Abstract
Maturational studies of the auditory-evoked brain response at the 50 ms latency provide an insight into why this response is aberrant in a number of psychiatric disorders that have developmental origin. Here, using intracranial recordings we found that neuronal activity of the primary contributors to this response can be localised at the lateral part of Heschl’s gyrus already at the age of 3.5 years. This study provides results to support the notion that deviations in cognitive function(s) attributed to the auditory P50 in adults might involve abnormalities in neuronal activity of the frontal lobe or in the interaction between the frontal and temporal lobes. Validation and localisation of progenitors of the adults’ P50 in young children is a much-needed step in the understanding of the biological significance of different subcomponents that comprise the auditory P50 in the adult brain. In combination with other approaches investigating neuronal mechanisms of auditory P50, the present results contribute to the greater understanding of what and why neuronal activity underlying this response is aberrant in a number of brain dysfunctions. Moreover, the present source localisation results of auditory response at the 50 ms latency might be useful in paediatric neurosurgery practice.
Keywords: Maturation, Auditory ERP, Intracranial recording, Temporal lobe, Source localization
Introduction
Auditory scalp-recorded Event Related Potentials (ERPs) in children are a promising tool that might elucidate the causes and origins (etiology) of diseases involving aberrations in auditory cognition (Picton and Taylor 2007).
The most conspicuous developmental changes in obligatory components of the auditory ERP recorded from the scalp are represented as a chronological transformation in the morphology of the dominating positive response labelled as “P1”. In the course of maturation, the earlier portion of this P1 response gradually splits into two identifiable components that are referred to as “P50 or P1” and “N1” responses in the adult ERP (Takeshita et al. 2002; Trainor et al. 2003; for review: Wunderlich and Cone-Wesson 2006).
Apart from the developmental perspective, the study of the auditory P50 response has become a primary interest in the investigation of schizophrenia (for meta-analysis see: (Bramon et al. 2004; Heinrichs 2004). In this context, any additional knowledge gained from developmental studies about neuronal mechanisms of auditory brain responses at the latency around 50 ms is crucial for adequate interpretation of P50-related findings in clinical research.
In the following text, for the sake of convenience, the large positive monophasic component dominating in young children’s fronto-central scalp electrodes and peaking at the latencies greater than 80 ms will be referenced as “P1”. When the maturational change in the latency of this response is approaching the adult range (45–75 ms), such a response is referred to as “P50”.
Results of scalp-recorded ERP in children (for review see: Wunderlich and Cone-Wesson 2006) aged from 4 years through adolescence indicate that stimulation rate is one of the major factors that determines distinctiveness (dissociability) of P1 as a separate ERP component in children from 7 to 10 years of age (as an example see: Gilley et al. 2005). Assuming that children’s P1 component (delayed in latency due to its immaturity) is a progenitor of the adults’ P50, it has been hypothesized that maturational separability of the P1 response is more likely due to emergence of N1 than maturation of P1 generators (Ceponiene et al. 2002). However, already in 2–6 months old infants, brain response around 50 ms has been described as a robust waveform (Jerger et al. 1987). Taken together, these empirical and theoretical considerations suggest quite a complex pattern of maturational changes of P50 neuronal sources (McGee and Kraus 1996).
Our present study was designed to test whether there is a brain response even in 3–5 year old children around 50 ms latency. We hypothesize that, due to immature status of neuronal generators, detection of adult-like P50 response in young children could be difficult, especially at the central midline scalp electrodes that are conventional sites for recording P50 in adults.
Methodologically, the majority of previous P50 brain localisation studies in adults used magnetoencephalography (MEG) as a tool that offers a high spatial resolution for localisation of neuronal generators. However, in children, MEG brain localisation of P50/P1 is a challenge, since getting a good signal-to-noise ratio in MEG data requires a child to keep their head in a steady position during data recording. Recent developments in MEG technology allow compensation of head movements and may open an avenue for reliable P50/P1 recordings in children (Wehner et al. 2008).
Another approach to P50 source localisation is represented by studies that evaluated auditory P50 responses in adult epilepsy patients using intracranial electrocorticography (ECoG) as a part of presurgical evaluation for epilepsy surgery (Godey et al. 2001; Korzyukov et al. 2007; Liegeois-Chauvel et al. 1994). In these studies, ECoG data were acquired from surgical candidates admitted to determine the epileptic focus using chronically (for several days) implanted subdural strip and grid electrodes (Spencer et al. 1997). Whereas the inherent spatial limitations of scalp ERP recordings make it difficult to completely isolate and study neuronal activity from distinct brain areas, intracranial ECoG recording limits the superimposition of distant electrical sources and, therefore, enhances the spatial resolution of the electrical fields that originate from P1/P50 generators.
In clinical practice, candidates for epilepsy surgery often have grid(s) of subdural electrodes kept in place for several days, in order to determine the seizure onset zone and to perform functional cortical mapping using cortical stimulation via subdural electrodes. Therefore, recording of auditory responses using intracranial electrodes in children with intractable epilepsy provides a unique opportunity to achieve the goals of the present study, which were: (1) to validate the presence of auditory responses elicited by simple sounds (tones) at the latency around 50 ms, and (2) to evaluate from maturational perspective the refined localisation of primary, temporal lobe contributors to these auditory responses.
Methods
From the study design perspective, adequate evaluation of maturation-related changes in P1/P50 response should include children who represent at least three chronological periods of P1/P50 maturation. The first period should be at age of 3–4 years, when P1/P50 is not observable as a separate, clearly distinguishable component on the central midline scalp electrodes independent of the stimulation rate (see: Gilley et al. 2005). The second period should be at the age of 7–10 years, when P1/P50 can be distinguished as a separate component but only at slow stimulation rates. The third period is the age of 12 years and above, when P1/P50 can be recorded at fast (inter-stimulus interval shorter than 1000 ms) and slow stimulation rates (see: Gilley et al. 2005).
Since results about lateralisation of 50 ms auditory-responses in healthy children were not available, and in order to be consistent with large scale study in healthy children (Ponton et al. 2000) only children with epilepsy who underwent subdural electrode placement predominantly involving the right hemisphere were included in this study. The following considerations were taken into account as the inclusion criteria in the present study: (i) age; (ii) absence of deviation in cognitive development and absence of neurological deficits; (iii) similarity (comparability) in localisation of electrode placement across all patients.
Participants
Data recorded from three patients of different ages were chosen for the present study. All patients had subdural electrodes covering the right lateral temporal cortex allowing the recording of neuronal activity originating from the superior temporal gyrus, the brain region where the largest portion of P50 is generated in adults (Godey et al. 2001; Huang et al. 2003; Liegeois-Chauvel et al. 1994).
All selected children underwent preoperative scalp video-electroencephalography (video-EEG), preoperative magnetic resonance imaging (MRI), 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography (FDG PET), preoperative neuropsychological examination, extraoperative intracranial ECoG and extraoperative functional cortical mapping using neurostimulation, as previously described (Fukuda et al. 2008).
Recording of auditory responses was performed on ECoG during the interictal state. The best estimate of children’s cognitive ability was determined using the appropriate measure after clinical review by neuropsychologist. The present study was approved by the Institutional Review Board at Wayne State University, and written informed consent was obtained from the parents or guardians of all subjects.
Patient No. 1 was a 197-month-old (16 years and 5 months) right-handed boy with intractable focal epilepsy with and without secondary generalization. MRI was normal. Ictal scalp EEG recording showed ictal onset arising from the right temporal region. FDG PET showed a mild glucose hypometabolism in the left temporal lobe. Thus, the patient underwent subdural electrode placement bilaterally; extraoperative ECoG showed independent seizure foci arising from the right and left medial temporal regions. Thus, the patient underwent placement of a vagus nerve stimulator instead of cortical resection. The patient was on Topiramate and Zonisamide preoperatively. Full-Scale IQ was 71 but Verbal IQ was 87 reflecting normal development of language skills despite the chronic epilepsy. Although this score is minimally outside the average range, it is possible that this partially reflects the negative impact of epilepsy medications (e.g. topiramate) on immediate test performance rather than a diminished language development. Furthermore, both verbally mediated learning and reading vocabulary were also in the average range further demonstrating this patient’s normal development of verbally mediated processing, memory, and written language.
Patient No. 2 was an 84-month-old (7 years) right-handed boy with intractable focal epilepsy with and without secondary generalization. MRI was normal. Ictal scalp EEG recording showed ictal onset arising from the right central region. FDG PET showed glucose hypometabolism in the right central region. The patient underwent subdural electrode placement on the right hemisphere, followed by cortical resection involving the right frontal-parietal region including the primary sensori-motor cortex. The patient was on Oxcarbamazepine, Valproate and Levetiracetam preoperatively. Full-scale IQ was 109.
Patient No. 3 was a 41-month-old (3 years and 5 months) right-handed girl with intractable focal epilepsy with and without secondary generalization. MRI was normal. Intracranial ECoG recording and FDG PET suggested the presence of epileptogenic zone in the right temporal region. The patient was on phenytoin preoperatively. The patient’s overall cognitive ability was best estimated by expressive language level, which showed good agreement between her laboratory assessment (IQ equivalent = 80) and parent rated performance.
Subdural Electrode Placement
For extraoperative video-ECoG recording, platinum grid electrodes (10 mm inter-contact distance; 4 mm diameter; Adtech, Racine, WI, USA) were surgically implanted on the presumed epileptogenic hemisphere. In all patients, electrodes also covered the lateral temporal region. One or more additional strips were also placed under the medial temporal region. The total number of electrode contacts ranged from 100 to 121. Antiepileptic medications were discontinued or reduced during ECoG monitoring until a sufficient number of habitual seizures were captured, and seizure onset zones were visually identified.
Stimuli
During the extraoperative ECoG recording, a conventional auditory oddball paradigm consisting of 1100 sounds were presented with an intensity of 85 dB binaurally via speakers with an inter-stimulus interval (onset-to-onset) of 800 ms. Brain responses elicited by repetitive (probability of occurrence 70%) sinusoidal (frequency 600 Hz) pure tones with a duration of 100 ms (including 10-ms rise and fall times) were analyzed in the present study.
Data Acquisition and Analysis
Extraoperative video-ECoG recordings were obtained for 3–5 days, using a 192-channel Nihon Kohden Neurofax 1100A Digital System (Nihon Kohden America Inc, Foothill Ranch, CA, USA), which has an input impedance of 200 MΩ, a common mode rejection ratio greater than 110 dB, an A/D conversion of 16 bits, a sampling frequency at 1000-Hz, and the amplifier band pass set at 0.08–300 Hz.
Recorded ECoG was segmented into epochs starting 100 ms before and ending 300 ms after each auditory stimulus onset and averaged across all epochs. Data were digitally filtered with a low-frequency cut-off of 1.0 Hz (with 12 dB/oct) and a high-frequency cut-off of 55.0 Hz (with 12 dB/oct). Epochs with artefacts defined as voltage variation exceeding 700 μV variation and epochs with a 600 Hz tone occurring right after a deviant tone were omitted from averaging. At least 300 acceptable trials had been collected for tones after rejection of trials with artefacts (Fig. 1f).
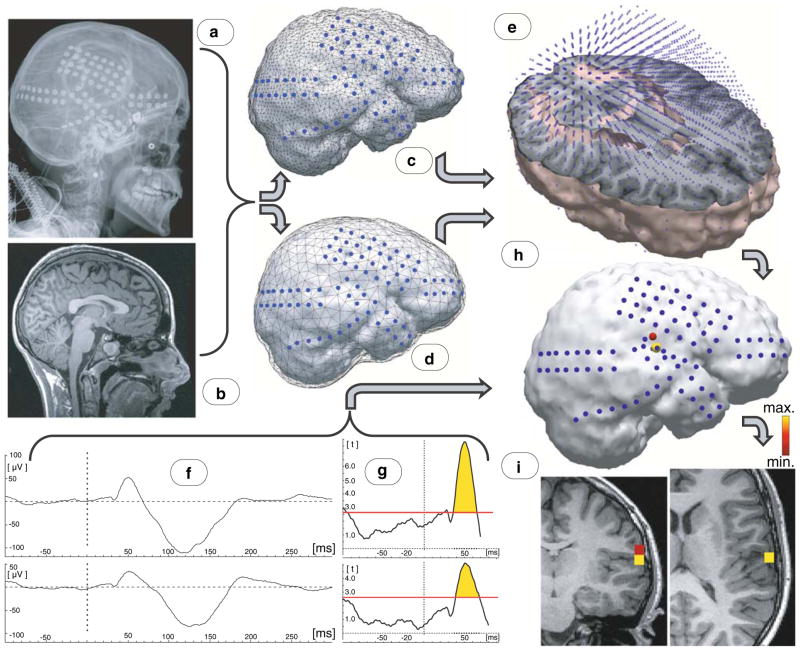
Fig. 1.
Schematic illustration of algorithm for data analyses. a X-ray images with the subdural electrodes; b Preoperative MRI brain image from the same patient; c Reconstructed electrodes position superimposed on a 3-dimensional reconstruction of individual cortex; d Electrodes superimposed on brain compartment of boundary element model of individual brain; e Source space model for individual brain; f Exemplary Event Related Potentials (ERP) recorded from grid electrodes with most prominent responses; g Results of t-test against zero calculated at each sampling point of the segmented interval; vertical axis: values from the t distribution; red horizontal line threshold for P = 0.01 for given degree of freedom; h Current density reconstruction (thresholded) results superimposed on the cortical reconstruction of individual brain; i Source localisation results superimposed onto the subject’s individual MRI brain image
In order to determine whether a statistically significant brain response was elicited, a t-test against zero was applied to the averaged amplitude values at each sampling point of the segmented interval, individually for every electrode, with a P-value threshold of 0.01 (Fig. 1g). The time window around 50 ms latency in which the auditory ERP response was significantly different from the zero was determined.
Auditory ERPs Source Analyses
To reconstruct the brain sources of these statistically significant auditory ERP responses at the 50 ms latency, data were submitted to the Minimum Norm Least Squares (MNLS) and sLORETA algorithms implemented in the CURRY software (Neuroscan, Compumedics USA Ltd, Charlotte, NC). These two so-called current density reconstruction methods were selected because they allow many distributed sources to be concurrently active as opposed to assuming a limited number of dipolar sources which are in general a too simple source model for grid recordings (Fuchs et al. 2007). For reviews on source reconstruction methods in general, see Fuchs et al. (1999) and Yao and Dewald (2005).
sLORETA (Pascual-Marqui 2002) is a post-processing step applied to the results of MNLS that computes (for each location) the squared current strength divided by its associated variance, yielding F-scores of activation. sLORETA has been shown to provide higher localization accuracy for point sources than MNLS (Wagner et al. 2004).
Magnetic Resonance Images (MRI) including a T1-weighted spoiled gradient echo (SPGR) image were obtained preoperatively (Fig. 1b). The SPGR sequence generates 124 contiguous 1.5 mm sections of the entire head in the coronal plane using a 35/5/1 (TR/TE/NEX) pulse sequence, flip angle of 35 degrees, matrix size of 256 × 128 and field of view of 240 × 240 mm2. For electrode localization on the brain surface, planar X-ray images (lateral and anteroposterior) were acquired with the subdural electrodes in place; three metallic fiducial markers were placed at anatomically well-defined locations on the patient’s head for co-registration of the X-ray image with the SPGR MRI (Fig. 1a) (Juhasz et al. 2009). A three-dimensional brain surface image was then created with the location of electrodes directly defined on the brain surface, as previously described (Muzik et al. 2007).
Physical anatomy along with positions of grid electrodes (Fig. 1c) was obtained from individual MRIs and X-ray images co-registered to these MRIs. For volume conductor modelling, different head tissue compartment borders (brain, skull and scalp) were obtained based on individual MRIs, using the automated tools implemented in the CURRY software. Within these compartments, conductivities may be assumed to be homogeneous, isotropic and ohmic. Therefore, the boundary element method (BEM) was used to solve the forward problem (Fuchs et al. 1998; Fuchs et al. 2001). However, in this study only one compartment (brain) was used (Fig. 1d), since data acquisition electrodes were placed directly on the surface of the brain (Fuchs et al. 2007).
Current density reconstruction algorithms compute brain activity on an exhaustive set of predefined anatomical locations (nodes), also called the source space. The subject-specific source space models utilized here are high-resolution (approximately 5,000 nodes) three-dimensional representations of the spatially smoothed cortical sheet, segmented from MRI data and triangulated by CURRY software (Wagner 1998). Such a spatially smoothed source space (as opposed to the individual folded cortical sheet) was used in order to avoid the still not established issue of whether grid recordings allow one to define source depth reliably at all. Consequently, source orientations were not anatomically constrained, accounting for the lack of information with regards to neuronal orientation contained in the smoothed source space model (Fig. 1e). Reconstructed source activity was thresholded to segregate the primary locus of neuronal activity that maximally contributed to the measured response at the latency of 50 ms (Fig. 1h). Source localisation results were finally superimposed onto the subjects’ individual MR (Fig. 1i).
Results
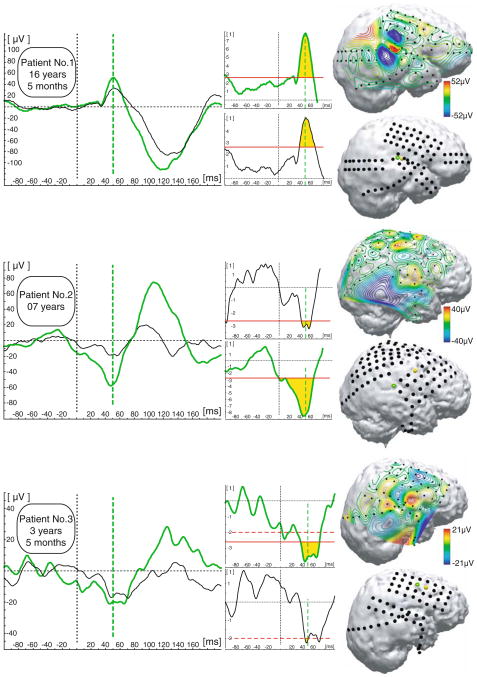
ERP analyses of brain responses to repetitive 600 Hz tones found statistically (P < 0.01) different from zero responses at the latency around 50 ms in all three subjects. A statistically significant P50 response was present in at least several electrode locations in every subject. Representative examples of the most prominent auditory ERPs occurring around 50 ms and their corresponding statistical evaluations are presented in Fig. 2. In the 16-year-old child, electrodes with the most prominent ERP deflection around 50 ms were clustered around the right superior temporal gyrus; whereas, in the 3-year-old child, the most prominent response was recorded over the right frontal lobe. The 7-year-old child had prominent responses recorded over both frontal and superior temporal areas (Fig. 2).
Fig. 2.
ERP results. Representative examples (left panel) of most prominent ERP responses recorded at the latency around 50 ms (marked as a green vertical dashed line). Middle panel is graphical representation of t-test against zero for two representative examples (vertical axis: values from the t distribution). Red horizontal line threshold for P = 0.01 (dashed P = 0.05) for given degree of freedom. Yellow area under the curve determine latency window in which ERP waveform is significantly different from the zero. Right panel voltage grid distribution and corresponding localisation of electrodes for each patient; yellow marks indicate positions of most prominent ERP responses for the representative examples at the left panel (green circle marks position of the electrode response from which response is depicted with green waveform)
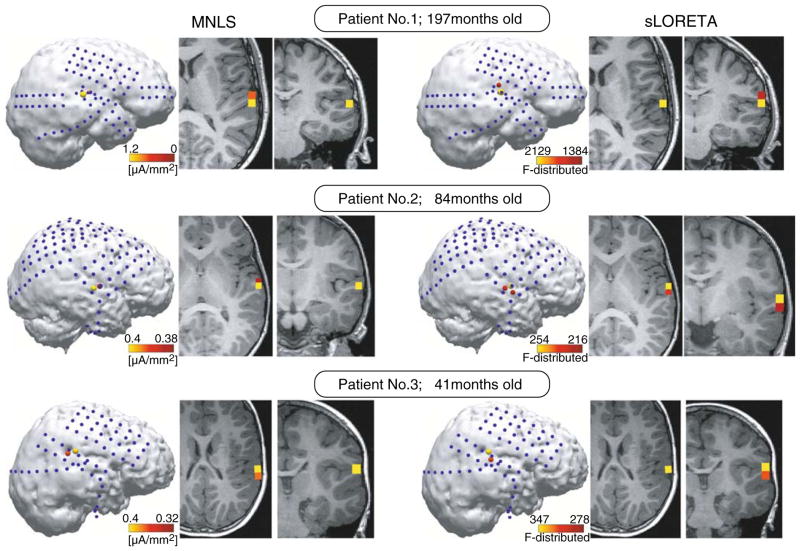
As shown in Fig. 3, both MNLS and sLORETA algorithms reconstructed neuronal sources at the same brain areas explaining 80–85% variance in the data. In all three children, the maximum of activity contributing to the response around 50 ms were localised at the lateral part of Heschl’s gyrus (HG).
Fig. 3.
Source reconstruction results of neuronal activity that maximally contributes the brain response at the 50 ms latency. Left three panels demonstrate MNLS source reconstruction results; right three panels demonstrate sLORETA results
Discussion
Several studies suggest that neuronal circuitry underlying auditory P50 in adults includes temporal and frontal generators contributing to scalp recorded P50 in adults (Weisser et al. 2001; Korzyukov et al. 2007; Knott et al. 2009).
Initial MEG attempts to localize P50/M50 sources in adults (Huotilainen et al. 1998; Reite et al. 1988) suggested that, along with the conventionally observable pair of bilateral supratemporal sources, one or more additional generator(s) may contribute to the P50 response recorded by scalp EEG in adults (Edgar et al. 2003). Indeed, studies suggested that the P50 is an overlapping potential (Onitsuka et al. 2000) that might receive contributions from another hypothesized source (presumably frontal; Grunwald et al. 2003; Mears et al. 2006; Weisser et al. 2001) that apparently is not detected reliably by MEG due to its radial orientations with respect to the MEG sensors. Our recent intracranial study (Korzyukov et al. 2007) in adults demonstrated that changes in frontal lobe activity are indeed associated with reduction of P50 amplitude when the same auditory stimulus is presented within 500 ms after the first one. Moreover it was shown that P50 reduction is significantly correlated with frontal functions (verbal fluency; complex working memory) assessed by neuropsychological tests (Thomas et al. 2009).
Study of auditory response around 50 ms in young children presented here provides new insights in the progenitors of primary, temporal lobe generators of P50 in adults. Although these data should be interpreted with caution, as more subjects are needed before any firm conclusions can be drawn, current results raise the possibility that human auditory system already at 3 year 5 months of age generates auditory processing related responses at the latencies between 35 and 60 ms. This suggests that biological functions that can be attributed to the P50 response in adults and its progenitors (at the latency ~50 ms) in young children are very essential (ontogenetically) for human survival and, therefore, are present (at least to some extent) in the auditory system at a young age.
Although the mainstream of maturational studies of scalp-recorded auditory ERP on healthy children is focused on the amplitude and latency changes of P1, in the results of a large scale study (total n = 137; see: Ponton et al. 2000), where auditory ERP in 12 homogeneous age groups ranging from 5 to 17 years were analysed, quite identifiable ERP responses can be seen (most prominent as a positive peak at the side contralateral to stimulation; see Ponton et al. (2000), Fig. 1, see C6 electrode) at the latency of 50 ms. These responses were present basically in all ages from 5 to 17 years. In line with this observation in healthy children, our results also demonstrate that even at the fast presentation rates (inter-stimulus interval less than 1 s) brain responses around 50 ms can be measured as a separate neurophysiological reaction to auditory stimuli beginning from 4 years of age.
As a noteworthy developmental distinction in Ponton et al. (2000) data, the response at 50 ms inverts its polarity on the corresponding mastoid electrode only in children 13 years of age and older (see age groups 13, 14, 15 and 16 years old; Ponton et al. (2000), Fig. 1). Similarly, Bishop et al. (2007) also showed that shapes of scalp-recorded auditory ERP waveform can be clearly distinguished between children before and after 12–13 years of age. These developmental changes might be explained by the results from anatomical studies. Investigations of axonal maturation demonstrate that by age 11 or 12, the density of mature axons in the auditory cortex has become equivalent to that seen in an adult. Moreover, neurons in the upper cortical layers also form and receive connections from adjacent areas, significantly broadening the scope of intracortical interaction (for review see: Moore and Linthicum 2007).
Consistent with these findings, the results of the present study showed gradual developmental shifts in localisation of most prominent responses around 50 ms from frontal lobe electrodes (in Patient 3; 3-year-old child) to the superior temporal area (in Patient 1; 16-year-old child). These developmental changes can be a manifestation of developmental changes in frontal lobe activity that contribute to P50 generation in adult brain.
Although the role of fronto-temporal interaction in P50 generation is matter of debate, a similar pattern of relationships can be seen in the present study and in studies of the adult P50 (Knott et al. 2009; Korzyukov et al. 2007): namely, changes in frontal lobe activity (caused by developmental changes or experimental manipulations in adults) affect auditory responses originating from the temporal lobe at around 50 ms.
Our brain localisation findings suggest that auditory processing at 50 ms latency in children likely occurs in the same neuronal networks of HG that are primary contributors to the temporal fraction of the P50 complex in adults.
Intracranial recordings in adults using cylindrical depth electrodes (with inversion of the response polarity used as a localisation criterion) suggested that auditory responses within a 35–60 ms latency range are distributed medio-laterally in HG (Godey et al. 2001; Liegeois-Chauvel et al. 1994). Maps derived from multi-contact subdural grid arrays have shown that the first positive auditory responses recorded in the adult posterior lateral superior temporal area (a portion of the “auditory parabelt” or a portion of Brodmann’s area 22) span the P50 peak (Howard et al. 2000). Distributed source estimates derived from deep cylindrical multi-contact electrodes implanted in HG, planum temporale, and superior temporal gyrus (STG) suggest that, after posterior–anterior and medio-lateral propagation of activity in HG and STG, the focal activity around 50 ms originated from several areas of the lateral belt region and possibly the parabelt in the STG (Yvert et al. 2005).
The cumulative findings from these studies are in accordance with source localisation of P50 to the lateral part of HG for the 16-year-old child (Patient 1) in the present study. Moreover, P50 localisation results from the oldest child (Patient 1) are in line with the results of an intracranial distributed source localisation study of P50 in adults (Korzyukov et al. 2007). The noteworthy finding in the present source localisation results is that children younger than 10 years of age have brain responses localised at approximately the same lateral part of HG as in the 16-year-old child.
It should be noted that, as with any other methodological approach, intracranial ECoG recording from the human cerebral cortex has certain limitations. For example, the recorded signals can characterize neuronal activity that mainly originated from or propagated through the cortical areas that are covered by the electrode array.
Although functional significance of P50 is not fully understood, several studies (Chait et al. 2004; Hertrich et al. 2000) have suggested that neuronal activity during the P50 time window might play a role in auditory input change detection and reflects brain response caused by any kind of incoming acoustic information. In contrast, successive brain activity (around the N1 time window) reflects later, more specialized stages of processing, possibly related to percept formation (Roberts et al. 2000), object discrimination (Murray et al. 2006) and memory (Conley et al. 1999; Lu et al. 1992).
Results of the present study give empirical support to the following hypotheses: (1) progenitors of the primary, temporal lobe neuronal generators of auditory P50 ERP component are functionally active at the age of 3–5 years; (2) biological mechanisms underlying cognitive auditory functions that can be attributed to this component in adults might be at least partially developed beginning from the age of 3–5 years; (3) some of the functional characteristics (for example orientation of neuronal generators or outcome of fronto-temporal interaction) of the 50 ms response might undergo maturational transformation after age 10–12 years; (4) frontal lobe activity might affect temporal lobe generators of auditory P50.
For elucidation of developmental mechanisms of central auditory function as well as for paediatric neurosurgery, it is essential to perform an age-specific, functionally characterized, refined anatomical mapping of auditory cortical areas that are involved in sequential stages of auditory processing. The initial step in making this mapping possible is identification and localisation of brain responses that, in spite of individual variability in brain geometry, can be used as age-independent, electrophysiological reference points for the localisation of any other event-related brain responses associated with auditory scene analyses.
The present results suggest that, during identification of auditory areas in paediatric neurosurgery practice, the auditory response originating from lateral HG at around 50 ms may be used as an age-independent landmark for refined cortical mapping of brain activity associated with auditory processing.
Contributor Information
Oleg Korzyukov, Email: okorzyuk@med.wayne.edu, Carman and Ann Adams Department of Pediatrics, Children’s Hospital of Michigan, Wayne State University, Detroit, MI 48201, USA. MRC Cognition & Brain Sciences Unit, 15 Chaucer Road, Cambridge CB2 7EF, UK.
Eishi Asano, Carman and Ann Adams Department of Pediatrics, Children’s Hospital of Michigan, Wayne State University, Detroit, MI 48201, USA. Department of Neurology, Children’s Hospital of Michigan, Wayne State University, Detroit, MI 48201, USA.
Valentina Gumenyuk, Department of Neurology, Henry Ford Hospital, Detroit, MI 48202, USA.
Csaba Juhász, Carman and Ann Adams Department of Pediatrics, Children’s Hospital of Michigan, Wayne State University, Detroit, MI 48201, USA. Department of Neurology, Children’s Hospital of Michigan, Wayne State University, Detroit, MI 48201, USA.
Michael Wagner, Compumedics Neuroscan, Heussweg 25, 20255 Hamburg, Germany.
Robert D. Rothermel, Department of Psychiatry and Psychology, Children’s Hospital of Michigan, Detroit, MI 48201, USA
Harry T. Chugani, Carman and Ann Adams Department of Pediatrics, Children’s Hospital of Michigan, Wayne State University, Detroit, MI 48201, USA. Department of Neurology, Children’s Hospital of Michigan, Wayne State University, Detroit, MI 48201, USA. Department of Radiology, Children’s Hospital of Michigan, Wayne State University, Detroit, MI 48201, USA
References
- Bishop DV, Hardiman M, Uwer R, von Suchodoletz W. Maturation of the long-latency auditory ERP: step function changes at start and end of adolescence. Dev Sci. 2007;10(5):565–575. doi: 10.1111/j.1467-7687.2007.00619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramon E, Rabe-Hesketh S, Sham P, Murray RM, Frangou S. Meta-analysis of the P300 and P50 waveforms in schizophrenia. Schizophr Res. 2004;70(2–3):315–329. doi: 10.1016/j.schres.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Ceponiene R, Rinne T, Naatanen R. Maturation of cortical sound processing as indexed by event-related potentials. Clin Neurophysiol. 2002;113(6):870–882. doi: 10.1016/s1388-2457(02)00078-0. [DOI] [PubMed] [Google Scholar]
- Chait M, Simon JZ, Poeppel D. Auditory M50 and M100 responses to broadband noise: functional implications. Neuroreport. 2004;15(16):2455–2458. doi: 10.1097/00001756-200411150-00004. [DOI] [PubMed] [Google Scholar]
- Conley EM, Michalewski HJ, Starr A. The N100 auditory cortical evoked potential indexes scanning of auditory short-term memory. Clin Neurophysiol. 1999;110(12):2086–2093. doi: 10.1016/s1388-2457(99)00183-2. [DOI] [PubMed] [Google Scholar]
- Edgar JC, Huang MX, Weisend MP, Sherwood A, Miller GA, Adler LE, Canive JM. Interpreting abnormality: an EEG and MEG study of P50 and the auditory paired-stimulus paradigm. Biol Psychol. 2003;65(1):1–20. doi: 10.1016/s0301-0511(03)00094-2. [DOI] [PubMed] [Google Scholar]
- Fuchs M, Drenckhahn R, Wischmann HA, Wagner M. An improved boundary element method for realistic volume-conductor modeling. IEEE Trans Biomed Eng. 1998;45(8):980–997. doi: 10.1109/10.704867. [DOI] [PubMed] [Google Scholar]
- Fuchs M, Wagner M, Kohler T, Wischmann HA. Linear and nonlinear current density reconstructions. J Clin Neurophysiol. 1999;16(3):267–295. doi: 10.1097/00004691-199905000-00006. [DOI] [PubMed] [Google Scholar]
- Fuchs M, Wagner M, Kastner J. Boundary element method volume conductor models for EEG source reconstruction. Clin Neurophysiol. 2001;112(8):1400–1407. doi: 10.1016/s1388-2457(01)00589-2. [DOI] [PubMed] [Google Scholar]
- Fuchs M, Wagner M, Kastner J. Development of volume conductor and source models to localize epileptic foci. J Clin Neurophysiol. 2007;24(2):101–119. doi: 10.1097/WNP.0b013e318038fb3e. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Nishida M, Juhasz C, Muzik O, Sood S, Chugani HT, Asano E. Short-latency median-nerve somatosensory-evoked potentials and induced gamma-oscillations in humans. Brain. 2008;131(Pt 7):1793–1805. doi: 10.1093/brain/awn100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilley PM, Sharma A, Dorman M, Martin K. Developmental changes in refractoriness of the cortical auditory evoked potential. Clin Neurophysiol. 2005;116(3):648–657. doi: 10.1016/j.clinph.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Godey B, Schwartz D, de Graaf JB, Chauvel P, Liegeois-Chauvel C. Neuromagnetic source localization of auditory evoked fields and intracerebral evoked potentials: a comparison of data in the same patients. Clin Neurophysiol. 2001;112(10):1850–1859. doi: 10.1016/s1388-2457(01)00636-8. [DOI] [PubMed] [Google Scholar]
- Grunwald T, Boutros NN, Pezer N, von Oertzen J, Fernandez G, Schaller C, Elger CE. Neuronal substrates of sensory gating within the human brain. Biol Psychiatry. 2003;53(6):511–519. doi: 10.1016/s0006-3223(02)01673-6. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW. Meta-analysis and the science of schizophrenia: variant evidence or evidence of variants? Neurosci Biobehav Rev. 2004;28(4):379–394. doi: 10.1016/j.neubiorev.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Hertrich I, Mathiak K, Lutzenberger W, Ackermann H. Differential impact of periodic and aperiodic speech-like acoustic signals on magnetic M50/M100 fields. Neuroreport. 2000;11(18):4017–4020. doi: 10.1097/00001756-200012180-00023. [DOI] [PubMed] [Google Scholar]
- Howard MA, Volkov IO, Mirsky R, Garell PC, Noh MD, Granner M, Damasio H, Steinschneider M, Reale RA, Hind JE, et al. Auditory cortex on the human posterior superior temporal gyrus. J Comp Neurol. 2000;416(1):79–92. doi: 10.1002/(sici)1096-9861(20000103)416:1<79::aid-cne6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Huang MX, Edgar JC, Thoma RJ, Hanlon FM, Moses SN, Lee RR, Paulson KM, Weisend MP, Irwin JG, Bustillo JR, et al. Predicting EEG responses using MEG sources in superior temporal gyrus reveals source asynchrony in patients with schizophrenia. Clin Neurophysiol. 2003;114(5):835–850. doi: 10.1016/s1388-2457(03)00041-5. [DOI] [PubMed] [Google Scholar]
- Huotilainen M, Winkler I, Alho K, Escera C, Virtanen J, Ilmoniemi RJ, Jaaskelainen IP, Pekkonen E, Naatanen R. Combined mapping of human auditory EEG and MEG responses. Electro-encephalogr Clin Neurophysiol. 1998;108(4):370–379. doi: 10.1016/s0168-5597(98)00017-3. [DOI] [PubMed] [Google Scholar]
- Jerger J, Chmiel R, Glaze D, Frost JD., Jr Rate and filter dependence of the middle-latency response in infants. Audiology. 1987;26(5):269–283. doi: 10.3109/00206098709081555. [DOI] [PubMed] [Google Scholar]
- Juhasz C, Asano E, Shah A, Chugani DC, Batista CE, Muzik O, Sood S, Chugani HT. Focal decreases of cortical GABA(A) receptor binding remote from the primary seizure focus: what do they indicate? Epilepsia. 2009;50(2):240–250. doi: 10.1111/j.1528-1167.2008.01721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott V, Millar A, Fisher D. Sensory gating and source analysis of the auditory P50 in low and high suppressors. Neuroimage. 2009;44(3):992–1000. doi: 10.1016/j.neuroimage.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Korzyukov O, Pflieger ME, Wagner M, Bowyer SM, Rosburg T, Sundaresan K, Elger CE, Boutros NN. Generators of the intracranial P50 response in auditory sensory gating. Neuroimage. 2007;35(2):814–826. doi: 10.1016/j.neuroimage.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liegeois-Chauvel C, Musolino A, Badier JM, Marquis P, Chauvel P. Evoked potentials recorded from the auditory cortex in man: evaluation and topography of the middle latency components. Electroencephalogr Clin Neurophysiol. 1994;92(3):204–214. doi: 10.1016/0168-5597(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Lu ZL, Williamson SJ, Kaufman L. Behavioral lifetime of human auditory sensory memory predicted by physiological measures. Science. 1992;258(5088):1668–1670. doi: 10.1126/science.1455246. [DOI] [PubMed] [Google Scholar]
- McGee T, Kraus N. Auditory development reflected by middle latency response. Ear Hear. 1996;17(5):419–429. doi: 10.1097/00003446-199610000-00008. [DOI] [PubMed] [Google Scholar]
- Mears RP, Klein AC, Cromwell HC. Auditory inhibitory gating in medial prefrontal cortex: single unit and local field potential analysis. Neuroscience. 2006;141(1):47–65. doi: 10.1016/j.neuroscience.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Moore JK, Linthicum FH., Jr The human auditory system: a timeline of development. Int J Audiol. 2007;46(9):460–478. doi: 10.1080/14992020701383019. [DOI] [PubMed] [Google Scholar]
- Murray MM, Camen C, Gonzalez Andino SL, Bovet P, Clarke S. Rapid brain discrimination of sounds of objects. J Neurosci. 2006;26(4):1293–1302. doi: 10.1523/JNEUROSCI.4511-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzik O, Chugani DC, Zou G, Hua J, Lu Y, Lu S, Asano E, Chugani HT. Multimodality data integration in epilepsy. Int J Biomed Imaging. 2007;2007:13963. doi: 10.1155/2007/13963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onitsuka T, Ninomiya H, Sato E, Yamamoto T, Tashiro N. The effect of interstimulus intervals and between-block rests on the auditory evoked potential and magnetic field: is the auditory P50 in humans an overlapping potential? Clin Neurophysiol. 2000;111(2):237–245. doi: 10.1016/s1388-2457(99)00241-2. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD. Standardized low-resolution brain electromagnetic tomography (sLORETA) technical details. Methods Find Exp Clin Pharmacol. 2002;24(Suppl D):5–12. [PubMed] [Google Scholar]
- Picton TW, Taylor MJ. Electrophysiological evaluation of human brain development. Dev Neuropsychol. 2007;31(3):249–278. doi: 10.1080/87565640701228732. [DOI] [PubMed] [Google Scholar]
- Ponton CW, Eggermont JJ, Kwong B, Don M. Maturation of human central auditory system activity: evidence from multi-channel evoked potentials. Clin Neurophysiol. 2000;111(2):220–236. doi: 10.1016/s1388-2457(99)00236-9. [DOI] [PubMed] [Google Scholar]
- Reite M, Teale P, Zimmerman J, Davis K, Whalen J. Source location of a 50 msec latency auditory evoked field component. Electroencephalogr Clin Neurophysiol. 1988;70(6):490–498. doi: 10.1016/0013-4694(88)90147-2. [DOI] [PubMed] [Google Scholar]
- Roberts TP, Ferrari P, Stufflebeam SM, Poeppel D. Latency of the auditory evoked neuromagnetic field components: stimulus dependence and insights toward perception. J Clin Neurophysiol. 2000;17(2):114–129. doi: 10.1097/00004691-200003000-00002. [DOI] [PubMed] [Google Scholar]
- Spencer SS, Sperling MR, Shewmon DA. Intracranial electrodes. In: Engel J, Pedley TA, editors. Epilepsy: a comprehensive textbook. Lippincott-Raven; New York: 1997. pp. 1719–1747. [Google Scholar]
- Takeshita K, Nagamine T, Thuy DH, Satow T, Matsuhashi M, Yamamoto J, Takayama M, Fujiwara N, Shibasaki H. Maturational change of parallel auditory processing in school-aged children revealed by simultaneous recording of magnetic and electric cortical responses. Clin Neurophysiol. 2002;113(9):1470–1484. doi: 10.1016/s1388-2457(02)00202-x. [DOI] [PubMed] [Google Scholar]
- Thomas C, Vom Berg I, Rupp A, Seidl U, Schroder J, Roesch-Ely D, Kreisel SH, Mundt C, Weisbrod M. P50 gating deficit in Alzheimer dementia correlates to frontal neuropsychological function. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2008.05.002. (in press) [DOI] [PubMed] [Google Scholar]
- Trainor LJ, Shahin A, Roberts LE. Effects of musical training on the auditory cortex in children. Ann N Y Acad Sci. 2003;999:506–513. doi: 10.1196/annals.1284.061. [DOI] [PubMed] [Google Scholar]
- Wagner M. Rekonstruktion neuronaler Ströme aus bioelektrischen und biomagnetischen Messungen auf der aus MR-Bildern segmentierten Hirnrinde. Shaker Verlag; Aachen: 1998. [Google Scholar]
- Wagner M, Fuchs M, Kastner J. Evaluation of sLORETA in the presence of noise and multiple sources. Brain Topogr. 2004;16(4):277–280. doi: 10.1023/b:brat.0000032865.58382.62. [DOI] [PubMed] [Google Scholar]
- Wehner DT, Hamalainen MS, Mody M, Ahlfors SP. Head movements of children in MEG: quantification, effects on source estimation, and compensation. Neuroimage. 2008;40(2):541–550. doi: 10.1016/j.neuroimage.2007.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisser R, Weisbrod M, Roehrig M, Rupp A, Schroeder J, Scherg M. Is frontal lobe involved in the generation of auditory evoked P50? Neuroreport. 2001;12(15):3303–3307. doi: 10.1097/00001756-200110290-00031. [DOI] [PubMed] [Google Scholar]
- Wunderlich JL, Cone-Wesson BK. Maturation of CAEP in infants and children: a review. Hear Res. 2006;212(1–2):212–223. doi: 10.1016/j.heares.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Yao J, Dewald JP. Evaluation of different cortical source localization methods using simulated and experimental EEG data. Neuroimage. 2005;25(2):369–382. doi: 10.1016/j.neuroimage.2004.11.036. [DOI] [PubMed] [Google Scholar]
- Yvert B, Fischer C, Bertrand O, Pernier J. Localization of human supratemporal auditory areas from intracerebral auditory evoked potentials using distributed source models. Neuroimage. 2005;28(1):140–153. doi: 10.1016/j.neuroimage.2005.05.056. [DOI] [PubMed] [Google Scholar]