Abstract
Oxidative stress increases with age and is postulated to be a major causal factor for sarcopenia in aging. Here, we examined whether the administration of a cystine-based antioxidant (F1) can alleviate/delay age-specific changes in skeletal muscles. C57BL6 male mice aged 17 months (middle aged) were fed with normal diet with or without supplementation of F1 (3mg/kg food) for 6 months. Compared with young (5 months old) mice old mice exhibited increased markers of oxidative stress, inflammation, and muscle cell apoptosis and decreased muscle weight. These age-related changes were further associated with inactivation of adenosine-5′-monophosphate–activated protein kinase (AMPK), increased lipogenesis, activation of c-Jun NH2-terminal kinase, and decreased expression of Delta 1, phospho-Akt, and proliferating cell nuclear antigen in aged skeletal muscle. Such alterations were significantly prevented by F1. These results demonstrate the beneficial effects of F1 to attenuate loss of muscle mass associated with aging.
Key Words: Antioxidant, Mice, Muscle cell apoptosis, Oxidative stress, Sarcopenia.
THE aging population is increasing dramatically throughout the world. Between 2000 and 2010, the population age 65 years and over increased at a faster rate (15.1%) than the total U.S. population (9.7%) (1). The rate of muscle loss is estimated to be ~1%–2% per year after the age of 50 years.Muscle loss can affect even healthy physically active adults (2). About 25% of people under the age of 70 years and approximately 40% of those who have reached the age of 80 years are clinically sarcopenic (3,4). Such age-related loss of muscle mass has a profound effect on the elderly population, manifested by decreased healing after injury, impaired physical function, and increased risk of falls, fractures, dependency, and death (5). The socioeconomic costs of these sequelae and the aging epidemic have underscored an urgent need for effective therapies.
Mechanisms that regulate age-related loss of skeletal muscle mass are not well defined, but the pathogenesis is likely multifactorial. With age, like many other tissues, there is a gradual decline of regenerative potential in skeletal muscle. This may in large part be due to a decline in Notch signaling, which is essential for activation, proliferation, and myogenic progression of satellite cells (6,7). Increased muscle cell death (apoptosis), like the decline in regenerative potential of satellite cells, also contributes to aging-associated sarcopenia (8–10). Thus, a combined approach targeting both diminished satellite cell regenerative potential and increased muscle cell apoptosis may present a framework for therapeutic intervention of aging-associated sarcopenia.
The amount of oxidative stress increases as an organism ages and is postulated to be a major causal factor of senescence (11). It also contributes to sarcopenia associated with aging (8–10,12). Thus, mitigating oxidative stress would be an important target in preventing sarcopenia in aging. The effect of antioxidants in delaying, preventing, or reversing age-associated sarcopenia is not well understood and is an active area of investigation (13–16). In vascular smooth muscle cells, we previously demonstrated that F1, a cystine-based glutathione (GSH) precursor, is more effective than GSH or N-acetylcysteine in preventing spermine (a uremic toxin)-induced oxidative stress, impairment of glucose metabolism, and apoptosis (17,18). We further demonstrated that this antioxidant is equally effective in preventing oxidative stress and hepatic steatosis in diet-induced obese mice (19).
The objective of this study was to determine whether dietary supplementation of F1 to middle-aged mice for 6 months would delay or prevent loss of muscle mass during aging, and if so to define the molecular mechanisms.
Methods
Antioxidant
F1 (FT061452/RE39734), a GSH precursor with cystine replacing cysteine and selenomethionine added, was obtained from Proimmune, Rhinebeck (20). The formulation (per 500mg) contains L-cystine (99.68mg), glycine (199.39mg), selenomethionine (1.54mg), and L-glutamine (199.39mg). This compound is tasteless and odorless.
Animals and Experimental Protocol
C57BL6J mice at various age groups were obtained from Harlan Laboratories (Indianapolis, IN). Mice were housed in a standard animal facility under controlled temperature (22°C) and photoperiod (12 hours of light, 12 hours of darkness) with food and water ad libitum. To investigate the molecular mechanisms by which F1 prevents aging-associated sarcopenia, groups of 10 middle-aged mice were fed either on a control diet of mouse chow or on an identical diet supplemented with F1 (3mg/kg food) for 6 months. Both diets were custom made and given as pellets (Research Diets, New Brunswick, NJ). Antioxidant dose is based on the results of our recent study, which showed that F1 at this dose levels significantly attenuates oxidative stress, hepatocellular apoptosis, and hepatic steatosis in diet-induced obese mice (19). Body weight and food intake were measured weekly. Food intake was measured per cage with three mice per cage to avoid the stress of individual housing (19). Six months after feeding with either control or F1-supplemented diet, when the mice in each group were of 23 months of age, they were euthanized with a lethal ip injection of sodium pentobarbital (200mg/kg body weight). Young mice aged 5 months were used as controls (N = 10).
Mice were fasted overnight before euthanasia. At autopsy, gastrocnemius muscles in each group were quickly removed and weighed. Portions of the tissues were frozen in liquid nitrogen and stored frozen for subsequent analysis by Western blotting and Enzyme Immunometric Assay. Additional portions were fixed in 4% paraformaldehyde for histological and immunohistochemical evaluation. The rationale for using gastrocnemius muscles was based on the results of several earlier studies, which show that this muscle exhibits a substantial decline in mass with age (8,9,12). Animal handling and experimentation were in accordance with the recommendation of the American Veterinary Medical Association and were approved by the Charles R. Drew University School of Medicine and Science Animal Care and Use Review Committee.
Muscle Fiber Cross-Sectional Area
Muscle fiber cross-sectional area (CSA) was determined in 5-µm paraffin-embedded and hematoxylin and eosin–stained sections of gastrocnemius muscles using the ImagePro Plus, version 5.1 software (Media Cybernetics, Silver Spring, MD) coupled to an Olympus BHS microscope equipped with a VCC video camera (8,9). For each animal, at least 100 fibers were measured.
Assessment of Apoptosis
In situ detection of cells with DNA strand breaks was performed in paraformaldehyde-fixed, paraffin-embedded muscle sections by the terminal deoxynucleotidyltransferase-mediated deoxyuridine triphosphate nick end labeling technique (8,9,21), using an ApopTag-peroxidase kit (Chemicon International, Inc., San Francisco, CA). Enumeration of terminal deoxynucleotidyltransferase-mediated deoxyuridine triphosphate nick end labeling-positive nuclei was carried out using an American Optical Microscope with a ×40 objective and a pair of ×10 eyepieces. A square grid fitted within one eyepiece provided a reference of 62,500 µm2. The rate of muscle cell apoptosis was expressed as the percentage of the terminal deoxynucleotidyltransferase-mediated deoxyuridine triphosphate nick end labeling-positive apoptotic nuclei per total nuclei (apoptotic plus nonapoptotic) present within the reference area.
Immunohistochemical Analysis
Paraformaldehyde-fixed, paraffin-embedded muscle sections were immunostained as described previously (8,19,21). Primary antibody included a mouse monoclonal 4-hydroxynonenal (4-HNE) protein adducts antibody (25mg/ml; Oxis International Inc., Foster City, CA). Immunoreactivity was detected using biotinylated antimouse IgG secondary antibody followed by avidin-biotinylated horseradish peroxidase complex, and visualized with diaminobenzidine tetrahydrochloride as per the manufacturer’s instructions (VECTASTAIN Elite ABC Mouse IgG kit, Burlingame, CA). Slides were counterstained with hematoxylin. Negative control was run for every assay and was processed in an identical manner, except that the primary antibody was substituted by the mouse IgG.
Measurements of Muscle-Reduced/Muscle-Oxidized Glutathione Ratio and Serum Interleukin-6 Levels
Muscle-reduced glutathione (GSH)/muscle-oxidized glutathione (GSSG) ratio was measured using a commercial kit (BIOXYTECH GSH/GSSG-412 assay kit (OXIS Research, a division of Oxis Health Product, Inc., Portland, OR), as described previously (18,19). This assay, using different sample preparations, measures both GSH and GSSG concentrations and the GSH/GSSG ratio. Serum interleukin-6 levels were measured by ELISA kit (BD Bioscience, San Jose, CA).
Western Blotting
Western blotting was performed using muscle lysates as described previously (8,9,21). No phospho inhibition cocktail was added in the tissue homogenization buffer. In brief, proteins (50–80 µg) were separated on a 4%–12% SDS-polyacrylamide gel with 2-(N-moroholine) ethane sulfonic acid or 3 (N-morpholine) propane sulfonic acid buffer purchased from Invitrogen (Carlsbad, CA) at 200 V. Gel was transferred on an Immunoblot polyvinylidenefluoride Membrane (Bio-Rad, Hercules, CA) overnight at 4°C. Membranes were blocked in blocking solution (0.3% Tween 20 in Tris-buffered saline and 10% nonfat dry milk) for 1 hour at room temperature and then probed using a mouse monoclonal 4-HNE (1:500) or rabbit polyclonal phospho-adenosine-5-monophosphate (AMP)–activated protein kinase (p-AMPK; 1:300); phospho-acetyl-CoA-carboxylase (p-ACC; 1:200), fatty acid synthase (FAS; 1:300), and phospho-c-Jun-NH2-terminal kinase (p-JNK; 1:400) from Cell Signaling Technology (Beverly, MA); glucose-6-phosphate dehydrogenase; 1:500; Abcam, Cambridge, MA), copper–zinc superoxide dismutase (SOD1; 1:200), manganese superoxide dismutase (SOD2; 1:200), proliferating cell nuclear antigen (1:100), Delta 1 (1:200), Notch 1 (1:200), and phospho-Akt, which detects Akt only when phosphorylated at threonine 308 (1:500) antibodies, from Santa Cruz Biotechnology, Inc., Santa Cruz, CA) for 1 hour at room temperature or overnight at 4°C with constant shaking. Following 3 × 10-min washes in TBS-T buffer, membranes were then incubated in antirabbit (Amersham Biosciences, Piscataway, NJ) or antimouse secondary antibody (Santa Cruz Biotechnology) at a 1:2000 dilution. All antibodies were diluted in blocking buffer. For immunodetection, membranes were washed three times in TBS-T wash buffer, incubated with enhanced chemiluminescence (ECL) solutions per the manufacturer’s specifications (Amersham Biosciences), and exposed to Hyper film ECL. The membranes were stripped and reprobed with a rabbit polyclonal glyceraldehydes-3-phosphate dehydrogenase (1:2000) for normalization of the loading. Band intensities were determined using Quantity One software from Bio-Rad (Hercules, CA).
Statistical Analysis
Statistical analyses were performed using the SigmaStat 2.0 Program (Jandel Corporation, San Rafael, CA). Data are presented as mean ± SE unless otherwise indicated. We used one-way ANOVA to compare group differences. If overall ANOVA revealed significant differences, post hoc (pairwise) comparisons were performed using Tukey’s test. Differences were considered significant if p < .05.
Results
Body and Muscle Weights, and Muscle Fiber CSA
Table1 summarizes the effects of dietary supplementation of F1 on body and gastrocnemius muscle weights and muscle fiber CSA. Old mice aged 23 months were heavier than young mice (p < .005). Dietary supplementation of F1 to middle-aged mice for 6 months was unable to suppress the body weight gain associated with aging. The weight of the gastrocnemius muscles was decreased significantly (p < .05) by 17.3% in the old mice compared with young mice. Antioxidant treatment attenuated such age-related decline in muscle mass to levels seen in young controls. However, the relative gastrocnemius muscle weight normalized to body weight was increased to 71% of the values measured in young mice.
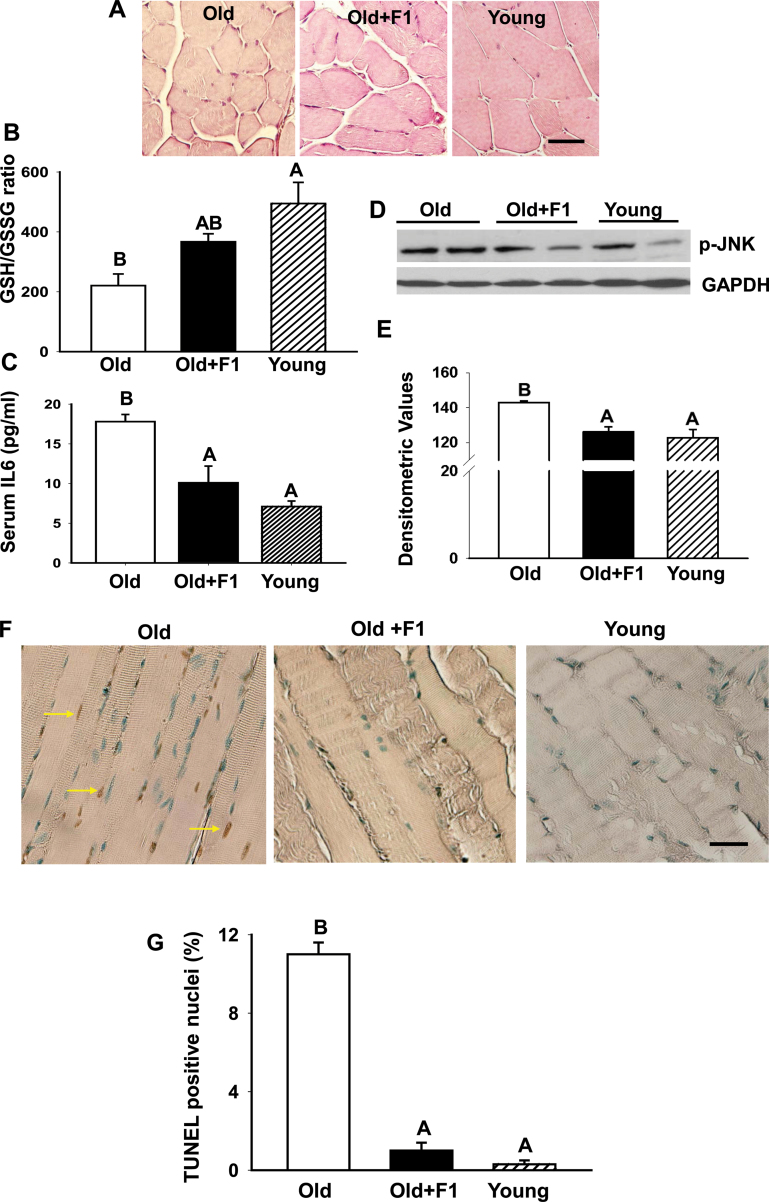
H&E stained muscle sections revealed smaller muscle fibers in old mice (Figure 1A) compared with young males (Figure 1A). Dietary supplementation of F1 to middle-aged mice for 6 months resulted in an apparent increase in muscle fiber size in the old mice similar to the phenotype observed in young controls.
Figure 1.
Dietary supplementation of F1 improves muscle morphology and suppresses oxidative stress, serum interleukin-6 levels, c-Jun NH2-terminal kinase activation, and apoptosis in old mice. (A) Dietary supplementation of F1 to middle-aged mice for 6 months results in an apparent increase in muscle fiber size in old mice similar to the phenotype observed in young controls. Old mice exhibit higher oxidative stress in the skeletal muscle, as indicated by low muscle-reduced glutathione/muscle-oxidized glutathione ratio (B) and increased circulating interleukin-6 levels (C) compared with young mice. These age-related changes are significantly (p < .05) attenuated by antioxidant treatment. Values are mean ± standard error of the mean (SEM). Means with unlike superscripts are significantly (p < .05) different. Means with superscripts A are different from means with superscripts B. However, means with superscripts AB are not different from means with either superscripts A or B. (D) Western blots of muscle lysates from old, F1-supplemented old and young mice show suppression of age-related increase in phospho-c-Jun NH2-terminal kinase levels by antioxidant treatment. The gels are representative of two mice belonging to each group from one of three separate experiments. Glyceraldehydes-3-phosphate dehydrogenase in the immunoblot is shown as a loading control. (E) Quantification of band intensities. Values are mean ± SEM. Means with unlike superscripts are significantly (p < .05) different. (F) Terminal deoxynucleotidyltransferase-mediated deoxyuridine triphosphate nick end labeling assay shows, compared with young muscles where no apoptosis is detected, a distinct increase in the incidence of muscle cell apoptosis in aged muscles, and that can be effectively prevented by F1 treatment. Scale bar = 25 µm. (G) Quantitation of muscle cell apoptosis in young, old, and F1-supplemented old mice. Apoptotic rate was expressed as the percentage of terminal deoxynucleotidyltransferase-mediated deoxyuridine triphosphate nick end labeling-positive nuclei per total nuclei (apoptotic plus nonapoptotic nuclei) counted in a unit reference area. Antioxidant treatment significantly prevents age-related increase in muscle cell apoptosis. Values are mean ± SEM. Means with unlike superscripts are significantly (p < .001) different.
Quantitative analysis revealed a significant (p < .001) reduction in individual fiber CSA in untreated old mice compared with young males (Table 1). F1 supplementation resulted in a significant (p < .001) increase in the mean fiber CSA compared with untreated old mice (Table 1). The observed increase in fiber CSA was comparable with the values measured in young mice. There was no significant difference in daily food intake (g/day/mouse) between aged controls (4.74±0.15) and aged F1-supplemented mice (4.93±0.18).
Table 1.
Effect of Dietary Supplementation of F1 on Body and Muscle Weights and Muscle Fiber Cross-Sectional Area
| Treatment | Old | Old + F1 | Young |
|---|---|---|---|
| Body weight (g) | 37.8±1.3A | 37.9±1.5A | 26.1±0.5B |
| Muscle weight (mg) | 134±7A | 165±5B | 162±4B |
| Muscle weight/100g body weight | 0.35±0.01A | 0.44±0.02B | 0.62±0.01C |
| Muscle fiber area (µm2) | 1322±28A | 1513±25B | 1603±40B |
Notes: Values are given as mean ± standard error of the mean.
Means with superscripts “A” are significantly (p < .05) different from means with superscripts “B” or “C.”
Oxidative Stress, Serum Interleukin-6 Levels, JNK Activation, and Muscle Cell Apoptosis
Figure 1B demonstrates that aging resulted in a significant (p < .05) increase in oxidative stress, indicated by low GSH/GSSG ratio, in aged muscles relative to young muscles. The GSH/GSSG ratio in aging mice treated with F1 did not differ from young mice. Aging also resulted in a significant increase (p < .05) in interleukin-6 levels in aged mice compared with their young counterparts and was attenuated by antioxidant treatment (Figure 1C).
We next examined the contribution of JNK signaling in aging and its intervention by F1 administration by immunoblotting. We found an increase in phospho-JNK levels in aged skeletal muscle compared with young muscle (Figure 1D). Dietary supplementation of F1 effectively prevented such age-related increase in phospho-JNK levels. Densitometric analysis revealed a significant (p < .05) increase in phospho-JNK expression in aged muscles compared with young muscles (Figure 1E). F1 significantly (p < .05) suppressed such age-related increase in phospho-JNK expression to levels seen in young controls (Figure 1E).
We then analyzed the incidence of muscle cell apoptosis in aged mice with or without antioxidant supplementation. Compared with young mice, where no apoptosis was detected, a distinct increase in the number of terminal deoxynucleotidyltransferase-mediated deoxyuridine triphosphate nick end labeling-positive nuclei was noted in gastrocnemius muscle of 23-month-old mice (Figure 1F). F1 treatment fully attenuated such age-related increase in muscle cell apoptosis. As shown in Figure 1G, apoptotic index in skeletal muscle was very low in young mice. Apoptotic index in skeletal muscle of aged mice was ~11%, but was fully prevented by dietary supplementation of F1 (Figure 1G).
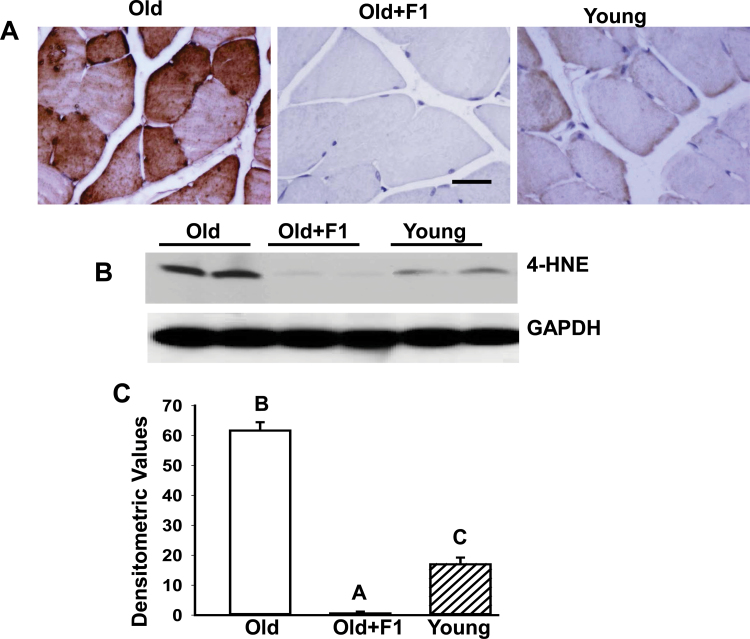
To determine whether F1-mediated mitigation of oxidative stress is further accompanied by alleviation of lipid peroxidation, we compared in vivo expression of 4-HNE in muscles. Indeed, aging resulted in a marked increase in 4-HNE expression in aged muscles, as evidenced by immunohistochemical staining of gastrocnemius muscle sections (Figure 2A) and by immunoblotting (Figure 2B and C) in aged mice in comparison with their young counterparts. Dietary supplementation of F1 significantly reduced such age-related increase in 4-HNE expression to levels even lower to that seen in young mice (Figure 2A–C).
Figure 2.
(A) Immunohistochemical analysis of gastrocnemius muscle sections shows a marked suppression of age-related increase in 4-hydroxynonenal immunoreactivity in muscles from aged mice supplemented with F1. (B) Western blot analysis of muscle lysates from young old, and old mice supplemented with F1 (old + F1) shows complete suppression of age-related increase in 4-hydroxynonenal levels by F1. The gels are representative of two mice belonging to each group from one of three separate experiments. Glyceraldehydes-3-phosphate dehydrogenase in the immunoblot is shown as a loading control. (C) Quantification of band intensities. Values are mean ± SEM. Means with unlike superscripts are significantly (p < .05).
SOD1 and SOD2 Expressions
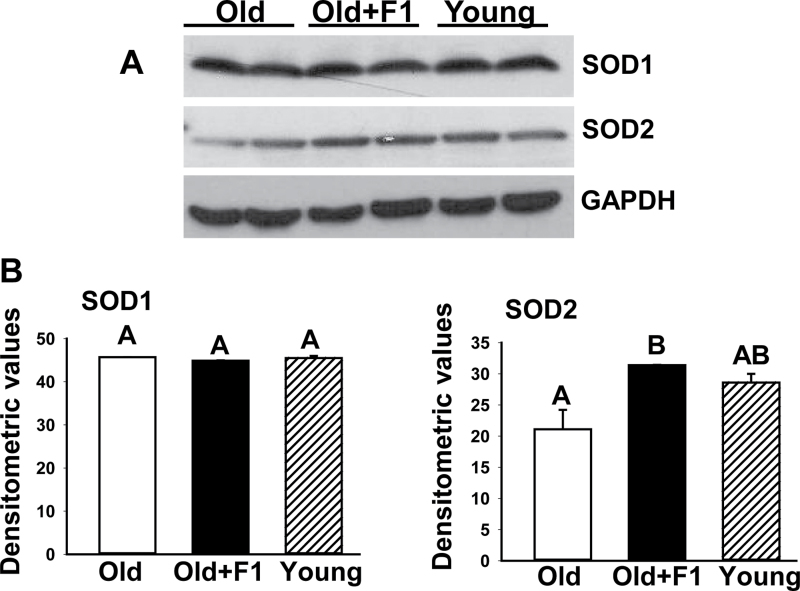
We used immunoblot analysis to examine the participation of SOD1 and SOD2 in F1-mediated mitigation of oxidative stress and loss of muscle mass in aging. There was no significant change in the expression of SOD1 in muscles among young, old, and F1-supplemented old mice (Figure 3A and B). Although there was also no change in the expression of SOD2 between young and aged muscles, muscles from old mice with supplementation of F1 had significantly (p < .05) elevated levels of SOD2 compared with old controls (Figure 3A and B).
Figure 3.
(A) Western blot analysis of muscle lysates from young old, and old mice supplemented with F1 (old + F1) shows upregulation of SOD2 expression in aged muscles by F1. The gels are representative of two mice belonging to each group from one of three separate experiments. Glyceraldehydes-3-phosphate dehydrogenase in the immunoblot is shown as a loading control. (B) Quantification of band intensities. Values are mean ± SEM. Means with unlike superscripts are significantly (p < .05).
AMPK Activation, Lipogenesis, and Glucose-6-Phosphate Dehydrogenase Expression
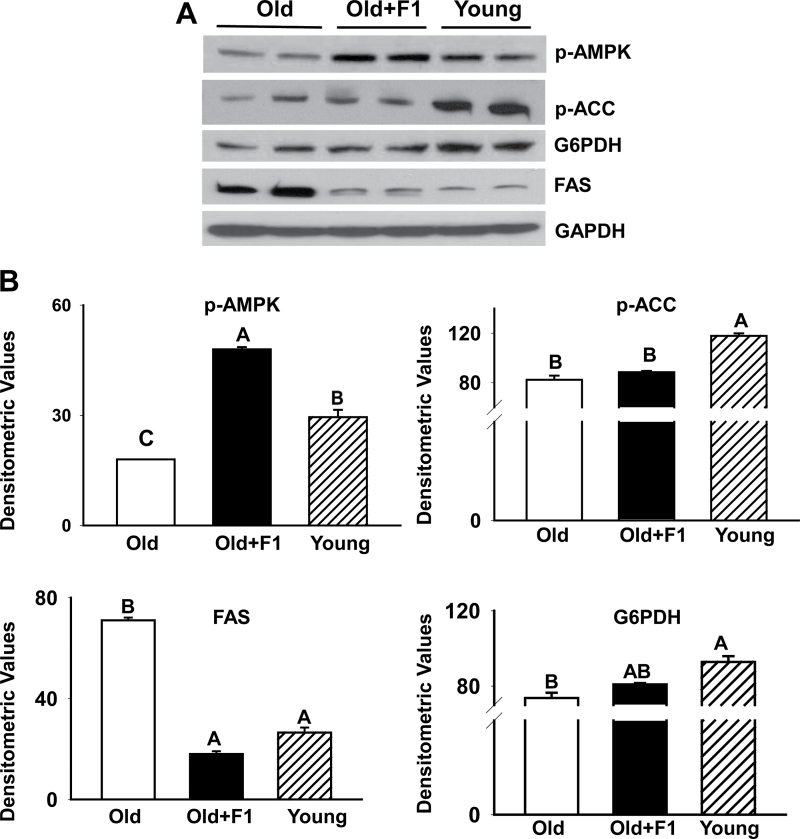
As shown in Figure 4A, there was a substantial reduction in phospho-AMPK levels in gastrocnemius muscles from old mice compared with that from young mice. Consistent with the finding of AMPK inactivation (dephosphorylation) in aging, markedly reduced levels of phospho-ACC was detected in aged muscles compared with that of young muscles (Figure 4A). Inactivation of AMPK was further associated with upregulation of FAS in aged muscles compared with young muscles. These findings were substantiated by densitometric evaluation (Figure 4B). Dietary supplementation of F1 effectively prevented AMPK inactivation and upregulation of FAS in aged muscles (Figure 4B). There was, however, no change in phospho-ACC expression between old and F1-supplemented old mice (Figure 4A and B). We also found a significant (p < .05) decrease in glucose-6-phosphate dehydrogenase expression in skeletal muscles of aged mice compared with that of young mice and that could be restored by antioxidant treatment (Figure 4A and B).
Figure 4.
(A) Western blot analysis shows a substantial reduction in phospho-AMPK levels (inactivation) in gastrocnemius muscles from old mice compared with that from young mice. Such age-related inactivation of AMPK is further associated with decreased phosphorylation and activation of acetyl-CoA-carboxylase and upregulation of fatty acid synthase, and decreased glucose-6-phosphate dehydrogenase levels in aged muscles compared with young muscles. Dietary supplementation of F1 effectively prevents AMPK inactivation and upregulation of fatty acid synthase in aged muscles but with no effect on phospho-acetyl-CoA-carboxylase expression. The gels are representative of two mice belonging to each group from one of three separate experiments. Glyceraldehydes-3-phosphate dehydrogenase in the immunoblot is shown as a loading control. (B) Quantification of band intensities. Values are mean ± SEM. Means with unlike superscripts are significantly (p < .05).
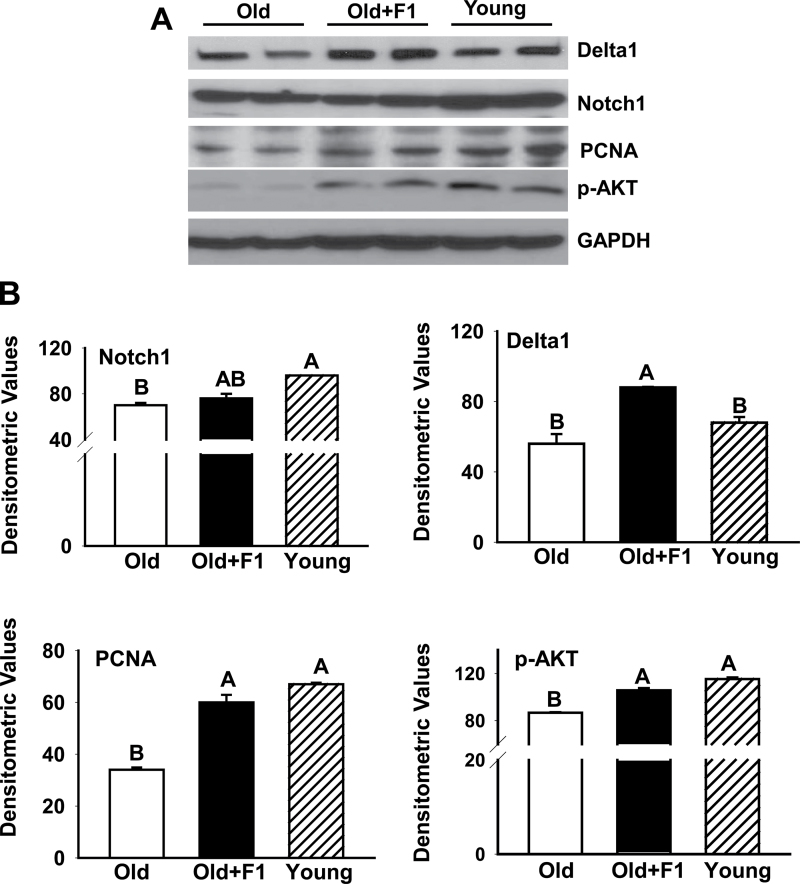
Notch and Akt Signaling
To examine the effects of F1 on Notch signaling, we analyzed expression of Notch1 and its ligand Delta1 by immunoblot analysis. Delta1 and Notch1 expressions were apparently decreased in aged muscles compared with their young counterparts (Figure 5A). Dietary supplementation of F1 significantly (p < .05) increased Delta1 but not Notch1 expression in aged muscles (Figure 5B). Antioxidant treatment was further associated with increased expression of proliferating cell nuclear antigen in muscle lysates (Figure 5A and B). As shown in Figure 5A and B, there was also a substantial reduction in phospho-Akt levels in gastrocnemius muscles from old mice compared with that of young mice. F1 treatment significantly (p < .05) prevented such age-related decrease in phospho-Akt levels (Figure 5A and B).
Figure 5.
(A) Western blots of muscle lysates show decreased levels of Delta 1, proliferating cell nuclear antigen, and phospho-Akt in aged muscles compared with young muscles. There is no change in the expression of Notch1 between young and aged muscles. These age-related changes are effectively prevented by dietary supplementation of F1. (B) Quantification of band intensities. Values are mean ± SEM. Means with unlike superscripts are significantly (p < .05) different.
Discussion
In this study, using a mouse model, we elucidated the molecular mechanisms by which a cystine-based antioxidant improves intramuscular lipid metabolism and regenerative potential of aged satellite cells, suppresses skeletal muscle apoptosis, and, in turn, prevents some of the biologic features of aging skeletal muscles.
Consistent with a pivotal role of muscle cell apoptosis in sarcopenia associated with aging (8–10), in this study, we found the muscle cell apoptotic index in the gastrocnemius muscles of old mice was about 11%. This is closely comparable with the reported values (~8%) obtained in the gastrocnemius muscles of mice of same age group (9) but is considerably higher than the calculated values reported for rat soleus muscles even when subjected to hind limb suspension (22,23). These observations led us to believe that the incidence of muscle cell apoptosis could vary depending on muscle types or even between species. This is consistent with earlier reports indicating higher muscle cell apoptotic index in plantaris muscles (9.9-fold) opposed to soleus muscles (3.2-fold) in rats following hind limb suspension in aged rats compared with ambulatory control rats (24). Notably, F1 treatment fully attenuated such age-related increase in muscle cell apoptosis.
Induction of high levels of reactive oxygen species subjects the cells to a state of oxidative stress, which is believed to play a pivotal role in aging-related deterioration of muscle mass and function (10,12,15,25). In this study, we found greater oxidative stress as evidenced by low GSH/GSSG ratio and increased levels of 4-HNE in the aged muscles relative to their younger counterparts, which were prevented by dietary supplementation with F1. Thus, it is likely that increased reactive oxygen species generation could contribute to increased muscle cell apoptosis and age-related deficits in muscle repair and regeneration, leading to loss of muscle mass. F1 supplementation was also able to increase SOD2 but not SOD1 expression in aged muscles. This is consistent with earlier reports indicating that antioxidant such as resveratrol is indeed capable of SOD2 upregulation in various cell systems (26,27), including in aged gastrocnemius muscles following hind limb suspension (14). One possible mechanism by which F1 can upregulate SOD2 in aged muscles is through activation of AMPK and silent information regulator T1 (SIRT1) axis (26,27). Indeed, in this study, we found that dietary supplementation of F1 is capable of restoring AMPK activation. Clearly, one implication of this observation is that F1 can alleviate oxidative stress in aging not only by improving GSH/GSSG ratio coupled with reducing 4-HNE levels but also by augmenting the expression of SOD2 in aged muscles.
Our findings are consistent with prior reports of oxidative stress-activating apoptotic signaling in aged skeletal myocytes (8–10,15). There is growing evidence to suggest that loss of muscle cells through increased apoptosis likely contributes to fiber atrophy and, in turn, sarcopenia associated with aging (10). We have previously demonstrated that the JNK signaling pathway, characterized by the perturbation of the B-cell lymphoma (BCL-2)/BCL-2 associated protein X (BAX) rheostat and activation of the initiator caspase 9 and the executioner caspase 3, constitutes a critical component of apoptotic signaling in skeletal muscles after injury (21) or in aging (8,9). In concert with these findings, here we show activation of JNK in aged muscles compared with that of young muscles. Most importantly, we further show that dietary supplementation of F1 significantly prevented such age-related increase in muscle cell apoptosis. This is consistent with our previous studies demonstrating that F1-mediated protection of spermine-induced vascular smooth muscle cell apoptosis (18) or HFD-induced hepatocellular apoptosis in apolipoprotein E knockout mice (19) involved suppression of JNK-mediated mitochondria-dependent apoptotic pathway. Therefore, it is possible that F1-mediated inhibition of JNK could lead to suppression of muscle cell apoptosis and, in turn, loss of muscle mass.
AMPK activity is decreased in aging and such inactivation of AMPK has been implicated as an important contributing factor in the reduced mitochondrial biogenesis, intramuscular lipid metabolism, inflammation, and sarcopenia associated with aging (28,29). Conversely increased AMPK activation can delay the aging process through modulation/activation of several longevity genes, including SIRT1, peroxisome-proliferator-activated receptor γcoactivator 1α, and forkhead box O (Foxo) factors (29). We are intrigued by the observation that dietary supplementation of F1 is capable of restoring AMPK activation. Indeed, we previously reported that F1 is capable of preventing hepatic steatosis in diet-induced obese mice through activation of AMPK coupled with suppression of de novo lipogenesis in the liver (19). These results are also consistent with earlier reports that resveratrol, a natural polyphenol antioxidant, is able to inhibit endotoxin-induced nitric oxide synthases in skeletal muscle and in L6 myocytes through AMPK activation (30). AMPK is a central regulator of lipid homeostasis and mediates suppression of lipogenic gene expression such as ACC and FAS through inhibition of sterol regulatory element binding protein-1c (31). ACC is a rate-determining enzyme for the synthesis of malonyl-CoA, both a critical substrate for fatty acid biosynthesis and a potent inhibitor of fatty acid oxidation (31). FAS catalyzes the last step in the fatty acid biosynthetic pathway and is believed to be a determinant of the maximal capacity of a tissue to synthesize fatty acid by de novo lipogenesis (32). The net effect of AMPK activation is reduced FAS levels, leading to suppression of intramyocellular lipogenesis. Despite AMPK activation in F1-supplemented old mice, no significant changes in the levels of phospho-ACC were noted between old and F1-supplemented mice, thus implying that AMPK-mediated inhibition of its downstream target ACC is blunted in the aged muscle. This is consistent with earlier works demonstrating that pharmacological stimulation of AMPK although effective in inhibiting (via phosphorylating) ACC in skeletal muscles of young rat failed to do so in the aged muscles (28). Thus, F1 may reverse age-specific deregulation of intramyocellular lipid metabolism through activation of AMPK. Given the critical role of AMPK in modulating an ever-expanding array of biological pathways, this study further underscores the potential ability of F1 to mediate a variety of signal transduction pathways in regulating cell fate.
The regenerative potential of skeletal muscle declines with age, which is largely due to a decline in Notch signaling, essential for activation, proliferation, and myogenic progression of satellite cells and muscle growth (6,7). A number of cell types have been reported to contribute to skeletal myogenesis; however, several lines of evidence suggest that satellite cells represent the predominant reservoir for muscle regeneration in adult mammals (for review, see Shadrach and Wagers, 2011) (33). Upon activation, satellite cells undergo proliferation and differentiation and commit to a myoblast cell fate (33). Finally, these cells fuse together to form multinucleated myotubes or nascent myofibers. In this study, consistent with a role for Notch signaling in muscle growth, we found increased expression of Delta1 in F1-supplemented aged skeletal muscle. We also found that upregulation of Delta1 is associated with muscle cell proliferation as demonstrated by increased expression of proliferating cell nuclear antigen in muscles from old mice following antioxidant treatment. Collectively, these data suggest that F1 may promote skeletal muscle growth in old mice through stimulation of Notch signaling. Our previous studies on aged mice indicate the involvement of Notch signaling in testosterone-mediated muscle growth (9). At present, we are unable to determine the contribution of various cell types, including satellite cells to F1-mediated muscle growth. Furthermore, we cannot rule out the possibility that prevention of satellite cell loss via apoptosis could also play a role in mitigating sarcopenia in aging (34,35).
In addition, we found F1-restored Akt signaling in aged muscles comparable to that seen in young muscles. Activation of Akt signaling promotes skeletal muscle hypertrophy (36), functioning through multiple signaling proteins involved in both survival and apoptotic pathways in skeletal muscle (9,37). Akt not only decreases skeletal muscle cell apoptosis but also promotes cell survival by directly regulating cellular glucose uptake by inducing expression of the glucose transporter, Glut 1, at the plasma membrane, targeting hexokinase activity to the mitochondria, and suppressing the caspase 2-mediated death pathway (38), the key signaling pathway in muscle cell apoptosis (8,21). There have also been studies indicating that AMPK activation can stimulate Akt singling in various cells, including cardiac and skeletal myocytes (39,40). Thus, the restoration of Akt signaling in aged muscles possibly emanates from F1-mediated activation of AMPK.
Our study has some limitations. Given that F1 supplementation was initiated in middle-aged mice, a potential shortfall of our study is the lack of middle-aged control data on various muscle parameters. However, there have been studies indicating that mice in these age groups do exhibit increased levels of oxidative stress coupled with cytochrome c release from mitochondria into cytosol (a key marker of apoptosis) and a decline in the relative gastrocnemius muscle weight normalized to body weight by 20.2% (41). Therefore, it is possible that the beneficial effects of F1 in delaying or preventing muscle mass that are so obvious when given to middle-aged mice with modest sarcopenia might not be so obvious if given to older mice with severe muscle loss. An additional short fall of our study is that, at present, we do not know whether F1-mediated protection of muscle loss is also associated with improved muscle strength in aged mice. In this context, it is pertinent to note here that dietary supplementation of resveratrol was indeed able to preserve isometric force output in gastrocnemius muscle following hind limb suspension in old rats (14). Earlier studies have also demonstrated beneficial role of various antioxidants in improving muscle function in both mice and rats following mechanical unloading (42) or fatiguing contractile activity (43). This merits further investigations. Further work is also needed to determine whether F1 is equally effective in preventing/reversing muscle atrophy and strength in older mice and the underlying mechanisms of F1-mediated protection of sarcopenia. Additionally, a large number of cross-sectional studies have shown loss of muscle mass in humans aged 18 to 80 years from 8% to 49% (for review, see Mitchell et al., 2012) (44), suggesting that elderly participants could experience varying degrees of muscle atrophy. Taken as a whole, the results of this study indicate that dietary supplementation of F1 may prove to be beneficial in preventive oxidative stress and muscle cell apoptosis and hence prevent age-related loss of muscle mass. Indeed, several preclinical studies suggest that targeting muscle cell apoptosis may provide novel and effective tools to combat sarcopenia in aging (for review, see Marzetti et al., 2012) (10). Although our study has important implications in the rapidly expanding field of possible use of antioxidants to combat sarcopenia in aging, future studies are clearly needed to determine whether F1 has the potential to be an effective therapeutic agent to mitigate oxidative stress and sarcopenia in elderly humans.
In summary, we have provided new insights into the molecular mechanisms by which dietary supplementation of a cystine-based antioxidant may delay or prevent loss of muscle mass in aging. F1 appears to work at various steps of signal transduction pathways involving suppression of oxidative stress, inhibition of JNK-mediated apoptotic signaling, suppression of intramyocellular lipogenesis together with restoration of AMPK activation, and stimulation of Akt and Notch signaling, which may explain why F1 has a protective effect in a wide range of cells. A deeper understanding of the F1-mediated mechanistic pathways that attenuate some of the biological changes in aged skeletal muscle may unveil novel targets for the development of anabolic therapies in aging.
Funding
This work was supported by a grant from the American Federation of Aging Research (I.S-H.). Additional support was provided by the National Institutes of Health grants (U54MD007598, formerly U54RR026138 to K.C.N. and I.S-H., P20MD00182 to K.C.N., 3P30AG021684 to K.C.N.).
Conflict of Interest
The authors have nothing to disclose.
References
- 1. Census briefs: the older population. Werner, CA: United States Census Bureau, US Department of Commerce; 2010. [Google Scholar]
- 2. Hughes VA, Frontera WR, Roubenoff R, Evans WJ, Singh MA. Longitudinal changes in body composition in older men and women: role of body weight change and physical activity. Am J Clin Nutr. 2002; 76: 473–481 [DOI] [PubMed] [Google Scholar]
- 3. Marzetti E, Leeuwenburgh C. Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp Gerontol. 2006; 41: 1234–1238 [DOI] [PubMed] [Google Scholar]
- 4. Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998; 147: 755–763 [DOI] [PubMed] [Google Scholar]
- 5. Glass D, Roubenoff R. Recent advances in the biology and therapy of muscle wasting. Ann N Y Acad Sci. 2010; 1211: 25–36 [DOI] [PubMed] [Google Scholar]
- 6. Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003; 302: 1575–1577 [DOI] [PubMed] [Google Scholar]
- 7. Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005; 433: 760–764 [DOI] [PubMed] [Google Scholar]
- 8. Braga M, Sinha Hikim AP, Datta S, et al. Involvement of oxidative stress and caspase 2-mediated intrinsic pathway signaling in age-related increase in muscle cell apoptosis in mice. Apoptosis. 2008; 13: 822–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kovacheva EL, Hikim AP, Shen R, Sinha I, Sinha-Hikim I. Testosterone supplementation reverses sarcopenia in aging through regulation of myostatin, c-Jun NH2-terminal kinase, Notch, and Akt signaling pathways. Endocrinology. 2010; 151: 628–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marzetti E, Calvani R, Bernabei R, Leeuwenburgh C. Apoptosis in skeletal myocytes: a potential target for interventions against sarcopenia and physical frailty - a mini-review. Gerontology. 2012; 58: 99–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005; 120: 483–495 [DOI] [PubMed] [Google Scholar]
- 12. Martin C, Dubouchaud H, Mosoni L, et al. Abnormalities of mitochondrial function can partly explain the metabolic disorders encountered in sarcopenic gastrocnemius. Aging cell. 2007; 1: 1–13 [DOI] [PubMed] [Google Scholar]
- 13. Bonetto A, Penna F, Muscaritoli M, et al. Are antioxidants useful for treating skeletal muscle atrophy? Free Radic Biol Med. 2009; 47: 906–916 [DOI] [PubMed] [Google Scholar]
- 14. Jackson JR, Ryan MJ, Hao Y, Alway SE. Mediation of endogenous antioxidant enzymes and apoptotic signaling by resveratrol following muscle disuse in the gastrocnemius muscles of young and old rats. Am J Physiol Regul Integr Comp Physiol. 2010; 299: R1572–R1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ryan MJ, Jackson JR, Hao Y, Leonard SS, Alway SE. Inhibition of xanthine oxidase reduces oxidative stress and improves skeletal muscle function in response to electrically stimulated isometric contractions in aged mice. Free Radic Biol Med. 2011; 51: 38–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Doria E, Buonocore D, Focarelli A, Marzatico F. Relationship between human aging muscle and oxidative system pathway. Oxid Med Cell Longev. 2012; 2012: 830257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sinha-Hikim I, Shen R, Kovacheva E, Crum A, Vaziri ND, Norris KC. Inhibition of apoptotic signalling in spermine-treated vascular smooth muscle cells by a novel glutathione precursor. Cell Biol Int. 2010; 34: 503–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sinha-Hikim I, Shen R, Paul Lee WN, Crum A, Vaziri ND, Norris KC. Effects of a novel cystine-based glutathione precursor on oxidative stress in vascular smooth muscle cells. Am J Physiol, Cell Physiol. 2010; 299: C638–C642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sinha-Hikim I, Sinha-Hikim AP, Shen R, et al. A novel cystine based antioxidant attenuates oxidative stress and hepatic steatosis in diet-induced obese mice. Exp Mol Pathol. 2011; 91: 419–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Crum A. Nutritional or therapeutic compositions to increase bodily glutathione levels. US RE39734E. 2007.
- 21. Sinha-Hikim I, Braga M, Shen R, Sinha Hikim AP. Involvement of c-Jun NH2-terminal kinase and nitric oxide-mediated mitochondria-dependent intrinsic pathway signaling in cardiotoxin-induced muscle cell death: role of testosterone. Apoptosis. 2007; 12: 1965–1978 [DOI] [PubMed] [Google Scholar]
- 22. Dupont-Versteegden EE, Strotman BA, Gurley CM, et al. Nuclear translocation of EndoG at the initiation of disuse muscle atrophy and apoptosis is specific to myonuclei. Am J Physiol Regul Integr Comp Physiol. 2006; 291: R1730–R1740 [DOI] [PubMed] [Google Scholar]
- 23. McKiernan SH, Bua E, McGorray J, Aiken J. Early-onset calorie restriction conserves fiber number in aging rat skeletal muscle. FASEB J. 2004; 18: 580–581 [DOI] [PubMed] [Google Scholar]
- 24. Hao Y, Jackson JR, Wang Y, Edens N, Pereira SL, Alway SE. β-Hydroxy-β-methylbutyrate reduces myonuclear apoptosis during recovery from hind limb suspension-induced muscle fiber atrophy in aged rats. Am J Physiol Regul Integr Comp Physiol. 2011; 301: R701–R715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meng SJ, Yu LJ. Oxidative stress, molecular inflammation and sarcopenia. Int J Mol Sci. 2010; 11: 1509–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tanno M, Kuno A, Yano T, et al. Induction of manganese superoxide dismutase by nuclear translocation and activation of SIRT1 promotes cell survival in chronic heart failure. J Biol Chem. 2010; 285: 8375–8382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim MY, Lim JH, Youn HH, et al. Resveratrol prevents renal lipotoxicity and inhibits mesengial cell glucotoxicity in a manner dependent on the AMPK-SIRT1-PGC1α axis in db/db mice. Dibetlogia. 2013; 56: 204–217 [DOI] [PubMed] [Google Scholar]
- 28. Reznick RM, Zong H, Li J, et al. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab. 2007; 5: 151–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Anderson RM, Weindruch R. Metabolic reprogramming, caloric restriction and aging. Trends Endocrinol Metab. 2010; 21: 134–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Centeno-Baez C, Dallaire P, Marette A. Resveratrol inhibition of inducible nitric oxide synthase in skeletal muscle involves AMPK but not SIRT1. Am J Physiol Endocrinol Metab. 2011; 301: E922–E930 [DOI] [PubMed] [Google Scholar]
- 31. Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest. 2008; 118: 829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang BB, Zhou G, Li C. AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 2009; 9: 407–416 [DOI] [PubMed] [Google Scholar]
- 33. Shadrach JL, Wagers AJ. Stem cells for skeletal muscle repair. Philos Trans R Soc Lond, B, Biol Sci. 2011; 366: 2297–2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alway SE, Siu PM. Nuclear apoptosis contributes to sarcopenia. Exerc Sport Sci Rev. 2008; 36: 51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pallafacchina G, Blaauw B, Schiaffino S. Role of satellite cells in muscle growth and maintenance of muscle mass. Nutr Metab Cardiovasc Dis. 2012; Epub ahead of print [DOI] [PubMed] [Google Scholar]
- 36. Izumiya Y, Hopkins T, Morris C, et al. Fast/Glycolytic muscle fiber growth reduces fat mass and improves metabolic parameters in obese mice. Cell Metab. 2008; 7: 159–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bassel-Duby R, Olson EN. Signaling pathways in skeletal muscle remodeling. Annu Rev Biochem. 2006; 75: 19–37 [DOI] [PubMed] [Google Scholar]
- 38. Robey RB, Hay N. Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene. 2006; 25: 4683–4696 [DOI] [PubMed] [Google Scholar]
- 39. Lieberthal W, Zhang L, Patel VA, Levine JS. AMPK protects proximal tubular cells from stress-induced apoptosis by an ATP-independent mechanism: potential role of Akt activation. Am J Physiol Renal Physiol. 2011; 301: F1177–F1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chopra I, Li HF, Wang H, Webster KA. Phosphorylation of the insulin receptor by AMP-activated protein kinase (AMPK) promotes ligand-independent activation of the insulin signalling pathway in rodent muscle. Diabetologia. 2012; 55: 783–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jackson JR, Ryan MJ, Alway SE. Long-term supplementation with resveratrol alleviates oxidative stress but does not attenuate sarcopenia in aged mice. J Gerontol A Biol Sci Med Sci. 2011; 66: 751–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Momken I, Stevens L, Bergouignan A, et al. Resveratrol prevents the wasting disorders of mechanical unloading by acting as a physical exercise mimetic in the rat. FASEB J. 2011; 25: 3646–3660 [DOI] [PubMed] [Google Scholar]
- 43. Pinheiro CH, Vitzel KF, Curi R. Effect of N-acetylcysteine on markers of skeletal muscle injury after fatiguing contractile activity. Scand J Med Sci Sports. 2012; 22: 24–33 [DOI] [PubMed] [Google Scholar]
- 44. Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol. 2012; 3: 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]