Abstract
7-Dehydrocholesterol (7-DHC) accumulates in tissues and fluids of patients with Smith-Lemli-Opitz syndrome (SLOS), which is caused by mutations in the gene encoding 3β-hydroxysterol-Δ7-reductase (DHCR7). We recently reported that 7-DHC is the most reactive lipid molecule toward free radical oxidation (lipid peroxidation) and 14 oxysterols have been identified as products of oxidation of 7-DHC in solution. As the high oxidizability of 7-DHC may lead to systemic oxidative stress in SLOS patients, we report here lipid biomarkers of oxidative stress in a Dhcr7-KO mouse model of SLOS, including oxysterols, isoprostanes (IsoPs), and neuroprostanes (NeuroPs) that are formed from the oxidation of 7-DHC, arachidonic acid and docosahexaenoic acid, respectively. In addition to a previously described oxysterol, 3β,5α-dihydroxycholest-7-en-6-one (DHCEO), we provide evidence for the chemical structures of three new oxysterols in the brain and/or liver tissue of Dhcr7-KO mice, two of which were quantified. We find that levels of IsoPs and NeuroPs are also elevated in brain and/or liver tissues of Dhcr7-KO mice relative to matching WT mice. While IsoPs and NeuroPs have been established as a reliable measurement of lipid peroxidation and oxidative stress in vivo, we show that in this genetic SLOS mouse model, 7-DHC-derived oxysterols are present at much higher levels than IsoPs and NeuroPs and thus are better markers of lipid oxidation and related oxidative stress.
Introduction
Free radical oxidation of lipids (lipid peroxidation or autoxidation) has been suggested to play an important role in the pathophysiology of many human diseases (Esterbauer et al 1992; Berliner and Heinecke 1996; Antczak et al 1997; Montuschi et al 1999a; Simonian and Coyle 1996; Sayre et al 1997; Fahn and Cohen 1992; Yoritaka et al 1996; Brown and Jessup 1999; Montine et al 2004; Bjorkhem et al 2009). The propensity for peroxidation depends on the rate constants for propagation of the chain reaction, which we recently measured for various polyunsaturated fatty acids (PUFAs) and sterols in solution and in model membranes employing a “peroxyl radical clock” method (Xu et al 2009; Yin et al 2011). We found that the rate constants of PUFA peroxidation depend on the number of bisallylic methylene (-CH2-) groups with docosahexaenoic acid (DHA) being the most reactive PUFA with five -CH2- groups, followed by eicosapentaenoic acid (EPA) with four, and arachidonic acid (AA) with three. Unexpectedly, we discovered that 7-dehydrocholesterol (7-DHC) has the largest propagation rate constant for oxidation of any lipid studied. The rate constant for 7-DHC, 2260 M−1 s−1 in solution, is 11 times that of AA, seven times that of DHA, and 200 times that of cholesterol (Xu et al 2009). Thus, it is reasonable to expect that a consequence of the unusual oxidizability of 7-DHC would be the formation of elevated levels of its oxidation products in tissues and fluids where local concentrations of this sterol are high.
Elevated levels of 7-DHC (along with reduced levels of cholesterol) are observed in tissues and fluids of patients with Smith-Lemli-Opitz syndrome (SLOS, OMIM 270400) (Tint et al 1994; Tint et al 1995; Haas et al 2007; Kelley 1995). SLOS is caused by mutations in the gene encoding 3β-hydroxysterol-Δ7-reductase (DHCR7; EC 1.3.1.21), the enzyme that catalyzes the reduction of 7-DHC to cholesterol in the last step of cholesterol biosynthesis (Tint et al 1994; Irons et al 1993; Krakowiak et al 2000; Kelley and Hennekam 2000; Porter and Herman 2011). This genetic defect is manifested as a broad spectrum of phenotypes, including multiple congenital malformations, neurological defects, photosensitivity, mental retardation, and autism-like behavior (Kelley and Hennekam 2000; Porter and Herman 2011; Charman et al 1998; Sikora et al 2006; Bukelis et al 2007). Presence of oxidative stress has been implicated in cell and animal models of SLOS (Richards et al 2006; Valencia and Kochevar 2006; Valencia et al 2006), which could be caused by the high oxidizability of 7-DHC. Our recent work has shown that one of the 7-DHC-derived oxysterols, 3β,5α-dihydroxy cholest-7-en-6-one (DHCEO), is present in cell and animal models of SLOS (including the Dhcr7-KO mouse that is used in this study) (Xu et al 2011a, b). We proposed that DHCEO is a good biomarker for the peroxidation of 7-DHC and we seek to determine if it is a good biomarker of endogenous oxidative stress in SLOS.
Isoprostanes (IsoPs) and neuroprostanes (NeuroPs) are well-established biomarkers of endogenous oxidative stress in tissues and fluids (Morrow 2000; Milne et al 2011). IsoPs are a class of chemically stable compounds that are formed from free radical oxidation of AA and these compounds are generally accepted as the “gold standard” for measurement of oxidative injury in vivo. NeuroPs are a similar class of compounds formed from DHA, and are thus important in assessing the oxidative injury in brains where DHA is a major component of the lipid pool (Milne et al 2011; Roberts et al 1998; Yin et al 2005). Elevated levels of IsoPs have been reported in a number of diseases or conditions, such as asthma (Montuschi et al 1999b), atherosclerosis (Pratico et al 1998a), Alzheimer (Pratico et al 1998b), Huntington (Montine et al 2004), and autism (Ming et al 2005). The facts that IsoPs and NeuroPs are generally considered to be good reporters of systemic oxidative stress and 7-DHC is highly oxidizable suggest that it is essential to assess IsoPs and NeuroPs as biomarkers for 7-DHC-induced oxidative injuries.
We report here analyses in the central nervous system (CNS) and liver of developing Dhcr7-KO mice for: 1) the 7-DHC-derived oxysterols (DHCEO, 4α-hydroxy-7-DHC, 4β-hydroxy-7-DHC, and 24-hydroxy-7-DHC); 2) the quantification of these 7-DHC-derived oxysterols; 3) the fatty acid profile and quantification of total phospholipid; 4) the measurements of IsoPs and NeuroPs; and 5) the evaluation of oxysterols, IsoPs and NeuroPs as lipid peroxidation biomarkers in this genetic model of SLOS.
Materials and methods
Materials
Unless otherwise noted, all chemicals were purchased from Sigma-Aldrich Co. HPLC grade solvents (hexanes and 2-propanol) were purchased from Thermo Fisher Scientific Inc. Syntheses of [25,26,26,26,27,27,27-d7]-7-DHC, [25,26,26,26,27,27,27-d7]-DHCEO, 4α-hydroxy-7-DHC, 4β-hydroxy-7-DHC, and 7-ketocholesterol were described elsewhere (Xu et al 2011a, b). NH2-SPE cartridges (55 μm, 70 Å, 500 mg/3 mL) were purchased from Phenomenex, Inc.
Dhcr7-KO Mice
Dhcr7-KO (Dhcr7tm1Gst/J) mice were purchased from Jackson Laboratories (catalogue # 007453). All experimental procedures were in accordance with the NIH guidelines for the use of live animals and were approved by the Vanderbilt University Institutional Animal Care and Use Committee. Genotyping and dissection of the tissues were performed as previously described (Xu et al 2011a). The brain and liver tissues were rapidly removed and frozen in pre-cooled methyl-butane and stored at −80°C until analysis of sterols, IsoPs and NeuroPs.
Analysis of fatty acid composition of phospholipids, IsoPs and NeuroPs
Fatty acids were analyzed by GC after lipid extraction, TLC separation, and methylation at the Vanderbilt Lipid Core for fatty acid analysis (Greene et al 1991). IsoPs and NeuroPs were quantified using gas chromatography with negative ionization mass spectrometry with selected ion monitoring in presence of deuterated 15-F2-IsoP (8-iso-PGF2α) and 18O-labelled 17-F4-NeuroP as internal standards at the Eicosanoid Core Laboratory of Vanderbilt University (Morrow et al 1999; Musiek et al 2004). Both IsoP and NeuroP were quantified in the same chromatographic run. The levels of IsoPs and NeuroPs in brain and liver were compared between wild type and Dhcr7-KO mice using student’s t-test.
Lipid extraction and HPLC-MS-MS analysis of sterols and oxysterols in brain and liver
The procedure was carried out as described previously (Xu et al 2011a, 2012a). Briefly, each collected tissue was homogenized in lysis buffer using blade homogenizer and the protein concentration was determined using Protein Dc photometric assay (BioRad). To the homogenate were added Folch’s solution (5 mL; chloroform/methanol=2/1 containing 0.001 M BHT and PPh3), aqueous NaCl solution (0.9 %, 1 mL), and an appropriate amount of d7-DHCEO standard. The bottom organic phase was collected, dried under nitrogen, re-dissolved in methylene chloride (500 μL) and subject to separation on NH2-SPE (500 mg column; condition with 4 mL of hexanes → load sample → elute with 4 mL of chloroform/2-propanol (2/1) to collect the neutral lipids containing oxysterols). The eluted fraction was then dried under SpeedVac Concentrator and re-constituted in methylene chloride (400 μL) for HPLC-APCI-MS-MS analyses (HPLC conditions: Silica 150× 4.6 mm column (Phenomenex, Inc.); 3 μm; 1.0 mL/min; elution solvent: 10 % 2-propanol in hexanes). Mass spectrometry analysis of oxysterol was carried out in the same way as described in detail previously (Xu et al 2011a, b, 2012a, 2010).
Results
Differential accumulation of 7-DHC-derived oxysterols in brain and liver of Dhcr7-KO mice
Using our established method of oxysterol analysis (Xu et al 2011a, 2012a), we determined the profile of 7-DHC-derived oxysterols in the developing Dhcr7-KO mouse brain (Fig. 1 and Fig. 2). Typically, sterol-containing lipid fractions of brain tissues of E20 or P0 mice were analyzed by normal phase (NP) HPLC-APCI-MS-MS. Representative chromatograms are shown in Fig. 2. By comparing the retention time (RT) and MS characteristics with the synthetic (4α-and 4β-hydroxy-7-DHC, and 7-kChol) or isolated standards (24-hydroxy-7-DHC; from brain tissues of AY9944-treated rats – a pharmacological animal model of SLOS) (Xu et al 2011b), we confirmed the presence of 4α- and 4β-hydroxy-7-DHC, and 24-hydroxy-7-DHC (structures shown in Fig. 1) in brain tissues of Dhcr7-KO mice while none of these oxysterols were observed in tissues of WT mice. An unknown product was observed at RT=5.97-min having a retention time and MS fragmentation pattern similar to 7-kChol, but 7-kChol elutes at 5.87-min under the same chromatography conditions, which suggests that 7-kChol is not present at a significant level. A photooxidation product of 7-DHC, 5α,8α-epidioxy-cholest-6-en-3β-ol (EnP(5,8)), was found in KO-samples, but it has been demonstrated to be a product of ex vivo oxidation (Xu et al 2011b).
Fig. 1.
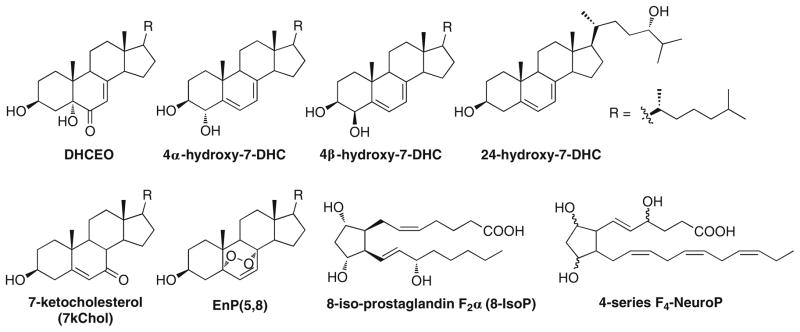
Structures of 7-DHC-derived oxysterols in this study and representative structures of isoprostanes and neuroprostanes. DHCEO, 4α-hydroxy-7-DHC, 4β-hydroxy-7-DHC, and 24-hydroxy-7-DHC are endogenously formed oxysterols observed in brain and/liver of Dhcr7-KO mice. 7-KChol was not observed in either tissue. EnP(5,8) was formed from ex vivo photooxidation of 7-DHC
Fig. 2.
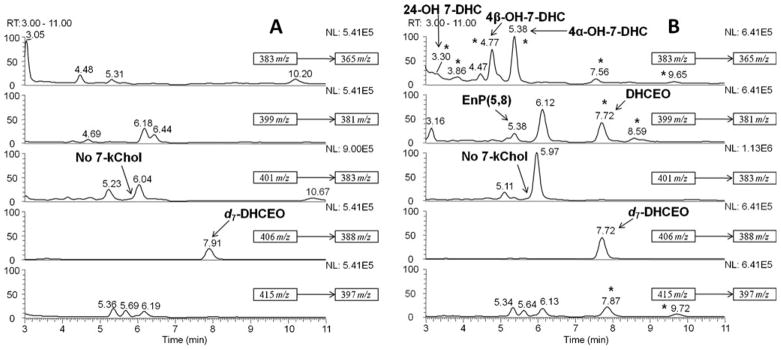
NP-HPLC-APCI-MS-MS (Silica 150×4.6 mm column; 3 μ; 1.0 mL/min; elution solvent: 10 % 2-propanol in hexanes) analysis of the oxysterols from (A) WT and (B) Dhcr7-KO mouse brains at P0. New peaks observed in KO-mice relative to WT are marked with “★”. DHCEO, 4α-hydroxy-7-DHC, 4β-hydroxy-7-DHC, and 24-hydroxy-7-DHC are identified in (B). Only the peaks with known identity were labeled in the figure legend
By the use of the same analytical method, the oxysterol profile of the liver from Dhcr7-KO mice was also elucidated (Fig. 3). 4α- and 4β-Hydroxy-7-DHC were observed as the major oxysterols in liver while DHCEO is a minor one. If present in liver, 24-hydroxy-7-DHC and 7-kChol were at concentrations below our limit of detection.
Fig. 3.
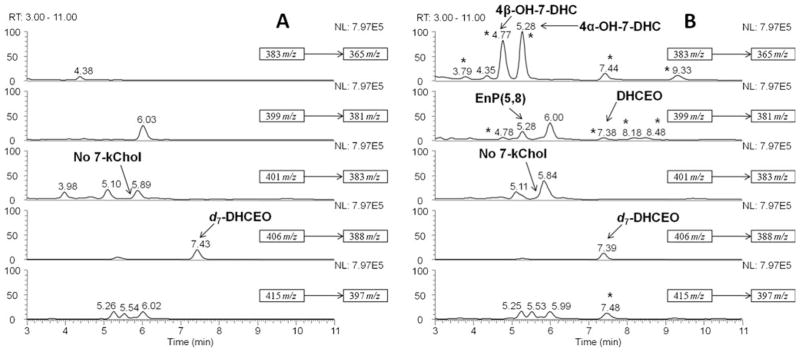
NP-HPLC-APCI-MS-MS (Silica 150×4.6 mm column; 3 μ; 1.0 mL/min; elution solvent: 10 % 2-propanol in hexanes) analysis of the oxysterols from (A) WT and (B) Dhcr7-KO mouse livers at P0. New peaks observed in KO-mice relative to WT are marked with “★”. DHCEO, 4α-hydroxy-7-DHC and 4β-hydroxy-7-DHC are identified in (B). Only the peaks with known identity were labeled in the figure legend
The levels of DHCEO, 4α- and 4β-hydroxy-7-DHC in the whole brain, brain regions, and liver of Dhcr7-KO mice (E20 or P0) were quantified by the same HPLC-MS method using d7-DHCEO as an internal standard. The corresponding levels of Chol and 7-DHC in each tissue were also quantified using d7-Chol and d7-7-DHC as external standards. The results are summarized in Table 1. Brain tissue has the highest cholesterol content when compared to other organs in the body (Chavko et al 1993). Our measurements show that in WT mice, brain tissue accumulates about four times higher levels of cholesterol than liver without a detectable amount of 7-DHC (15 vs. 4.3 μg/mg, Table 1). In KO mice, the levels of 7-DHC in brain are about three times those in liver. While 7-DHC-derived oxysterols are present in both organs of the KO mice, their levels are different. DHCEO is a major 7-DHC-derived oxysterol in the brain, but it is present at much lower levels in the liver. Furthermore, 4α- (16 ng/mg) and 4β-hydroxy-7-DHC (19 ng/mg) show similar levels to those of DHCEO in the brain (20 ng/mg), but both oxysterols are present at much higher levels (40 and 54 ng/mg, respectively) than DHCEO (5 ng/mg) in the liver.
Table 1.
Levels of 4α-hydroxy-7-DHC, 4β-hydroxy-7-DHC, DHCEO, Chol, and 7-DHC in the whole brain, whole liver and different brain regions of E20 or P0 WT or Dhcr7-KO micea
| Whole brain b
|
Brain regions (KO) c
|
Liver b
|
||||||
|---|---|---|---|---|---|---|---|---|
| WT | KO | Cortex | Midbrain | Hippocampus | Cerebellum | WT | KO | |
| 4α-OH-7-DHC-(ng/mg) | 0 | 16±2 | 33±7 | 39±5 | 11±2 | 32±9 | 0 | 40±10 |
| 4β-OH-7-DHC (ng/mg) | 0 | 19±2 | 41±8 | 51±5 | 13±5 | 38±11 | 0 | 54±13 |
| DHCEO (ng/mg) | 0 | 20±3 | 17±2 d | 22±2 d | 5±1 d | 10±2 d | 0 | 5±2 |
| Chol (μg/mg) | 15±3 | 1.7±0.3 | 4.8±0.7 d | 4.2±0.7 d | 6.9±1.7 d | 5.8±0.8 d | 4.3±0.9 | 1.4±0.3 |
| 7-DHC (μg/mg) | 0 | 21±3 | 22±3 d | 37±13 d | 12±3 d | 11±2 d | 0 | 6.3±1.6 |
Standard deviation shown; normalized to per mg of protein.
n=3.
n=4.
from reference Xu et al 2012a
In addition to tissue specific accumulation, there are differences in oxysterol profile between specific brain regions. As shown in Table 1, oxysterols accumulate to different levels in different brain regions, suggesting that the extent of oxidation or sterol metabolism varies between regions. Accumulation of 7-DHC-derived oxysterols in the liver is more pronounced than in the brain, i.e., when normalized to the levels of 7-DHC, total oxysterol levels are ca. 2.6 ng/μg of 7-DHC in brain and ca. 15.7 ng/μg in liver. This observed difference might reflect the intrinsic differences in cholesterol metabolism between liver and the CNS, which are known to have distinct and independent cholesterol metabolisms (Dietschy and Turley 2004; Kalaany and Mangelsdorf 2006).
Biomarkers of endogenous oxidative stress (IsoPs and NeuroPs) are elevated in Dhcr7-KO mice
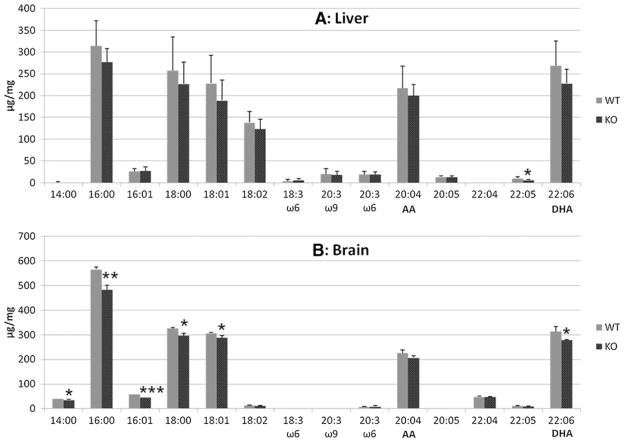
Arachidonic acid (AA) and docosahexaenoic acid (DHA), both PUFAs, are major targets for oxidative damage and their oxidation products can serve as biomarkers of oxidative stress in the tissue. Therefore, we first examined the fatty acid composition of total phospholipids in brain and liver tissues of WT and Dhcr7-KO mice. The results are summarized in Fig. 4. As seen in the figure, overall fatty acid levels appear to be lower in KO-samples than in WT. Even though most of the changes in liver are not statistically significant, there are some statistically significant changes in several fatty acids within brain: 14:0, 16:0, 16:01, 18:0, 18:01, and 22:6. Particularly, DHA (22:6) is lower in brains of KO-mice compared to its level in WT mice.
Fig. 4.
Fatty acid composition of the phospholipids from (A) brains (n=3) and (B) livers (n=7) of WT and Dhcr7-KO P0 mice. The x-axis represents different fatty acids measured with the first number denoting the carbon number of the fatty acid and the second number denoting the number of C=C double bonds. The y-axis represents the levels of each fatty acid that are normalized to wet tissue weight. Statistical analyses were performed with t-test (two-tailed distribution). ★, p< 0.05; ★★, p<0.005; ★★★, p<0.0001
We next evaluated if AA and DHA oxidation products, IsoPs and NeuroPs (representative structures shown in Fig. 1), are different between WT and KO mice. The results are presented in Fig. 5. Overall, levels of IsoPs and NeuroPs were elevated in the livers of Dhcr7-KO mice, suggesting elevated oxidative stress in general in these tissues. In the brain, only the levels of NeuroPs were significantly higher in KO mice than in WT. However, this increase in NeuroPs levels is particularly significant considering that the levels of DHA is in fact lower in the brain of KO mice, further supporting increased oxidative stress in tissues of the KO mice.
Fig. 5.
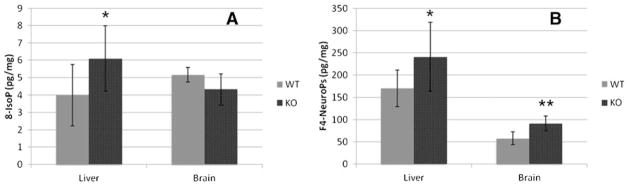
Levels of (A) isoprostanes (IsoPs) and (B) neuroprostanes (NeuroPs) in brains (n=7) and livers (n=12) of WT and Dhcr7-KO P0 mice. The levels of IsoPs and NeuroPs are normalized to wet tissue weight. Statistical analyses were performed with t-test (two-tailed distribution). ★, p=0.01; ★★, p<0.005
Discussion
In the embryonic developing rodent model of SLOS, Dhcr7-KO mice, we report in this study the 7-DHC-derived oxy-sterol profiles for DHCEO, 4α-hydroxy-7-DHC, 4β-hydroxy-7-DHC, and 24-hydroxy-7-DHC in the central nervous system (CNS) and liver. We also report an assessment of IsoP and NeuroP levels in the brain and liver of the Dhcr7-KO mice. The presence and elevation of these lipid oxidation biomarkers strongly suggest that SLOS is a disorder that is characterized by elevated oxidative stress. The Dhcr7-KO mice used here have replacement of exon 8 with the PGK-neo cassette and this results in deletion of 1/3 of the protein from amino acids 318–471. This mouse model mimics the most frequent mutation observed in SLOS patients (IVS8-1G > C) (Fitzky et al 2001). This mutation is not compatible with life in humans in the homozygous state. Similarly, it is lethal in Dhcr7-KO mice and newborn pups die shortly after birth (Fitzky et al 2001; Wassif et al 2001). Highly elevated levels of 7-DHC are observed in tissues of these mice (8-DHC was not detected at a significant level), e.g., the 7-DHC/Chol ratios reach as high as 12 in KO brain tissues and 4–5 in liver (see Table 1), but 7-DHC is not detectable in WT brains (Xu et al 2011a). While the free radical oxidation of 7-DHC in solution gives over a dozen oxidation products (i.e., oxysterols) with novel structures (Xu et al 2010), it was not known which of these oxysterols, if any, form in vivo during the development of the mouse brain.
In addition to the previously reported oxysterol, DHCEO (Xu et al 2011a), we found three other 7-DHC-derived oxysterols in the developing Dhcr7-KO mouse brain. Interestingly, this oxysterol profile is similar to the profile seen in the brain tissue of adult AY9944 (an inhibitor of Dhcr7)-treated rats (Xu et al 2011b). When normalized to tissue weight, the level of DHCEO in Dhcr7-KO mouse brain is ca. 1/3 of the level in AY9944-treated rat brain (Xu et al 2011a, b). On the other hand, the level of 4α-hydroxy-7-DHC or 4β-hydroxy-7-DHC is similar to that of DHCEO in the mouse brain (Table 1), but is ten times that of DHCEO in the rat brain (Xu et al 2011b). In addition to the unique 7-DHC-derived oxysterols that are present in both rodent models and absent in controls, we found highly elevated levels of 7-kChol in AY9944 rats but not in Dhcr7-KO mice. Although 7-kChol is an autoxidation product of cholesterol, it can be formed via enzymatic oxidation of 7-DHC that is catalyzed by cytochrome P450 (CYP) 7A1 (Shinkyo et al 2011). Therefore, although 7-kChol was observed at very low levels in tissues of control rats, this oxysterol is present at significantly higher levels in tissues of AY9944-treated rats (Xu et al 2011b, 2012b). Future studies will inquire if these findings reflect different developmental stages (embryonic vs. adult) and/or difference in metabolism between mouse and rat and/or difference between genetic (mouse) and pharmacological inhibition of Dhcr7 (rat). Regardless of these differences, the fact that those oxysterols were found in both animal models suggests that these compounds can be used as biomarkers of 7-DHC peroxidation or enzymatic oxidation during the early development of brain.
From earlier cell culture experiments we determined that DHCEO is formed from a primary product of 7-DHC peroxidation, 7-DHC-5α,6α-epoxide (Xu et al 2011a). 24-Hydroxy-7-DHC is apparently an enzymatic oxidation product of 7-DHC that is formed via the catalysis of CYP 46A1 (Xu et al 2011b; Lund et al 1999). There is, however, no evidence concerning the mechanism of formation of 4α- and 4β-hydroxy-7-DHC, which could be either an enzymatic or a free radical oxidation (Xu et al 2011b; Bodin et al 2001; Bodin et al 2002; Breuer et al 1996). As shown in Table 1, levels of 7-DHC in the brain are some three times that found in the liver yet there is over twice the amount of 4α- and 4β-hydroxy-7-DHC in the liver than in the brain. This is in contrast to the levels of DHCEO found in the brain and liver of the KO-mice, where the levels of DHCEO are proportional to the levels of 7-DHC. While DHCEO is an apparent peroxidation product of 7-DHC and levels of product correlate with precursor concentrations, the lack of such a correlation for 4α- and 4β-hydroxy-7-DHC suggests a likely enzymatic mechanism for their formation. Interestingly, a small amount of 4β-hydroxycholesterol (the cholesterol analogue of 4β-hydroxy-7-DHC) was also detected in plasma of normal human population (Quehenberger et al 2010). When normalized to the levels of corresponding precursors (7-DHC or cholesterol), the levels of 4β-hydroxy-7-DHC in liver of Dhcr7-KO mice (8.6 μg/mg of 7-DHC) is over 500 times higher than that of 4β-hydroxycholesterol in plasma of normal individuals (0.015 μg/mg of cholesterol), further supporting the high oxidizability of 7-DHC. Future studies will address if these specific 7-DHC-derived oxysterols are found in increased concentrations in persons with SLOS.
In addition to tissue specific differences, the analyses identified region-specific differences in oxysterol accumulation within the developing mouse brain. Cortex and midbrain accumulate more DHCEO than hippocampus and cerebellum. Cortex, midbrain and cerebellum accumulate more 4α- and 4β-hydroxy-7-DHC than hippocampus. From our previous studies we know that DHCEO reduces the neuronal viability and increases arborization of neuronal processes, the biological effects of 4α- and 4β-hydroxy-7-DHC on neurons are unknown and are currently under investigation in our laboratory. What is clear from the current study is that there is significant accumulation of several 7-DHC-derived oxysterols that may significantly alter normal developmental processes in the brain and lead to permanent changes in neuronal connectivity and metabolism. The differential accumulation of oxysterols in different brain regions might shed light on the different neurological defects observed in SLOS patients.
IsoPs and NeuroPs vs. oxysterols as lipid peroxidation biomarkers in SLOS
IsoPs and NeuroPs are minor peroxidation products of their lipid precursors and are formed at very low levels (1–300 pg/mg of tissue), which are 10–1000 times less than those of oxysterols in tissues of the rodent models of SLOS (e.g., 1.4 ng/mg of DHCEO in brain tissue of Dhcr7-KO mice) (Xu et al 2011a, b). Oxysterols can be isolated from a small amount of tissue following straightforward procedures that do not involve derivatization of the analytes. The observation of the much higher levels of metabolically stable oxidation products from 7-DHC than those found from AA and DHA may be a reflection of the relative reactivities of these lipids. In solution, 7-DHC undergoes lipid peroxidation 11 and seven times faster than AA and DHA, respectively (Xu et al 2009). Thus, oxysterols would appear to be preferred biomarkers of oxidative stress in SLOS animal models compared to IsoPs and NeuroPs.
Oxysterols also serve as reliable oxidative stress biomarkers in a number of diseases, such as atherosclerosis (Brown et al 1997), cataract (Pratico et al 1998a), age-related macular degeneration (Rodriguez and Larrayoz 2010), and Niemann-Pick type C1 disease (Porter et al 2010), and they also can exert a variety of biological activities by themselves (Schroepfer 2000; Javitt 2007; Vejux and Lizard 2009; Brown and Jessup 2009; Olkkonen and Hynynen 2009). 7-DHC-derived oxysterols, in particular, have been shown to be toxic to Neuro2a cells and induce similar gene expression changes in normal Neuro2a cells as those observed in Dhcr7-deficient Neuro2a cells (Korade et al 2010). One of the oxysterols that is observed in vivo, DHCEO, was found to be toxic to primary cortical neuronal and glial cells in vitro and to accelerate differentiation and arborization of cortical neurons (Xu et al 2012a). There are other potentially detrimental effects of these 7-DHC-derived oxysterols. For example, structures of 4α and 4β-hydroxy-7-DHC are similar to that of 4-carboxyl sterol and therefore these two oxysterols may interfere with the activities of C4 demethylation complex in the cholesterol biosynthesis pathway (Miller and Gaylor 1970a; Miller and Gaylor 1970b). More importantly, 4β-hydroxycholesterol was reported to be a good ligand for LXRα in an in vitro test, indicating a critical role of the 4-hydroxylated oxysterols in cholesterol homeostasis (Janowski et al 1996).
Clinical implications of oxidative stress biomarkers
While there is compelling evidence of increased oxidative stress in the SLOS rodent models, including reports by Fliesler and co-workers (Richards et al 2006; Vaughan et al 2006) and our recently published studies (Xu et al 2011a, b), there is lack of such studies on SLOS patients. Based on the identification of oxysterols in the serum of the rat SLOS model (Xu et al 2011b), it is very likely that 7-DHC-derived oxysterols will become important biomarkers to monitor the progression of the disease and potential therapeutic outcomes. Recent studies in another disorder of cholesterol metabolism, Niemann-Pick disease, have shown that oxysterols in blood samples from affected individuals are reliable markers of the disease severity and will allow the monitoring of clinical progression and potential beneficial effects of therapies (Porter et al 2010). Therefore, in addition to profiling oxysterols in the blood of individuals affected by SLOS, it is essential to identify the best antioxidant(s) and evaluate their efficacy in the rodent models. While oxysterol profile of serum samples reflects liver metabolism, it is important to analyze the similarities and differences in cholesterol metabolism and oxysterol formation between liver and brain. In addition to being promising biomarkers, reduction of the levels of these oxysterols may lead to improvement of brain function since these oxysterols are detrimental to neuronal and glial cells (Xu et al 2012a) and may cross blood-brain barrier (e.g., 27-hydroxycholesterol can cross the blood-brain barrier to the brain in human) (Leoni et al 2003; Heverin et al 2005). From this aspect, antioxidants that can cross the blood-brain barrier may be particularly beneficial in alleviating the brain-related symptoms in SLOS patients (Packer et al 1997; Agus et al 1997; Wang et al 2002; Yang et al 2005). Based on the current report on the elevated levels of multiple oxysterols during embryonic development in the SLOS mice, future research efforts might be directed toward detecting SLOS at an early embryonic stage and initiating antioxidant treatment in utero.
In summary, the accumulation of high levels of 7-DHC in developing brain of Dhcr7-KO mice results in enzymatic and free radical oxidation of 7-DHC, which in turn leads to the formation of detrimental oxysterols. Furthermore, altered cholesterol biosynthesis and/or resulting accumulation of 7-DHC-derived oxysterols disturbs the typical phospholipid composition of CNS and leads to increased in vivo oxidation of AA and DHA in the liver and/or brain tissues of this genetic SLOS model. Therefore, our current findings suggest that future SLOS therapeutical approaches should include reducing 7-DHC oxidation, as well as reducing 7-DHC accumulation. We suggest that the measurement of oxysterol accumulation may provide a valuable tool for monitoring potential treatment efficacy.
Acknowledgments
The National Institutes of Health (ES013125) (HD064727) (MH079299) supported this work. Z.K appreciates support from the Vanderbilt Kennedy Center for Research on Human Development. The authors thank Vanderbilt Eicosanoid Core Laboratory for the analysis of isoprostanes and neuroprostanes (Dr. Ginger L. Milne) and the MMPC (DK59637) Lipid Core for fatty acid analysis (Carla Harris and Dr. Larry L. Swift).
Abbreviations
- APCI
atmospheric pressure chemical ionization
- BHT
butylated hydroxytoluene
- Chol
cholesterol
- CYP
cytochrome P450
- 7-DHC
7-dehydrocholesterol
- DHCEO
3β,5α-dihydroxycholest-7-en-6-one
- Dhcr7 or DHCR7
7-dehydrocholesterol reductase
- EnP(5,8)
5α,8α-epidioxy-cholest-6-en-3β-ol
- IsoP
isoprostane
- NeuroP
neuroprostane
- 8-IsoP
8-iso-prostaglandin F2α (8-iso-PGF2α)
- 7-kChol
7-ketocholesterol
- KO
knock out
- NP
normal phase
- 4α-OH-7-DHC
4α-hydroxy-7-DHC
- 4β-OH-7-DHC
4β-hydroxy-7-DHC
- 24-OH-7-DHC
24-hydroxy-7-DHC
- PPh3
triphenylphosphine
- SLOS
Smith-Lemli-Opitz syndrome
- SRM
selective reaction monitoring
- WT
wild type
Footnotes
Conflict of interest None.
Contributor Information
Zeljka Korade, Department of Psychiatry and Vanderbilt Kennedy Center for Research on Human Development, Vanderbilt University, Nashville, TN 37235, USA.
Libin Xu, Department of Chemistry and Vanderbilt Institute of Chemical Biology, Nashville, TN 37235, USA.
Karoly Mirnics, Department of Psychiatry and Vanderbilt Kennedy Center for Research on Human Development, Vanderbilt University, Nashville, TN 37235, USA.
Ned A. Porter, Email: n.porter@vanderbilt.edu, Department of Chemistry and Vanderbilt Institute of Chemical Biology, Nashville, TN 37235, USA. Department of Chemistry, 7962 Stevenson Center, Vanderbilt University, Nashville, TN 37235, USA
References
- Agus DB, Gambhir SS, Pardridge WM, et al. Vitamin C crosses the blood-brain barrier in the oxidized form through the glucose transporters. J Clin Invest. 1997;100:2842–2848. doi: 10.1172/JCI119832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antczak A, Nowak D, Shariati B, Krol M, Piasecka G, Kurmanowska Z. Increased hydrogen peroxide and thiobarbituric acid-reactive products in expired breath condensate of asthmatic patients. Eur Respir J. 1997;10:1235–1241. doi: 10.1183/09031936.97.10061235. [DOI] [PubMed] [Google Scholar]
- Berliner JA, Heinecke JW. The role of oxidized lipoproteins in atherogenesis. Free Radical Biol Med. 1996;20:707–727. doi: 10.1016/0891-5849(95)02173-6. [DOI] [PubMed] [Google Scholar]
- Bjorkhem I, Cedazo-Minguez A, Leoni V, Meaney S. Oxysterols and neurodegenerative diseases. Mol Aspects Med. 2009;30:171–179. doi: 10.1016/j.mam.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Bodin K, Bretillon L, Aden Y, et al. Antiepileptic drugs increase plasma levels of 4beta-hydroxycholesterol in humans: evidence for involvement of cytochrome p450 3A4. J Biol Chem. 2001;276:38685–38689. doi: 10.1074/jbc.M105127200. [DOI] [PubMed] [Google Scholar]
- Bodin K, Andersson U, Rystedt E, et al. Metabolism of 4 beta -hydroxycholesterol in humans. J Biol Chem. 2002;277:31534–31540. doi: 10.1074/jbc.M201712200. [DOI] [PubMed] [Google Scholar]
- Breuer O, Dzeletovic S, Lund E, Diczfalusy U. The oxysterols cholest-5-ene-3 beta,4 alpha-diol, cholest-5-ene-3 beta,4 beta-diol and cholestane-3 beta,5 alpha,6 alpha-triol are formed during in vitro oxidation of low density lipoprotein, and are present in human atherosclerotic plaques. Biochim Biophys Acta. 1996;1302:145–152. doi: 10.1016/0005-2760(96)00052-5. [DOI] [PubMed] [Google Scholar]
- Brown AJ, Jessup W. Oxysterols and atherosclerosis. Atherosclerosis. 1999;142:1–28. doi: 10.1016/s0021-9150(98)00196-8. [DOI] [PubMed] [Google Scholar]
- Brown AJ, Jessup W. Oxysterols: Sources, cellular storage and metabolism, and new insights into their roles in cholesterol homeostasis. Mol Aspects Med. 2009;30:111–122. doi: 10.1016/j.mam.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Brown AJ, Leong SL, Dean RT, Jessup W. 7-Hydroperoxycholesterol and its products in oxidized low density lipoprotein and human atherosclerotic plaque. J Lipid Res. 1997;38:1730–1745. [PubMed] [Google Scholar]
- Bukelis I, Porter FD, Zimmerman AW, Tierney E. Smith-Lemli-Opitz syndrome and autism spectrum disorder. Am J Psychiatry. 2007;164:1655–1661. doi: 10.1176/appi.ajp.2007.07020315. [DOI] [PubMed] [Google Scholar]
- Charman CR, Ryan A, Tyrrell RM, et al. Photosensitivity associated with the Smith-Lemli-Opitz syndrome. Br J Dermatol. 1998;138:885–888. doi: 10.1046/j.1365-2133.1998.02231.x. [DOI] [PubMed] [Google Scholar]
- Chavko M, Nemoto EM, Melick JA. Regional lipid composition in the rat brain. Mol Chem Neuropathol. 1993;18:123–131. doi: 10.1007/BF03160026. [DOI] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD. Thematic review series: brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res. 2004;45:1375–1397. doi: 10.1194/jlr.R400004-JLR200. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Gebicki J, Puhl H, Jurgens G. The role of lipid-peroxidation and antioxidants in oxidative modification of Ldl. Free Radical Biol Med. 1992;13:341–390. doi: 10.1016/0891-5849(92)90181-f. [DOI] [PubMed] [Google Scholar]
- Fahn S, Cohen G. The oxidant stress hypothesis in Parkinson’s disease - evidence supporting it. Ann Neurol. 1992;32:804–812. doi: 10.1002/ana.410320616. [DOI] [PubMed] [Google Scholar]
- Fitzky BU, Moebius FF, Asaoka H, et al. 7-Dehydrocholesterol-dependent proteolysis of HMG-CoA reductase suppresses sterol biosynthesis in a mouse model of Smith-Lemli-Opitz/RSH syndrome. J Clin Invest. 2001;108:905–915. doi: 10.1172/JCI12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene HL, Swift LL, Knapp HR. Hyperlipidemia and fatty acid composition in patients treated for type IA glycogen storage disease. J Pediatr. 1991;119:398–403. doi: 10.1016/s0022-3476(05)82052-9. [DOI] [PubMed] [Google Scholar]
- Haas D, Garbade SF, Vohwinkel C, et al. Effects of cholesterol and simvastatin treatment in patients with Smith-Lemli-Opitz syndrome (SLOS) J Inherit Metab Dis. 2007;30:375–387. doi: 10.1007/s10545-007-0537-7. [DOI] [PubMed] [Google Scholar]
- Heverin M, Meaney S, Lutjohann D, Diczfalusy U, Wahren J, Bjorkhem I. Crossing the barrier: net flux of 27-hydroxycholesterol into the human brain. J Lipid Res. 2005;46:1047–1052. doi: 10.1194/jlr.M500024-JLR200. [DOI] [PubMed] [Google Scholar]
- Irons M, Elias ER, Salen G, Tint GS, Batta AK. Defective cholesterol biosynthesis in Smith-Lemli-Opitz syndrome. Lancet. 1993;341:1414. doi: 10.1016/0140-6736(93)90983-n. [DOI] [PubMed] [Google Scholar]
- Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature. 1996;383:728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- Javitt NB. Oxysterols: functional significance in fetal development and the maintenance of normal retinal function. Curr Opin Lipidol. 2007;18:283–288. doi: 10.1097/MOL.0b013e328133851e. [DOI] [PubMed] [Google Scholar]
- Kalaany NY, Mangelsdorf DJ. LXRS and FXR: the yin and yang of cholesterol and fat metabolism. Annu Rev Physiol. 2006;68:159–191. doi: 10.1146/annurev.physiol.68.033104.152158. [DOI] [PubMed] [Google Scholar]
- Kelley RI. Diagnosis of Smith-Lemli-Opitz syndrome by gas chromatography/mass spectrometry of 7-dehydrocholesterol in plasma, amniotic fluid and cultured skin fibroblasts. Clin Chim Acta. 1995;236:45–58. doi: 10.1016/0009-8981(95)06038-4. [DOI] [PubMed] [Google Scholar]
- Kelley RI, Hennekam RC. The Smith-Lemli-Opitz syndrome. J Med Genet. 2000;37:321–335. doi: 10.1136/jmg.37.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korade Z, Xu L, Shelton R, Porter NA. Biological activities of 7-dehydrocholesterol-derived oxysterols: implications for Smith-Lemli-Opitz syndrome. J Lipid Res. 2010;51:3259–3269. doi: 10.1194/jlr.M009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakowiak PA, Nwokoro NA, Wassif CA, et al. Mutation analysis and description of sixteen RSH/Smith-Lemli-Opitz syndrome patients: polymerase chain reaction-based assays to simplify genotyping. Am J Med Genet. 2000;94:214–227. [PubMed] [Google Scholar]
- Leoni V, Masterman T, Patel P, Meaney S, Diczfalusy U, Bjorkhem I. Side chain oxidized oxysterols in cerebrospinal fluid and the integrity of blood-brain and blood-cerebrospinal fluid barriers. J Lipid Res. 2003;44:793–799. doi: 10.1194/jlr.M200434-JLR200. [DOI] [PubMed] [Google Scholar]
- Lund EG, Guileyardo JM, Russell DW. cDNA cloning of cholesterol 24-hydroxylase, a mediator of cholesterol homeostasis in the brain. Proc Natl Acad Sci U S A. 1999;96:7238–7243. doi: 10.1073/pnas.96.13.7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WL, Gaylor JL. Investigation of the component reactions of oxidative sterol demethylation. Oxidation of a 4 alpha-methyl sterol to a 4 alpha-carboxylic acid during cholesterol biosynthesis. J Biol Chem. 1970a;245:5369–5374. [PubMed] [Google Scholar]
- Miller WL, Gaylor JL. Investigation of the component reactions of oxidative sterol demethylation. Oxidation of a 4,4-di-methyl sterol to a 4 beta-methyl-4 alpha-carboxylic acid during cholesterol biosynthesis. J Biol Chem. 1970b;245:5375–5381. [PubMed] [Google Scholar]
- Milne GL, Yin H, Hardy KD, Davies SS, Roberts LJ. Isoprostane generation and function. Chem Rev. 2011;111:5973–5996. doi: 10.1021/cr200160h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming X, Stein TP, Brimacombe M, Johnson WG, Lambert GH, Wagner GC. Increased excretion of a lipid peroxidation biomarker in autism. Prostaglandins Leukot Essent Fatty Acids. 2005;73:379–384. doi: 10.1016/j.plefa.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Montine KS, Quinn JF, Zhang J, et al. Isoprostanes and related products of lipid peroxidation in neurodegenerative diseases. Chem Phys Lipids. 2004;128:117–124. doi: 10.1016/j.chemphyslip.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Montuschi P, Corradi M, Ciabattoni G, Nightingale J, Kharitonov SA, Barnes PJ. Increased 8-isoprostane, a marker of oxidative stress, in exhaled condensate of asthma patients. Am J Respir Crit Care Med. 1999a;160:216–220. doi: 10.1164/ajrccm.160.1.9809140. [DOI] [PubMed] [Google Scholar]
- Montuschi P, Corradi M, Ciabattoni G, Nightingale J, Kharitonov SA, Barnes PJ. Increased 8-isoprostane, a marker of oxidative stress, in exhaled condensate of asthma patients. Am Respir Crit Care Med. 1999b;160:216–220. doi: 10.1164/ajrccm.160.1.9809140. [DOI] [PubMed] [Google Scholar]
- Morrow JD. The isoprostanes: their quantification as an index of oxidant stress status in vivo. Drug Metab Rev. 2000;32:377–385. doi: 10.1081/dmr-100102340. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Zackert WE, Yang JP, et al. Quantification of the major urinary metabolite of 15-F2t-isoprostane (8-iso-PGF2alpha) by a stable isotope dilution mass spectrometric assay. Anal Biochem. 1999;269:326–331. doi: 10.1006/abio.1999.4008. [DOI] [PubMed] [Google Scholar]
- Musiek ES, Cha JK, Yin H, et al. Quantification of F-ring isoprostane-like compounds (F4-neuroprostanes) derived from docosahexaenoic acid in vivo in humans by a stable isotope dilution mass spectrometric assay. J Chromatogr B. 2004;799:95–102. doi: 10.1016/j.jchromb.2003.10.036. [DOI] [PubMed] [Google Scholar]
- Olkkonen VM, Hynynen R. Interactions of oxysterols with membranes and proteins. Mol Aspects Med. 2009;30:123–133. doi: 10.1016/j.mam.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Packer L, Tritschler HJ, Wessel K. Neuroprotection by the metabolic antioxidant alpha-lipoic acid. Free Radical Biol Med. 1997;22:359–378. doi: 10.1016/s0891-5849(96)00269-9. [DOI] [PubMed] [Google Scholar]
- Porter FD, Herman GE. Malformation syndromes caused by disorders of cholesterol synthesis. J Lipid Res. 2011;52:6–34. doi: 10.1194/jlr.R009548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter FD, Scherrer DE, Lanier MH, et al. Cholesterol oxidation products are sensitive and specific blood-based biomarkers for Niemann-Pick C1 disease. Sci Transl Med. 2010;2:56ra81. doi: 10.1126/scitranslmed.3001417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratico D, Tangirala RK, Rader DJ, Rokach J, FitzGerald GA. Vitamin E suppresses isoprostane generation in vivo and reduces atherosclerosis in ApoE-deficient mice. Nat Med. 1998a;4:1189–1192. doi: 10.1038/2685. [DOI] [PubMed] [Google Scholar]
- Pratico D, Lee VM-Y, Trojanowski JQ, Rokach J, Fitzgerald GA. Increased F2-isoprostanes in Alzheimer’s disease: evidence for enhanced lipid peroxidation in vivo. FASEB J. 1998b;12:1777–1783. doi: 10.1096/fasebj.12.15.1777. [DOI] [PubMed] [Google Scholar]
- Quehenberger O, Armando AM, Brown AH, et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res. 2010;51:3299–3305. doi: 10.1194/jlr.M009449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards MJ, Nagel BA, Fliesler SJ. Lipid hydroperoxide formation in the retina: correlation with retinal degeneration and light damage in a rat model of Smith-Lemli-Opitz syndrome. Exp Eye Res. 2006;82:538–541. doi: 10.1016/j.exer.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LJ, 2nd, Montine TJ, Markesbery WR, et al. Formation of isoprostane-like compounds (neuroprostanes) in vivo from docosahexaenoic acid. J Biol Chem. 1998;273:13605–13612. doi: 10.1074/jbc.273.22.13605. [DOI] [PubMed] [Google Scholar]
- Rodriguez IR, Larrayoz IM. Cholesterol oxidation in the retina: implications of 7KCh formation in chronic inflammation and age-related macular degeneration. J Lipid Res. 2010;51:2847–2862. doi: 10.1194/jlr.R004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayre LM, Zelasko DA, Harris PLR, Perry G, Salomon RG, Smith MA. 4-hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer’s disease. J Neurochem. 1997;68:2092–2097. doi: 10.1046/j.1471-4159.1997.68052092.x. [DOI] [PubMed] [Google Scholar]
- Schroepfer GJ., Jr Oxysterols: modulators of cholesterol metabolism and other processes. Physiol Rev. 2000;80:361–554. doi: 10.1152/physrev.2000.80.1.361. [DOI] [PubMed] [Google Scholar]
- Shinkyo R, Xu L, Tallman KA, Cheng Q, Porter NA, Guengerich FP. Conversion of 7-dehydrocholesterol to 7-ketocholesterol is catalyzed by human cytochrome P450 7A1 and occurs by direct oxidation without an epoxide intermediate. J Biol Chem. 2011;286:33021–33028. doi: 10.1074/jbc.M111.282434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikora D, Pettit-Kekel K, Penfield J, Merkens L, Steiner R. The near universal presence of autism spectrum disorders in children with Smith-Lemli-Opitz syndrome. Am J Med Genet. 2006;140A:1511–1518. doi: 10.1002/ajmg.a.31294. [DOI] [PubMed] [Google Scholar]
- Simonian NA, Coyle JT. Oxidative stress in neurodegenerative diseases. Annu Rev Pharmacol Toxicol. 1996;36:83–106. doi: 10.1146/annurev.pa.36.040196.000503. [DOI] [PubMed] [Google Scholar]
- Tint GS, Irons M, Elias ER, et al. Defective cholesterol biosynthesis associated with the Smith-Lemli-Opitz syndrome. N Engl J Med. 1994;330:107–113. doi: 10.1056/NEJM199401133300205. [DOI] [PubMed] [Google Scholar]
- Tint GS, Seller M, Hughes-Benzie R, et al. Markedly increased tissue concentrations of 7-dehydrocholesterol combined with low levels of cholesterol are characteristic of the Smith-Lemli-Opitz syndrome. J Lipid Res. 1995;36:89–95. [PubMed] [Google Scholar]
- Valencia A, Kochevar IE. Ultraviolet A induces apoptosis via reactive oxygen species in a model for Smith-Lemli-Opitz syndrome. Free Radical Biol Med. 2006;40:641–650. doi: 10.1016/j.freeradbiomed.2005.09.036. [DOI] [PubMed] [Google Scholar]
- Valencia A, Rajadurai A, Carle AB, Kochevar IE. 7-Dehydrocholesterol enhances ultraviolet A-induced oxidative stress in keratinocytes: Roles of NADPH oxidase, mitochondria, and lipid rafts. Free Radical Biol Med. 2006;41:1704–1718. doi: 10.1016/j.freeradbiomed.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan DK, Peachey NS, Richards MJ, Buchan B, Fliesler SJ. Light-induced exacerbation of retinal degeneration in a rat model of Smith-Lemli-Opitz syndrome. Exp Eye Res. 2006;82:496–504. doi: 10.1016/j.exer.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vejux A, Lizard G. Cytotoxic effects of oxysterols associated with human diseases: induction of cell death (apoptosis and/or oncosis), oxidative and inflammatory activities, and phospholipidosis. Mol Aspects Med. 2009;30:153–170. doi: 10.1016/j.mam.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Wang Q, Xu J, Rottinghaus GE, et al. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res. 2002;958:439–447. doi: 10.1016/s0006-8993(02)03543-6. [DOI] [PubMed] [Google Scholar]
- Wassif CA, Zhu P, Kratz L, et al. Biochemical, phenotypic and neurophysiological characterization of a genetic mouse model of RSH/Smith–Lemli–Opitz syndrome. Hum Mol Genet. 2001;10:555–564. doi: 10.1093/hmg/10.6.555. [DOI] [PubMed] [Google Scholar]
- Xu L, Davis TA, Porter NA. Rate constants for peroxidation of polyunsaturated fatty acids and sterols in solution and in liposomes. J Am Chem Soc. 2009;131:13037–13044. doi: 10.1021/ja9029076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Korade Z, Porter NA. Oxysterols from free radical chain oxidation of 7-dehydrocholesterol: product and mechanistic studies. J Am Chem Soc. 2010;132:2222–2232. doi: 10.1021/ja9080265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Korade Z, Rosado DA, Liu W, Lamberson CR, Porter NA. An oxysterol biomarker for 7-dehydrocholesterol oxidation in cell/mouse models for Smith-Lemli-Opitz syndrome. J Lipid Res. 2011a;52:1222–1233. doi: 10.1194/jlr.M014498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Liu W, Sheflin LG, Fliesler SJ, Porter NA. Novel oxy-sterols observed in tissues and fluids of AY9944-treated rats - a model for Smith-Lemli-Opitz Syndrome. J Lipid Res. 2011b;52:1810–1820. doi: 10.1194/jlr.M018366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Mirnics K, Bowman AB, et al. DHCEO accumulation is a critical mediator of pathophysiology in a Smith-Lemli-Opitz syndrome model. Neurobiol Dis. 2012a;45:923–929. doi: 10.1016/j.nbd.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Sheflin LG, Porter NA, Fliesler SJ. 7-Dehydrocholesterol-derived oxysterols and retinal degeneration in a rat model of Smith-Lemli-Opitz syndrome. Biochim Biophys Acta. 2012b;1821:877–883. doi: 10.1016/j.bbalip.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Lim GP, Begum AN, et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- Yin H, Musiek E, Gao L, Porter NA, Morrow JD. Regiochemistry of neuroprostanes generated from the peroxidation of docosahexaenoic acid in vitro and in vivo. J Biol Chem. 2005;280:26600–26611. doi: 10.1074/jbc.M503088200. [DOI] [PubMed] [Google Scholar]
- Yin H, Xu L, Porter NA. Free radical lipid peroxidation: mechanisms and analysis. Chem Rev. 2011;111:5944–5972. doi: 10.1021/cr200084z. [DOI] [PubMed] [Google Scholar]
- Yoritaka A, Hattori N, Uchida K, Tanaka M, Stadtman ER, Mizuno Y. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc Natl Acad Sci U S A. 1996;93:2696–2701. doi: 10.1073/pnas.93.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]