SD: 100±6; aged + SD: 151±11; p < .05 vs young). HFD-induced obesity was also associated with significant increases in cellular 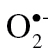 production in perivascular adipose tissue (young + HFD: 200±10; aged + HFD: 296±16; p < .05 vs young + HFD). To analyze macrophage infiltration, we assessed CD68+ cells in the perivascular adipose tissue surrounding aorta segments from the same anatomical location. Periaortic adipose tissue surrounding the distal portion of the thoracic aortas differs in gross appearance among the four groups. We found that in SD-fed young mice adipose tissue surrounding the distal portion of the thoracic aorta was primarily composed of multilocular brown adipocytes (Figure 2B, upper left panel). In contrast, in aged mice surrounding the distal portion of the thoracic aorta adipose tissues consisting of both multilocular brown adipocytes and unilocular white adipocytes could be observed (Figure 2B, lower panels). Note that HFD feeding resulted in significant hypertrophy of the white adipocytes. We found that obesity in aged mice was associated with a significantly increased presence of CD68+ cells in the perivascular adipose tissue of HFD-fed aged mice (Figure 2B and C). Obesity-related changes in macrophage infiltration in perivascular adipose tissue in young mice statistically were not significant.
production in perivascular adipose tissue (young + HFD: 200±10; aged + HFD: 296±16; p < .05 vs young + HFD). To analyze macrophage infiltration, we assessed CD68+ cells in the perivascular adipose tissue surrounding aorta segments from the same anatomical location. Periaortic adipose tissue surrounding the distal portion of the thoracic aortas differs in gross appearance among the four groups. We found that in SD-fed young mice adipose tissue surrounding the distal portion of the thoracic aorta was primarily composed of multilocular brown adipocytes (Figure 2B, upper left panel). In contrast, in aged mice surrounding the distal portion of the thoracic aorta adipose tissues consisting of both multilocular brown adipocytes and unilocular white adipocytes could be observed (Figure 2B, lower panels). Note that HFD feeding resulted in significant hypertrophy of the white adipocytes. We found that obesity in aged mice was associated with a significantly increased presence of CD68+ cells in the perivascular adipose tissue of HFD-fed aged mice (Figure 2B and C). Obesity-related changes in macrophage infiltration in perivascular adipose tissue in young mice statistically were not significant.
Figure 2.
Aging exacerbates high-fat diet (HFD)–induced oxidative stress and inflammation in perivascular adipose tissue. (Panel A) Representative micrographs showing nuclear dihydroethidium (DHE) fluorescence (white), representing cellular 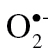 production in sections of perivascular adipose tissue isolated from standard diet (SD)–fed and HFD-fed young and aged mice. Note that tissues derived from HFD-fed aged mice exhibit the most intense DHE fluorescence. Original magnification: 20×. (Panel B) Representative micrographs showing brown immunostaining for CD68 in sections of perivascular adipose tissues isolated from SD- and HFD-fed young and aged mice. Tissues derived from HFD-fed aged mice exhibit the greatest infiltration by CD68+ macrophages (arrows). In SD-fed young mice adipose tissue surrounding the distal portion of the thoracic aorta was primarily composed of multilocular brown adipocytes. In contrast, in aged mice surrounding the distal portion of the thoracic aorta adipose tissues consisting of both multilocular brown adipocytes and unilocular white adipocytes could be observed. Note that HFD feeding resulted in significant hypertrophy of the white adipocytes. (Panel C) Summary data for relative age- and HFD-induced changes in CD68+ macrophage content in perivascular adipose tissue. Data are mean ± SEM; *p < .05 vs control.
production in sections of perivascular adipose tissue isolated from standard diet (SD)–fed and HFD-fed young and aged mice. Note that tissues derived from HFD-fed aged mice exhibit the most intense DHE fluorescence. Original magnification: 20×. (Panel B) Representative micrographs showing brown immunostaining for CD68 in sections of perivascular adipose tissues isolated from SD- and HFD-fed young and aged mice. Tissues derived from HFD-fed aged mice exhibit the greatest infiltration by CD68+ macrophages (arrows). In SD-fed young mice adipose tissue surrounding the distal portion of the thoracic aorta was primarily composed of multilocular brown adipocytes. In contrast, in aged mice surrounding the distal portion of the thoracic aorta adipose tissues consisting of both multilocular brown adipocytes and unilocular white adipocytes could be observed. Note that HFD feeding resulted in significant hypertrophy of the white adipocytes. (Panel C) Summary data for relative age- and HFD-induced changes in CD68+ macrophage content in perivascular adipose tissue. Data are mean ± SEM; *p < .05 vs control.
Effects of Obesity and Aging on Cytokine Secretion Profile of Perivascular Adipose Tissue
Secretomes obtained by incubating nonobese and obese perivascular adipose tissues in serum-free medium for 24 hours were analyzed by a multiplex protein array system. Obesity was associated with marked increases in the secretion of inflammatory mediators both in the young and the aged mice (Figure 3A). We found that several inflammatory factors were secreted in higher quantities from the perivascular adipose tissue derived from HFD-fed aged mice as compared with perivascular adipose tissues isolated from HFD-fed young mice (Figure 3A). These factors included several cytokines (eg, TNFα, IL-6) and various chemokines (eg, eotaxin, MIP-1α). There was a positive correlation (p < .05, r2 = .26) between levels of inflammatory mediators in the perivascular adipose tissue–conditioned media and in the sera obtained from the same animals (Figure 3B). We found that secretion of adiponectin from the perivascular adipose tissue was significantly decreased by consumption of a HFD (Figure 3C).
Figure 3.
(Panel A) Proteomic profiles of perivascular adipose tissue secretomes. Perivascular adipose tissues, derived from young (Y) and aged (A) mice fed a high-fat diet (HFD) or standard diet (SD), were kept in organoid culture and cytokine concentrations were assessed in the conditioned media (see Methods section). The heat map is a graphic representation of normalized cytokine concentration values in perivascular adipose tissue–conditioned media, depicted by color intensity, from highest (bright red) to lowest (bright green) expression. Values represent average secreted protein levels (log2 [fold change, normalized to the respective control mean value]) in replicate samples (n = 6 in each group). Perivascular adipose tissues from aged mice on a HFD secrete the highest levels of inflammatory cytokines and chemokines. (Panel B) Correlation between cytokines secreted from the perivascular adipose tissue and cytokine levels in the sera from the same animals (p < .05, r2 = .26). (Panel C) Relative levels of adiponectin secretion from perivascular adipose tissue derived from young and aged SD- or HFD-fed animals (see Methods section). Data are means ± SEM (n = 10 in each group); *p < .05 vs SD.
Prooxidative and Proinflammatory Vascular Effects of Aged Obese Perivascular Adipose Tissue Secretome
We used an organoid culture-based bioassay approach utilizing aorta segments isolated from young control mice, which were treated with perivascular adipose tissue–conditioned media (Figure 4A). In vitro exposure of aorta segments of young mice to the young obese perivascular adipose tissue secretory products induced oxidative stress, as indicated by the increased vascular H2O2 production (Figure 4B). Vascular oxidative stress induced by aged obese perivascular adipose tissue secretome was significantly increased (Figure 4B). In vitro exposure of detector vessels to the aged obese perivascular adipose tissue secretome resulted in upregulation of Nox2, Nox1 (Figure 4C and D, respectively), TNFα, IL-6, IL-1β, Nlrp3 (Figure 5), ICAM-1, Panx1, and P2rx7 (data not shown), and downregulation of endothelial nitric oxide synthase (Figure 4E). Changes in vascular expression of Nox1 (Figure 4D), TNFα, IL-6, IL-1β, and Nlrp3 (Figure 5) were significantly smaller after in vitro exposure of detector vessels to the young obese perivascular adipose tissue secretome.
Figure 4.
(Panel A) Experimental design depicting the ex vivo organoid culture-based bioassay approach utilizing aortas isolated from control mice treated with perivascular adipose tissue–conditioned media. (Panel B) Production of H2O2 by detector vessels induced by proinflammatory factors secreted from the perivascular adipose tissue derived from young and aged standard diet (SD)–fed or high-fat diet (HFD)–fed mice. The Amplex Red/horseradish peroxidase assay was used to assess vascular reactive oxygen species (ROS) production. (Panels C and E) Quantitative real-time RT-PCR data showing mRNA expression of Nox2 (C), Nox1 (D), and Nos3 (E) in detector vessels cultured in the presence of perivascular adipose tissue–conditioned media. Data are means ± SEM (n = 10 in each group); *p < .05 vs young SD-fed, #p < .05 vs young HFD-fed, &p < .05 vs aged SD-fed.
Figure 5.
Quantitative real-time RT-PCR data showing mRNA expression of TNF-α (A), IL-6 (B), IL-1Β (C), and Nlrp3 (D) in detector vessels cultured in the presence of factors secreted from the perivascular adipose tissue derived from young and aged standard diet (SD)–fed or high-fat diet (HFD)–fed mice. Data are mean ± SEM (n = 10 in each group); *p < .05 vs young SD-fed, #p < .05 vs young HFD-fed, &p < .05 vs aged SD-fed.
Discussion
Previous clinical and experimental studies demonstrate that obesity and metabolic syndrome promote vascular oxidative stress, endothelial dysfunction, and vascular inflammation, which have a key role in the development of vascular disease (2,40,49–52). Our present study provides evidence that HFD-induced vascular ROS generation substantially increases in old age, which is associated with exacerbation of endothelial dysfunction and vascular inflammation (Figure 1). Previous studies demonstrate that activation of NADPH oxidases contribute to vascular oxidative stress in obesity and metabolic syndrome (53,54), although one cannot exclude the contribution of other cellular sources of ROS, including mitochondrial dysfunction (55) and uncoupling of endothelial nitric oxide synthase (15). In accordance with this view, our findings suggest that increased oxidative stress in the aorta of HFD-fed aged mice may be due, at least in part, to upregulation of NADPH oxidase (Figure 1D). Consuming a HFD also exacerbates NADPH oxidase activity in the brain of aged mice (42). Increased cellular oxidative stress, chronic low-grade vascular inflammation, and increased apoptotic cell death in aging are causally related (31,33,46,56,57). This concept is further supported by the finding that exacerbation of vascular oxidative stress in obese aged mice was associated with an upregulation of inflammatory mediators and increased apoptosis in the vascular wall (Figure 1F–H).
Oxidative stress-induced cellular replicative senescence is associated with aging and it is believed that increased presence of endothelial cells with senescence-associated phenotypes in the vascular wall promote atherogenesis (58,59). Our findings give further support to the concept that cellular senescence may be an important consequence of obesity and contribute to obesity-induced vascular complications (47). Importantly, our findings suggest that aged vessels are significantly more vulnerable to the senescence-inducing effects of obesity than young arteries (Figure 1, Panel I). Previous studies demonstrated that the process of cellular senescence entails a striking increase in the secretion of proinflammatory proteins (60), thus obesity-related vascular senescence might be an important additional contributor to chronic vascular inflammation. We propose that age-related exacerbation of obesity-induced vascular oxidative stress and the resulting inflammation and increased cellular senescence increase the risk for the development of vascular diseases in the elderly individuals. The mechanisms underlying the increased vulnerability of aged arteries to the deleterious effects of obesity are likely multifaceted and are the focus of our ongoing investigations (20,36,61). In the present study, we focused on the role of age-related changes in the perivascular adipose tissue.
HFD-induced obesity in young animals promotes oxidative stress in perivascular adipose tissue, which is associated with increased macrophage infiltration (Figure 2A and B) and results in a marked proinflammatory shift in the profile of cytokines and chemokines secreted from the perivascular adipose tissue (Figure 3A), extending previous findings (4). Here, we provide evidence that aging exacerbates obesity-induced oxidative stress and inflammation in periaortic adipose tissue (Figure 2A and B), which results in the increased secretion of a wide range of inflammatory chemokines and cytokines (Figure 3A). There are multiple mechanisms by which obesity and aging alter the secretome of the perivascular adipose tissue. First, it is likely that both obesity and aging alter the cytokine expression in the adipocytes per se. Second, adipose tissue infiltrating macrophages in aged obese mice is also likely a significant source of cytokines, including TNF-α and IL-6, which likely confer important pathophysiological effects. Previous studies show that inflammatory cytokines secreted from infiltrating macrophages can induce insulin resistance in adipocytes (62) and are also thought to promote adipocyte death. Further, it is likely that increased levels of macrophage-derived cytokines and chemokines in obese aged mice have a role in the propagation of macrophage recruitment and exacerbate vascular inflammation. Third, previous studies in young animals suggest that the microvascular cells within adipose tissue are also an important source of chemokine secretions (11,63). Further studies are warranted to determine the role of age-related alterations of the microcirculation in exacerbation of obesity-induced inflammation in perivascular adipose tissue. Over the past few years, inflamma- somes have emerged as central regulators of obesity-induced inflammation (revised recently in [64]). Inflammasomes are multiprotein platforms that orchestrate the activation and secretion of IL-1β, as well as other cytokines, in response to danger-associated signals. In that regard, it is important that obesity is associated with a substantial increase in IL-1β levels in the secretome of perivascular adipose tissue. Thus, further studies are warranted to test the hypothesis that in aged mice obesity-induced inflammasome activation contribute to the inflammatory alterations of the perivascular adipose tissue.
Inflammatory cytokines secreted form the perivascular adipose tissue likely reach the cells in the vessel wall altering their function and phenotype. To elucidate some of the potential mechanisms by which factors secreted from perivascular adipose tissue contribute to obesity-related development of atherosclerosis, we used an ex vivo organoid culture-based bioassay approach utilizing aortas isolated from control mice treated with perivascular adipose tissue–conditioned media. Here, we demonstrate for the first time that inflammatory factors secreted from the perivascular adipose tissue from obese aged mice elicit increased oxidative stress in the vascular wall, as compared with the effects of the young obese secretome (Figure 4B). There are likely multiple cellular mechanisms involved in the prooxidative effects of the obese secretome, including upregulation of NADPH oxidases (Figure 4C and D) and downregulation of endothelial nitric oxide synthase (Figure 4E). Further studies are needed to identify the paracrine factors present in the aged obese secretome that are responsible for the upregulation of vascular ROS production. Likely, candidates include TNFα, which is known to activate NADPH oxidase-dependent ROS generation in aged vessels (31), and leptin, which was shown to impair endothelial function in coronary arteries in a paracrine manner (6). We also demonstrate that obesity is associated with a decline in adiponectin secretion from perivascular adipose tissue (Figure 3C) (15). Because adiponectin is known to confer antioxidative effects, a decline in its level may also contribute to increased vascular oxidative stress in obese aged mice.
We found that increased prooxidative effects of the aged obese secretome are associated with activation of inflammatory processes in the vascular wall, including upregulation of cytokines and adhesion molecules (Figure 5). Our findings extend the results of previous studies showing that exposure of cultured endothelial cells to the secretomes of visceral and subcutaneous adipose tissue from obese individuals results in endothelial activation and activation of NF-κB (51). We found that the young obese secretome upregulated the vascular expression of some of the inflammatory factors (eg, TNFα) and this effect was further augmented by aging. Further studies are warranted to identify the factors underlying these phenomena and to characterize age- and obesity-related effects. Studies published over the past few years suggest a prominent role for inflammasomes in both the induction and perpetuation of obesity-driven inflammation (64,65). In particular, in obesity, the NLRP3 inflammasome is thought to function as a sensor to detect danger signals and induce downstream inflammatory signaling including activation of NF-κB that contributes to obesity-associated pathophysiological conditions. The factors responsible for inflammasome activation in obesity may include ROS, fatty acid metabolites, and damage signals emanating from injured cells. In that regard, it is potentially important that vascular NLRP3 expression is upregulated by factors secreted from the perivascular adipose tissue of aged obese mice (Figure 5D). Future investigations that address the role of inflammasome pathways in age-related exacerbation of obesity-induced vascular inflammation will probably greatly enhance the understanding of the etiology of obesity-associated cardiovascular diseases.
Although obesity may alter the secretome of adipose tissues in a depot-specific manner (10,11,49), a similar robust increase in inflammatory cytokines can be generally observed in each adipose depot (9,63,66,67). We found that the inflammatory cytokines and chemokines that are upregulated in the perivascular adipose tissue in obese aged mice are also present at elevated levels in the circulation of the same animals (Figure 3B), likely due to heightened state of inflammation of the visceral adipose tissue. Thus, we propose that in aged obese mice circulating proinflammatory factors (22) derived mainly from visceral adipose depots act in synergy with locally produced proinflammatory cytokines secreted by the perivascular adipose tissue to promote vascular oxidative stress and inflammation. Adipose-derived proinflammatory cytokines are also thought to modulate insulin sensitivity of the tissues (68), which may also affect vascular function in aged obese mice.
Both clinical observations and experimental studies support the concept that perivascular adipose tissue plays a role in atherogenesis. First, in the human heart the quantity of perivascular adipose tissue is correlated with the extent of coronary atherosclerosis (8). Segments of the human coronary arteries, which are surrounded by myocardium, are not exposed to inflammatory factors secreted from the perivascular adipose tissue. Importantly, these intramyocardial segments of the coronary arteries appear to be protected from atherosclerosis. Furthermore, expression of proinflammatory cytokines and chemokines is increased in epicardial adipose tissue of patients with coronary artery disease as compared with disease-free participants. The quantity of perivascular adipose tissue in the coronary circulation in humans is thought to correlate with total body fat content (and its proxy measure, increased waist circumference). Thus, both the increased quantity and the inflammatory status of the perivascular adipose tissue in obesity likely have a role in development of vascular diseases. The pathophysiological importance of perivascular adipose tissue inflammation in laboratory animals has been established by the demonstration of increased atherosclerosis in the carotid artery of Apo−/− mice after perivascular transplantation of visceral adipose tissue (5,69). Recent studies also suggest that activation of the NLRP3 inflammasome and release of IL-1β, which are upregulated in obese mice, have a role in atherogenesis (70), including promotion of the development of lipid plaques and destabilizion of plaques in mice. Furthermore, conditioned medium from periaortic adipose tissue was demonstrated to stimulate proliferation of smooth muscle cells, an effect that is significantly enhanced in aged rats and in rats fed a HFD (71). Importantly, smooth muscle cell proliferation is known to play a role in atherogenesis. Taken together, the existing data from clinical and experimental studies support the concept that the perivascular adipose tissue promotes vascular inflammation and the process of atherosclerosis (8).
Our findings suggest that obesity in aging is associated with exacerbated inflammation in perivascular adipose tissue and thus represents a more significant cardiovascular risk factor in the elderly participants than in younger participants. The available data suggest that increased secretion of inflammatory factors from perivascular adipose tissue may be involved in local stimulation of atherosclerotic plaque formation in obese elderly patients. It can also be hypothesized that obesity- and age-related alterations in the secretion of perivascular adipose-derived adipokines (eg, leptin, adiponectin) may contribute to the initiation and expansion of coronary disease in the obese elderly patients. Furthermore, in obese aged individuals perivascular adipose tissue likely constitutes a reservoir for inflammatory cells, which can migrate into the vascular wall. Because vascular expression of adhesion molecules and chemokines increase with age (46) and is induced by obesity (12), it is likely that inflammatory cells migrating from the perivascular adipose tissue into the vascular wall will contribute to the development of obesity-induced vascular diseases in aging. Clinical studies also demonstrate that obesity significantly increases the risk for abdominal aortic aneurysms in the elderly individuals (50,72). These conclusions are supported by experimental studies that obesity promotes formation of abdominal aortic aneurysms in mice, at least in part, by inducing inflammation in the periaortic adipose tissue (4,73). Age-related exacerbation of obesity-induced perivascular adipose tissue inflammation may lead to increased periadventitial entry of leukocytes into the vascular wall leading to medial accumulation of macrophages in abdominal aortic aneurysms-prone areas (74,75).
In conclusion, our studies provide evidence that aging exacerbates obesity-induced oxidative stress and inflammation in the perivascular adipose tissue. Our findings support a role for localized perivascular tissue inflammation in exacerbation of vascular oxidative stress and inflammation in aging, an effect that likely enhances the risk for development of cardiovascular diseases from obesity in the elderly individuals.
Acknowledgment
The authors would like to express their gratitude for the support of the Donald W. Reynolds Foundation, which funds aging research at the University of Oklahoma Health Sciences Center under its Aging and Quality of Life Program.
Footnotes
Funding
This work was supported by grants from the American Heart Association to Z.U., P.T., and A.C., American Federation for Aging Research to A.C., the Oklahoma Center for the Advancement of Science and Technology to A.C. and Z.U., the National Institutes of Health (AG031085 to A.C.; AT006526 to Z.U.; AG038747, NS056218, and P01 AG11370 to W.E.S.), the Ellison Medical Foundation and the Arkansas Claude Pepper Older Americans Independence Center at University of Arkansas Medical Center to A.C., and the Nemzeti Fejlesztési Ügynökség (TÁMOP-4.2.1/b-10/2/KONV-2010-0012 to ZU).
References
- 1. Wang YC, Colditz GA, Kuntz KM. Forecasting the obesity epidemic in the aging U.S. population. Obesity (Silver Spring). 2007;15:2855–2865 [DOI] [PubMed] [Google Scholar]
- 2. Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67:968–977 [DOI] [PubMed] [Google Scholar]
- 3. Henrichot E, Juge-Aubry CE, Pernin A, et al. Production of chemokines by perivascular adipose tissue: a role in the pathogenesis of atherosclerosis? Arterioscler Thromb Vasc Biol. 2005;25:2594–2599 [DOI] [PubMed] [Google Scholar]
- 4. Police SB, Thatcher SE, Charnigo R, Daugherty A, Cassis LA. Obesity promotes inflammation in periaortic adipose tissue and angiotensin II-induced abdominal aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2009;29:1458–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Öhman MK, Luo W, Wang H, et al. Perivascular visceral adipose tissue induces atherosclerosis in apolipoprotein E deficient mice. Atherosclerosis. 2011;219:33–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Payne GA, Borbouse L, Kumar S, et al. Epicardial perivascular adipose-derived leptin exacerbates coronary endothelial dysfunction in metabolic syndrome via a protein kinase C-beta pathway. Arterioscler Thromb Vasc Biol. 2010;30:1711–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Payne GA, Kohr MC, Tune JD. Epicardial perivascular adipose tissue as a therapeutic target in obesity-related coronary artery disease. Br J Pharmacol. 2012;165:659–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Verhagen SN, Visseren FL. Perivascular adipose tissue as a cause of atherosclerosis. Atherosclerosis. 2011;214:3–10 [DOI] [PubMed] [Google Scholar]
- 9. Surmi BK, Hasty AH. Macrophage infiltration into adipose tissue: initiation, propagation and remodeling. Future Lipidol. 2008;3:545–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fain JN. Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitam Horm. 2006;74:443–477 [DOI] [PubMed] [Google Scholar]
- 11. Hocking SL, Wu LE, Guilhaus M, Chisholm DJ, James DE. Intrinsic depot-specific differences in the secretome of adipose tissue, preadipocytes, and adipose tissue-derived microvascular endothelial cells. Diabetes. 2010;59:3008–3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nishimura S, Manabe I, Nagasaki M, et al. In vivo imaging in mice reveals local cell dynamics and inflammation in obese adipose tissue. J Clin Invest. 2008;118:710–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Starr ME, Evers BM, Saito H. Age-associated increase in cytokine production during systemic inflammation: adipose tissue as a major source of IL-6. J Gerontol A Biol Sci Med Sci. 2009;64:723–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu D, Ren Z, Pae M, et al. Aging up-regulates expression of inflammatory mediators in mouse adipose tissue. J Immunol. 2007;179:4829–4839 [DOI] [PubMed] [Google Scholar]
- 15. Marchesi C, Ebrahimian T, Angulo O, Paradis P, Schiffrin EL. Endothelial nitric oxide synthase uncoupling and perivascular adipose oxidative stress and inflammation contribute to vascular dysfunction in a rodent model of metabolic syndrome. Hypertension. 2009;54:1384–1392 [DOI] [PubMed] [Google Scholar]
- 16. Hua K, Forbes ME, Lichtenwalner RJ, Sonntag WE, Riddle DR. Adult-onset deficiency in growth hormone and insulin-like growth factor-I alters oligodendrocyte turnover in the corpus callosum. Glia. 2009;57:1062–1071; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ramsey MM, Ingram RL, Cashion AB, et al. Growth hormone-deficient dwarf animals are resistant to dimethylbenzanthracine (DMBA)-induced mammary carcinogenesis. Endocrinology. 2002;143:4139–4142 [DOI] [PubMed] [Google Scholar]
- 18. Carter CS, Ramsey MM, Ingram RL, et al. Models of growth hormone and IGF-1 deficiency: applications to studies of aging processes and life-span determination. J Gerontol A Biol Sci Med Sci. 2002;57:B177–B188 [DOI] [PubMed] [Google Scholar]
- 19. Nieves-Martinez E, Sonntag WE, Wilson A, et al. Early-onset GH deficiency results in spatial memory impairment in mid-life and is prevented by GH supplementation. J Endocrinol. 2010;204:31–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bailey-Downs LC, Sosnowska D, Toth P, et al. Growth hormone and IGF-1 deficiency exacerbate high-fat diet-induced endothelial impairment in obese Lewis dwarf rats: implications for vascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:553–564; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fisher G, Hunter GR, Glasser SP. Associations Between Arterial Elasticity and Markers of Inflammation in Healthy Older Women. J Gerontol A Biol Sci Med Sci. 2012;; doi:10.1093/gerona/gls188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brinkley TE, Hsu FC, Beavers KM, et al. Total and abdominal adiposity are associated with inflammation in older adults using a factor analysis approach. J Gerontol A Biol Sci Med Sci. 2012;67:1099–1106.; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jenny NS, French B, Arnold AM, et al. Long-term assessment of inflammation and healthy aging in late life: the Cardiovascular Health Study All Stars. J Gerontol A Biol Sci Med Sci. 2012;67:970–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kizer JR, Arnold AM, Jenny NS, et al. Longitudinal changes in adiponectin and inflammatory markers and relation to survival in the oldest old: the Cardiovascular Health Study All Stars study. J Gerontol A Biol Sci Med Sci. 2011;66:1100–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Crasto CL, Semba RD, Sun K, et al. Endogenous secretory receptor for advanced glycation end products is associated with low serum interleukin-1 receptor antagonist and elevated IL-6 in older community-dwelling adults. J Gerontol A Biol Sci Med Sci. 2011;66:437–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bailey-Downs LC, Mitschelen M, Sosnowska D, et al. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:313–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Csiszar A, Labinskyy N, Orosz Z, Xiangmin Z, Buffenstein R, Ungvari Z. Vascular aging in the longest-living rodent, the naked mole rat. Am J Physiol Heart Circ Physiol. 2007;293:H919–H927 [DOI] [PubMed] [Google Scholar]
- 28. Ungvari Z, Csiszar A, Huang A, Kaminski PM, Wolin MS, Koller A. High pressure induces superoxide production in isolated arteries via protein kinase C-dependent activation of NAD(P)H oxidase. Circulation. 2003;108:1253–1258 [DOI] [PubMed] [Google Scholar]
- 29. Csiszar A, Labinskyy N, Perez V, et al. Endothelial function and vascular oxidative stress in long-lived GH/IGF-deficient Ames dwarf mice. Am J Physiol Heart Circ Physiol. 2008;295:H1882–H1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Csiszar A, Ungvari Z, Edwards JG, et al. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166 [DOI] [PubMed] [Google Scholar]
- 31. Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti-tumor necrosis factor-alpha treatment in aging. Am J Pathol. 2007;170:388–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ungvari Z, Labinskyy N, Gupte S, Chander PN, Edwards JG, Csiszar A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am J Physiol Heart Circ Physiol. 2008;294:H2121–H2128 [DOI] [PubMed] [Google Scholar]
- 33. Ungvari Z, Orosz Z, Labinskyy N, et al. Increased mitochondrial H2O2 production promotes endothelial NF-kappaB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293:H37–H47 [DOI] [PubMed] [Google Scholar]
- 34. Csiszar A, Smith K, Labinskyy N, Orosz Z, Rivera A, Ungvari Z. Resveratrol attenuates TNF-alpha-induced activation of coronary arterial endothelial cells: role of NF-kappaB inhibition. Am J Physiol Heart Circ Physiol. 2006;291:H1694–H1699 [DOI] [PubMed] [Google Scholar]
- 35. Csiszar A, Sosnowska D, Wang M, Lakatta EG, Sonntag WE, Ungvari Z. Age-associated proinflammatory secretory phenotype in vascular smooth muscle cells from the nonhuman primate Macaca mulatta: reversal by resveratrol treatment. J Gerontol A Biol Sci Med Sci. 2012;67:811–820; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ungvari Z, Bailey-Downs L, Gautam T, et al. Age-associated vascular oxidative stress, Nrf2 dysfunction, and NF-{kappa}B activation in the nonhuman primate Macaca mulatta. J Gerontol A Biol Sci Med Sci. 2011;66:866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Valcarcel-Ares MN, Gautam T, Warrington JP, et al. Disruption of Nrf2 signaling impairs angiogenic capacity of endothelial cells: implications for microvascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:821–829; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Csiszar A, Sosnowska D, Tucsek Z, et al. Circulating factors induced by caloric restriction in the non-human primate Macaca mulatta activate angiogenic processes in endothelial cells. J Gerontol A Biol Sci Med Sci. 2012; doi:10.1093/gerona/gls193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ungvari Z, Orosz Z, Rivera A, et al. Resveratrol increases vascular oxidative stress resistance. Am J Physiol Heart Circ Physiol. 2007;292:H2417–H2424 [DOI] [PubMed] [Google Scholar]
- 40. Reckless J, Rubin EM, Verstuyft JB, Metcalfe JC, Grainger DJ. Monocyte chemoattractant protein-1 but not tumor necrosis factor-alpha is correlated with monocyte infiltration in mouse lipid lesions. Circulation. 1999;99:2310–2316 [DOI] [PubMed] [Google Scholar]
- 41. Ungvari Z, Ridgway I, Philipp EE, et al. Extreme longevity is associated with increased resistance to oxidative stress in Arctica islandica, the longest-living non-colonial animal. J Gerontol A Biol Sci Med Sci. 2011;66:741–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bruce-Keller AJ, White CL, Gupta S, et al. NOX activity in brain aging: exacerbation by high fat diet. Free Radic Biol Med. 2010;49:22–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Morrison CD, Pistell PJ, Ingram DK, et al. High fat diet increases hippocampal oxidative stress and cognitive impairment in aged mice: implications for decreased Nrf2 signaling. J Neurochem. 2010;114:1581–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ungvari Z, Bailey-Downs L, Sosnowska D, et al. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of NRF2-mediated antioxidant response. Am J Physiol Heart Circ Physiol. 2011;301:H363–H372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ungvari Z, Parrado-Fernandez C, Csiszar A, de Cabo R. Mechanisms underlying caloric restriction and lifespan regulation: implications for vascular aging. Circ Res. 2008;102:519–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci. 2010;65:1028–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang CY, Kim HH, Hiroi Y, et al. Obesity increases vascular senescence and susceptibility to ischemic injury through chronic activation of Akt and mTOR. Sci Signal. 2009;2:ra11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Findeisen HM, Pearson KJ, Gizard F, et al. Oxidative stress accumulates in adipose tissue during aging and inhibits adipogenesis. PLoS ONE. 2011;6:e18532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fantuzzi G, Mazzone T. Adipose tissue and atherosclerosis: exploring the connection. Arterioscler Thromb Vasc Biol. 2007;27:996–1003 [DOI] [PubMed] [Google Scholar]
- 50. Golledge J, Clancy P, Jamrozik K, Norman PE. Obesity, adipokines, and abdominal aortic aneurysm: Health in Men study. Circulation. 2007;116:2275–2279 [DOI] [PubMed] [Google Scholar]
- 51. Hanzu FA, Palomo M, Kalko SG, et al. Translational evidence of endothelial damage in obese individuals: inflammatory and prothrombotic responses. J Thromb Haemost. 2011;9:1236–1245 [DOI] [PubMed] [Google Scholar]
- 52. Sloboda N, Fève B, Thornton SN, et al. Fatty acids impair endothelium-dependent vasorelaxation: a link between obesity and arterial stiffness in very old Zucker rats. J Gerontol A Biol Sci Med Sci. 2012;67:927–938; [DOI] [PubMed] [Google Scholar]
- 53. Ketonen J, Shi J, Martonen E, Mervaala E. Periadventitial adipose tissue promotes endothelial dysfunction via oxidative stress in diet-induced obese C57Bl/6 mice. Circ J. 2010;74:1479–1487 [DOI] [PubMed] [Google Scholar]
- 54. Miller AA, De Silva TM, Judkins CP, Diep H, Drummond GR, Sobey CG. Augmented superoxide production by Nox2-containing NADPH oxidase causes cerebral artery dysfunction during hypercholesterolemia. Stroke. 2010;41:784–789 [DOI] [PubMed] [Google Scholar]
- 55. Ruggiero C, Ehrenshaft M, Cleland E, Stadler K. High-fat diet induces an initial adaptation of mitochondrial bioenergetics in the kidney despite evident oxidative stress and mitochondrial ROS production. Am J Physiol Endocrinol Metab. 2011;300:E1047–E1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Csiszar A, Labinskyy N, Jimenez R, et al. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev. 2009;130:518–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics. 2004;17:21–30 [DOI] [PubMed] [Google Scholar]
- 58. Minamino T, Komuro I. Vascular cell senescence: contribution to atherosclerosis. Circ Res. 2007;100:15–26 [DOI] [PubMed] [Google Scholar]
- 59. Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation. 2002;105:1541–1544 [DOI] [PubMed] [Google Scholar]
- 60. Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010;16:238–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ungvari Z, Bailey-Downs L, Gautam T, et al. Adaptive induction of NF-E2-related factor-2-driven antioxidant genes in endothelial cells in response to hyperglycemia. Am J Physiol Heart Circ Physiol. 2011;300:H1133–H1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Eringa EC, Bakker W, Smulders YM, Serné EH, Yudkin JS, Stehouwer CD. Regulation of vascular function and insulin sensitivity by adipose tissue: focus on perivascular adipose tissue. Microcirculation. 2007;14:389–402 [DOI] [PubMed] [Google Scholar]
- 63. Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kanneganti TD, Dixit VD. Immunological complications of obesity. Nat Immunol. 2012;13:707–712 [DOI] [PubMed] [Google Scholar]
- 65. Horng T, Hotamisligil GS. Linking the inflammasome to obesity-related disease. Nat Med. 2011;17:164–165 [DOI] [PubMed] [Google Scholar]
- 66. Weisberg SP, Hunter D, Huber R, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kanda H, Tateya S, Tamori Y, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ohman MK, Wright AP, Wickenheiser KJ, Luo W, Russo HM, Eitzman DT. Monocyte chemoattractant protein-1 deficiency protects against visceral fat-induced atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:1151–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Duewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Barandier C, Montani JP, Yang Z. Mature adipocytes and perivascular adipose tissue stimulate vascular smooth muscle cell proliferation: effects of aging and obesity. Am J Physiol Heart Circ Physiol. 2005;289:H1807–H1813 [DOI] [PubMed] [Google Scholar]
- 72. Alcorn HG, Wolfson SK, Jr, Sutton-Tyrrell K, Kuller LH, O’Leary D. Risk factors for abdominal aortic aneurysms in older adults enrolled in The Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 1996;16:963–970 [DOI] [PubMed] [Google Scholar]
- 73. Police SB, Putnam K, Thatcher S, Batifoulier-Yiannikouris F, Daugherty A, Cassis LA. Weight loss in obese C57BL/6 mice limits adventitial expansion of established angiotensin II-induced abdominal aortic aneurysms. Am J Physiol Heart Circ Physiol. 2010;298:H1932–H1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cassis LA, Rateri DL, Lu H, Daugherty A. Bone marrow transplantation reveals that recipient AT1a receptors are required to initiate angiotensin II-induced atherosclerosis and aneurysms. Arterioscler Thromb Vasc Biol. 2007;27:380–386 [DOI] [PubMed] [Google Scholar]
- 75. Daugherty A, Cassis L. Chronic angiotensin II infusion promotes atherogenesis in low density lipoprotein receptor -/- mice. Ann N Y Acad Sci. 1999;892:108–118 [DOI] [PubMed] [Google Scholar]







