Abstract
Neurocognitive late effects are common sequelae of cancer in children, especially in those who have undergone treatment for brain tumors or in those receiving prophylactic cranial radiation therapy to treat leukemia. Neurocognitive morbidity in attention, executive functioning, processing speed, working memory, and memory frequently occurs and contributes to declines in intellectual and academic abilities. Oncologists are faced with the challenge of using the most effective, often the most intense, therapy to achieve the primary goal of medical success, balanced with the desire to prevent adverse late effects. Not all children with similar diagnoses and treatment have identical neurocognitive outcomes; some do very poorly and some do well. Attention now turns to the reliable prediction of risk for poor outcomes and then, using risk-adapted therapy, to preserve neurocognitive function. Prevention of late effects through rehabilitative strategies, continuation of school, and pharmacotherapy will be explored.
Introduction
Although rare, cancer in children is the leading cause of death after accidents among those younger than 15 years of age. In 2007, 10,400 children in this age range were diagnosed with cancer, and it is estimated that almost 15% will die of their disease.1 Approximately 1 child in 5000 will be diagnosed with cancer every year.2 Despite the discouraging statistics, this still represents a significant improvement in the survival rate compared with just a decade ago. This improvement has occurred because of advances in treatments, primarily the use of multidrug regimens and the way in which treatments are delivered. As a result, more children are surviving and living complete lives. The number of postcancer life-years is much greater for surviving children than for adults, and for children these years often include major life milestones such as education, career, and reproduction decisions. Clinicians and researchers are now observing the long-term effects of cancer and treatments on these survivors’ lives and attempting to discover ways to decrease their impact. However, the ultimate neurocognitive outcome is very complex and depends on a number of factors that interact in unpredictable ways (see Figure 1).
Figure 1.
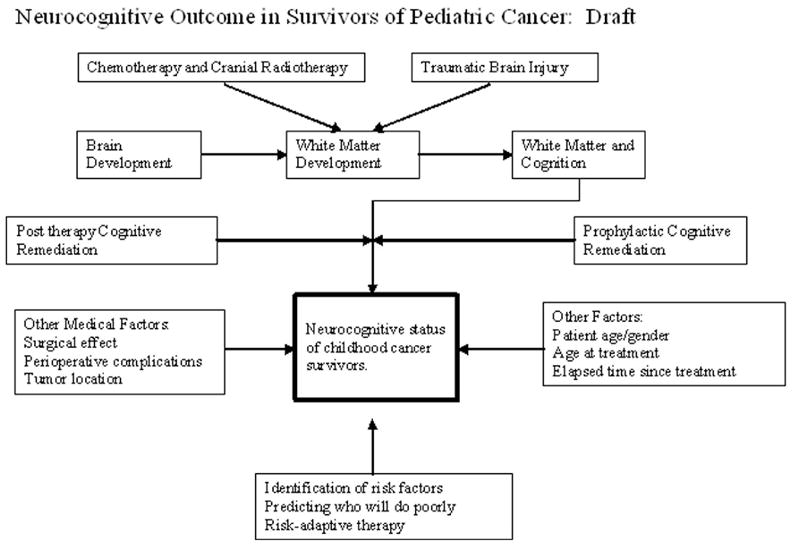
Schematic showing factors that contribute to the neurocognitive outcome of children with cancer.
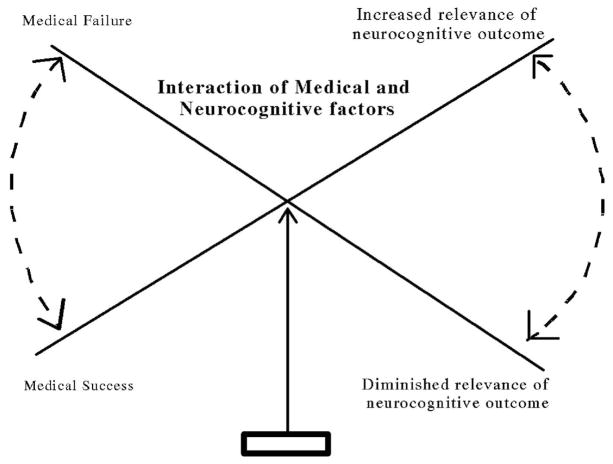
The ultimate goal of cancer therapy is to cure the patient’s disease, but effective treatments are not always without costs. Oncologists and others involved in the treatment of childhood cancer must maintain a delicate balance between effective therapy and acceptable toxicity. In some cases higher doses of therapy (eg, chemotherapy or radiation therapy) or more aggressive surgery are associated both with better cure rates and greater morbidity. In contrast, therapeutic regimens that seek to minimize toxicity may increase the chance of relapse, disease progression, metastasis, or death (Figure 2).
Figure 2.
Balancing medical success and neurocognitive outcome.
Leukemia and brain tumors or cancers of the central nervous system account for the majority of childhood cancers. Dramatic improvement in disease control has been achieved for leukemias (5-year survival for children diagnosed today may be as high as 90%), but similar success has not been realized for brain tumors. Medulloblastoma is the most common malignant brain tumor in children, and approximately 70% of average-risk patients will survive long-term.4 Those with poor risk disease have a 5-year survival rate of between 30% and 40%. Craniospinal irradiation combined with chemotherapy and surgery is currently the mainstay of treatment but leaves children at risk for late neurocognitive sequelae.
Although methotrexate and craniospinal irradiation are generally associated with poor neurocognitive outcome, this is not true for all children. Currently we do not have the ability to reliably predict in advance which patients will develop significant cognitive impairment and which will not. The mechanisms by which neurocognitive changes occur are also not well-understood. Knowledge of both of these is crucial if the neurocognitive sequelae are to be reduced or prevented.
Neurocognitive Late Effects
Newer aggressive, more effective medical treatments directed at the child’s brain are often associated with neurocognitive morbidity. The functional neurocognitive domains that are affected the most by cancer treatments are attention, executive functioning, processing speed, working memory, and ability to learn, which in turn adversely affect the academic performance of pediatric cancer patients and childhood cancer survivors.3 It is well-established now that children with brain tumors demonstrate declines in neurocognitive functioning and academic achievement over time.3,5,6 Younger age at diagnosis and female gender place children at great risk for neurocognitive and academic declines.7,8
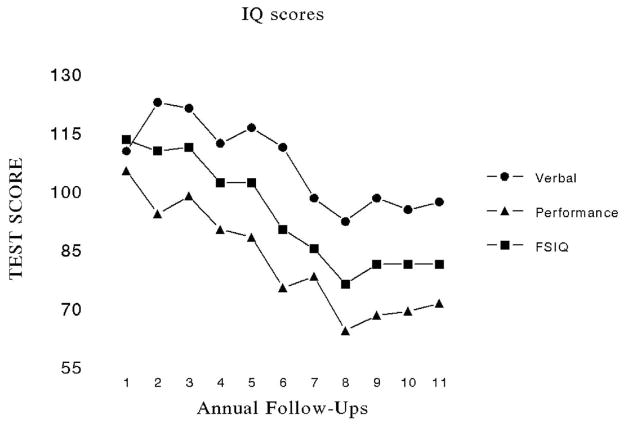
In some children, symptoms consistent with attention-deficit disorder are present. Impairments in these cognitive functions resulting from cancer therapy are responsible for declines in intelligence quotient (IQ) and in academic achievement. IQ may decline 1 or more standard deviations (average IQ is 100 with a standard deviation of 15); some children experience a drop of 3 to 4 points a year, perhaps reaching a plateau, or not. Figure 3 demonstrates the neurocognitive decline of a patient diagnosed with medulloblastoma at 9 months of age. She was treated initially with nine cycles of MOPP chemotherapy (mechlorethamine, vincristine, procarbazine, and prednisone) in an attempt to delay craniospinal irradiation because of her young age.9 But when she progressed at about 19 months of age she was treated with craniospinal irradiation to 35 Gy. Beginning 2 years after diagnosis, she was evaluated with a battery of age-appropriate developmental and neuropsychological tests. She received a total of 11 annual evaluations (Figure 3).
Figure 3.
Intellectual decline of a patient treated for medulloblastoma at 19 months of age.
Not every child who is treated in a similar manner will suffer the same degree of neurocognitive morbidity as displayed in Figure 3. Some children will remain relatively intact, whereas others will suffer marked declines in their intellectual and cognitive abilities. In the case of the latter, children often fail to fully attain their academic and career potential.
In a comprehensive meta-analysis, Mulhern and colleagues10 reviewed 18 studies with a total of 403 patients ranging in age up to 18 years. Mean IQ was 91.0 (SD = 24.1). Of particular interest is that the standard deviation of this large group substantially exceeds that of the normative sample. This suggests there is a great range of variability. Some of the risk factors identified were traditional and well-known, such as patient age, tumor location, type of surgery, hydrocephalus, dose of radiation therapy, chemotherapy, and time since treatment, seizures, and presence or absence of recurrent disease.
Neurocognitive sequelae are most apparent in attention, memory, visuospatial abilities, executive functioning, and cognitive processing speed. Because of deficits in these important functional domains, survivors experience declines in IQ and academic achievement relative to same-age peers. This does not mean that cognitive growth is arrested or declines as in dementia, but that growth rate is reduced compared with same-age peers. Therefore, as elapsed time since treatment increases, the gap in abilities between survivors and the general population increases. This presents challenges for some survivors in problem solving, academic attainment, independent living, and general quality of life.
Causes
Neurocognitive late effects can result from any of the major treatment modalities: surgery, chemotherapy, or craniospinal irradiation. Surgical resection of brain tumors that infiltrate or impinge upon areas of the brain critical for language, memory, attention, executive functioning, or other higher-order cognitive skills may lead to neurocognitive morbidity. In addition, severity of preoperative and perioperative neurological status may result in persistent neurocognitive sequelae.11 These risks are mitigated to some extent by advanced neurosurgical techniques.
White matter implicated
Treatments for brain tumors and high-risk acute lymphoblastic leukemia frequently involve high-dose chemotherapy and radiation therapy delivered to the brain. Although different mechanisms have been postulated to explain the underlying neurological basis of neurocognitive dysfunction, damage to cortical and subcortical white matter has received the most attention.12–14 Up to 50% of patients treated with cranial radiation therapy show changes in white matter that is generally progressive and does not resolve (Figure 4). Intracerebral calcifications are associated with the intensity of chemotherapy regimens that include intrathecal methotrexate, and with neurocognitive performance.15 Because the axons of the projection and association areas of the cerebral cortex do not become fully myelinated until the early adult years,16 the brain remains vulnerable to neurotoxic agents during the prime learning period of a child’s life. If white matter development is disrupted through disease, injury, genetic abnormality, or exposure to neurotoxic agents, cortical and cognitive development will most likely be affected.17,18
Figure 4.
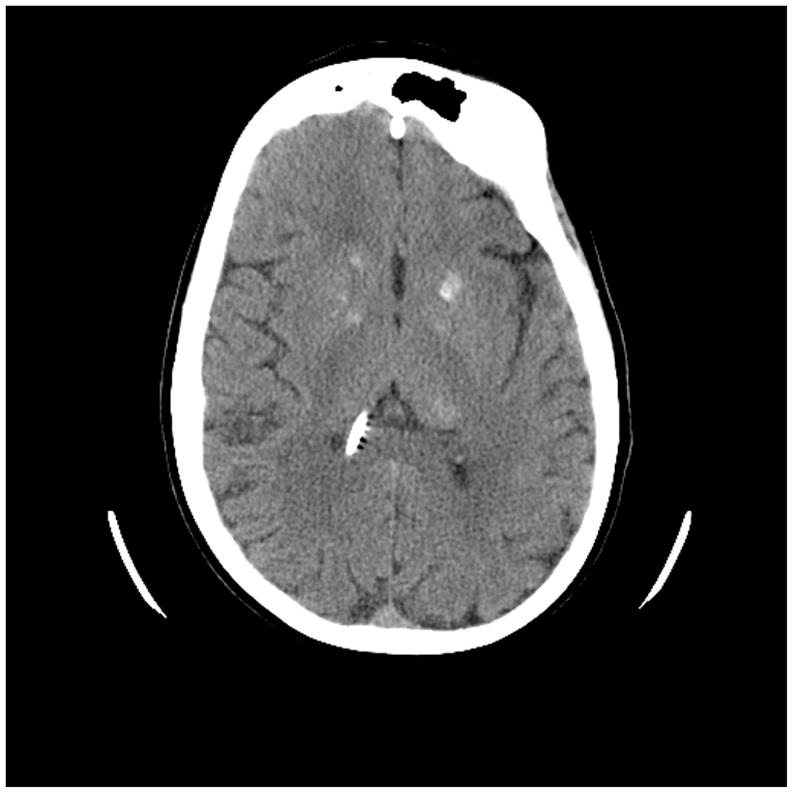
Computed tomography (CT) scan of the brain of a 21-year-old patient treated with chemotherapy and craniospinal irradiation for medulloblastoma at 30 months of age. Note areas of calcification (and shunt). This patient’s intellectual progress is seen in Figure 3.
Mulhern analyzed quantitative magnetic resonance imaging (MRI) scans in 40 radiated (+/− chemotherapy) long-term survivors of pediatric brain tumors at a median of 5.7 years posttherapy.19 Significant associations were found between normal-appearing white matter volumes and decreased attention abilities. Adequate attention and concentration abilities are essential for normal academic achievement, and these results suggest that a viable avenue of remediation would be to target attention, either behaviorally or pharmacologically (see below).
The importance of white matter to cognition has been documented by numerous studies of traumatic brain injury in which cognitive deficits have been correlated with the extent of white matter damage.20 The neurocognitive deficits commonly resulting from traumatic brain injury are similar to those of pediatric cancer survivors (attention, executive functioning, processing speed, working memory, and memory). White matter changes in aging have also been associated with the cognitive decline leading to dementia.21
Reddick used longitudinal MRI during therapy to assess the effect of two craniospinal irradiation doses (conventional [36 Gy] and reduced [23.4 Gy]) on volumes of normal-appearing white matter volume4 in 26 children and young adults with medulloblastoma. Medulloblastoma patients treated with craniospinal irradiation had a significant loss of normal-appearing white matter volume compared with untreated individuals of the same age. Reduced dose of craniospinal irradiation, but not younger age, was associated with less white matter change.
Late effects of cranial radiation therapy are characterized by various neurological deficits and neuroanatomical changes and are largely thought to be responsible for the gradual neurocognitive decline. Demyelination and necrosis are the primary morphological changes observed on brain MRI scans.22 Although demyelination is seen as the end result of white matter damage secondary to cranial radiation therapy, vascular lesions appear to cause the demyelization.22 Vascular injury resulting in white matter changes is most likely due to a combination of damage to oligodendrocytes and to vascular endothelial cells; however, the exact mechanism for this is not clearly understood.22 Cerebral lacunes, foci of white matter loss usually associated with ischemic infarcts, may also be associated with central nervous system treatments in children. Fouladi and colleagues documented a relatively low incidence (6%) in a group of 421 children treated with cranial radiation therapy for brain tumors.23 Lacunes were more likely to be associated with young age (< 5 years) at time of treatment, but were not associated with clinical deficits.
In children with acute lymphoblastic leukemia and even those with brain tumors, treatment regimens consisting of chemotherapy alone remain an option in an effort to spare the patient from the neurocognitive late effects of craniospinal irradiation,24 or at least delay it until the child is older.9,25 Lacaze and colleagues24 examined 27 patients between 1.5 and 15.7 years who had optic gliomas and who were treated with only chemotherapy as front-line treatment. Eight subsequently had to be treated with cranial radiation therapy as salvage therapy. Children treated with only chemotherapy had normal IQ, with an advantage of more than a standard deviation over those treated with cranial radiation therapy. Von der Weid and colleagues26 compared 132 acute lymphoblastic leukemia survivors treated with chemotherapy alone to 100 children with non-central nervous system tumors who did not receive chemotherapy on standardized neuropsychological measures. Intellectual abilities were within the normal range and were comparable between the groups, suggesting that chemotherapy alone did not have an additional adverse effect on neurocognitive functioning above the cancer experience itself. Moleski27 reviewed literature pertaining to neurocognitive outcome in children with acute lymphoblastic leukemia treated with intrathecal chemotherapy and concluded that their intellectual consequences, while not benign, were less severe and more subtle than the effects of cranial radiation therapy.
Copeland and colleagues28 arrived at a conclusion similar to Moleski’s27 in a longitudinal study of 99 long-term cancer survivors treated with either intrathecal chemotherapy or no central nervous system therapy (no child had been treated with cranial radiation therapy). The sample was diverse in terms of diagnoses, including acute lymphoblastic leukemia, Hodgkin’s disease, osteosarcoma, Ewing’s sarcoma, and others. Seventy-three percent had a diagnosis of leukemia or lymphoma. Patients treated with intrathecal chemotherapy received methotrexate, cytarabine, and hydrocortisone. They also received systemic chemotherapy. Using a comprehensive battery of neuropsychological tests, these children were assessed four times between 5 and 11 years after they had been diagnosed. Mean scores for both the intrathecal chemotherapy and the No-intrathecal chemotherapy groups were within the average range, and there were no statistically significant between-group differences. There was a significant group X time interaction whereby the group receiving intrathecal chemotherapy declined slightly on perceptual motor skills and those in the No-intrathecal chemotherapy group improved. Copeland and colleagues28 concluded that chemotherapy had only a slight effect on neurocognitive status and was confined to perceptual motor skills. However, much like von der Weid,26 Copeland and colleagues28 found age effects on performance IQ and perceptual motor skills; younger children performed more poorly. No effects of gender were observed, however.
The evidence that chemotherapy alone causes lasting neurocognitive late effects is not consistent. A number of studies have concluded that chemotherapy effects are negligible and not clinically significant compared to when craniospinal irradiation is involved.26–28 In one of these studies,28 children treated with intrathecal methotrexate were not significantly different from those who did not receive any chemotherapy. Unlike acute lymphoblastic leukemia, the treatment regimen for children with brain tumors usually includes both chemotherapy and craniospinal irradiation.
Timing of chemotherapy
The timing of chemotherapy in relation to cranial radiation therapy may also have an effect on eventual neurocognitive outcome, at least in girls. When methotrexate is administered concurrently with 24 Gy cranial radiation therapy, IQ scores were lower, especially for young girls.29 In a follow-up to that study, Balsom, Bleyer, Robison, and colleagues30 found that when methotrexate was administered prior to treatment with 24 Gy of cranial radiation therapy, IQ scores 2 to 11 years later were significantly higher than the standard timing of therapies. When children were young (< 5 years), the effect at follow-up was profound, with IQ being 25 to 29 points higher in those receiving pre-irradiation methotrexate.
How does cancer therapy affect white matter?
Treatments for brain tumors and acute lymphoblastic leukemia frequently involve high-dose chemotherapy and radiation therapy delivered to the brain. Up to 50% of patients treated with cranial radiation therapy show changes in white matter that are generally progressive.31 Intracerebral calcifications are associated with the intensity of chemotherapy regimens that include intrathecal methotrexate and with neurocognitive performance.15 Calcification was detected in 24%, and almost 30% showed signs of leukoencephalopathy or cerebral atrophy.
White matter changes associated with chemotherapy are not always permanent. Wilson and colleagues reported that transient white matter changes on MR images in children with acute lymphoblastic leukemia who were undergoing chemotherapy correlated with neurocognitive deficiencies.19,32 MRI of 25 children with acute lymphoblastic leukemia who underwent chemotherapy showed transient white matter abnormalities in 70% during consolidation therapy.19 Twelve of 20 children showed neuropsychological deficits. In the group as a whole, there was no correlation between white matter changes and neuropsychological deficits, but in the subgroup of children under age 5 years at the time of diagnosis, 90% showed neuropsychological deficits and 73% had white matter changes. Children under age 5 who undergo chemotherapy for acute lymphoblastic leukemia are at risk of developing white matter changes, and neuropsychological deficiencies and surveillance with MRI may help predict those who are most likely to be affected.
Predictors of Late Neurocognitive Outcome
Prevention or remediation of neurocognitive late effects requires, in part, knowledge of the predictors responsible for the variability in the severity of late effects; in other words, who will do poorly and who will do well. There are a number of well-known risk factors for poorer neurocognitive outcomes, including demographic, medical, and treatment variables. Other predictors include genetic polymorphisms, neuroimaging, and acute sequelae of therapy.
Demographic factors
Young age has been consistently been implicated in poor neurocognitive outcomes after treatment for cancers that involve the central nervous system.14,25,33–35 This is logical in light of what is known about the development of the nervous system and, in particular, of cortical and subcortical white matter. Substituting or delaying the use of cranial radiation therapy in very young children may lessen the neurocognitive morbidity without compromising the medical outcome in infants with brain tumors.9 In children with brain tumors who were under 3 years of age when diagnosed, those who were treated without cranial radiation therapy had scores within the average range of intellectual functioning and academic achievement, but those who were treated with cranial radiation therapy had significant deficits in verbal and performance IQ, academic achievement, memory, visuospatial, fine motor, and attentional abilities.36
Time since treatment
Although cross-sectional studies have provided important information suggesting that neurocognitive status declines with increasing time since treatment with cranial radiation therapy,14 only a few longitudinal studies have been conducted for children with brain tumors.33,37,38 Copeland, deMoor, Moore, and Ater33 used growth curve analyses to characterize the change in neurocognitive functioning of 27 children diagnosed during infancy with posterior fossa tumors. The time since diagnosis ranged from 2 to 13 years (mean, 7 years). The results suggest that in the absence of cranial radiation therapy, children with cerebellar tumors can have a positive neurocognitive outcome. Other longitudinal studies of children treated with cranial radiation therapy have concluded that IQ declines 2 or more points per year.39
Medical factors (perioperative neurological severity)
Neurological severity in the perioperative period is related to neurocognitive late effects in children operated for brain tumors. Ater and colleagues examined various perioperative complications as predictors of neurocognitive outcome; for example, prior to diagnosis (eg, seizures), pre-existing (eg, Down syndrome), perioperative events (eg, hydrocephalus), and postoperative events (eg, ataxia). By scoring these factors, the total neurological severity score was significantly correlated with visuospatial skills, memory, attention, and Performance IQ.40
Advances in neuroimaging
Part of assessing response to therapy in patients with a brain tumor involves assessing tumor shrinkage or progression, which relies greatly on precise quantitative measurement. Magnetic resonance spectroscopy and positron emission tomography (PET) give information regarding the chemical composition and metabolic activity, respectively, of a tumor and also provide insight into its malignant state and response to therapy, thus helping to guide therapy and avoid potentially unnecessary additional neurotoxic therapy. Functional MRI also guides surgeons in the precise localization of critical areas of the brain in relation to the tumor, allowing them to perform more complete tumor resections while reducing the potential for neurocognitive morbidity.
White matter fractional anisotropy
Using diffusion tensor imaging, Khong demonstrated a significant decline white matter diffusion anisotropy and IQ. Decreased anisotropy (a measure of the microstructural integrity of white matter tracts) was significantly correlated with Verbal, Performance, and Full-Scale IQ.41 White matter anisotrophy has demonstrated the acute effects of stroke42 and mild traumatic brain injury43 on cerebral white matter,42 raising the possibility that diffusion tensor imaging might be useful during the acute stage of treatment to predict those patients who are at increased risk for progressive white matter injury and, therefore, late neurocognitive effects. Diffusion tensor imaging may prove more sensitive than conventional imaging methods in detecting subtle but clinically meaningful changes after craniospinal irradiation and may be crucial in refining prognosis and medical management.
Methotrexate-induced neurotoxicity may be a result of methotrexate-induced folate depletion leading to homocysteinemia. Krajinovic and colleagues studied 93 pediatric patients with acute lymphoblastic leukemia, assessing a number of common polymorphisms to determine whether any were active in modulating homocysteinemia and if they were associated with changes in neurocognitive functioning at diagnosis over the subsequent 4 years.44 The NOS3 894TT genotype was the only one that had a significant relationship with change in IQ. Those with this genotype who received cranial radiation therapy declined an average of 0.5 standard deviations within the first 3 years after diagnosed, whereas those treated with cranial radiation therapy but without the 894TT genotype did not decline.
White matter changes occur in some but not all of the patients who get treatment with methotrexate. Methotrexate inhibits folate pathway enzymes (dihydrofolate reductase and thymidilate synthase) and causes folate depletion in tumor cells, thus inhibiting DNA synthesis. It is transported into cell by the reduced folate carrier. Dihydrofolate reductase and thymidilate synthase enzymes are polymorphic, and such polymorphisms alter the function of the enzymes, which possibly may modify the effect of methotrexate. Moreover, within the folate pathway, an additional polymorphic enzyme, 5,10-methylenetetrahydroreductase, plays a role in levels of available folate for DNA synthesis and methylation. Therefore, polymorphisms of the folate pathway enzymes, either individually or in coordination, may determine the risk for development of neurocognitive decline after methotrexate therapy in childhood acute lymphoblastic leukemia.
Because the prefrontal cortex is so heavily involved in higher cognition, and because the prefrontal cortex is primarily dopaminergic, genes regulating dopamine circuitry may play an important role in cognition, at least in the general population.45 Impairments in dopamine are also implicated in patients with cognitive deficits, such as Parkinson’s disease. Administration of L-dopa to patients with Parkinson’s disease alleviates at least some of their deficits in working memory and executive functions.46,47
Evidence also supports the influence of catechol-O-methyltransferase and brain-derived neurotrophic factor on cognition.45 In an extensive review of the relation between catechol-O-methyltransferase and brain-derived neurotrophic factor with cognition, a large number of studies support the notion that those who possess the catechol-O-methyltransferase val158met polymorphism perform better on a variety of cognitive tests, including making use of feedback to shift mental set, one aspect of executive functioning,48,49 visual memory,50 as well as episodic and semantic memory.51,52 In fact, 20 to 26 studies reviewed reported an association between the catechol-O-methyltransferase polymorphism and cognitive ability. If the markers discussed above can be identified, existing regimens can be tailored for patients with increased risk for neurocognitive impairment, or to implement preventive measures.
Preventing Neurocognitive Late Effects
Advanced radiotherapy techniques
The use of fractionated cranial radiation therapy to deliver a greater number of small doses effectively reduces toxicity to surrounding tissue. Stereotactic radiosurgery precisely targets a tumor by the use of very high-resolution neuroimaging scans coupled with 3-dimensional computer guided radiotherapy, so that the beam of ionizing radiation converges on the tumor while surrounding tissues receive only minimal exposure.
Conformal cranial radiation therapy is also effective at successfully treating patients while decreasing neurocognitive late effects.53,54 Merchant55 conducted a phase 2 trial of conformal cranial radiation therapy in young patients with localized ependymoma. The 3-year progression-free survival estimate was high (about 75%) and scores on all neurocognitive measures were within normal limits, demonstrating that good disease control with minimal neurocognitive late effects can be achieved by focusing therapy on the tumor and limiting the volume to normal brain.
The most promising advance at this time may be proton beam radiotherapy.53 With proton beam radiotherapy, almost all of the energy is focused into the tumor, thereby sparing surrounding tissues most of the toxic effects.56–58 This has obvious implications for sparing neurocognitive functioning in the treatment of pediatric brain tumors. However, for some tumors such as medulloblastoma, craniospinal irradiation or whole-brain irradiation is still necessary. There are only five centers in the United States with proton beam facilities at this time.
Advanced chemotherapy regimens
Certain tumors are known to be chemoresistant or chemoresponsive, and designing therapies for specific genotypes can result in better treatment outcome with less neurotoxicity.59 While some regimens seek to reduce therapies to an acceptable toxicity level while maintaining therapeutic efficacy, others seek to increase dose beyond the toxic level by the use of cytoenhancers and chemoprotectants.59 Radioenhancers can make cancer cells more sensitive to the ionizing effect of radiation, allowing lower doses to be delivered and thus sparing other tissues.
Early educational, cognitive, behavior, and pharmacological interventions
Cognitive functioning and academic achievement are important components of quality of life after successful treatment for pediatric cancer. Mabbott and colleagues60 demonstrated that survivors of medulloblastoma and ependymoma who have undergone cranial radiation show a reduced rate of skill acquisition and fall progressively behind their peers in reading, spelling, and mathematics achievement. Interestingly, poorer academic outcomes were not solely accounted for by decline in intelligence scores. The remaining variability in academic performance may be associated with factors such as fatigue and time away from school due to medical treatment, follow-up appointments, and/or health concerns. Overall, these studies have shown that children start to fail to advance in their neurocognitive and academic achievement soon after the diagnosis of cancer; thus it is imperative to institute intervention programs early to attenuate these learning and academic problems. Indeed, studies that have examined head injury rehabilitation in children suggest the importance of early intervention for restoration of impaired functions via relearning and practice.61 There may be an optimal or critical period during brain development and/or rehabilitation when cognitive and environmental stimulation are required for the brain to maximize its potential for recovery from the insults associated with central nervous system treatment. Three types of early interventions for children with cancer include hospital school programs, cognitive training, and pharmacotherapy.
Continuation of academic instruction
Successful completion of school and the acquisition of academic concepts and information is the foundation for adult productivity. School is an essential part of a child’s life, even more so while undergoing cancer treatment. School can provide a sense of normalcy, comfort, and hope, which are healing experiences for children and families during an otherwise tumultuous and uncertain time. In addition, promoting the child’s academic development during cancer treatment engenders a positive sense of self-efficacy, which may counteract the feelings of helplessness that can accompany cancer and cancer treatment. Enrollment in a school program allows for progress in children’s academic development and helps children remain on grade level while receiving treatment for cancer.
Options for educational placement during cancer treatment include continuation in one’s own community school, homebound education, or hospital school.62 It is common for pediatric patients to access support from different educational settings across different time points in treatment. Ideally, hospital-based educational professionals will work with parents to coordinate the transitions between settings to ensure continuity of instruction, course credit, and effective implementation of individualized education plans. If children participate in education programs during cancer treatment, they are often able to re-enter their community schools without losing academic credit or falling behind in grades. Although hospital schools represent a relatively new concept nationwide, most children’s hospitals now offer some type of education program for pediatric patients. Some hospital school programs work in conjunction with local school districts to provide curricula and instruction, while others function independently. The Pediatric Education Program at the Children’s Cancer Hospital at M.D. Anderson has a number of components that contribute to a comprehensive educational experience for patients.63 These include classroom and bedside instruction, academic enrichment activities such as creative arts and physical education, consultation-liaison and school reentry services, and career counseling. National organizations such as the Association for the Education of Children with Medical Needs are developing standard practices to aid in the uniform development of high-quality hospital school programs. The Children’s Oncology Group has recently published Guidelines for Identification of, Advocacy for, and Interventions in Neurocognitive Problems in Survivors of Childhood Cancer, which presents recommendations for the screening and management of neurocognitive late effects and outlines important areas of school and legal advocacy for survivors with disabilities.63
Cognitive training
Based on the cognitive training to help remediate children and adults with brain injuries, Butler and Copeland conducted some of the first studies with childhood cancer survivors.64,65 Their model of training uses techniques and methods from three disciplines: brain injury rehabilitation, special education/educational psychology, and clinical psychology. From brain injury rehabilitation, mass practice is used, building on Sohlberg and Mateer’s (2001) Attention Process Training, which exercises attentional processes in the areas of sustained, selective, divided, and executive attentional control.66 These tasks are monotonous; thus an alternating approach is supported where Attention Process Training exercises, administered 15 minutes at a time, are interspersed with more intrinsically motivating activities such as interactive games and computer games that promote development of attention skills. This alternating approach helps maintain the child’s stamina over the 20 2-hour sessions that comprise the cognitive remediation program. The program uses a 50% to 80% rule in which children must achieve a minimum of 80% correct before progressing to more difficult work. Until that time, strategies are used to help the child master the given task. If less than 50% mastery is obtained on a given task, then a simplified version of the task is substituted. This is done to minimize frustration and to help children attempt challenges that are “just right” for them.
The second aspect of the tripartite model borrows techniques from special education/educational psychology. Using metacognitive strategies, children learn to organize their approach to schoolwork (task preparedness), remain on task, and review their work in an effort to improve academic outcomes. Each participant has an individual therapist who supports the child or adolescent’s performance during training. Fifteen strategies are taught, one at a time, and individualized for each participant. Additional strategies are frequently added as participants develop their own unique methods for improving their work efforts.
The third component of the cognitive remediation program employs cognitive-behavioral strategies67 designed to help participants maintain a positive attitude by reframing struggles into positives, providing psychotherapeutic support, acknowledging weaknesses and roadblocks to successful improvement, acknowledging learning strengths, monitoring internal dialogue, stress inoculation, and becoming one’s own “best friend.” The cognitive-behavioral approach helps promote a positive, realistic, and hopeful therapeutic context for strategy and skill acquisition. During the course of the 20-session training, the participants’ teachers are contacted a minimum of three times by the therapist and more often by the caregiver, to help generalize strategies learned in cognitive remediation to the classroom setting.
Results of Butler and Copeland’s pilot study68 showed that those who participated in cognitive training improved significantly on the measure considered the most sensitive to attentional disturbances found in the childhood cancer population — a computerized continuous performance test. Data analyses from the larger clinical trial have documented significant increments in academic achievement, development of metacognitive learning strategies, and parent reports of enhanced attention functioning (Butler et al, 2008, in press). In addition to massed practice, enhancing the child’s repertoire of organizational skills, and creating a positive mindset, perhaps one reason for the success of the cognitive remediation program is that the parent, therapist, and school professionals work together form a support team for the child or adolescent. This team coordinates efforts among members and clearly communicates to the child that his or her academic efforts and emotional well-being are valued. We ourselves are currently conducting a study to examine the potential benefit of providing Butler and Copeland’s cognitive training intervention at the time of treatment to determine whether a prophylactic application of cognitive training may help prevent or attenuate the academic declines observed in children and adolescents who undergo central nervous system treatment.
Pharmacotherapy
Pharmacological approaches are currently being used to enhance attention and executive functioning among survivors of childhood cancer, because these abilities are prerequisite for learning and successful academic development. Whereas no evidence exists that childhood cancer survivors have neurotransmitter system deficiencies, some survivors of malignant brain tumors and acute lymphoblastic leukemia exhibit behavioral symptoms similar to those of children with attention-deficit disorder.5 The explanation for this may be found in emerging imaging studies, which are now correlating microscopic damage in normal-appearing white matter and lower volumes of normal-appearing white matter with poorer intellectual and academic outcomes for survivors of brain tumor.4,69,70 Normal-appearing white matter appears to be an important substrate for treatment-induced neurocognitive problems among survivors of childhood brain tumors. To help improve neurocognitive functioning among survivors, stimulant medications such as methylphenidate hydrochloride are now being studied.71–73 The clinical trial conducted by Conklin and colleagues73 of childhood survivors of acute lymphoblastic leukemia and brain tumors found a significant positive effect for methylphenidate hydrochloride versus placebo on attention, cognitive flexibility, and processing speed as measured by the Stroop Word-Color Association Test. Male gender, older age at treatment, and higher intelligence were predictive of better medication responses. The medication was well-tolerated by most children, with no differences found for number or severity of adverse side effects as a function of active medication. The randomized clinical trial conducted by Mulhern and colleagues72 not only showed a beneficial effect of methylphenidate hydrochloride versus placebo for attentional deficits, but demonstrated a significant improvement in social skills among survivors of acute lymphoblastic leukemia and brain tumor as reported by teachers and parents on the Conners’ Rating Scales and by teachers on the Social Skills Rating System. This study also found that low and moderate doses of methylphenidate hydrochloride offered the same benefit.
Newer stimulant medications such as modafinil that are structurally and pharmacologically different from other agents used for the treatment of attention-deficit hyperactivity disorder have not yet been studied in childhood cancer survivors, but have established efficacy and safety in use with children with attention-deficit hyperactivity disosrder.74,75 Modafinil selectively activates the cortex and has a low potential for abuse, according to Biederman and colleagues.74 Thus, newer stimulant medications may also hold promise for ameliorating the cognitive late effects of childhood cancer.
Discussion
Children treated for cancer are at risk for neurocognitive late effects that produce declines in IQ, academic skills, and career attainment. The use of intensive chemotherapy (eg, methotrexate) and radiation therapy are thought to cause damage to cortical and subcortical white matter, resulting in these late effects. Symptoms consistent with attention-deficit disorder and deficits in mental processing speed, working memory, executive functioning, and memory combine to leave survivors intellectually and academically disadvantaged. In some children, IQ drops by as much as 3 to 4 points per year (approximately 1 standard deviation every 5 years). Brain calcifications, leukoencephalopathy, and declines in the volume of white matter correlate with these declines in neurocognitive functioning.
Preventing these late effects is a challenge for both the medical team and for psychologists and rehabilitation specialists. Advances in neurosurgery, chemotherapy, and radiotherapy techniques are helping to a great extent, but may not be totally successful at preventing these late effects. Prevention depends in part on being able to predict those at greatest risk. Factors that are known to predict poor neurocognitive outcome include a young age at treatment; the use of steroids, methotrexate, and craniospinal irradiation; and medical and neurological complications. Advances in neuroimaging, including diffusion tensor imaging of white matter tracts, magnetic resonance spectrography, PET, and functional MRI, hold promise for helping to predict during the acute phase of treatment those patients who may suffer the greatest white matter changes and hence neurocognitive declines. Consequently, these patients may have risk-adapted therapy that seeks to lessen their morbidity. However, using genetic markers such as polymorphisms for DNA repair and methotrexate metabolism may actually help predict those at risk before therapy even begins.
For survivors who show neurocognitive decline following cancer treatment, rehabilitation similar to that used for survivors of traumatic brain injury have shown some effectiveness. However, for those newly diagnosed patients who are identified as being at risk by one of the above methods, prophylactic interventions such cognitive training and maintenance of academic growth may offer the best hope of preventing late effects.
Cancer is the No. 2 cause of death (after accidents) in children under the age of 15. Nevertheless, the survival rate approaches 90% for those diagnosed with acute lymphoblastic leukemia today, although less for children with brain tumors. A child cured of cancer may have many more decades of life ahead of them than adults cured of their cancer. In addition, the child is in the formative years of school, career and social development and is therefore at risk for falling short of their potential due to neurocognitive late effects of their cancer and its treatment. As cure rates for cancer and brain tumors climb, prevention of these late effects becomes more of a priority.
Acknowledgments
This article was presented at the Neurobiology of Disease in Children: Symposium on Central Nervous System Tumors, in conjunction with the 36th annual meeting of the Child Neurology Society, Quebec City, Quebec, October 10, 2007.
References
- 1.Cancer Facts and Figures. Atlanta, GA: American Cancer Society; 2007. [Google Scholar]
- 2.Ries LAG, Reichman ME, Lewis DR, et al. Cancer survival and incidence from the surveillance, epidemiology, and end results (SEER) program. Oncologist. 2003;8:541–552. doi: 10.1634/theoncologist.8-6-541. [DOI] [PubMed] [Google Scholar]
- 3.Moore BD. Neurocognitive outcomes in survivors of childhood cancer. J Pediatr Psychol. 2005;30:51–63. doi: 10.1093/jpepsy/jsi016. [DOI] [PubMed] [Google Scholar]
- 4.Reddick WE, Russell JM, Glass JO, et al. Subtle white matter volume differences in children treated for medulloblastoma with conventional or reduced dose craniospinal irradiation. Magnt Reson Imaging. 2000;18:787–793. doi: 10.1016/s0730-725x(00)00182-x. [DOI] [PubMed] [Google Scholar]
- 5.Butler RW, Mulhern RK. Neurocognitive interventions for children and adolescents surviving cancer. J Pediatr Psychol. 2005;30:65–78. doi: 10.1093/jpepsy/jsi017. [DOI] [PubMed] [Google Scholar]
- 6.Palmer SL, Reddick WE, Gajjar A. Understanding the cognitive impact on children who are treated for medulloblastoma. J Pediatr Psychol. 2007;32:1040–1049. doi: 10.1093/jpepsy/jsl056. [DOI] [PubMed] [Google Scholar]
- 7.Fouladi M, Gilger E, Kocak M, et al. Intellectual and functional outcome of children 3 years old or younger who have CNS malignancies. J Clin Oncol. 2005;23:7152–7160. doi: 10.1200/JCO.2005.01.214. [DOI] [PubMed] [Google Scholar]
- 8.Lahteenmaki PM, Harila-Saari A, Pukkala EI, et al. Scholastic achievements of children with brain tumors at the end of comprehensive education - A nationwide, register-based study. Neurology. 2007;69:296–305. doi: 10.1212/01.wnl.0000265816.44697.b4. [DOI] [PubMed] [Google Scholar]
- 9.Ater JL, van EJ, Woo SY, et al. MOPP chemotherapy without irradiation as primary postsurgical therapy for brain tumors in infants and young children. J Neurooncol. 1997;32:243–252. doi: 10.1023/a:1005744527443. [DOI] [PubMed] [Google Scholar]
- 10.Mulhern RK, Hancock J, Fairclough D, Kun L. Neuropsychological status of children treated for brain-tumors: a critical-review and integrative analysis. Med Pediatr Oncol. 1992;20:181–191. doi: 10.1002/mpo.2950200302. [DOI] [PubMed] [Google Scholar]
- 11.Mazzocco MM, Pennington BF, Hagerman RJ. The neurocognitive phenotype of female carriers of fragile X: additional evidence for specificity. J Dev Behavl Pediatr. 1993;14:328–335. [PubMed] [Google Scholar]
- 12.Steen RG, Koury M, Granja CI, et al. Effect of ionizing radiation on the human brain: White matter and gray matter T1 in pediatric brain tumor patients treated with conformal radiation therapy. Int J Radiat Oncol Biol Phys. 2001;49:79–91. doi: 10.1016/s0360-3016(00)01351-1. [DOI] [PubMed] [Google Scholar]
- 13.Mulhern RK, Palmer SL, Reddick WE, et al. Quantitative white matter loss explains risks of young age for neurocognitive deficits in medulloblastoma survivors. Arch Clin Neuropsychol. 2000;15:791–792. [Google Scholar]
- 14.Mulhern RK, Palmer SL, Reddick WE, et al. Risks of young age for selected neurocognitive deficits in medulloblastoma are associated with white matter loss. J Clin Oncol. 2001;19:472–479. doi: 10.1200/JCO.2001.19.2.472. [DOI] [PubMed] [Google Scholar]
- 15.Iuvone L, Mariotti P, Colosimo C, et al. Long-term cognitive outcome, brain computed tomography scan, and magnetic resonance imaging in children cured for acute lymphoblastic leukemia. Cancer. 2002;95:2562–2570. doi: 10.1002/cncr.10999. [DOI] [PubMed] [Google Scholar]
- 16.Netter FH. The CIBA collection of medical illustrations. Summit, NJ: Ciba-Geigy Corporation; 1983. Volume 1, Nervous System. Part I: Anatomy and Physiology. [Google Scholar]
- 17.Meyn RE, Milas L, Stephens C. Programmed cell death in normal development and disease. Cancer Bull. 1994;46:120–124. [Google Scholar]
- 18.Baron IS, Fennell EB, Voeller KKS. Pediatric Neuropsychology in the Medical Setting. New York, NY: Oxford University Press; 1995. Neurodevelopmental and genetic syndromes; pp. 64–113. [Google Scholar]
- 19.Wilson DA, Nitschke R, Bowman ME, et al. Transient white matter changes on MR images in children undergoing chemotherapy for acute lymphocytic leukemia: correlation with neuropsychological deficiencies. Radiology. 1991;180:205–209. doi: 10.1148/radiology.180.1.2052695. [DOI] [PubMed] [Google Scholar]
- 20.Levin HS. Neuroplasticity following non-penetrating traumatic brain injury. Brain Inj. 2003;17:665–674. doi: 10.1080/0269905031000107151. [DOI] [PubMed] [Google Scholar]
- 21.Wolf H, Ecke GM, Bettin S, et al. Do white matter changes contribute to the subsequent development of dementia in patients with mild cognitive impairment? A longitudinal study. Int J Geriatr Psychiatry. 2000;15:803–812. doi: 10.1002/1099-1166(200009)15:9<803::aid-gps190>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 22.Schultheiss TE, Kun LE, Ang KK, Stephens LC. Radiation response of the central nervous-system. Int J Radiat Oncol Biol Physics. 1995;31:1093–1112. doi: 10.1016/0360-3016(94)00655-5. [DOI] [PubMed] [Google Scholar]
- 23.Fouladi M, Langston J, Mulhern R, et al. Silent lacunar lesions detected by magnetic resonance imaging of children with brain tumors: a late sequela of therapy. J Clin Oncol. 2000;18:824–831. doi: 10.1200/JCO.2000.18.4.824. [DOI] [PubMed] [Google Scholar]
- 24.Lacaze E, Kieffer V, Streri A, et al. Neuropsychological outcome in children with optic pathway tumours when first-line treatment is chemotherapy. Br J Cancer. 2003;89:2038–2044. doi: 10.1038/sj.bjc.6601410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore BD, Ater JL, Copeland DR. Improved neuropsychological outcome in children with brain tumors diagnosed during infancy and treated without cranial irradiation. J Child Neurol. 1992;7:281–290. doi: 10.1177/088307389200700308. [DOI] [PubMed] [Google Scholar]
- 26.der Weid N, Mosimann I, Hirt A, et al. Intellectual outcome in children and adolescents with acute lymphoblastic leukaemia treated with chemotherapy alone: age- and sex-related differences. Eur J Cancer. 2003;39:359–365. doi: 10.1016/s0959-8049(02)00260-5. [DOI] [PubMed] [Google Scholar]
- 27.Moleski M. Neuropsychological, neuroanatomical, and neurophysiological consequences of CNS chemotherapy for acute lymphoblastic leukemia. Arch Clin Neuropsychol. 2000;15:603–630. [PubMed] [Google Scholar]
- 28.Copeland DR, Moore BD, Francis DJ, et al. Neuropsychologic effects of chemotherapy on children with cancer: a longitudinal study. J Clin Oncol. 1996;14:2826–2835. doi: 10.1200/JCO.1996.14.10.2826. [DOI] [PubMed] [Google Scholar]
- 29.Bleyer WA, Fallavollita J, Robison L, et al. Influence of age, sex, and concurrent intrathecal methotrexate therapy on intellectual function after cranial irradiation during childhood: a report from the Children’s Cancer Study Group. Pediatr Hematol Oncol. 1990;7:329–338. doi: 10.3109/08880019009033410. [DOI] [PubMed] [Google Scholar]
- 30.Balsom WR, Bleyer WA, Robison LL, et al. Intellectual function in long-term survivors of childhood acute lymphoblastic leukemia: protective effect of pre-irradation methotrexate? A Childrens Cancer Study Group Study. Med Pediatr Oncol. 1991;19:486–492. doi: 10.1002/mpo.2950190607. [DOI] [PubMed] [Google Scholar]
- 31.Constine LS, Konski A, Ekholm S, et al. Adverse effects of brain irradiation correlated with MR and CT imaging. Int J Radiat Oncol Biol Physics. 1988;15:319–330. doi: 10.1016/s0360-3016(98)90011-6. [DOI] [PubMed] [Google Scholar]
- 32.Paakko E, Vainionpaa L, Lanning M, et al. White matter changes in children treated for acute lymphoblastic leukemia. Cancer. 1992;70:2728–2733. doi: 10.1002/1097-0142(19921201)70:11<2728::aid-cncr2820701126>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 33.Copeland DR, deMoor C, Moore BD, III, Ater JL. Neurocognitive development of children after a cerebellar tumor in infancy: a longitudinal study. J Clin Oncol. 1999;17:3476–3486. doi: 10.1200/JCO.1999.17.11.3476. [DOI] [PubMed] [Google Scholar]
- 34.Radcliffe J, Bunin GR, Sutton LN, et al. Cognitive deficits in long-term survivors of childhood medulloblastoma and other noncortical tumors: age-dependent effects of whole brain radiation. Int J Dev Neuroscience. 1994;12:327–334. doi: 10.1016/0736-5748(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 35.Walter AW, Mulhern RK, Gajjar A, et al. Survival and neurodevelopmental outcome of young children with medulloblastoma at St Jude Children’s Research Hospital. J Clin Oncol. 1999;17:3720–3728. doi: 10.1200/JCO.1999.17.12.3720. [DOI] [PubMed] [Google Scholar]
- 36.Cichowski K, Shih TS, Schmitt E, et al. Mouse models of tumor development in neurofibromatosis type 1. Science. 1999;286:2172–2176. doi: 10.1126/science.286.5447.2172. [DOI] [PubMed] [Google Scholar]
- 37.Palmer SL, Gajjar A, Reddick WE, et al. Predicting intellectual outcome among children treated with 35–40 Gy craniospinal irradiation for medulloblastoma. Neuropsychology. 2003;17:548–555. doi: 10.1037/0894-4105.17.4.548. [DOI] [PubMed] [Google Scholar]
- 38.Anderson VA, Godber T, Smibert E, et al. Cognitive and academic outcome following cranial irradiation and chemotherapy in children: a longitudinal study. Br J Cancer. 2000;82:255–262. doi: 10.1054/bjoc.1999.0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spiegler BJ, Bouffet E, Greenberg ML, et al. Change in neurocognitive functioning after treatment with cranial radiation in childhood. J Clin Oncol. 2004;22:706–713. doi: 10.1200/JCO.2004.05.186. [DOI] [PubMed] [Google Scholar]
- 40.Ater JL, Moore BD, Francis DJ, et al. Correlation of medical and neurosurgical events with neuropsychological status in children at diagnosis of astrocytoma: utilization of a neurological severity score. J Child Neurol. 1996;11:462–469. doi: 10.1177/088307389601100610. [DOI] [PubMed] [Google Scholar]
- 41.Khong PL, Leung LH, Fung AS, et al. White matter anisotropy in post-treatment childhood cancer survivors: Preliminary evidence of association with neurocognitive function. J Clin Oncol. 2006;24:884–890. doi: 10.1200/JCO.2005.02.4505. [DOI] [PubMed] [Google Scholar]
- 42.Mukherjee P. Diffusion tensor imaging and fiber tractography in acute stroke. Neuroimaging Clin N Am. 2005;15:655–665. xii. doi: 10.1016/j.nic.2005.08.010. Review. [DOI] [PubMed] [Google Scholar]
- 43.Wilde HE, McCauley S, Hunter JV, et al. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology. 2008;70:948–955. doi: 10.1212/01.wnl.0000305961.68029.54. [DOI] [PubMed] [Google Scholar]
- 44.Krajinovic M, Robaey P, Chiasson S, et al. Polymorphisms of genes controlling homocysteine levels and IQ score following the treatment for childhood ALL. Pharmacogenomics. 2005;6:293–302. doi: 10.1517/14622416.6.3.293. [DOI] [PubMed] [Google Scholar]
- 45.Savitz J, Solms M, Ramesar R. The molecular genetics of cognition: dopamine, COMT and BDNF. Genes Brain Behav. 2006;5:311–328. doi: 10.1111/j.1601-183X.2005.00163.x. [DOI] [PubMed] [Google Scholar]
- 46.Mattay VS, Tessitore A, Callicott JH, et al. Dopaminergic modulation of cortical function in patients with Parkinson’s disease. Ann Neurol. 2002;51:156–164. doi: 10.1002/ana.10078. [DOI] [PubMed] [Google Scholar]
- 47.Cools R, Stefanova E, Barker R, et al. Dopaminergic modulation of high-level cognition in Parkinson’s disease: The role of pre-frontal cortex and basal ganglia revealed by PET. Neuroimage. 2001;13:S652. doi: 10.1093/brain/awf052. [DOI] [PubMed] [Google Scholar]
- 48.Malhotra AK, Egan M, Lipsky RH, et al. The COMT Val158Met polymorphism and human cognitive function. Biol Psychiatry. 2002;51:95S. [Google Scholar]
- 49.Malhotra AK, Kestler LJ, Mazzanti C, et al. A functional polymorphism in the COMT gene and performance on a test of prefrontal cognition. Am J Psychiatry. 2002;159:652–654. doi: 10.1176/appi.ajp.159.4.652. [DOI] [PubMed] [Google Scholar]
- 50.Bates JA, Goldman D, Malhotra AK. COMT and neurocognition: new evidence for a role in visual memory. Schizophr Res. 2003;60:123. [Google Scholar]
- 51.de Frias CM, Annerbrink K, Westberg L, et al. COMT gene polymorphism is associated with declarative memory in adulthood and old age. Behav Genet. 2004;34:533–539. doi: 10.1023/B:BEGE.0000038491.06972.8c. [DOI] [PubMed] [Google Scholar]
- 52.de Frias CM, Annerbrink K, Westberg L, et al. COMT gene polymorphism is associated with cognitive functioning in adulthood and old age. Behav Genet. 2004;34:635. doi: 10.1023/B:BEGE.0000038491.06972.8c. [DOI] [PubMed] [Google Scholar]
- 53.Kirsch DG, Tarbell NJ. Conformal radiation therapy for childhood CNS tumors. Oncologist. 2004;9:442–450. doi: 10.1634/theoncologist.9-4-442. [DOI] [PubMed] [Google Scholar]
- 54.Kirsch DG, Tarbell NJ. New technologies in radiation therapy for pediatric brain tumors: the rationale for proton radiation therapy. Pediatr Blood Cancer. 2004;42:461–464. doi: 10.1002/pbc.10471. [DOI] [PubMed] [Google Scholar]
- 55.Merchant TE, Mulhern RK, Krasin MJ, et al. Preliminary results from a phase II trial of conformal radiation therapy and evaluation of radiation-related CNS effects for pediatric patients with localized ependymoma. J Clin Oncol. 2004;22:3156–3162. doi: 10.1200/JCO.2004.11.142. [DOI] [PubMed] [Google Scholar]
- 56.Schneider U, Lomax A, Lombriser N. Comparative risk assessment of secondary cancer incidence after treatment of Hodgkin’s disease with photon and proton radiation. Radiat Res. 2000;154:382–388. doi: 10.1667/0033-7587(2000)154[0382:craosc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 57.Suit H. The Gray Lecture 2001: coming technical advances in radiation oncology. Int J Radiat Oncol Biol Physics. 2002;53:798–809. doi: 10.1016/s0360-3016(02)02851-1. [DOI] [PubMed] [Google Scholar]
- 58.Paganetti H, Niemierko A, Ancukiewicz M, et al. Relative biological effectiveness (RBE) values for proton beam therapy. Int J Radiat Oncol Biol Physics. 2002;53:407–421. doi: 10.1016/s0360-3016(02)02754-2. [DOI] [PubMed] [Google Scholar]
- 59.Doolittle ND, Anderson CP, Bleyer WA, et al. Importance of dose intensity in neuro-oncology clinical trials: summary report of the Sixth Annual Meeting of the Blood-Brain Barrier Disruption Consortium. Neurooncol. 2001;3:46–54. doi: 10.1093/neuonc/3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mabbott DJ, Spiegler BJ, Greenberg ML, et al. Serial evaluation of academic and behavioral outcome after treatment with cranial radiation in childhood. J Clin Oncol. 2005;23:2256–2263. doi: 10.1200/JCO.2005.01.158. [DOI] [PubMed] [Google Scholar]
- 61.Baron I, Fennell EB, Voeller KKS. Pediatric Neuropsychology in the Medical Setting. New York, NY: Oxford University Press; 1995. [Google Scholar]
- 62.Searle NS, Askins M, Bleyer WA. Adolescent cancer patients’ perspectives on their educational experiences: ten case studies. Psychooncol. 2003;12:S78. [Google Scholar]
- 63.Copeland DR, Askins MA. Behavioral medicine in cancer care. In: Chan K, Raney RB, editors. Pediatric Oncology. New York, NY: Springer Science and Business Media, Inc; 2005. pp. 244–255. [Google Scholar]
- 64.Nathan PC, Patel SK, Dilley K, et al. Guidelines for identification of, advocacy for, and intervention in neurocognitive problems in survivors of childhood cancer: a report from the Children’s Oncology Group. Arch Pediatr Adolesc Med. 2007;161:798–806. doi: 10.1001/archpedi.161.8.798. [DOI] [PubMed] [Google Scholar]
- 65.Butler RW. Attentional processes and their remediation in childhood cancer. Med Pediatr Oncol. 1998:75–78. doi: 10.1002/(sici)1096-911x(1998)30:1+<75::aid-mpo11>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 66.Sohlberg MM, Mateer CA. Improving attention and managing attentional problems. Adapting rehabilitation techniques to adults with ADD. Ann N Y Acad Sci. 2001;931:359–375. [PubMed] [Google Scholar]
- 67.Meichenbaum D. Cognitive-behavior modification: An integrative approach. New York, NY: Plenum Press; 2008. [Google Scholar]
- 68.Butler RW, Copeland DR. Attentional processes and their remediation in children treated for cancer: a literature review and the development of a therapeutic approach. J Int Neuropsychol Soc. 2002;8:115–124. [PubMed] [Google Scholar]
- 69.Mabbott DJ, Noseworthy MD, Bouffet E, et al. Diffusion tensor imaging of white matter after cranial radiation in children for medulloblastoma: correlation with IQ. Neurooncology. 2006;8:244–252. doi: 10.1215/15228517-2006-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mulhern RK, White HA, Glass JO, et al. Attentional functioning and white matter integrity among survivors of malignant brain tumors of childhood. J Int Neuropsycholl Soc. 2004;10:180–189. doi: 10.1017/S135561770410204X. [DOI] [PubMed] [Google Scholar]
- 71.Thompson SJ, Leigh L, Christensen R, et al. Immediate neurocognitive effects of methylphenidate on learning-impaired survivors of childhood cancer. J Clin Oncol. 2001;19:1802–1808. doi: 10.1200/JCO.2001.19.6.1802. [DOI] [PubMed] [Google Scholar]
- 72.Mulhern RK, Khan R, Kaplan S, et al. A randomized, double-blind, placebo-controlled trial of methylphenidate for attentional problems in survivors of childhood cancer. J Clin Oncol. 2004;22:801S. doi: 10.1200/JCO.2004.04.128. [DOI] [PubMed] [Google Scholar]
- 73.Conklin HM, Khan RB, Reddick WE, et al. Acute neurocognitive response to methylphenidate among survivors of childhood cancer: A randomized, double-blind, cross-over trial. J Pediatr Psychol. 2007;32:1127–1139. doi: 10.1093/jpepsy/jsm045. [DOI] [PubMed] [Google Scholar]
- 74.Biederman J, Swanson JM, Wigal SB, et al. Efficacy and safety of modafinil film-coated tablets in children and adolescents with attention-deficit/hyperactivity disorder: results of a randomized, double-blind, placebo-controlled, flexible-dose study. Pediatrics. 2005;116:E777–E784. doi: 10.1542/peds.2005-0617. [DOI] [PubMed] [Google Scholar]
- 75.Rugino TA, Copley TC. Effects of modafinil in children with attention-deficit/hyperactivity disorder: an open-label study. J Am Acad Child Adolesc Psychiatry. 2001;40:230–235. doi: 10.1097/00004583-200102000-00018. [DOI] [PubMed] [Google Scholar]