Abstract
This study focuses on the participants of the Long Life Family Study to elucidate whether biogenetic mechanisms underlying relationships among heritable complex phenotypes in parents function in the same way for the same phenotypes in their children. Our results reveal 3 characteristic groups of relationships among phenotypes in parents and children. One group composed of 3 pairs of phenotypes confirms that associations among some phenotypes can be explained by the same biogenetic mechanisms working in parents and children. Two other groups including 9 phenotype pairs show that this is not a common rule. Our findings suggest that biogenetic mechanisms underlying relationships among different phenotypes, even if they are causally related, can function differently in successive generations or in different age groups of biologically related individuals. The results suggest that the role of aging-related processes in changing environment may be conceptually underestimated in current genetic association studies using genome wide resources.
Key Words: Heritability, Longevity regulation, Aging, Disease, Genetics of healthspan
POPULATION aging raises serious concerns about potential expansion of morbidity and disability especially in developed societies (1–3). Analyses of various geriatric traits and longevity show that these phenotypes tend to cluster in families (4,5) implying that they are heritable (6–9) and, thus, that they can be genetically regulated (10–12). If so, discovering genetic variants, which can be involved in regulation of health in late life and life span, could be a major breakthrough in addressing the problem of extending healthspan. Following this logic, genome-wide association studies have been thought to substantially advance discovering genes predisposing to complex phenotypes in late life, that is, phenotypes with pronounced expression in the postreproductive period. Heritability of such phenotypes drives genome-wide association studies strategies on discovering new genes and their replication in different population settings. The lack of replication is often considered as a false-positive finding.
The problem is that connections of genes with phenotypes in late life can be very complex and highly sensitive to the aging-related processes in a given environment (13). This complexity is largely due to the lack of a global genetic program similar to, for example, the program of development, which can directly drive selection of genes involved in regulation of health effects in late life (11,14). Most familiar among complex modalities of gene action is probably antagonistic pleiotropy (postulated by G. Williams [15,16]), wherein the same gene can be advantageous in early life (ie, during reproduction) but become detrimental in late life (13,16–19). Another example involves genes that can predispose to some diseases and protect against the others (20–23), exhibiting the so-called genetic trade-offs (20,24). These complexities may well explain the lack of replication of associations at the same variant with the same allele in different settings (25–28) that strengthen the role of aging-related processes in changing environment.
One way to deal with the complexity of gene action on heterogeneous phenotypes in late life is to focus on less heterogeneous intermediate phenotypes (called endophenotypes), which can be in a causative pathway to a phenotype in question (29,30). The idea is that if an endophenotype and a phenotype are causally related (eg, obesity and diabetes), then genes predisposing to an endophenotype (eg, obesity) can also predispose to a downstream phenotype (eg, diabetes) through a common network of molecular and biological processes at subcellular, cellular, tissue, and organismal levels; the network (called biogenetic mechanism) that links genes with phenotypic expression.
This idea implicitly assumes that if the traits in late life are heritable, a common biogenetic mechanism for them should work in the same way in successive generations. The problem is that the concept of heritability has been originally developed for a very narrow range of circumstances having nothing to do with human diseases and related traits in late life. This is the reason for criticizing this concept because differences between so-called genetic and environmental components in predisposition to aging-related diseases are elusive (31). For example, environment directly causes human health phenotype in very specific circumstances (eg, a car accident). Mostly, environment works through some sort of biogenetic mechanisms and, thus, this process eventually involves genes. Further, given that the original genome-wide association studies hypothesis of common disease—common variant becomes discouraging, an alternative hypothesis assumes that genes rather confer risks of complex common diseases in late life than cause them (32,33). This hypothesis is based on a principle of allelic equivalence, that is, that different alleles can cause the same disease. Accordingly, because heritability is evaluated using phenotypic markers, these estimates say little about whether the same genes can cause a phenotype in question in different family members.
Given the earlier posed concerns, in this study, we focus on the participants of the Long Life Family Study (LLFS) to address the question whether biogenetic mechanisms underlying relationships among different aging-related traits in parents function in the same way for the same traits in their children.
Methods
Long Life Family Study
The LLFS consists of families selected for exceptional familial longevity in four field centers (three in the United States, ie, Boston, New York, and Pittsburg and one in Denmark). The study eligibility criteria were described elsewhere (5,34,35). Briefly, in the United States, the family eligible for the LLFS must have two living siblings aged 80 years and older, two living offspring of one or more of the siblings, and a living spouse of one of the offspring, who were considered as controls. In addition, the family must demonstrate exceptional longevity based on a Family Longevity Selection Score, which is a summary measure based on the survival experience of the oldest living generation of siblings relative to what would be expected based on birth cohort life tables (5). Families with members of this generation who are still alive and larger sibships are given higher priorities. Finally, an eligible family is enrolled in the LLFS if at least three family members (the proband, at least one sibling of the proband, and one offspring of the proband or the sibling) indicate their willingness to participate.
In Denmark, the identification of potentially eligible probands and their families is as follows. First, individuals who would be aged 90 years and older during the study recruitment period are identified in the Danish National Register of Persons (34). Second, using information on the place of birth and the names, parish registers available in regional archives are searched to locate the parents of the elderly individuals in order to identify sibships. Based on the aforementioned information, potentially eligible families are identified and contact is made with potential probands to further assess the family’s eligibility for and willingness to participate in the LLFS using criteria parallel to that used in the United States.
Once enrolled in the LLFS, information from the U.S. and Danish participants was collected using similar questionnaires and in-home physical examinations, covering such topics as sociodemographic characteristics, physical activity and functioning, health and medical history, cognitive functioning, mood and personality, anthropometry, blood pressure, and spirometry. In this study, we use data on 4,954 participants who had completed the first examination by April 9, 2010.
Analyses
To address the goal of this work, we adopt the logic that if the biogenetic mechanisms connecting heritable aging-related traits are the same in parents and offspring, then we should observe significant cross-associations among traits measured in different family groups. Following this logic, we should assess (i) associations among parental trait in question and the same trait measured in their children, (ii) association of a given trait with alternative trait(s) in each family group, and (iii) association of a given trait measured in parents with alternative trait(s) measured in their children. Given elusive nature of difference between “genetic” and “environmental” contributions to human diseases in late life (see the introductory section), we evaluate heritability in terms of regression of a parental phenotype on a phenotype in their children (36) without specification of “genetic” and “environmental” contributions.
Because we evaluate associations among multiple traits measured in different family members, different models were used to ensure correct interpretation of the results. Specifically, because we want to know whether a trait in parents is associated with the same trait in their children, only the trait in question has being used in the analyses at Stage 1. At Stage 2, we evaluate associations among different traits in each family group separately. These analyses included multiple traits in question to correctly compare the effects of different trait predictors on a trait outcome. At Stage 3, we analyze the association of a trait outcome measured in children with traits of interest measured in the same children and their parents.
To perform these analyses, we stratified our sample according to family relatedness. The LLFS cohorts includes 590 extended families, which are made up of any related nuclear families, comprising a sample of N = 4,934 participants with currently known pedigree. This sample includes 1,500 long-living participants (probands and siblings), 191 spouses of probands and siblings, 2,433 offspring (composed of children, nieces, and nephews of probands), and 810 spouses of children. Due to limited number of spouses of probands and siblings making the analyses of both parents unfeasible, all spouses have been pooled in a single group of biologically unrelated individuals because there was no information to associate them with nuclear families.
We selected systolic (SBP) and diastolic (DBP) blood pressures (mm Hg), blood glucose (BG; mg/dL), body mass index (BMI; kg/m2), high-density lipoprotein cholesterol (HDL-C; mg/dL; directly measured), low-density lipoprotein cholesterol (LDL-C; mg/dL; calculated by the Friedewald equation), and triglycerides (TG; mg/dL) as quantitative phenotypes of health as well as type 2 diabetes and hypertension. Measurements of BG, HDL-C, TG, and SBP were log transformed in the regression models to correct for deviation from normality of their frequency distributions. Measurements of LDL-C and DBP were normally distributed, but they were divided by a factor of 10 for better visibility of the effect size in regression models. Although currently the LLFS data have no explicit information on medication use, diabetes and hypertension were defined conditional on such information at in-home examinations. Specifically, diabetes was defined as self-reported history of diabetes and taking hypoglycemic medication or glucose greater than or equal to 126mg/dL. Hypertension was defined as self-reported history of high blood pressure and taking antihypertensive medication or SBP greater than or equal to 140mm Hg or DBP greater than or equal to 90mm Hg. The majority of the sample (89.6%) was fasting before blood chemistry for more than 8 hours. The results of our analyses did not show any qualitative difference for the entire sample and the sample with participants fasting less than 8 hours excluded. Accordingly, all participants were retained, but the analyses were adjusted for fasting status.
For all analyses, we used a mixed effect regression model (SAS; release 9.3, Cary, NC) with the most general unstructured correlation matrix to account for correlation within extended families. This model was used for both quantitative and dichotomous traits to keep all estimates in consistent units because differences between logistic and linear models make no qualitative differences. The interpretation of the associations in the case of dichotomous traits is similar to that in the case of quantitative ones with coefficient beta considered as a probability of a dichotomous trait. All statistical tests were adjusted for age, sex, field center (ie, Boston, New York, Pittsburg, or Denmark), fasting status (<8 hours or not), and smoking (defined in the LLFS as ever smoked more than 100 cigarettes through the entire life or not).
Demographic and health information related to this study for the overall sample and for each family group is shown in Table 1.
Table 1.
Baseline Demographic and Health-Related Characteristics of the Long Life Family Study (LLFS) Participants
| Phenotypes | All (N * = 4,934) | Parents (N * = 1,500) | Offspring N * = 2,433) | Spouses (N * = 1,001) |
|---|---|---|---|---|
| Adjustments | ||||
| Age, y; mean (SD) | 70.6 (15.9) | 90.4 (6.4) | 60.5 (8.3) | 65.3 (12.2) |
| Sex, men; N (%) | 2,211 (44.8) | 710 (47.3) | 1,033 (42.5) | 468 (46.8) |
| BU center, N (%) | 1,272 (25.8) | 364 (24.3) | 643 (26.4) | 265 (26.5) |
| NY center, N (%) | 1,083 (21.9) | 466 (31.1) | 462 (19.0) | 155 (15.5) |
| PT center, N (%) | 1,310 (26.6) | 438 (29.2) | 701 (28.8) | 171 (17.1) |
| DK center, N (%) | 1,269 (25.7) | 232 (15.5) | 627 (25.3) | 410 (41.0) |
| Fasting <8hr, N (%) | 523 (10.8) | 184 (12.5) | 266 (11.2) | 73 (7.4) |
| Smoking >100 cigarettes/life (%) | 2,110 (42.9) | 560 (37.4) | 1,104 (45.6) | 446 (44.6) |
| Phenotypes used as predictors and outcomes | ||||
| Diabetes type 2, yes (%) | 346 (7.0) | 137 (9.1) | 134 (5.5) | 75 (7.5) |
| Hypertension, yes (%) | 2519 (51.1) | 988 (65.9) | 1030 (42.3) | 501 (50.0) |
| BMI, kg/m2; mean (SD) | 27.1 (4.9) | 26.0 (4.2) | 27.7 (5.3) | 27.4 (4.6) |
| BG, mg/dL; mean (SD) | 95.3 (20.7) | 96.0 (21.8) | 94.4 (20.0) | 96.7 (20.4) |
| HDL-C, mg/dL; mean (SD) | 58.8 (17.3) | 55.9 (16.1) | 60.5 (17.9) | 59.1 (16.8) |
| LDL-C†, mg/dL; mean (SD) | 118.5 (35.7) | 109.3 (35.8) | 122.4 (34.6) | 122.5 (35.9) |
| TG, mg/dL; mean (SD) | 113.3 (72.7) | 110.0 (59.0) | 115.0 (78.6) | 114.2 (72.7) |
| SBP, mm Hg; mean (SD) | 131.9 (22.3) | 138.3 (25.6) | 128.0 (19.5) | 131.4 (21.3) |
| DBP, mm Hg; mean (SD) | 77.2 (11.5) | 73.3 (12.0) | 79.0 (10.8) | 78.5 (11.1) |
Notes: Field centers were coded as BU = Boston, NY = New York, PT = Pittsburg, and DK = Denmark.
BMI = body mass index; BG = blood glucose; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; TG = triglycerides; SBP = systolic blood pressure; DBP = diastolic blood pressure; SD = standard deviation.
Percentage is within the entire sample (all) and family groups.
*Maximal sample size; actual size can be up to 6% smaller for certain factors due to missing values.
†LDL-C has been calculated by the Friedewald equation. For all participants, LDL-C was less than 300mg/dL.
Results
When parental phenotype is regressed on the same phenotype in their children (Stage 1, see Methods), Table 2 shows that the association for each selected phenotype is significant, implying that these phenotypes predominantly cluster in families, that is, that they are heritable (see the introductory section). These estimates qualitatively agree with the traditional estimates of the narrow-sense heritability (9). The most significant associations are seen for BMI and HDL-C. For example, the analyses show that the expected change in BMI in children for a one-unit change in BMI in parents is β = 0.259 with significance p = 3.3×10–13 (provided that all other covariates are fixed). Of note, Supplementary Table 1 ensures that excluding participants fasting less than 8 hours makes no difference.
Table 2.
Associations of Each Phenotype Measured in Parents With the Same Phenotype Measured in Their Children
| Phenotypes | N | β | SE | p Value |
|---|---|---|---|---|
| T2D | 1709 | 0.054 | 0.025 | 3.6E-02 |
| Htn | 1709 | 0.095 | 0.024 | 8.8E-05 |
| BMI | 1531 | 0.259 | 0.035 | 3.3E-13 |
| BG | 1569 | 0.127 | 0.027 | 2.3E-06 |
| HDL-C | 1578 | 0.199 | 0.027 | 2.6E-13 |
| LDL-C | 1556 | 0.097 | 0.026 | 2.6E-04 |
| TG | 1579 | 0.162 | 0.030 | 1.4E-07 |
| SBP | 1627 | 0.081 | 0.020 | 4.9E-05 |
| DBP | 1625 | 0.062 | 0.024 | 8.9E-03 |
Notes: T2D = type 2 diabetes; Htn = Hypertension; BMI = body mass index; BG = blood glucose; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; TG = triglycerides; SBP = systolic blood pressure; DBP = diastolic blood pressure; SE = standard error.
All analyses were adjusted for age, sex, field center, fasting status (less than 8h or not), and smoking (ever smoked more than 100 cigarettes through the entire life or not).
To characterize connections among different phenotypes in the same family groups, we selected phenotypes with associations among them documented in prior studies. Specifically, we evaluated associations of (i) BMI with quantitative components of metabolic syndrome (37), that is, BG, HDL-C, TG, and SBP; (ii) hypertension with four quantitative components of metabolic syndrome, that is, BMI, BG, HDL-C, and TG; and (iii) diabetes with cardiovascular physiological markers (38–40) including BMI, LDL-C, TG, SBP, and DBP. Multivariate analyses at Stage 2 with all selected phenotypes included in each model (see Methods) were performed for the entire sample, as well as for each family group (Table 3 and Supplementary Table 2).
Table 3.
Multivariate Associations of a Phenotype in Question (outcome) With Physiological Indices (predictors) in Parents, Their Children, and All Offspring
| Model | Phenotypes | Parents | Offspring* | Children† | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Predictors | β | SE | p Value | β | SE | p Value | β | SE | p Value | |
| Model 1 | BMI | N = 1,277 | N = 2,159 | N = 1,400 | |||||||
| BG | 7.40 | 1.41 | 2.0E-7 | 12.24 | 1.384 | 1.3E-17 | 11.88 | 1.74 | 2.6E-11 | ||
| HDL-C | −6.39 | 1.07 | 4.8E-9 | −10.12 | 0.959 | 5.1E-23 | −9.98 | 1.19 | 2.0E-15 | ||
| TG | 3.35 | 0.62 | 2.1E-7 | 3.76 | 0.502 | 9.0E-13 | 3.69 | 0.63 | 2.0E-8 | ||
| SBP | 3.90 | 1.42 | 7.6E-3 | 10.69 | 1.609 | 2.8E-10 | 11.86 | 2.04 | 5.0E-8 | ||
| Model 2 | Htn | N = 1,281 | N = 2,164 | N = 1,410 | |||||||
| BMI | 9.2E-3 | 3.4E-3 | 6.1E-3 | 1.6E-2 | 2.2E-3 | 4.5E-13 | 1.7E-2 | 2.7E-3 | 4.6E-10 | ||
| BG | 1.8E-1 | 1.7E-1 | 3.0E-1 | 4.6E-1 | 1.4E-1 | 1.7E-03 | 5.0E-1 | 1.8E-1 | 5.5E-3 | ||
| HDL-C | 1.5E-1 | 1.3E-1 | 2.5E-1 | 1.0E-1 | 1.0E-1 | 3.1E-01 | 1.4E-1 | 1.2E-1 | 2.6E-1 | ||
| TG | 8.8E-2 | 7.5E-2 | 2.4E-1 | 2.0E-1 | 5.2E-2 | 1.3E-04 | 2.4E-1 | 6.3E-2 | 2.3E-4 | ||
| Model 3 | T2D | N = 1,274 | N = 2,142 | N = 1,389 | |||||||
| BMI | 5.6E-3 | 2.0E-3 | 6.5E-3 | 6.9E-3 | 1.0E-3 | 5.4E-11 | 7.4E-3 | 1.2E-3 | 4.0E-9 | ||
| LDL-C | −1.1E-2 | 2.4E-3 | 6.2E-6 | −1.0E-2 | 1.4E-3 | 1.1E-11 | −8.7E-3 | 1.8E-3 | 1.5E-6 | ||
| TG | 1.3E-1 | 4.4E-2 | 2.8E-3 | 8.9E-2 | 2.3E-2 | 1.6E-04 | 9.7E-2 | 2.7E-2 | 5.0E-4 | ||
| SBP | 5.4E-3 | 1.3E-1 | 9.7E-1 | 4.3E-1 | 1.2E-1 | 3.4E-04 | 4.1E-1 | 1.5E-1 | 7.7E-3 | ||
| DBP | 1.3E-3 | 8.6E-3 | 8.8E-1 | −3.2E-2 | 6.9E-3 | 7.3E-06 | −3.2E-2 | 8.6E-3 | 4.1E-4 | ||
Notes: See notations and the models adjustments in Table 2.
*“Offspring” denotes children, nieces, and nephews of long-living individuals shown as “Parents.”
†“Children” denotes children of long-living individuals shown as “Parents.” Children with phenotypes presented for each model were excluded from the analyses if these phenotypes were not measured in their parents.
BMI is highly significantly associated with physiological markers (ie, BG, HDL-C, TG, and SBP) in multivariate analyses in the entire sample as well as in the samples of parents, offspring, children (see Table 3), and spouses (Table 3 and Supplementary Table 2; Model 1). These results show that individuals having higher levels of BG, TG, and SBP and lower levels of HDL-C are more likely to have higher BMI. The strength (ie, beta) of the associations of SBP, BG, and HDL-C with BMI is higher (in absolute units) in offspring than in parental generation. The effect sizes for the associations in offspring resemble those in children despite about 1/3 smaller sample size of children.
In the entire sample, hypertension is highly significantly associated with BMI and TG, whereas its associations with BG and HDL-C are much less significant (Supplementary Table 2; Model 2). In parents, the only significant association is seen for hypertension and BMI (Table 3; Model 2). The BMI, BG, and TG show significant additive contributions to hypertension in offspring and children (Table 3; Model 2). The effect sizes for these associations are virtually the same in offspring and children, and they are larger than in parents. In spouses, we can see significant association for BMI and TG (Supplementary Table 2; Model 2).
Diabetes is significantly associated with all physiological markers in the entire sample (Supplementary Table 2; Model 3). In parents (Table 3; Model 3), diabetes is significantly associated with BMI, TG, and LDL-C; no effect is seen for SBP and DBP. All physiological markers, including SBP and DBP, are associated with diabetes in offspring and children (Table 3; Model 3). The strengths of the associations of diabetes with BMI, LDL-C, and TG in parents resemble those in offspring and children. The analyses support associations of diabetes with BMI, LDL-C, TG, and DBP in spouses (Supplementary Table 2; Model 3).
Because each phenotype measured in parents is predictive of the same phenotype measured in their children (Table 2) and because different phenotypes can correlate in parents and children (Table 3), it can be expected that parental phenotypes can predict cross phenotypes in children (see Stage 3 in Methods). If so, this would suggest that biogenetic mechanisms underlying relationships among different traits in parents can function in the same way for the same traits in their children.
First, we consider the associations of the same phenotypes as in the model for BMI (Table 3; Model 1) but measured in parents, that is, BGP, HDL-CP, TGP, and SBPP (subscript “P” denotes phenotype measured in parents), with BMIC measured in their children (subscript “C” denotes phenotype measured in children). Table 4 (Models 1 and A, abbreviated as Model 1A) shows that HDL-CP and SBPP are associated with BMIC, whereas BGP and TGP do not. Including BMIP (Table 4; Model 1B) attenuates associations for the HDL-CP and SBPP because BMI is associated with HDL-C and SBP in parents (Table 3; Model 1, Parents). Nevertheless, BMIP does not absorb entirely the associations of HDL-CP and SBPP with BMIC implying contribution of another (not related to BMIP) mechanism(s). When we add phenotypes measured in children (ie, BGC, HDL-CC, TGC, and SBPC; Supplementary Table 3; Model C), they absorb association signals from the same parental phenotypes, as expected, because they are connected (Table 2). They do not, however, alter the association for BMIP. The estimates of the associations of BMIC with BGC, HDL-CC, TGC, and SBPC (Supplementary Table 3; Model C) resemble those in Table 3 (Model 1) for children. These results confirm presence of concurrent mechanisms for BMIC (ie, BMIP related and BMIP nonrelated).
Table 4.
Multivariate Associations of a Phenotype in Question Measured in Children (outcome) With Physiological Indices Measured in Their Parents (predictors)
| Model | Phenotypes | Model A | Model B | |||||
|---|---|---|---|---|---|---|---|---|
| Outcome | Predictors | β | SE | p Value | β | SE | p Value | |
| Model 1 | BMIC | BGP | −0.72 | 2.00 | 7.2E-1 | −2.32 | 2.06 | 2.6E-1 |
| HDL-CP | −4.05 | 1.33 | 2.6E-3 | −3.06 | 1.36 | 2.5E-2 | ||
| TGP | 0.75 | 0.77 | 3.4E-1 | −0.16 | 0.79 | 8.4E-1 | ||
| SBPP | 5.37 | 1.84 | 7.0E-3 | 3.66 | 1.89 | 6.5E-2 | ||
| BMIP | N/I | N/I | N/I | 0.24 | 0.04 | 8.8E-5 | ||
| Model 2 | HtnC | BMIP | 7.7E-3 | 3.0E-3 | 1.1E-2 | 7.1E-3 | 3.0E-3 | 1.9E-2 |
| BGP | 2.2E-2 | 1.8E-1 | 9.0E-1 | −8.5E-4 | 1.8E-1 | 1.0E+0 | ||
| HDL-CP | 3.9E-2 | 1.2E-1 | 7.4E-1 | 3.1E-2 | 1.1E-1 | 7.9E-1 | ||
| TGP | 7.6E-2 | 6.8E-2 | 2.7E-1 | 5.9E-2 | 6.7E-2 | 3.9E-1 | ||
| HtnP | N/I | N/I | N/I | 8.1E-2 | 2.6E-2 | 7.6E-3 | ||
| Model 3 | T2DC | BMIP | 2.1E-3 | 1.4E-3 | 1.3E-1 | 1.8E-3 | 1.4E-3 | 1.8E-1 |
| LDL-CP | −2.5E-3 | 1.8E-3 | 1.6E-1 | −1.6E-3 | 1.8E-3 | 3.6E-1 | ||
| TGP | 3.6E-2 | 3.2E-2 | 2.6E-1 | 3.5E-2 | 3.1E-2 | 2.7E-1 | ||
| SBPP | 4.9E-2 | 9.6E-2 | 6.1E-1 | 4.0E-2 | 9.6E-2 | 6.8E-1 | ||
| DBPP | −2.2E-3 | 6.3E-3 | 7.4E-1 | −3.0E-3 | 6.3E-3 | 6.5E-1 | ||
| T2DP | N/I | N/I | N/I | 5.8E-2 | 2.8E-2 | 1.3E-1 | ||
Notes: Subscript “P” means parents and subscript “C” means their children.
N/I = not included in the model; other notations and the models adjustments are in Table 2.
The sample size for all models is about N = 1,539 with the exception of N = 1,459 for Model 1B.
Table 4 (Model 2A) shows that parental traits BGP, HDL-CP, and TGP do not contribute to hypertension in their children, whereas the BMIP does. Parental hypertension is a significant predictor of hypertension in their children but its contribution is BMIP independent (Table 4; Model 2B). The effect of BMIP is largely attributed to direct inheritance of this phenotype (Supplementary Table 4; Model C and Table 1). The estimates of the associations of BMIC, BGC, HDL-CC, and TGC with hypertension in parents (Supplementary Table 4; Model D) resemble those in Table 3 (Model 2) implying independent contribution of parental hypertension.
Despite the association of BMI, LDL-C, and TG with diabetes in parents (Table 3; Model 3), these parental traits do not contribute to diabetes in children (Table 4; Model 3A). Neither diabetes in parents is a significant predictor of diabetes in their children (Table 4; Model 3B). Strength of the associations of BMIC, LDL-CC, TGC, SBPC, and DBPC with diabetes in children (Supplementary Table 4; Model 3B) is the same as in Table 3 for children.
Discussion and Conclusions
Numerous studies of heritability demonstrate that aging-related diseases, health traits, life span, biological age, and physical functioning can have genetic origin (41,42). For example, studies show substantial heritability of blood pressure (43), pulmonary function (44), BG levels (45), diabetes (46), bone degeneration (47), cognitive health (48) including Alzheimer’s disease (49), muscle strength (50), walking speed (51), and so on. Analysis of the narrow-sense heritability of diseases and related traits in the LLFS (9) shows that such phenotypes are heritable as well; the results of our analyses presented in Table 2 are in agreement with the estimates by Matteini and colleagues (9).
The traditional heritability estimates are particularly useful in the case when phenotypes are controlled by a small number of genes. Then, heritability analysis can suggest existence of genetic mutations causing such a phenotype (eg, as in the case of autosomal dominant form of Alzheimer’s disease; [52]). However, in majority of cases of various common aging-related phenotypes (eg, the late-onset Alzheimer’s disease), genes rather confer risks than cause them (32,33). Then, traditional estimates of heritability can be misleading (53) because different alleles can cause the same common phenotype in different environments (eg, in different birth cohorts or human generations) and/or chronological ages. The results of our analyses illustrate this logic.
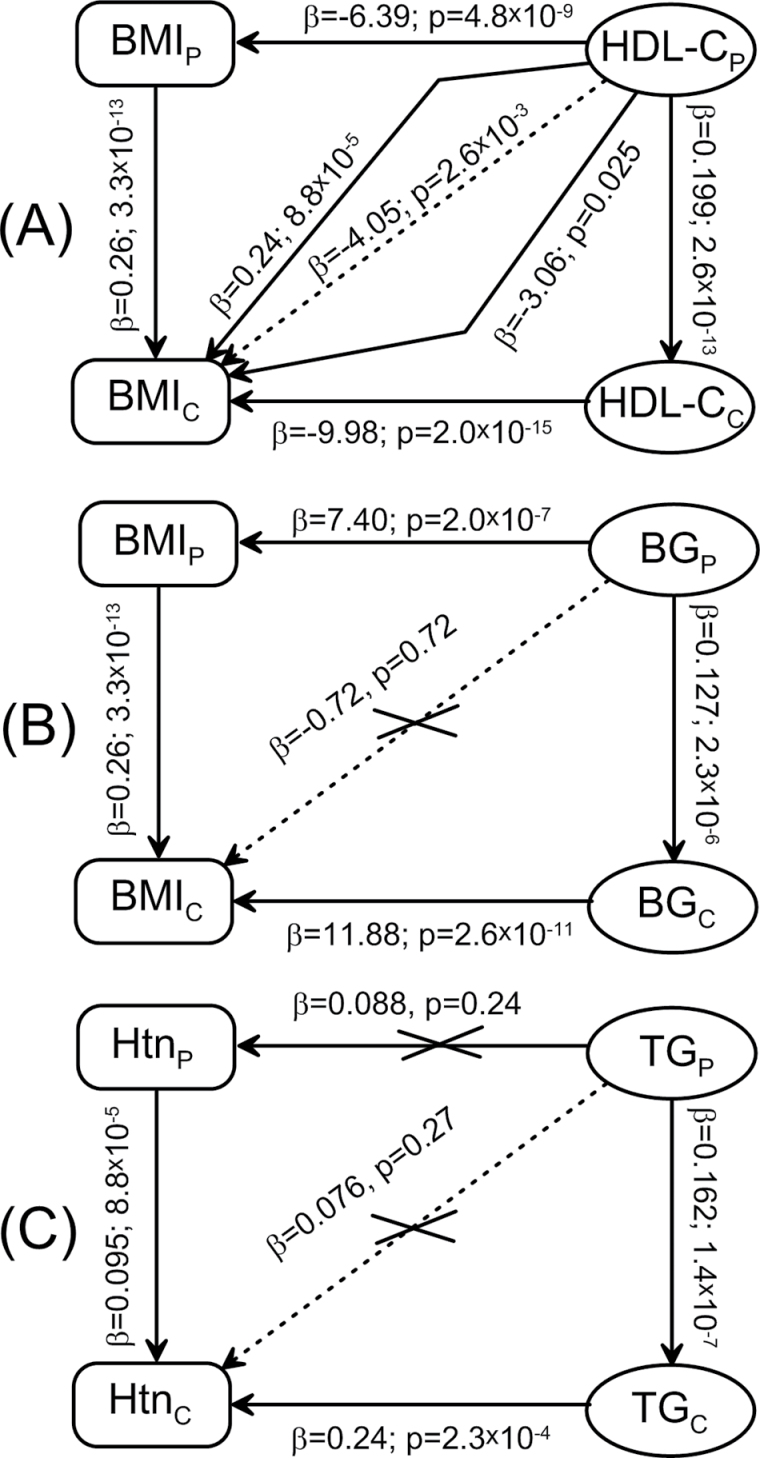
Specifically, our analyses reveal three characteristic groups of relationships among the same 12 pairs of traits in the parental and offspring generations of the participants of the LLFS, which are illustrated in Figure 1.
Figure 1.
Summary of associations among selected phenotypes representing three qualitatively different patterns of relationships. (A) Associations among body mass index (BMI) and high-density lipoprotein cholesterol (HDL-C) measured in parents (subscript “P”) and their children (subscript “C”). Other two phenotype pairs from this group are BMI and systolic blood pressure (SBP) and hypertension (Htn) and BMI. (B) Associations among BMI and blood glucose (BG) measured in parents and their children. Other four phenotype pairs from this group are BMI and triglycerides (TG), diabetes and BMI, diabetes and low-density lipoprotein cholesterol (LDL-C), and diabetes and TG. (C) Associations among Htn and TG measured in parents and their children. Other three phenotype pairs from this group are Htn and BG, diabetes and SBP, and diabetes and diastolic blood pressure (DBP). Arrowheads pinpoint an outcome. Vertical (horizontal) arrows denote associations from Table 2 (Table 3). Inclined arrows denote associations from Table 4: dashed (Model A) and solid (Model B) lines.
The first group (Figure 1A) illustrates common expectation that if associations among different traits are observed in parents and their children and if these traits are heritable, then it is likely that the biogenetic mechanisms (originated at specific genes) underlying relationships between such traits are the same in parents and children, for example, the same genes confer risks of the same traits in parents and children. If this is indeed the case, then phenotypes measured in parents should be predictive of cross phenotypes measured in their children (Figure 1A). Our analyses show that such cross-associations between traits can indeed exist and they can be a superposition of complementary mechanisms. For example, Figure 1A illustrates that associations between HDL-CP and BMIC are explained by the HDL-CP–BMIP–BMIC and the HDL-CP–HDL-CC–BMIC pathways. Importantly, analysis of other two pairs of cross-associations from this group (ie, BMI–SBP and hypertension–BMI) shows that the strength and significance of the associations among different traits in each generation and between the same traits in different generations play at most a minor role in explaining the strength of cross-associations and preferred pathways of inheritance.
Figure 1B illustrates the second group (composed of five pairs of traits listed in Figure 1) with a counter-intuitive finding that despite associations among different traits (even causally related) and despite their heritability, there are no cross-trait associations. This means that genes underlying biogenetic mechanisms connecting health traits in parents either can be not transmitted to or do not play the same role in their children. Accordingly, connections among the parental traits in children can be regulated by different biogenetic mechanism(s). This is an important finding implying that biogenetic mechanisms underlying relationships among different traits in parents may not function in the same way for the same traits in their children.
The third characteristic group composed of four pairs of traits is illustrated in Figure 1C. These findings show that even if the associations among health traits are lacking in parents, this does not necessarily mean the lack of these associations in their children. Then, it is clear that in this case, the lack of cross-associations between traits in parents and their children implies that genetic variants inherited by children from parents are not the risk variants for given traits for those children.
Therefore, in general, we may not expect that genetic effects can be the same even in successive generations or in different age groups of biologically related individuals. Indeed, our observations can be explained by two factors assuming that the parental risk alleles are transmitted to progeny.
The first is that connections among different health traits, even if they are causally related, can be under strong environmental pressure, which can dramatically change the risk genetic profiles even in the same populations of successive generations. From the evolutionary point of view, this may be because the human genome was established to provide better robustness under adverse environmental conditions, which are markedly distinct from modern conditions especially in developed populations (54). For example, evolutional strategy worked to conserve genes (eg, the insulin and insulin-like signaling) when the periods of high and low food supply were irregular. This means that selective advantage was better for individuals who could store energy in the period with high food supply and effectively use it during starvation. In modern conditions, when food is abundant and sedentary lifestyle is prevalent, this strategy can lead to increased incidence of, for example, storage diseases such as diabetes and obesity (55,56) associated with new roles for even the same allelic variants in new environment (so-called thrifty genes hypothesis [57]).
The second is that genetic variants can change their role with age (58–62). This explanation is in line with the antagonistic pleiotropy hypothesis (15) and supported by recent findings (16–19). Similarly, it is in line with the mutation accumulation hypothesis (63) and the maintenance hypothesis of aging (64). This explanation is, however, less realistic and it is not immediately obvious.
Specifically, detrimental genetic variants transmitted from parents to children (say alleles of group “Old”) may not work in children because of their younger ages. These variants can show, however, detrimental effect late in life when either strength of mechanisms counterbalancing negative effect of these genes declines (eg, buffering genes [65]) and/or such genes change their expression with age (eg, genetic underpinnings of secondary hypertension can be silent until old age). Although at a first glance, this explanation looks plausible, it is against the observations that the strength of the associations (beta) in children is either more pronounced (in absolute units) or of the same magnitude than that in their parents for all traits (Table 3). Note also that this strength is virtually not sensitive to the sample size of offspring (Table 3). However, according to the aforementioned hypotheses, the strength of the associations should show opposite trends, that is, it should increase with advanced age and, therefore, should be larger in parents than in offspring.
Because offspring are younger than individuals from the parental generation (see Table 1), it might be argued that the observed phenomena could be due to survival selection of the most robust parents. In this case, we have to assume that all offspring carrying detrimental alleles working at younger ages (say alleles of group “Young”) have to die before they can become as old as their parents in order to explain potential decline in the effect sizes for the remaining survivors as they are getting older. This is, however, unlikely a realistic scenario.
Finally, both mechanisms, that is, selective survival and change of the role of genes with age, can work together. In this case, some individuals carrying detrimental alleles of group “Young” can die, whereas the other alleles from this group in survivors may lose their detrimental role at advanced ages. Instead, however, the alleles of group “Old” (ie, those which are characteristic for the parents) will start to work at those ages.
Thus, our results indicate that complex, aging-related traits are unlikely to be genetically modulated in a straightforward fashion (66–68). The role of aging-related processes in changing environment may be underestimated in currently prevailing genetic association studies. As a consequence, existing strategies for technical replication of genetic associations may be a major limiting factor in accelerating progress in understanding biogenetic mechanisms driving healthspan. Future progress will require the collaborative multidisciplinary and interdisciplinary input of specialists from diverse disciplines, including biogenetics, evolutionary biology, epidemiology, biodemography, and aging.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
The Long Life Family Study is funded by U01AG023749, U01AG023744, and U01AG023712 from the National Institute on Aging . The analyses were supported by 2U01AG023712.
Conflict of interest
The authors declare no conflict of interest.
Supplementary Material
Acknowledgments
A.M. Kulminski contributed to the study conception, design, statistical analysis, interpretation of the results, and writing the manuscript. K.G. Arbeev contributed to statistical analyses. K. Christensen, I. Miljkovic, and M. Barmada contributed to discussion of the results and to writing the final version. E. Stallard contributed to assessing the logic of the analysis and to writing the final version. A.I. Yashin contributed to discussion and interpretation of the results and to drafting the manuscript.
References
- 1. Sierra F, Hadley E, Suzman R, Hodes R. Prospects for life span extension. Annu Rev Med. 2009; 60: 457–469 [DOI] [PubMed] [Google Scholar]
- 2. Olshansky SJ, Perry D, Miller RA, Butler RN. Pursuing the longevity dividend: scientific goals for an aging world. Ann N Y Acad Sci. 2007; 1114: 11–13 [DOI] [PubMed] [Google Scholar]
- 3. Robine J-M. Determining health expectancies. Chichester, West Sussex, UK; Hoboken, NJ: Wiley; 2003. [Google Scholar]
- 4. Cournil A, Kirkwood TB. If you would live long, choose your parents well. Trends Genet. 2001; 17: 233–235 [DOI] [PubMed] [Google Scholar]
- 5. Sebastiani P, Hadley EC, Province M, et al. A family longevity selection score: ranking sibships by their longevity, size, and availability for study. Am J Epidemiol. 2009; 170: 1555–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown WM, Beck SR, Lange EM, et al. Age-stratified heritability estimation in the Framingham Heart Study families. BMC Genet. 2003; 4 Suppl 1: S32 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Herskind AM, McGue M, Holm NV, Sørensen TI, Harvald B, Vaupel JW. The heritability of human longevity: a population-based study of 2872 Danish twin pairs born 1870-1900. Hum Genet. 1996; 97: 319–323 [DOI] [PubMed] [Google Scholar]
- 8. Iachine IA, Holm NV, Harris JR, et al. How heritable is individual susceptibility to death? The results of an analysis of survival data on Danish, Swedish and Finnish twins. Twin Res. 1998; 1: 196–205 [DOI] [PubMed] [Google Scholar]
- 9. Matteini AM, Fallin MD, Kammerer CM, et al. Heritability estimates of endophenotypes of long and health life: the Long Life Family Study. J Gerontol A Biol Sci Med Sci. 2010; 65: 1375–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martin GM, Bergman A, Barzilai N. Genetic determinants of human health span and life span: progress and new opportunities. PLoS Genet. 2007; 3: e125 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vijg J, Suh Y. Genetics of longevity and aging. Annu Rev Med. 2005; 56: 193–212 [DOI] [PubMed] [Google Scholar]
- 12. Finch CE, Tanzi RE. Genetics of aging. Science. 1997; 278: 407–411 [DOI] [PubMed] [Google Scholar]
- 13. Martin GM. Modalities of gene action predicted by the classical evolutionary biological theory of aging. Ann N Y Acad Sci. 2007; 1100: 14–20 [DOI] [PubMed] [Google Scholar]
- 14. Di Rienzo A, Hudson RR. An evolutionary framework for common diseases: the ancestral-susceptibility model. Trends Genet. 2005; 21: 596–601 [DOI] [PubMed] [Google Scholar]
- 15. Williams GC. Pleiotropy, natural-selection, and the evolution of senescence Evolution. 1957; 11: 398–411 [Google Scholar]
- 16. Williams PD, Day T. Antagonistic pleiotropy, mortality source interactions, and the evolutionary theory of senescence. Evolution. 2003; 57: 1478–1488 [DOI] [PubMed] [Google Scholar]
- 17. Kulminski AM, Culminskaya I, Ukraintseva SV, Arbeev KG, Land KC, Yashin AI. Beta2-adrenergic receptor gene polymorphisms as systemic determinants of healthy aging in an evolutionary context. Mech Ageing Dev. 2010; 131: 338–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Summers K, Crespi BJ. Xmrks the spot: life history tradeoffs, sexual selection and the evolutionary ecology of oncogenesis. Mol Ecol. 2010; 19: 3022–3024 [DOI] [PubMed] [Google Scholar]
- 19. Alexander DM, Williams LM, Gatt JM, et al. The contribution of apolipoprotein E alleles on cognitive performance and dynamic neural activity over six decades. Biol Psychol. 2007; 75: 229–238 [DOI] [PubMed] [Google Scholar]
- 20. Kulminski AM, Culminskaya I, Ukraintseva SV, et al. Trade-off in the effects of the apolipoprotein E polymorphism on the ages at onset of CVD and cancer influences human lifespan. Aging Cell. 2011; 10: 533–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Finch CE. Evolution in health and medicine Sackler colloquium: Evolution of the human lifespan and diseases of aging: roles of infection, inflammation, and nutrition. Proc Natl Acad Sci USA. 2010; 107 Suppl 1: 1718–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Charlesworth B. Evolution of senescence: Alzheimer’s disease and evolution. Curr Biol. 1996; 6: 20–22 [DOI] [PubMed] [Google Scholar]
- 23. Martin GM. APOE alleles and lipophylic pathogens. Neurobiol Aging. 1999; 20: 441–443 [DOI] [PubMed] [Google Scholar]
- 24. Kulminski A, Culminskaya I, Arbeev K, Ukraintseva S, Arbeeva L, Yashin AI. Trade-off in the effect of the apoe gene on the ages at onset of cvd and cancer across ages, gender, and human generations. Rejuvenation Res. 2012; October 25. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Murk W, Walsh K, Hsu LI, Zhao L, Bracken MB, Dewan AT. Attempted replication of 50 reported asthma risk genes identifies a SNP in RAD50 as associated with childhood atopic asthma. Hum Hered. 2011; 71: 97–105 [DOI] [PubMed] [Google Scholar]
- 26. Assimes TL, Hólm H, Kathiresan S, et al. Lack of association between the Trp719Arg polymorphism in kinesin-like protein-6 and coronary artery disease in 19 case-control studies. J Am Coll Cardiol. 2010; 56: 1552–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dahabreh IJ, Murray S. Lack of replication for the association between HER2 I655V polymorphism and breast cancer risk: a systematic review and meta-analysis. Cancer Epidemiol. 2011; 35: 503–509 [DOI] [PubMed] [Google Scholar]
- 28. Day-Williams AG, Zeggini E. The effect of next-generation sequencing technology on complex trait research. Eur J Clin Invest. 2011; 41: 561–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arking DE, Chakravarti A. Understanding cardiovascular disease through the lens of genome-wide association studies. Trends Genet. 2009; 25: 387–394 [DOI] [PubMed] [Google Scholar]
- 30. Bloss CS, Pawlikowska L, Schork NJ. Contemporary human genetic strategies in aging research. Ageing Res Rev. 2011; 10: 191–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rose SP. Commentary: heritability estimates–long past their sell-by date. Int J Epidemiol. 2006; 35: 525–527 [DOI] [PubMed] [Google Scholar]
- 32. Plomin R, Haworth CM, Davis OS. Common disorders are quantitative traits. Nat Rev Genet. 2009; 10: 872–878 [DOI] [PubMed] [Google Scholar]
- 33. Fisher RA. The correlation between relatives on the supposition of mendelian inheritance Transact Royal Soc Edinburgh. 1918; 52: 399–433 [Google Scholar]
- 34. Pedersen CB, Gøtzsche H, Møller JO, Mortensen PB. The Danish Civil Registration System. A cohort of eight million persons. Dan Med Bull. 2006; 53: 441–449 [PubMed] [Google Scholar]
- 35. Yashin AI, Arbeev KG, Kulminski A, et al. “Predicting” parental longevity from offspring endophenotypes: data from the Long Life Family Study (LLFS). Mech Ageing Dev. 2010; 131: 215–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pierce BA. Transmission and population genetics. 3rd ed New York; 2009. New York: W. H. Freeman [Google Scholar]
- 37. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002; 287: 356–359 [DOI] [PubMed] [Google Scholar]
- 38. Fox CS. Cardiovascular disease risk factors, type 2 diabetes mellitus, and the Framingham Heart Study. Trends Cardiovasc Med. 2010; 20: 90–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Preis SR, Pencina MJ, Hwang SJ, et al. Trends in cardiovascular disease risk factors in individuals with and without diabetes mellitus in the Framingham Heart Study. Circulation. 2009; 120: 212–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wilson PW Meigs JB Sullivan L Fox CS Nathan DM D’Agostino RBSr. Prediction of incident diabetes mellitus in middle-aged adults: the Framingham Offspring Study. Arch Intern Med. 2007; 167: 1068–1074 [DOI] [PubMed] [Google Scholar]
- 41. Melzer D, Hurst AJ, Frayling T. Genetic variation and human aging: progress and prospects. J Gerontol A Biol Sci Med Sci. 2007; 62: 301–307 [DOI] [PubMed] [Google Scholar]
- 42. Pilling LC, Harries LW, Powell J, Llewellyn DJ, Ferrucci L, Melzer D. Genomics and successful aging: grounds for renewed optimism? J Gerontol A Biol Sci Med Sci. 2012; 67: 511–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kraft P, Bauman L, Yuan JY, Horvath S. Multivariate variance-components analysis of longitudinal blood pressure measurements from the Framingham Heart Study. BMC Genet. 2003; 4 Suppl 1: S55 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wilk JB, DeStefano AL, Joost O, et al. Linkage and association with pulmonary function measures on chromosome 6q27 in the Framingham Heart Study. Hum Mol Genet. 2003; 12: 2745–2751 [DOI] [PubMed] [Google Scholar]
- 45. Mathias RA, Roy-Gagnon MH, Justice CM, et al. Comparison of year-of-exam- and age-matched estimates of heritability in the Framingham Heart Study data. BMC Genet. 2003; 4 Suppl 1: S36 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wienke A, Herskind AM, Christensen K, Skytthe A, Yashin AI. The heritability of CHD mortality in danish twins after controlling for smoking and BMI. Twin Res Hum Genet. 2005; 8: 53–59 [DOI] [PubMed] [Google Scholar]
- 47. Karasik D, Demissie S, Cupples LA, Kiel DP. Disentangling the genetic determinants of human aging: biological age as an alternative to the use of survival measures. J Gerontol A Biol Sci Med Sci. 2005; 60: 574–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Greenwood PM, Parasuraman R. Normal genetic variation, cognition, and aging. Behav Cogn Neurosci Rev. 2003; 2: 278–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gatz M, Pedersen NL, Berg S, et al. Heritability for Alzheimer’s disease: the study of dementia in Swedish twins. J Gerontol A Biol Sci Med Sci. 1997; 52: M117–M125 [DOI] [PubMed] [Google Scholar]
- 50. Tiainen K, Sipilä S, Alen M, et al. Heritability of maximal isometric muscle strength in older female twins. J Appl Physiol. 2004; 96: 173–180 [DOI] [PubMed] [Google Scholar]
- 51. Carmelli D, Kelly-Hayes M, Wolf PA, et al. The contribution of genetic influences to measures of lower-extremity function in older male twins. J Gerontol A Biol Sci Med Sci. 2000; 55: B49–B53 [DOI] [PubMed] [Google Scholar]
- 52. Liddell MB, Lovestone S, Owen MJ. Genetic risk of Alzheimer’s disease: advising relatives. Br J Psychiatry. 2001; 178: 7–11 [DOI] [PubMed] [Google Scholar]
- 53. Lewontin RC. Annotation: the analysis of variance and the analysis of causes. Am J Hum Genet. 1974; 26: 400–411 [PMC free article] [PubMed] [Google Scholar]
- 54. Kuningas M, Mooijaart SP, van Heemst D, Zwaan BJ, Slagboom PE, Westendorp RG. Genes encoding longevity: from model organisms to humans. Aging Cell. 2008; 7: 270–280 [DOI] [PubMed] [Google Scholar]
- 55. Neel JV, Weder AB, Julius S. Type II diabetes, essential hypertension, and obesity as “syndromes of impaired genetic homeostasis”: the “thrifty genotype” hypothesis enters the 21st century. Perspect Biol Med. 1998; 42: 44–74 [DOI] [PubMed] [Google Scholar]
- 56. Fox CS, Pencina MJ, Meigs JB, Vasan RS, Levitzky YS, D’Agostino RB., Sr. Trends in the incidence of type 2 diabetes mellitus from the 1970s to the 1990s: the Framingham Heart Study. Circulation. 2006; 113: 2914–2918 [DOI] [PubMed] [Google Scholar]
- 57. Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet. 1962; 14: 353–362 [PMC free article] [PubMed] [Google Scholar]
- 58. Shi G, Gu CC, Kraja AT, et al. Genetic effect on blood pressure is modulated by age: the Hypertension Genetic Epidemiology Network Study. Hypertension. 2009; 53: 35–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. De Benedictis G, Carotenuto L, Carrieri G, et al. Age-related changes of the 3’APOB-VNTR genotype pool in ageing cohorts. Ann Hum Genet. 1998; 62:(Pt 2):115–122 [DOI] [PubMed] [Google Scholar]
- 60. Ilveskoski E, Perola M, Lehtimäki T, et al. Age-dependent association of apolipoprotein E genotype with coronary and aortic atherosclerosis in middle-aged men: an autopsy study. Circulation. 1999; 100: 608–613 [DOI] [PubMed] [Google Scholar]
- 61. Yashin AI, Ukraintseva SV, De Benedictis G, et al. Have the oldest old adults ever been frail in the past? A hypothesis that explains modern trends in survival. J Gerontol A Biol Sci Med Sci. 2001; 56: B432–B442 [DOI] [PubMed] [Google Scholar]
- 62. Jarvik GP, Goode EL, Austin MA, et al. Evidence that the apolipoprotein E-genotype effects on lipid levels can change with age in males: a longitudinal analysis. Am J Hum Genet. 1997; 61: 171–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Medawar PB. An unsolved problem of biology: an inaugural lecture delivered at University College, London, 6 December, 1951. London, UK: H.K. Lewis and Co.; 1952. [Google Scholar]
- 64. Holliday R. Aging is no longer an unsolved problem in biology. Ann N Y Acad Sci. 2006; 1067: 1–9 [DOI] [PubMed] [Google Scholar]
- 65. Bergman A, Atzmon G, Ye K, MacCarthy T, Barzilai N. Buffering mechanisms in aging: a systems approach toward uncovering the genetic component of aging. PLoS Comput Biol. 2007; 3: e170 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kirkwood TB. Systems biology of ageing and longevity. Philos Trans R Soc Lond, B, Biol Sci. 2011; 366: 64–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Goh KI, Cusick ME, Valle D, Childs B, Vidal M, Barabási AL. The human disease network. Proc Natl Acad Sci USA. 2007; 104: 8685–8690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kirkwood TB, Cordell HJ, Finch CE. Speed-bumps ahead for the genetics of later-life diseases. Trends Genet. 2011; 27: 387–388 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.