Abstract
Relapse is a highly prevalent phenomenon in addiction. This paper examines the new research on identifying biological factors that contribute to addiction relapse risk. Prospective studies examining relapse risk are reviewed, and clinical, biological, and neural factors that predict relapse risk are identified. Clinical factors, patient-related factors, and subjective and behavioral measures such as depressive symptoms, stress, and drug craving all predict future relapse risk. Among biological measures, endocrine measures such as cortisol and cortisol/corticotropin (ACTH) ratio as a measure of adrenal sensitivity and serum brain-derived neurotrophic factor were also predictive of future relapse risk. Among neural measures, brain atrophy in the medial frontal regions and hyperreactivity of the anterior cingulate during withdrawal were identified as important in drug withdrawal and relapse risk. Caveats pertaining to specific drug abuse type and phase of addiction are discussed. Finally, significant implications of these findings for clinical practice are presented, with a specific focus on determining biological markers of relapse risk that may be used to identify those individuals who are most at risk of relapse in the clinic. Such markers may then be used to assess treatment response and develop specific treatments that will normalize these neural and biological sequelae so as to significantly improve relapse outcomes.
Keywords: Addiction relapse, Stress dysregulation, Drug craving, Cortisol, Cortisol/ACTH ratio, Serum BDNF, Anterior cingulate, Biomarkers, Human studies, Biological factors, Vulnerability
Introduction
Addictions are among the most prevalent psychiatric disorders in the world. Nicotine smoking and excessive alcohol use are the top behavioral conditions causing high levels of global disease burden. The chronic, relapsing nature of addictive disorders is a key factor contributing to high disease burden. Although we have US Food and Drug Administration–approved treatments for nicotine, alcohol, and opioid addiction, more than two thirds of individuals are known to relapse after initiating treatment for substance use disorders. Furthermore, there are no validated biological markers to identify those at high risk of relapse. However, several new research advances in the past decade have moved the field closer to understanding the biology of relapse risk. The purpose of this paper is to describe these advances and to indicate the goals for developing indices of relapse risk that may be utilized in the clinic.
Addiction Relapse Vulnerability
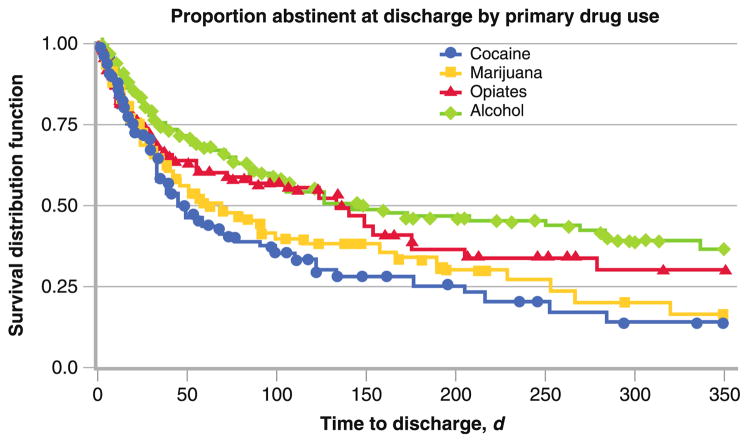
It has long been known that addictive disorders are chronic and relapsing in nature [1, 2]. Recent estimates from clinical treatment studies suggest that more than two thirds of individuals relapse within weeks to months of initiating treatment [3, 4•, 5]. For 1-year outcomes across alcohol, nicotine, weight, and illicit drug abuse, studies show that more than 85% of individuals relapse and return to drug use within 1 year of treatment [2]. Data from 878 patients entering a large, publicly funded, Yale University–affiliated addiction treatment facility in the New Haven, Connecticut, area acquired over a 1-year period were assessed for proportion of patients who were abstinent at discharge. Patients are classified here on the basis of their primary drug of abuse (eg, alcohol, opiates, cocaine, marijuana), excluding nicotine, as described in Dodge et al. [6]. Patients participated in state-of-the-art, empirically based behavioral and pharmacologic therapies. Proportion abstinent at discharge was assessed based on most recent urine toxicology screening and patient and clinician reports. Findings indicated that less than 25% of primary marijuana- and cocaine-dependent patients were abstinent at discharge, while less than 35% were abstinent from alcohol and opiates over the course of a 1-year period (Fig. 1). The latter rates were higher, as non-agonist medications for alcohol and opioids were actively used in treatment in conjunction with behavioral relapse prevention approaches. These findings are consistent with previously reported observations on relapse rates, and suggest a critical need to understand the mechanisms that make addiction relapse likely, identify sensitive and specific biomarkers for relapse risk, and develop therapies to target relapse risk in order to improve addiction relapse outcomes.
Fig. 1.
Data on proportion of patients remaining abstinent and surviving relapse at discharge from a large, publicly funded addiction treatment clinic (N=878). Patients are classified on the basis of primary drug of abuse (cocaine, marijuana, opioid, alcohol). Survival distribution function is shown on the y axis, which represents the proportion of patients surviving relapse and remaining abstinent during the assessment period of 350 days shown on the x axis environmental stimuli and interoceptive cues, it is important to examine the psychobiological consequences of chronic drug use and assess whether such changes are involved in increasing relapse risk.
Is There a Biology of Relapse?
Because addiction relapse is a common phenomenon, research in the past decade has focused on whether there is a biology underlying relapse susceptibility, and if so, whether it is possible to develop new treatments to decrease relapse risk [7, 8]. The most common reasons for relapse given by substance-abusing patients include stress, negative mood and anxiety, drug-related cues, temptations and boredom, and lack of positive environmental contingencies (eg, job, family relationships, responsibilities) [9]. To understand how and why recovering addicted individuals succumb to relapse, particularly in the context of external
Chronic Substance Use, Stress, and Associated Subjective and Behavioral Changes
High levels of stress and trauma exposure are commonly associated with substance use disorders [10, 11•]. Increases in irritability, anxiety, emotional distress, sleep problems, dysphoria, aggressive behaviors, and drug craving are common during early abstinence from alcohol, cocaine, opiates, nicotine, and marijuana [10]. The dependent state is marked by negative affect, distress, and anhedonia during early abstinence, which relates to neuroadaptations in brain reward and stress pathways [7, 10, 12–15].
Chronic abuse of substances also results in greater incentive salience such that there is an increased “wanting” of drug, particularly in stress- and drug-related contexts [16]. Thus, acute stress exposure in the laboratory increases drug craving and anxiety in individuals dependent on opiates, alcohol, nicotine, cocaine, and marijuana [17, 18••, 19••, 20]. Similarly, substance abusers report significantly higher levels of drug-related and drug cue–related craving and attentional bias than healthy controls [21–24].
Prediction of Subsequent Relapse Risk
Several treatment studies have shown that higher levels of psychological withdrawal or “abstinence” symptoms, such as subjective distress, irritability, drug craving, sleep, and cognitive problems, occurring during early drug abstinence, even beyond the acute withdrawal phase, are associated with worse treatment outcomes among smokers, cocaine addicts, heroin-dependent individuals, and alcoholics [10]. In general, findings indicate that the greater the severity of dependence and of such drug abstinence symptoms, the worse the treatment outcomes will be.
Many human studies have shown that stress and trauma are associated with drug relapse [4•, 7, 25–29, 30••, 31•, 32]. While an important strength of these studies over previous correlational studies of stress and relapse has been the prospective assessment of drug use for a follow-up period in order to predict future relapse risk, studies have varied in their assessment of stressful life events, the time period of follow-up to assess future relapse risk, and in the methods used for relapse assessment, which may have led to some negative results [33].
Prospective studies of relapse risk show that several clinical variables, such as depressive symptoms and drug craving, are predictive of subsequent relapse risk. Higher depression scores predicted shorter time to relapse and less likelihood of abstinence [6, 34]. Higher craving levels during abstinence and in outpatient treatment are known to predict relapse and return to drug use [3, 29, 31•]. Several studies have shown that exposure to certain stressors in the laboratory, including guided imagery stress scripts, Trier Social Stress Task [35], and systemic injections of the stress hormone corticotropin-releasing factor (CRF), increases subjective self-reports of drug craving [7, 17, 36–38]. These studies elegantly show a cause-and-effect relationship between stress and drug cue exposure and drug craving [7, 17]. Limited cause-and-effect evidence exists for stress exposure and relapse in laboratory studies [19••]. Other research combining the laboratory-based provocation of stress, negative affect, and drug craving with a prospective assessment of relapse in the real world have shown that stress-induced and cue-induced craving and stress and lower positive emotional responses are predictive of subsequent time to relapse and drug use outcomes [5, 18••, 39–41•]. Additionally, ecological momentary assessment (EMA) techniques have been used recently to assess daily stressors and negative affect and their association with both drug use and non–drug use events in a within-subjects case-crossover design in the real world. These studies have also shown that stress and negative affect are predictive of day-to-day drug use episodes monitored in the real world setting [29, 30••, 32, 42].
Chronic Substance Use and Biological Changes
A growing body of evidence is documenting alterations in the dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, autonomic nervous system changes, and alterations in brain dopaminergic and emotion and motivational systems of addicted individuals. Research has shown that both acute and protracted withdrawal from psychoactive substances are associated with overactivity of the CRF systems as documented in preclinical studies [13] and in clinical studies showing CRF-HPA disturbances in alcoholics and opiate-, cocaine-, and nicotine-addicted individuals [18••, 19••, 21, 22, 43–48]. In cocaine-dependent women, higher levels of daily measured morning sex hormone progesterone and plasma cortisol were found during the first month of abstinence as compared to healthy controls [49]. Autonomic and noradrenergic abnormalities also have been well-documented with overactivity of these systems during acute and protracted withdrawal from opiates, alcohol, and cocaine [48, 50–53]. These findings indicate that the CRF, and CRF-related HPA and hypothalamic-pituitary-gonadal axes and the autonomic/noradrenergic systems are dysregulated during acute withdrawal, and that mild to moderate alterations exist past acute withdrawal during protracted abstinence for at least 4 to 12 weeks. This may contribute to the abstinence symptoms (discussed in the previous section), addictive behaviors, and relapse susceptibility.
Biological Changes and Subsequent Relapse
Several studies have used a prospective design to examine whether changes in biological stress responses are predictive of future relapse. In alcoholics, blunted stress- and cue-induced cortisol responses have been associated with poor alcohol relapse outcomes [40, 46, 54, 55]. Nicotine-deprived smokers who were exposed to a series of stressors showed blunted corticotropin (ACTH), cortisol, and blood pressure responses to stress, but increased nicotine withdrawal and craving scores, and these responses were predictive of poor nicotine relapse outcomes [45].
In our research, inpatient treatment–engaged, recovering cocaine- and alcohol-dependent individuals completed research participation in experimental studies during which they were exposed to stress, drug cues, and neutral relaxing scenarios and assessed for drug craving, anxiety, and stress responses. After completion of the laboratory study, the patients were discharged from inpatient treatment and observed repeatedly for 90 days to assess relapse outcomes. For the cocaine group, in which we found altered stress responses compared with controls [21, 48], higher stress-induced ACTH and cortisol responses were not associated with time to relapse, but these responses were predictive of greater amounts of cocaine consumed during follow-up [5]. In abstinent, treatment-engaged, recovering alcohol-dependent individuals, we found higher basal ACTH levels and also blunted stress- and cue-induced ACTH and cortisol responses. Relating to the higher basal ACTH levels, we also found that individuals with high cortisol/ACTH ratios (a measure of sensitivity of the adrenal glands to release cortisol in response to the ACTH signal) were more likely to relapse quickly after discharge from inpatient treatment. In fact, high cortisol/ACTH ratios more than doubled the risk of shorter time to relapse [18••]. In a recent laboratory study modeling nicotine relapse in early abstinent nicotine-dependent individuals, stress-induced increases in cortisol were associated with shorter time to resisting smoking [19••]. Finally, in opiate-abstinent, methadone- or buprenorphine- maintained individuals, higher cortisol levels during drug cue reactivity were predictive of higher relapse outcomes [56]. Thus, across abstinent, recovering cocaine-, alcohol-, opiate-, and nicotine-dependent individuals, upregulation of the HPA axis with altered responsivity of the HPA axis has been associated with poor relapse outcomes. Thus, all of these addicted groups show both higher compulsive motivation for drug (craving), along with poor stress regulatory responses as measured by higher cortisol levels and/or altered glucocorticoid feedback, which results in an enhanced susceptibility to subsequent addiction relapse.
Evidence from preclinical studies shows chronic drug-related central changes in brain-derived neurotrophic factor (BDNF) and other growth factors during abstinence that have been associated with reinstatement of drug seeking in animal models of relapse [8, 57, 58]. In a recent study, we found morning serum BDNF levels to be significantly higher in abstinent cocaine abusers [59••] and alcoholics compared with controls (unpublished data). Furthermore, higher serum BDNF levels were highly predictive of shorter time to cocaine relapse and higher amounts of cocaine used, as well as greater number of days of cocaine use over a 90-day follow-up period [59••].
Human Neuroimaging Studies Documenting Chronic Substance-Related Brain Changes
Several studies have documented lower gray matter volume in cortical, thalamic, and cerebellar brain regions in individuals with substance use disorders [60–63]. More severe gray matter deficits have been reported in relapsers than in abstainers [64, 65]. Assessing volumes in specific regions of the amygdala, hippocampus, and ventral striatum in alcoholics after only 1 week of alcohol abstinence, Wrase and colleagues [66] recently reported lower amygdala volumes in those who relapsed compared with those who remained abstinent. In a comprehensive analysis using voxel-based morphometry, we examined changes in gray and white matter volume in abstinent, recovering alcoholics compared with controls and assessed whether volume changes predicted time to alcohol relapse and heavy drinking relapse [67]. We found that lower medial frontal cortical and parietal-occipital volumes in recovering alcoholics significantly predicted shorter time to alcohol relapse.
Using functional neuroimaging technology and a variety of cue induction procedures, many studies have examined brain regions associated with craving in addicted individuals. Exposure to drug cues known to increase craving increases activity in the amygdala and regions of the frontal cortex [68–70]. Gender differences also have been reported with cue-related activation in the amygdala and frontal cortex of cocaine-dependent individuals [71, 72]. Cue-induced craving for nicotine, methamphetamine, and opiates also activates regions of the prefrontal cortex, amygdala, hippocampus, insula, and ventral tegmental area [73]. We also examined brain activation during stress and exposure to neutral imagery in a functional MRI study. Although healthy controls and cocaine-dependent individuals showed similar levels of anxiety (using a verbal 10-point analogue scale) and pulse changes during stress exposure, brain response to emotional stress in paralimbic regions such as the anterior cingulate cortex, hippocampus, and parahippocampal regions was observed in healthy controls, while cocaine-dependent patients showed a striking absence of such activation [74]. In contrast, patients had increased activity in the caudate and dorsal striatum region during stress activation that was significantly associated with stress-induced cocaine craving ratings. Similarly, stress, alcohol cue, and neutral imagery exposure were assessed in social drinkers, and robust and similar activation of medial prefrontal, anterior cingulate cortex, insula, amygdala, hippocampus, and ventral and dorsal striatal regions was seen with stress and alcohol cue exposure. Alcohol cue-induced ventral and dorsal striatal activity correlated with alcohol cue-induced craving in men [75].
Recent positron emission tomography studies have also shown significant positive correlations between the dorsal striatum and drug cue-induced cocaine craving [76, 77]. These findings are consistent with the results of imaging studies, with alcoholic patients showing an increased association between dorsal striatum regions and alcohol craving in response to presentation of alcohol-related stimuli [78, 79]. Using positron emission tomography imaging with alcoholics and cocaine patients, research has shown a significant association between dopamine D2 receptor binding in the ventral striatum and drug craving, as well as motivation for self-administration [80–82].
Neuropsychological and imaging studies examining prefrontal executive functions, including impulse control, decision making, and set shifting, have shown executive function deficits and hypo-frontal responses in addicted individuals as compared with control volunteers [83–89]. Together, these data show a distinct pattern of findings indicating that increased stress- and cue-induced craving and compulsive drug-seeking states in addicted individuals are associated with greater activity in the striatum, but decreased activity in specific regions of the cingulate and prefrontal cortex and related regions involved in controlling impulses and emotions [88].
Neural Correlates of Addiction Relapse
In a study by Paulus et al. [90], recovering methamphet-amine abusers who were studied in a decision-making task during a functional MRI session early in their recovery were then assessed 1 year after treatment to determine neural correlates of methamphetamine relapse. Findings indicated an important role for the middle frontal, posterior cingulate, and insula in predicting relapse to methamphet-amine. In a preliminary study, Grusser and colleagues [78] reported that alcohol cue-induced activation in the putamen (striatum), anterior cingulate, and medial prefrontal cortex was more pronounced in alcoholic patients who subsequently relapsed compared with those who had not. Kosten and colleagues [91] assessed drug cue-induced brain activation in recently abstinent cocaine-dependent patients prior to initiation in a double-blind, placebo-controlled, 12-week treatment trial of sertraline. Cocaine-dependent patients who relapsed showed greater activation in the sensory association cortex, motor cortex, and the posterior cingulate during exposure to cocaine-related videotapes.
In a preliminary study, we examined whether brain activity changes during exposure to stress imagery and stress-induced cocaine craving were associated with cocaine relapse outcomes in 31 treatment-engaged, abstinent, cocaine-dependent individuals (20 men and 11 women) [73]. Findings indicated that increased activity in the medial prefrontal cortex (Brodmann area [BA] 9, 10) was associated with a shorter time to cocaine relapse and with a higher number of days of cocaine use during the 90-day period. The medial prefrontal cortex (BA 10) is involved in emotional and autonomic regulation and with suppression of negative affect. The current findings extend its regulatory function and suggest that in abstinent drug abusers, activity in this region may represent a coping response (albeit maladaptive) in the face of emotional distress.
In two studies using different methods, brain regions important for nicotine withdrawal and smoking cessation were identified. Azizian et al. [92•] conducted a functional MRI of the Stroop task to assess cognitive control and reported that hyperreactivity of the right anterior cingulate cortex was associated with acute nicotine withdrawal. However, it is not known whether such hyperactivation in these regions associated with nicotine withdrawal predicted subsequent relapse in these individuals. Naqvi et al. [93] have shown that smokers with brain damage involving the insula, a region implicated in conscious urges, were more likely than smokers with brain damage not involving the insula to undergo a disruption of smoking addiction, characterized by the ability to quit smoking easily, immediately, without relapse, and without persistence of the urge to smoke. This result suggests that the insula is a critical neural substrate in the addiction to smoking.
Summary and Conclusions
The previous sections outline previous and recent findings on the growing research to identify sensitive markers of addiction relapse. There have been rapid changes in the technology available to assess neural and biological changes related to addictive disorders. For example, novel biochemicals such as serum BDNF can be measured reliably and have been shown to predict future cocaine relapse. Neuroimaging technologies are available to assess neural changes associated with chronic drug use and their impact on assessing relapse risk. Improvements also have been made in methods to assess drug craving, relapse, and drug use. For example, while early studies examined relapse status over a longer period, such as 1-year outcomes [33], it is now clearly evident that addiction relapse is a common and rapid process, and more frequent assessment of early relapse within weeks to months of treatment completion is important to identify high susceptibility to relapse. Several studies have shown relapse rates as high as 65% to 70% in the 90-day period following treatment. Laboratory-based assessments of provoked drug craving and EMA methods of drug craving and stress have shown high accuracy for predicting lapse to drug use in the real world [5, 29, 30••, 31•, 94]. All these developments have begun to generate clinical outcome data that are sensitive to identifying future relapse.
While clinical symptoms such as depressive symptoms, history of trauma and high stress, and drug craving represent phenotypes important in identifying individuals entering treatment who may be most susceptible to relapse, exciting new data have begun to identify biological correlates of future relapse. These include high cortisol levels at baseline, at resting state, and with drug cue challenge. High levels of serum BDNF and high levels of adrenal sensitivity (cortisol/ACTH ratio) were also found to be predictive of relapse. Using neuroimaging, brain atrophy in the medial frontal brain region was found to predict alcohol relapse risk, and hyperreactivity of the anterior cingulate cortex was found to be associated with withdrawal and relapse risk. These findings suggest that there are key neural and biological changes associated with chronic alcohol and drug abuse that are also important as clinical predictors of relapse. With further validation of these measures in future research, it may be possible to identify an “endophenotype,” or biological profile of relapse risk, that can be used to assess relapse susceptibility in the clinic. Such markers could be highly useful not only in identifying individuals who are at risk of relapse and treatment failure, but to target specific treatments for those high relapse risk individuals.
In conclusion, it is well-known that addictions are chronic, relapsing illnesses, but systematic study to identify biological markers of addiction relapse risk has been rare. Clinical observations have shown high rates of relapse in treatment-seeking individuals within weeks and months of entering and completing treatment. Recent neural and biological evidence from clinical studies using prospective designs to assess relapse was examined to identify specific measures that show sensitivity in predicting relapse risk. Studies from cocaine-, alcohol-, nicotine-, and opiate-dependent individuals are reviewed to identify clinical, biological, and neural measures that are predictive of addiction relapse. Stress, depressive symptoms, drug craving, cortisol and adrenal sensitivity, serum BDNF, medial frontal gray matter volume, and functional response in the anterior cingulate cortex were all identified as significant predictors of addiction relapse. Further validation of these measures along with identification of new measures could lead to the development of an endopheno-type for relapse risk that may be used to screen and identify those most susceptible to relapse in the clinic. Such markers have the potential to be used as outcome measures to assess treatment response, and in the development of new treatments that reverse and normalize these biological responses to improve relapse outcomes in addiction.
Acknowledgments
Preparation of this review was supported by grants from the National Institutes of Health (P50-DA165556, R01-AA13892, R01-DA27230, UL1-DE019589, PL1-DA024859).
Footnotes
Disclosure: No potential conflict of interest relevant to this article was reported.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Brownell KD, Marlatt GA, Lichtenstein E, Wilson GT. Understanding and preventing relapse. Am Psychol. 1986;41:765–82. doi: 10.1037//0003-066x.41.7.765. [DOI] [PubMed] [Google Scholar]
- 2.Brandon TH, Vidrine JI, Litvin EB. Relapse and relapse prevention. Annu Rev Clin Psychol. 2007;3:257–84. doi: 10.1146/annurev.clinpsy.3.022806.091455. [DOI] [PubMed] [Google Scholar]
- 3.Paliwal P, Hyman SM, Sinha R. Craving predicts time to cocaine relapse: further validation of the now and brief versions of the cocaine craving questionnaire. Drug Alcohol Depend. 2008;93:252–9. doi: 10.1016/j.drugalcdep.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4•.Hyman SM, Paliwal P, Chaplin TM, et al. Severity of childhood trauma is predictive of cocaine relapse outcomes in women but not men. Drug Alcohol Depend. 2008;92:208–16. doi: 10.1016/j.drugalcdep.2007.08.006. This paper is the first to show sex differences in the association of severity of childhood trauma and cocaine relapse outcomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sinha R, Garcia M, Paliwal P, et al. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatr. 2006;63:324–31. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- 6.Dodge R, Sindelar J, Sinha R. The role of depressive symptoms in predicting drug abstinence in outpatient substance abuse treatment. J Subst Abus Treat. 2005;28:189–96. doi: 10.1016/j.jsat.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–59. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- 8.Shaham Y, Shalev U, Lu L, et al. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharma-cology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 9.McKay JR, Rutherford MJ, Alterman AI, et al. An examination of the cocaine relapse process. Drug Alcohol Depend. 1995;38:35–43. doi: 10.1016/0376-8716(95)01098-j. [DOI] [PubMed] [Google Scholar]
- 10.Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N YAcad Sci. 2008;1141:105–30. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11•.Enoch MA. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology (Berl) 2011;214:17–31. doi: 10.1007/s00213-010-1916-6. This is an excellent recent review documenting high rates of early trauma in addictive disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–8. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- 13.Koob GF, Ahmed SH, Boutrel B, et al. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2004;27:739–49. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cerebral Cortex. 2000;10:318–25. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- 15.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatr. 2005;162:1403–13. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 16.Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95 (Suppl 2):S91–S117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- 17.Sinha R. Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addict Biol. 2009;14:84–98. doi: 10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18••.Sinha R, Fox HC, Hong KI, et al. Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Arch Gen Psychiatr. 2011 doi: 10.1001/archgenpsychiatry.2011.49. (epub online). This is the first study to show that provoked alcohol craving and adrenal sensitivity are predictive of subsequent alcohol relapse. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19••.McKee S, Sinha R, Weinberger AH, et al. Stress decreases the ability to resist smoking and potentiates smoking intensity and reward. J Psychopharmacol. 2011;25(4):490–502. doi: 10.1177/0269881110376694. This is the first laboratory demonstration of stress-induced nicotine relapse. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McRae-Clark A, Carter R, Price K, et al. Stress and cue-elicited craving and reactivity in marijuana-dependent individuals. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-011-2376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fox HC, Hong KI, Siedlarz K, Sinha R. Enhanced sensitivity to stress and drug/alcohol craving in abstinent cocaine-dependent individuals compared to social drinkers. Neuropsychopharmacology. 2008;33:796–805. doi: 10.1038/sj.npp.1301470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinha R, Fox HC, Hong KA, et al. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34:1198–208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Field M, Wiers RW, Christiansen P, et al. Acute alcohol effects on inhibitory control and implicit cognition: implications for loss of control over drinking. Alcohol Clin Exp Res. 2010;34(8):1346–52. doi: 10.1111/j.1530-0277.2010.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Constantinou N, Morgan CJ, Battistella S, et al. Attentional bias, inhibitory control and acute stress in current and former opiate addicts. Drug Alcohol Depend. 2010;109:220–5. doi: 10.1016/j.drugalcdep.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Baker TB, Piper ME, McCarthy DE, et al. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- 26.Brown SA, Vik PW, McQuaid JR, et al. Severity of psychosocial stress and outcome of alcoholism treatment. J Abnorm Psychol. 1990;99:344–8. doi: 10.1037//0021-843x.99.4.344. [DOI] [PubMed] [Google Scholar]
- 27.Brown SA, Vik PW, Patterson TL, et al. Stress, vulnerability and adult alcohol relapse. J Stud Alcohol. 1995;56:538–45. doi: 10.15288/jsa.1995.56.538. [DOI] [PubMed] [Google Scholar]
- 28.Greenfield SF, Kolodziej ME, Sugarman DE, et al. History of abuse and drinking outcomes following inpatient alcohol treatment: a prospective study. Drug Alcohol Depend. 2002;67:227–34. doi: 10.1016/s0376-8716(02)00072-8. [DOI] [PubMed] [Google Scholar]
- 29.Epstein DH, Marrone GF, Heishman SJ, et al. Tobacco, cocaine, and heroin: craving and use during daily life. Addict Behav. 2009;35:318–24. doi: 10.1016/j.addbeh.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30••.Epstein DH, Willner-Reid J, Vahabzadeh M, et al. Real-time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Arch Gen Psychiatr. 2009;66:88–94. doi: 10.1001/archgenpsychiatry.2008.509. This is the first study to use EMA to demonstrate the effects of real world cue and stress exposure on subsequent drug use. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31•.Preston KL, Epstein DH. Stress in the daily lives of cocaine and heroin users: relationship to mood, craving, relapse triggers, and cocaine use. Psychopharmacology. 2011 doi: 10.1007/s00213-011-2183-x. (in press). This is an excellent paper illustrating of the use of recent methodologic advances with monitoring drug use daily in the real world and demonstrating how stress is associated with drug use. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shiffman S. Dynamic influences on smoking relapse process. J Personal. 2005;73:1–34. doi: 10.1111/j.0022-3506.2005.00364.x. [DOI] [PubMed] [Google Scholar]
- 33.Hall SM, Havassy BE, Wassermann DA. Commitment to abstinence and acute stress in relapse to alcohol, opiates and nicotine. J Counsel Clin Psychol. 1990;58:175–81. doi: 10.1037//0022-006x.58.2.175. [DOI] [PubMed] [Google Scholar]
- 34.Greenfield SF, Weiss RD, Muenz LR, et al. The effect of depression on return to drinking: a prospective study. Arch Gen Psychiatr. 1998;55:259–65. doi: 10.1001/archpsyc.55.3.259. [DOI] [PubMed] [Google Scholar]
- 35.Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 36.Back SE, Hartwell K, DeSantis SM, et al. Reactivity to laboratory stress provocation predicts relapse to cocaine. Drug Alcohol Depend. 2010;106:21–7. doi: 10.1016/j.drugalcdep.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coffey SF, Saladin ME, Drobes DJ, et al. Trauma and substance cue reactivity in individuals with comorbid posttraumatic stress disorder and cocaine or alcohol dependence. Drug Alcohol Depend. 2002;65:115–27. doi: 10.1016/s0376-8716(01)00157-0. [DOI] [PubMed] [Google Scholar]
- 38.Sinha R, Catapano D, O’Mally S. Stress-induced craving and stress responses in cocaine dependent individuals. Psychopharmacology. 1999;142:343–51. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- 39.Cooney NL, Litt MD, Morse PA, et al. Alcohol cue reactivity, negative-mood reactivity, and relapse in treated alcoholic men. J Abnorm Psychol. 1997;106:243–50. doi: 10.1037//0021-843x.106.2.243. [DOI] [PubMed] [Google Scholar]
- 40.Breese GR, Chu K, Dayas CV, et al. Stress enhancement of craving during sobriety and the risk of relapse. Alcohol Clin Exp Res. 2005;29(2):185–95. doi: 10.1097/01.alc.0000153544.83656.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41•.Lubman DI, Yucel M, Kettle JW, et al. Responsiveness to drug cues and natural rewards in opiate addiction: associations with later heroin use. Arch Gen Psychiatr. 2009;66:205–12. doi: 10.1001/archgenpsychiatry.2008.522. This is the first study to show that reduced positive affect to drug cues and natural rewards is associated with later drug use. [DOI] [PubMed] [Google Scholar]
- 42.Shiffman S, Paty JA, Gnys M, et al. First lapses to smoking: within-subjects analysis of real-time reports. J Consult Clin Psychol. 1996;64:366–79. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- 43.Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51:23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- 44.Contoreggi C, Herning RI, Na P, et al. Stress hormone responses to corticotropin-releasing hormone in substance abusers without severe comorbid psychiatric disease. Biol Psych. 2003;54:873–8. doi: 10.1016/s0006-3223(03)00167-7. [DOI] [PubMed] [Google Scholar]
- 45.Al’absi M, Hatsukami DK, Davis G. Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacology (Berlin) 2005;181:107–17. doi: 10.1007/s00213-005-2225-3. [DOI] [PubMed] [Google Scholar]
- 46.Adinoff B, Junghanns K, Kiefer F, Krishnan-Sarin S. Suppression of the HPA axis stress-response: implications for relapse. Alcohol Clin Exp Res. 2005;29:1351–5. doi: 10.1097/01.ALC.0000176356.97620.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sinha R, Talih M, Malison R, et al. Hypothalamic-pituitary-adrenal axis and sympatho-adrenomedullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl) 2003;170:62–72. doi: 10.1007/s00213-003-1525-8. [DOI] [PubMed] [Google Scholar]
- 48.Fox HC, Hong KI, Siedlarz KM, et al. Sex-specific dissociations in autonomic and HPA responses to stress and cues in alcohol-dependent patients with cocaine abuse. Alcohol Alcohol. 2009;44:575–85. doi: 10.1093/alcalc/agp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fox HC, Hong KH, Paliwal P, et al. Altered levels of sex and stress steroid hormones assessed daily over a 28-day cycle in early abstinent cocaine dependent females. Psychopharmacology. 2007;195:527–36. doi: 10.1007/s00213-007-0936-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McDougle CJ, Black JE, Malison RT, et al. Noradrenergic dysregulation during discontinuation of cocaine use in addicts. Arch Gen Psychiatr. 1994;51:713–9. doi: 10.1001/archpsyc.1994.03950090045007. [DOI] [PubMed] [Google Scholar]
- 51.Krystal JH, Webb E, Cooney NL, et al. Serotonergic and noradrenergic dysregulation in alcoholism: M-chrophenylpiperazine and yohimbine effects in recently detoxified alcoholics and healthy comparison subjects. Am J Psychiatr. 1996;153:83–92. doi: 10.1176/ajp.153.1.83. [DOI] [PubMed] [Google Scholar]
- 52.Bar KJ, Boettger MK, Neubauer R, et al. Heart rate variability and sympathetic skin response in male patients suffering from acute alcohol withdrawal syndrome. Alcohol Clin Exp Res. 2006;30:1592–8. doi: 10.1111/j.1530-0277.2006.00191.x. [DOI] [PubMed] [Google Scholar]
- 53.Ingjaldsson JT, Thayer JF, Laberg JC. Craving for alcohol and pre-attentive processing of alcohol stimuli. Int J Psychophysiol. 2003;49:29–39. doi: 10.1016/s0167-8760(03)00075-8. [DOI] [PubMed] [Google Scholar]
- 54.Junghanns K, Backhaus J, Tietz U. Impaired serum cortisol stress response is a predictor of early relapse. Alcohol Alcohol. 2003;38:189–93. doi: 10.1093/alcalc/agg052. [DOI] [PubMed] [Google Scholar]
- 55.Brady KT, Back SE, Waldrop AE, et al. Cold pressor task reactivity: predictors of alcohol use among alcohol-dependent individuals with and without comorbid posttraumatic stress disorder. Alcohol Clin Exp Res. 2006;30:938–46. doi: 10.1111/j.1530-0277.2006.00097.x. [DOI] [PubMed] [Google Scholar]
- 56.Fatseas M, Denis C, Massida Z, et al. Cue-induced reactivity, cortisol response and substance use outcome in treated heroin dependent individuals. Biol Psychiatr. 2011 Jul 7; doi: 10.1016/j.biopsych.2011.05.015. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 57.Schoenbaum G, Stalnaker TA, Shaham Y. A role for BDNF in cocaine reward and relapse. Nat Neurosci. 2007;10:935–6. doi: 10.1038/nn0807-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nestler EJ. Is there a common molecular pathway for addiction. Nat Neurosci. 2005;8:1445–9. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- 59.D’Sa C, Fox HC, Hong K, et al. Increased serum brain-derived neurotrophic factor (BDNF) is predictive of cocaine relapse outcomes: a prospective study. Biological Psychiatr. 2011 doi: 10.1016/j.biopsych.2011.05.013. (in press). This is the first study to identify a biomarker in the basal state that is significantly higher during early recovery from cocaine, and also to predict time to cocaine relapse. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pfefferbaum A, Sullivan EV, Mathalon DH, et al. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcohol Clin Exp Res. 1995;19:1177–91. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- 61.Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcohol Clin Exp Res. 2000;24:611–21. [PubMed] [Google Scholar]
- 62.Fein G, Di Sclafani V, Meyerhoff DJ. Prefrontal cortical volume reduction associated with frontal cortex function deficit in 6-week abstinent crack-cocaine dependent men. Drug Alcohol Depend. 2002;68:87–93. doi: 10.1016/s0376-8716(02)00110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tanabe J, Tregellas JR, Dalwani M, et al. Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biol Psychiatr. 2009;65:160–4. doi: 10.1016/j.biopsych.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pfefferbaum A, Sullivan EV, Rosenbloom MJ, et al. A controlled study of cortical gray matter and ventricular changes in alcoholic men over a 5-year interval. Arch Gen Psychiatr. 1998;55:905–12. doi: 10.1001/archpsyc.55.10.905. [DOI] [PubMed] [Google Scholar]
- 65.Gazdzinski S, Durazzo TC, Meyerhoff DJ. Temporal dynamics and determinants of whole brain tissue volume changes during recovery from alcohol dependence. Drug Alcohol Depend. 2005;78:263–73. doi: 10.1016/j.drugalcdep.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 66.Wrase J, Makris N, Braus DF, et al. Amygdala volume associated with alcohol abuse relapse and craving. Am J Psychiatr. 2008;165:1179–84. doi: 10.1176/appi.ajp.2008.07121877. [DOI] [PubMed] [Google Scholar]
- 67.Rando K, Hong KI, Bhagwagar Z. Association of frontal and posterior cortical gray matter volume with time to alcohol relapse: a prospective study. Am J Psychiatr. 2011;168:183–92. doi: 10.1176/appi.ajp.2010.10020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Childress A, Mozely PD, McElgin W, et al. Limbic activation during cue-induced cocaine craving. Am J Psychiatr. 1999;156:11–8. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grant S, London ED, Newlin DB, et al. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci USA. 1996;93:12040–5. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kilts C, Schweitzer JB, Quinn CK, et al. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatr. 2001;58:334–41. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- 71.Kilts C, Gross RE, Ely TD, Drexler KP. The neural correlates of cue-induced craving in cocaine-dependent women. Am J Psychiatr. 2004;161:233–41. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- 72.Li C-S, Kemp KA, Milivojevic V, Sinha R. Neuroimaging reveals sex differences in the neuropathology of cocaine abuse. J Gend Med. 2005;2:174–82. doi: 10.1016/s1550-8579(05)80046-4. [DOI] [PubMed] [Google Scholar]
- 73.Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007;26:25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- 74.Sinha R, Lacadie C, Skudlarski P, et al. Neural activity associated with stress-induced cocaine craving: an fMRI study. Psychophar-macology. 2005;183(2):171–80. doi: 10.1007/s00213-005-0147-8. [DOI] [PubMed] [Google Scholar]
- 75.Seo D, Jia Z, Lacadie CM, et al. Sex differences in neural responses to stress and alcohol context cues. Hum Brain Mapp. 2010 doi: 10.1002/hbm.21165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Volkow ND, Wang GJ, Telang F, et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–8. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wong MM, Nigg JT, Zucker RA, et al. Behavioral control and resiliency in the onset of alcohol and illicit drug use: a prospective study from preschool to adolescence. Child Dev. 2006;77:1016–33. doi: 10.1111/j.1467-8624.2006.00916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grusser S, Wrase J, Klein S, et al. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- 79.Wrase J, Grusser S, Klein S, et al. Development of alcohol-associated cues and cue-induced brain activation in alcoholics. Biol Psych. 2002;17:287–91. doi: 10.1016/s0924-9338(02)00676-4. [DOI] [PubMed] [Google Scholar]
- 80.Heinz A, Siessmeier T, Wrase J, et al. Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatr. 2004;161:1783–9. doi: 10.1176/appi.ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- 81.Martinez D, Gil R, Slifstein M, et al. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatr. 2005;58:779–86. doi: 10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 82.Martinez D, Kim JH, Krystal J, Abi-Dargham A. Imaging the neurochemistry of alcohol and substance abuse. Neuroimaging Clin N Am. 2007;17:539–55. doi: 10.1016/j.nic.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 83.Ersche KD, Clark L, London M, et al. Profile of executive and memory function associated with amphetamine and opiate dependence. Neuropsychopharmacology. 2006;31:1036–47. doi: 10.1038/sj.npp.1300889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ersche KD, Fletcher PC, Lewis SJ, et al. Abnormal frontal activations related to decision-making in current and former amphetamine and opiate dependent individuals. Psychopharmacology (Berl) 2005;180:612–23. doi: 10.1007/s00213-005-2205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ersche KD, Roiser JP, Robbins TW, Sahakian BJ. Chronic cocaine but not chronic amphetamine use is associated with perseverative responding in humans. Psychopharmacology (Berl) 2008;197:421–31. doi: 10.1007/s00213-007-1051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004;24:11017–22. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kaufman J, Ross TJ, Stein EA, Garavan H. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. J Neurosci. 2003;23:7839–43. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li CS, Sinha R. Inhibitory control and emotional stress regulation: neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addiction. Neurosci Biobehav Rev. 2008;32:581–97. doi: 10.1016/j.neubiorev.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Noel X, Bechara A, Dan B, et al. Response inhibition deficit is involved in poor decision making under risk in nonamnesic individuals with alcoholism. Neuropsychology. 2007;21:778–86. doi: 10.1037/0894-4105.21.6.778. [DOI] [PubMed] [Google Scholar]
- 90.Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Arch Gen Psychiatr. 2005;62:761–8. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- 91.Kosten TR, Scanley BE, Tucker KA, et al. Cue-induced brain activity changes and relapse in cocaine dependent patients. Neuropsychopharm. 2006;31:644–50. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- 92•.Azizian A, Nestor LJ, Payer D, et al. Smoking reduces conflict-related anterior cingulate activity in abstinent cigarette smokers performing a Stroop task. Neuropsychopharmacology. 2010;35:775–82. doi: 10.1038/npp.2009.186. This paper identified the specific effect of nicotine withdrawal and smoking on anterior cingulate function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315 (5811):531–4. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sinha R, Shaham Y, Heilig M. Translational and reverse translational research on the role of stress in drug craving and relapse. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-011-2263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]