Summary
Previous studies of epileptic spasms reported that ictal events were associated with high-frequency oscillations (HFOs) or delta waves involving widespread regions. We determined whether ictal HFOs at 80–200 Hz were coupled with a phase of slow-wave, whether ictal slow-waves were diffusely or locally synchronous signals, and whether the mode of coupling between HFOs and slow-wave phases differed between ictal and interictal states. We studied 11 children who underwent extraoperative electrocorticography (ECoG) recording. The phases and amplitudes of slow-waves were measured at the peak of ictal and interictal HFOs in the seizure-onset sites. Ictal HFOs were locked tightly to the phase of slow-wave at ≤1 Hz. Ictal slow-waves propagated from the seizure-onset site to other regions. In contrast, interictal HFOs in the seizure-onset site were loosely locked to the phase of slow-wave at ≤1 Hz but tightly to that of ≥3-Hz. Ictal slow-waves coupled with HFOs can be explained as near-field and locally synchronized potentials generated by the neocortex rather than far-field potentials generated by subcortical structures. Ictal slow-waves in epileptic spasms may be generated by a mechanism different from what generates interictal HFOs–slow-wave complexes.
Keywords: Pediatric epilepsy surgery, Ripples, Infantile spasms, West syndrome, Intracranial EEG recording
Epileptic spasms are seizures frequently seen during infancy and early childhood and characterized by periodic muscle contractions of variable intensity. Investigators have attempted to determine the pathophysiology of epileptic spasms using ictal electroencephalography (EEG) reflecting the dynamic brain activities related to spasms. Scalp EEG studies found that ictal signal changes consisted of brief widespread delta waves or fast wave bursts (Kellaway et al., 1979; Fusco & Vigevano, 1993). Intracranial electrocorticography (ECoG) studies demonstrated that high-frequency oscillations (HFOs) with frequency of >80 Hz and duration of >500 ms were generated by the neocortex and that propagation of such ictal HFOs to the sensorimotor cortex was associated with body jerking; furthermore, surgical resection of the cortical region generating ictal HFOs was associated with a good seizure outcome (RamachandranNair et al., 2008; Nariai et al., 2011). Although fast wave bursts on ictal EEG are of cortical origin in epileptic spasms, the origin of ictal delta waves is still debated. Some speculated that such ictal delta waves might be far-field potentials generated by subcortical structures such as brainstem (Fusco & Vigevano, 1993), whereas others suggested that these waves might be near-field potentials generated by neocortex (Panzica et al., 1999; Kobayashi et al., 2005).
In this ECoG study, we determined how tightly a phase of slow-wave was coupled with ictal HFOs at 80–200 Hz in epileptic spasms. Observation of cross-frequency coupling between HFOs and slow-waves as well as propagation of such slow-waves from the seizure-onset site to the surrounding areas would support the hypothesis that ictal slow-waves are of neocortical origin. Signal sampling at 1-cm intervals would determine more accurately whether ictal slow-waves are diffusely or locally synchronous signals. We also compared the mode of cross-frequency coupling between ictal and interictal states. We hypothesized that ictal and interictal HFOs would be coupled with different types of slow-waves.
Methods
Patients
We studied the same 11 children (age 1.3–8.8 years) reported in our previous study (Nariai et al., 2011). The study was approved by the institutional review board, and written informed consent was obtained from the guardians.
Video-ECoG recording
Platinum electrodes (intercontact distance: 10 mm; contacts: 104–148 per patient) were placed in the subdural space over cortical regions (Nariai et al., 2011). Signals were obtained with a sampling rate at 1,000 Hz and an amplifier band pass at 0.08–300 Hz. The averaged voltage of signals derived from the fifth and sixth intracranial electrodes of the amplifier was used as the original reference; signals were then re-montaged to a common average reference. Channels contaminated with large interictal epileptiform discharges or artifacts were visually identified and excluded from the common average reference.
Analysis of slow oscillations
We determined the amplitude and phase of slow-wave at the peak of ictal and interictal HFOs at the 18 seizure-onset sites reported in our previous study (Nariai et al., 2011). Ictal and interictal ECoG traces were assessed visually with a low-frequency filter of 53 Hz and a sensitivity of 10 μV/ mm. Ictal HFOs during 636 spasm events (Nariai et al., 2011) were used for further analysis. A total of 546 interictal HFOs, defined as oscillatory events of ≥6 cycles with a frequency of ≥80 Hz, were identified visually at the seizure-onset sites at least 30 s prior to spasms. The average interval between interictal HFOs and the onset of spasms was 634 s (standard error 136 s). The largest negative peak of ictal and interictal HFOs at the seizure-onset site was then marked as a trigger point (Nagasawa et al., 2011).
Ictal and interictal HFO events were transformed into the time-frequency domain using complex demodulation, using BESA® software (BESA GmbH, Gräfelfing, Germany; Hoechstetter et al., 2004). The low-pass filter used in the complex demodulation process was a finite impulse response (FIR) filter of Gaussian shape, making the complex demodulation effectively equivalent to a Gabor transform. The signal was assigned an amplitude (μV) and phase as a function of frequency and time. The size of each time-frequency bin was “0.5-Hz and 100-ms” for frequencies between 1 and 10 Hz (time-frequency resolution defined as the 50% power drop of the FIR filter: ±0.71 Hz and ±158 ms), while being “0.05 Hz and 1,000 ms” for frequencies between 0.1 and 2.0 Hz (time-frequency resolution: ±0.071 Hz and ±1,580 ms).
We determined whether the distribution of slow-wave phases at the peak of ictal HFOs differed from a uniform distribution using the Rayleigh’s test of nonuniformity. A larger z-value on Rayleigh’s test indicates a tighter cross-frequency coupling between the peak of HFOs and a slow-wave phase. Phase angles were computed in the range between 0 degrees and 360 degrees, where 0 degrees reflects a peak and 180 degrees reflects a trough of the slow-wave at the trigger point. We determined the coherence of slow-wave activity in the sites surrounding the seizure-onset site and tested how consistently slow-waves coupled with ictal HFOs propagated to the surrounding areas. Finally, we determined whether ictal and interictal HFOs in the 18 seizure-onset sites were coupled with different types of slow-waves.
Results
Cross-frequency coupling between ictal HFOs and slow-wave phase
We found that each spasm was associated with a slow-wave at ≤1 Hz in all types of spasms (Figs 1, S1 and S2). Ictal HFOs at ≥80 Hz were superimposed on such slow-waves, and the onset of slow-waves corresponded to the onset of ictal HFOs. Ictal slow-waves originated from the same region as the ictal HFOs. Ictal slow-waves propagated to surrounding widespread regions (Video S1). At the peak of ictal HFOs, slow-wave coherence with the seizure-onset site was high in the surrounding regions but not diffusely (Fig. 1).
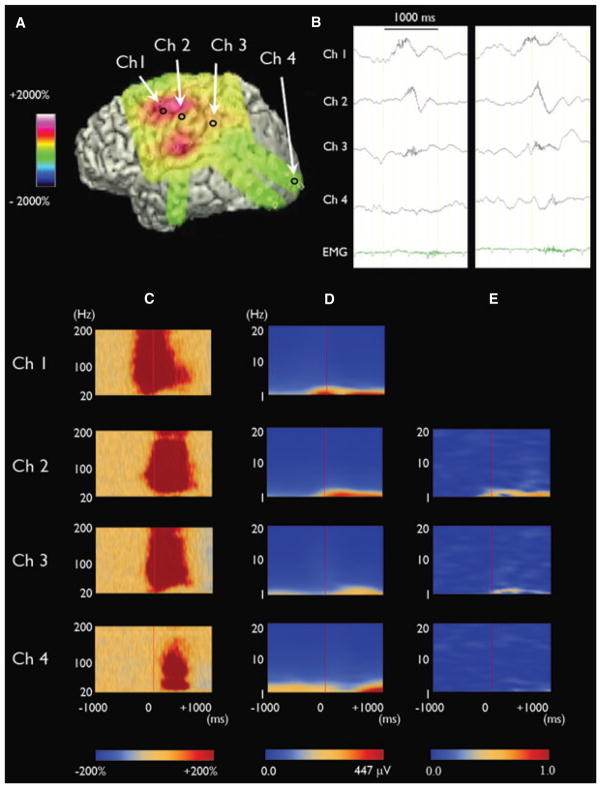
Figure 1.
Cross-frequency coupling between ictal HFOs and slow-wave phase in a 2-year and 6-month-old boy with epileptic spasms. (A) Three-dimensional brain surface image shows the location of seizure-onset site (channel 1). (B) ECoG traces show ictal HFOs superimposed on a slow-wave. Both ictal HFOs and slow-waves propagated from the seizure-onset site to the surrounding sites. (C) Time-frequency matrixes demonstrate that augmentation of HFOs occurred at the seizure-onset site earlier than those in the surrounding sites (Nariai et al., 2011). (D) Amplitude augmentation of slow-waves at 1 Hz occurred at the seizure-onset site earlier than those in the surrounding sites. (E) When the amplitude of ictal HFOs reached the peak (i.e., at 0 ms), average slow-wave coherence (at 1 Hz) with the seizure-onset site was high in some surrounding sites (0.74 at channel 2; 0.33 at channel 3) but minimal in other sites (0.07 at channel 4).
Epilepsia © ILAE
Difference in cross-frequency coupling between ictal and interictal events
Ictal HFOs in the seizure-onset site were coupled tightly with the phase of slow-wave at ≤1 Hz (Fig. 2). The tightest coupling between HFOs and slow-wave phase was noted around 0.3 Hz, according to the mean z-value on the Rayleigh’s test. Conversely, interictal HFOs in the seizure-onset site were loosely locked to the phase of slow-wave at ≤1 Hz but tightly to that of ≥3-Hz. For example, the degree of cross-frequency coupling with 0.3-Hz slow-wave phase was larger on ictal HFOs (grand mean z-value: 18.7 vs. 4.0; p = 0.001 on the Wilcoxon signed-rank test), whereas that with 5-Hz theta phase was larger on interictal HFOs (grand mean z-value: 1.9 vs. 15.7; p = 0.005).
Figure 2.
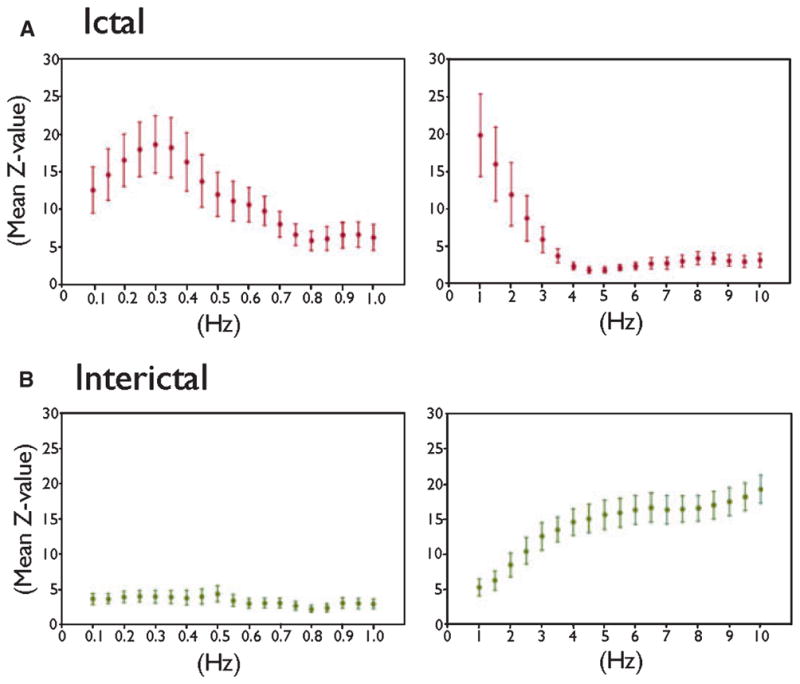
Difference in cross-frequency coupling between ictal and interictal events. (A) Upper row. Ictal HFOs in the seizure-onset site were tightly coupled with the phase of slow-wave at ≤1 Hz. X-axis: frequency of phase of slow-oscillation. Y-axis: mean z-value on Ray-leigh’s test of nonuniformity with error bars. A larger z-value on Rayleigh’s test indicates a tighter cross-frequency coupling between the peak of HFOs and a given phase of slow-wave. The size of each time-frequency bin was “0.05 Hz and 1,000 ms” for frequencies between 0.1 and 1.0 Hz (graphs on the left side), while being “0.5-Hz and 100-ms” for frequencies between 1 and 10 Hz (graphs on the right side). (B) Lower row. Interictal HFOs in the seizure-onset site were only loosely coupled with the phases of slow-oscillations at ≤1 Hz but tightly coupled with those at ≥3 Hz.
Epilepsia © ILAE
Discussion
Ictal HFOs at the seizure-onset site were tightly locked to the phase of slow-wave at ≤1 Hz. Ictal slow-waves were consistently noted regardless of the severity of body movements during spasms, out of phase across regions, and propagated from the seizure-onset site to the surrounding regions in a stereotypic fashion. Therefore, such ictal slow-waves can be explained as near-field and locally synchronized potentials generated by the neocortex rather than far-field potentials generated by subcortical structures or artifacts induced by body movement. The overall degree of measured coherence could have been biased toward higher values by the use of a common average reference, but the effect of reference cannot explain propagation of ictal slow-waves or double dissociation between the frequencies of slow-waves coupled with ictal and interictal HFOs. Further studies are warranted to determine the localization value of ictal slow-wave on scalp EEG in patients with epileptic spasms.
The mechanism of such ictal slow-waves at ≤1 Hz in spasms is unknown. A recent study of a patient with focal neocortical epilepsy showed that ictal slow-waves at <0.1 Hz co-occurred with HFOs lasting >10 s in the seizure-onset site (Imamura et al., 2011). A plausible hypothesis is that such ictal slow-waves might reflect passive depolarization of glial cells co-occurring with ictal neuronal firing (Kuffler et al., 1966). Our results did not prove or disprove involvement of subcortical structures during spasms. A previous study using ictal single-photon emission computed tomography (SPECT) indicated that thalamus and brainstem showed increased blood flow during spasms in some patients (Kakisaka et al., 2009).
Interictal HFOs at the seizure-onset site were tightly coupled with slow-wave phases at ≥3 Hz, which were much steeper than those coupled with ictal HFOs in the same site. Such slow-waves co-occurring with interictal HFOs might reflect hyperpolarization of cortical neurons (Steriade & Amzica, 1999). The duration of HFOs differed between interictal and ictal states. Further studies considering clinically available measures are warranted to determine the mechanism that alters the frequency of slow-waves coupled with HFOs.
Supplementary Material
Acknowledgments
This work was supported by NIH grants NS47550 and NS64033 (to E. Asano).
Footnotes
Disclosure
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Additional Supporting Information may be found in the online version of this article:
Figure S1. Cross-frequency coupling between ictal HFOs and slow-wave phase in a 6-year-old girl with epileptic spasms.
Figure S2. Cross-frequency coupling between ictal HFOs and slow-wave phase in a 3-year-old girl with epileptic spasms.
Video S1. Cross-frequency coupling between ictal HFOs and delta phase in a 2-year and 6-month-old boy with epileptic spasms.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Fusco L, Vigevano F. Ictal clinical electroencephalographic findings of spasms in West syndrome. Epilepsia. 1993;34:671–678. doi: 10.1111/j.1528-1157.1993.tb00445.x. [DOI] [PubMed] [Google Scholar]
- Hoechstetter K, Bornfleth H, Weckesser D, Ille N, Berg P, Scherg M. BESA source coherence: a new method to study cortical oscillatory coupling. Brain Topogr. 2004;16:233–238. doi: 10.1023/b:brat.0000032857.55223.5d. [DOI] [PubMed] [Google Scholar]
- Imamura H, Matsumoto R, Inouchi M, Matsuhashi M, Mikuni N, Takahashi R, Ikeda A. Ictal wideband ECoG: direct comparison between ictal slow shifts and high frequency oscillations. Clin Neurophysiol. 2011 doi: 10.1016/j.clinph.2010.12.060. [DOI] [PubMed] [Google Scholar]
- Kakisaka Y, Haginoya K, Ishitobi M, Togashi N, Kitamura T, Wakusawa K, Sato I, Hino-Fukuyo N, Uematsu M, Munakata M, Yokoyama H, Iinuma K, Kaneta T, Higano S, Tsuchiya S. Utility of subtraction ictal SPECT images in detecting focal leading activity and understanding the pathophysiology of spasms in patients with West syndrome. Epilepsy Res. 2009;83:177–183. doi: 10.1016/j.eplepsyres.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Kellaway P, Hrachovy RA, Frost JD, Zion T. Precise characterization and quantification of infantile spasms. Ann Neurol. 1979;6:214–218. doi: 10.1002/ana.410060306. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Oka M, Inoue T, Ogino T, Yoshinaga H, Ohtsuka Y. Characteristics of slow waves on EEG associated with epileptic spasms. Epilepsia. 2005;46:1098–1105. doi: 10.1111/j.1528-1167.2005.63004.x. [DOI] [PubMed] [Google Scholar]
- Kuffler SW, Nicholls JG, Orkand RK. Physiological properties of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966;29:768–787. doi: 10.1152/jn.1966.29.4.768. [DOI] [PubMed] [Google Scholar]
- Nagasawa T, JuhQsz C, Rothermel R, Hoechstetter K, Sood S, Asano E. Spontaneous and visually driven high-frequency oscillations in the occipital cortex: intracranial recording in epileptic patients. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nariai H, Nagasawa T, JuhQsz C, Sood S, Chugani HT, Asano E. Statistical mapping of ictal high-frequency oscillations in epileptic spasms. Epilepsia. 2011;52:63–74. doi: 10.1111/j.1528-1167.2010.02786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzica F, Franceschetti S, Binelli S, Canafoglia L, Granata T, Avanzini G. Spectral properties of EEG fast activity ictal discharges associated with infantile spasms. Clin Neurophysiol. 1999;110:593–603. doi: 10.1016/s1388-2457(98)00031-5. [DOI] [PubMed] [Google Scholar]
- RamachandranNair R, Ochi A, Imai K, Benifla M, Akiyama T, Holowka S, Rutka JT, Snead OC, III, Otsubo H. Epileptic spasms in older pediatric patients: MEG and ictal high-frequency oscillations suggest focal-onset seizures in a subset of epileptic spasms. Epilepsy Res. 2008;78:216–224. doi: 10.1016/j.eplepsyres.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Steriade M, Amzica F. Intracellular study of excitability in the seizure-prone neocortex in vivo. J Neurophysiol. 1999;82:3108–3122. doi: 10.1152/jn.1999.82.6.3108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.