Abstract
Objectives
Cocaine dependence is a chronic stress state. Furthermore, both stress and substance abuse have robust and reciprocal effects on immune system cytokines, which are known to be powerful modulators of mood. We therefore examine basal and provoked changes in peripheral cytokines in cocaine dependent individuals to better understand their role in the negative reinforcing effects of cocaine.
Methods
Twenty-eight (16 F/12 M) treatment-seeking cocaine dependent individuals and 27 (14 F/13 M) social drinkers were exposed to three 5-min guided imagery conditions (stress, drug cue, relaxing) presented randomly across consecutive days. Measures of salivary cortisol, tumor necrosis factor alpha (TNFα), interleukin-10 (IL-10), and interleukin-1 receptor antagonist (IL-1ra) were collected at baseline and various post-imagery time-points.
Results
Cocaine abusers demonstrated decreased basal IL-10 compared with social drinkers. They also showed significant elevations in pro-inflammatory TNFα when exposed to stress compared with when they were exposed to relaxing imagery. This was not observed in the social drinkers. Conversely, social drinkers demonstrated increases in the anti-inflammatory markers, IL-10 and IL-1ra, following exposure to cue, which were not seen in the dependent individuals.
Conclusions
Cocaine dependent individuals demonstrate an elevated inflammatory state both at baseline and following exposure to the stress imagery condition. Cytokines may reflect potentially novel biomarkers in addicted populations for treatment development.
Keywords: cocaine dependence, HPA axis, cytokines, TNFα, IL-10, IL-1ra
INTRODUCTION
Cocaine dependence has been characterized as a chronic stress state marked by generalized enhanced stress system function (Sinha, 2001) for which there is currently no Food and Drug Administration (FDA) approved medication. This may be because of the fact that many potential therapeutic agents do not specifically target stress arousal systems or account for the prevalence of co-morbid psychological and somatic health issues, including depressive symptomatology (Falck et al., 2002), co-morbid alcohol abuse (McCance-Katz et al., 2005), and nicotine dependence (Mello and Newman, 2011). As immune system cytokines may have a neuromodulatory function that promotes deleterious moods associated with chronic illness and stress (Dantzer and Kelley, 2007 for review), peripheral immune system adaptations may play an integral role in contributing to the negative reinforcing effects of cocaine. In the current study, we highlight how findings from a laboratory-based situation may be used to identify changes in immune system cytokines in primarily cocaine dependent individuals. Findings may expose new biomarkers with potential utility in the development of new stress-based treatments.
Peripheral immune system cytokines may represent novel biomarkers in substance abusers for treatment development for several reasons. First, inextricable and reciprocal associations exist between relapse-related stress system activation and the immune system (Butts and Sternberg, 2008 for review). A wealth of prior research has shown that glucocorticoid stress system hormones are potent modulators of immune responses and inflammatory processes (Turnbull and Rivier, 1999). For example, acute psychosocial stress increases levels of Type helper 1 (Th1) pro-inflammatory cytokines in the brain (Anisman et al., 2007; Gibb et al., 2008), and this is associated with activation of the hypothalamic-pituitary-adrenal (HPA) axis and sympathetic stress systems (Tilders et al., 1993; Dunn et al., 2005). Increased glucocorticoids and catecholamines then mediate a feedback “shift” or down-regulation in pro-inflammatory cytokine cascades, as well as an up-regulation of healing Type helper 2 (Th2) anti-inflammatory cytokine cascades (Calcagni and Elenkov, 2006; Qin et al., 2008). As Th1 and Th2 responses are mutually inhibitory (Elenkov, 2008) and stress/immune system homeostasis is dependent upon activation being appropriate to the stimulus, it is possible that chronic stress system perturbations in substance dependent individuals (Fox and Sinha, 2009 for review) will be associated with disrupting the homeostatic balance between PI and AI cytokine cascades.
Second, in recent years, the neuromodulatory role of immune system cytokines has become a major focus with regard to the development of co-morbid affective disorders during chronic substance abuse, systemic illness, or stress (Dantzer et al., 2008; McAfoose and Baune, 2009). Peripheral inflammatory cytokines may therefore play an important role in underpinning the negative reinforcing effects of cocaine associated with chronic stress system up-regulation (Sinha et al., 2003; Fox et al., 2005; Fox and Sinha, 2009) and drug maintenance (de Jong and de Kloet, 2004; Sinha et al., 2006, 2011; Koob, 2009). For example, although acute immune system activation may be associated with adaptive behaviors necessary for recuperation, such as fatigue, social withdrawal, and immobility (Dantzer and Kelley, 1989; Kent et al., 1992), chronic immune system changes may be associated with more “mal-adaptive” elevations in negative mood and stress system dysregulation (Grippo and Johnson, 2009; McAfoose and Baune, 2009) typical of that associated with craving and relapse in chronic substance abusers (Stewart, 2003; Fox and Sinha, 2009, Preston and Epstein, 2011).
In the current study, we assess stress and immune system response to a stress-related and cue-related imagery scenario relative to relaxation imagery in a group of treatment-seeking cocaine dependent individuals and a group of socially drinking controls. We assess tonic and phasic changes in the pro-inflammatory cytokine TNFα, and the anti-inflammatory cytokines, IL-10 and IL1-ra. TNFα has been frequently associated with mood disorders in chronic illnesses (Dantzer and Kelley, 2007; Howren et al., 2009; Liu et al., 2010), as well as prolifically researched in terms of the assessment of the emotional response to psychological stress (Gaab et al., 2005; Weinstein et al., 2010) and the pathophysiology of substance dependence (Emanuele et al., 2005; Yamada, 2008). The roles of anti-inflammatory cytokines have been less extensively examined in terms of their effect on mood, despite evidence showing that IL-10 reduces depressive symptoms in rats (Leon et al., 1999; Mesquita et al., 2008) and that cocaine increases the expression of IL-10 at acute doses (Kubera et al., 2004; Dhillon et al., 2007). In order to more thoroughly assess the role of anti-inflammatory markers, we also assess adaptations in IL-1ra, which is a naturally occurring anti-inflammatory IL-1 regulator (Dinarello, 2000) and is also known to moderate the development of depression, increased stress perception (Maes et al., 1998), and anxiety (Kubera et al., 2000; Maes et al., 2000; Lehto et al., 2010).
METHOD
Participants
Twenty-eight treatment-seeking cocaine dependent (16 F/12 M) individuals and 27 socially drinking (14 F/13 M) individuals participated in the current study. All participants were recruited via advertisements placed either on-line or in local newspapers and magazines. Current dependence was determined with the use of the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders IV (SCID IV—First et al., 1997). All participants were also tested for positive urine toxicology screens upon admission to inpatient treatment at the Connecticut Mental Health Center (CMHC). Exclusion criteria for cocaine dependent patients included Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV) dependence for any drug other than cocaine, alcohol, or nicotine. All social drinkers were excluded if they met current or lifetime dependence criteria for alcohol or any other illicit drug. All participants using prescribed medications or failing to meet health requirements were also ineligible. Participants underwent stringent medical assessments that included electrocardiography and laboratory tests of renal, hepatic, pancreatic, hematopoietic, and thyroid function to ensure good physical health. All participants gave written and verbal consent, and the Human Investigation Committee of the Yale University School of Medicine approved the study.
All controls were light social drinkers (25 drinks or less per month), as classified by the Cahalan Quantity Frequency Variability Index (Cahalan et al., 1969). A socially drinking, rather than a drug-naïve comparison group, was used in the current design to allow a more thorough examination of the stress-related craving state in both a substance dependent and non-dependent group. Previous findings, with the use of our current imagery paradigm, have shown that both stress-related and cue-related imagery induce alcohol craving in light social drinkers (Chaplin et al., 2008; Fox et al., 2008; Sinha et al., 2009).
Design
In the current study, we used a mixed design, where the drug group (cocaine dependent/ social drinkers) represented the between subjects factor, and the imagery condition (stress, drug/acohol cue, relaxing) and time-points (repeated assessments in each laboratory session), the within subjects factor. During three laboratory sessions, participants were exposed to all three personalized guided imagery conditions (stress, drug/ alcohol cue, relaxing) across consecutive days, one imagery condition per day in a randomized and counterbalanced order. Research staff was blind to imagery condition and the content of the scripts assigned to each laboratory session. Subjects also remained blind until imagery presentation.
Salivary cortisol, plasma TNFα (pro-inflammatory cytokine), and plasma IL-10 and IL-1ra (anti-inflammatory markers) represented the dependent variables. Cocaine craving, mood, and physiological responses were all presented previously as part of a larger study (Fox et al., 2008).
General procedures (see Figure 1)
Figure 1.
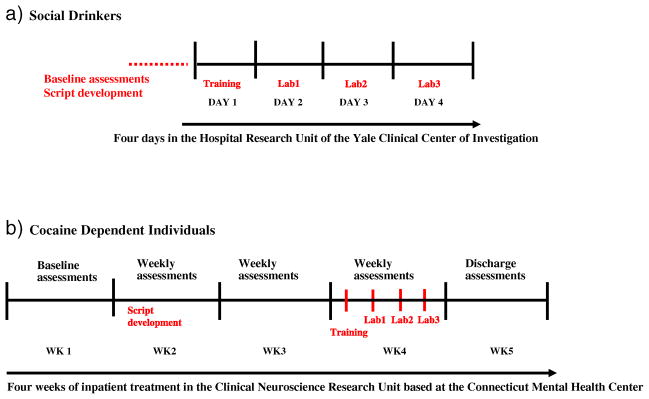
Overview of study schedules for (a) socially drinking controls and (b) cocaine dependent individuals
Cocaine dependent participants were admitted to the Clinical Neuroscience Research Unit (CNRU) of the Connecticut Mental Health Center (CMHC) for approximately 4 weeks of inpatient treatment and study participation. The CNRU is a locked inpatient treatment research facility with no access to alcohol or drugs, although participants were allowed four smoke breaks per day to avoid nicotine withdrawal. Participants have limited access to visitors, and drug testing is conducted regularly to ensure drug abstinence. Because subjects were treatment-seeking, they participated in 4 weeks of group counseling treatment for cocaine addiction with the use of the standard drug counseling manual as a guide (Mercer and Woody, 1992). During the first week of inpatient stay, cocaine dependent participants were administered structured baseline assessments measuring psychiatric and substance use history. In the second week, scripts for the guided imagery induction were developed, as described in previous studies (Sinha et al., 2003; Bergquist et al., 2010). All laboratory sessions were conducted approximately 23 days after admission to allow for normalization of neurobiological changes associated with acute cocaine abstinence.
Socially drinking participants were admitted to the Hospital Research Unit (HRU) of the Yale Clinical Center of Investigation (YCCI) located a block away at Yale/New Haven hospital for a 4-day stay. Within that time, they were required to remain in the hospital unit, within a controlled environment similar to that of the substance abusing participants’. They were given a similar diet, allowed limited access to visitors, and limited staff-accompanied smoke breaks. Baseline demographics, psychiatric, and substance use assessments, as well as imagery scripts, were prepared prior to their admission to the HRU. All socially drinking controls were exposed to an alcohol-related script for the drug cue condition.
Imagery script development—for presentation in the laboratory sessions
Briefly, the stress imagery script was based on individual subjects’ description of a personal stressful event that had occurred in the last year and was experienced as being “most stressful”. “Most stressful” was determined by having each subject rate their perceived stress on a ten-point Likert scale, where 1 = “not at all stressful” and 10 = “the most stress they felt recently in their life”. Only situations rated as 8 or above on the ten-point scale were accepted as appropriate for script development. The drug cue scripts were developed by having participants identify a recent situation that involved the anticipatory excitement of wanting cocaine or alcohol. The scenarios incorporated drug-related imagery, such as being at a bar or watching others smoke crack and drink alcohol, and had to result in subsequent drug use. The cocaine dependent group was presented with cocaine cue imagery, and the social drinkers with an alcohol cue. A neutral script was developed from the subjects’ description of a personal non-drug-related relaxing situation to represent an intra-individuals baseline or control condition. All scripts were then recorded onto an audiotape to be played in the laboratory sessions.
Training
On a day prior to the laboratory sessions, subjects were brought into the testing room to acclimatize them to specific aspects of the study procedures including IV insertion, as well as relaxation and imagery procedures, as previously described in Sinha et al. (2003).
Laboratory sessions (see Figure 2)
Figure 2.
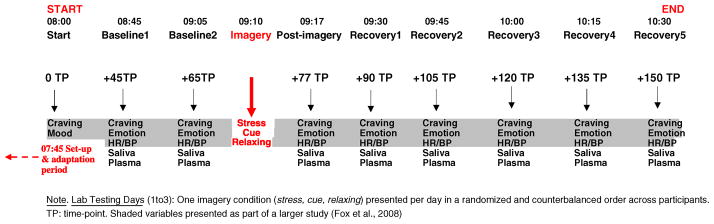
Laboratory schedule (identical for all 3 days, with the exception of the imagery condition presented)
On each testing day, subjects abstained from breakfast and were brought into the testing room at 7:45 AM. All subjects were allowed an initial smoke break at 7:30 AM to reduce nicotine craving. After settling in a sitting position on a hospital bed, a heparin-treated catheter was inserted by the research nurse in the ante-cubital region of the subject’s non-preferred arm to periodically obtain blood samples. A blood pressure cuff was placed on the subject’s preferred arm to monitor blood pressure (systolic blood pressure and diastolic blood pressure), and a pulse sensor was placed on the subject’s forefinger to obtain a measure of heart rate. Self-reports of craving and mood were completed after set up at 08:00 AM. This was followed by a 45-minute adaptation period during which the subjects were instructed to practice relaxation. Baseline measures of subjective reports, heart rate and blood pressure, saliva, and plasma were taken at two time-points: one at 08:45 (+45) and one at 9:05 (+65). At 9:10 AM, participnats were provided with headphones and given the following instructions for the imagery procedure: “Close your eyes and imagine the situation being described, ‘as if’ it were happening right now. Let your body and mind get completely involved in the situation, doing what you would do in the real situation”. The length of each script was approximately 5 min. Heart rate and blood pressure was continuously monitored during the imagery period.
Subjective ratings of craving, emotion, and anxiety, as well as heart rate, blood pressure, saliva, and plasma, were collected at the following time-points: +45 and +65 (baseline time-points), immediately following imagery exposure (+ 77 time-point), and subsequently at regular 15-min intervals +90, +105, +120, +135, +150 (recovery time-points). After the final assessments, the IV line, blood pressure cuff, and pulse sensor were removed, and breakfast was served.
All subjective measures (craving, anxiety, and emotion) and cardiovascular measures (heart rate and blood pressure) are presented as part of a larger study in a separate publication (Fox et al., 2008). In the current study, we present salivary cortisol and plasma cytokine data collected from a sub-sample of the participant population (28 cocaine dependent individuals and 27 social drinkers).
Laboratory assessments
Saliva measures for cortisol
Participants placed a cotton roll between their tongue and cheek for approximately 2–3 min until the swab was completely saturated (Salivette Sarstedt, Inc., Newton NC). Participants were required to focus their gaze on a segment of lemon being squeezed 2 ft away from them to stimulate saliva flow. Samples were then immediately placed in ice and subsequently stored in a −20°C freezer. All samples were assayed in duplicate following standard radioimmunoassay kits with no modifications (Diagnostic Products Corporation, CA) at the YCCI Core Laboratories. The intra assay coefficients of variation ranged from 3.0% to 5.1%.
Plasma measures for cytokines
Immediately following collection, the tubes were placed on ice. Plasma was subsequently separated by centrifugation at 4°C for 15 min at 1000 g. Plasmas were then aliquoted and stored in polypropylene tubes at −80°C until the time of the assay. TNF-α, IL-10, and IL-1ra concentrations were quantitatively determined by enzyme-linked immunesorbent assays using the DuoSet ELISA Development Kit from R&D systems (Minneapolis, MN, USA). Assaying of all plasma cytokines was conducted at Microgen Laboratories, La Marque, TX, USA under the direction of Dr. Raymond Stowe.
Statistical analysis
The drug groups were compared in terms of their demographics and substance use measures with the use of either T-tests or chi-square, and measures in which groups differed were included as covariates in all analyses.
Linear mixed effect models (Laird and Ware 1982) were implemented to analyze baseline (+65 time-point) data and response data with the use of SPSS software (version 17 SPSS Inc., Chicago, IL). The between subjects factors of the group (cocaine dependent individuals vs social drinkers), the within subjects factors of the condition (stress, drug/alcohol cue, and relaxation), and time-points (varying levels) were the fixed effect factors. Participants represented the random effect factor. To account for baseline variability across each testing day, the researchers used the change from the baseline of all measures to assess response to the imagery exposure. Bonferroni tests were used as adjustments for all multiple comparisons.
RESULTS
Participants
Both cocaine dependent individuals and socially drinking controls were statistically matched for race, gender, and IQ. However, the cocaine dependent group was significantly older than the control group and was comprised of individuals with a higher number of both regular smokers and those with current and lifetime history of alcohol abuse. Therefore, age, smoking status, and amount of alcohol consumed prior to inpatient treatment were used as covariates in all analyses (Table 1).
Table 1.
Participant demographic and clinical characteristics (means and standard deviations are shown)
| N = 55 | Socially drinking group n = 27 | Cocaine dependent group n = 28 | p |
|---|---|---|---|
| Gender—No. of males (%) | 13 (48.1%) | 12 (42.9%) | ns |
| Race | |||
| - No. of African American participants (%) | 7 (25.9%) | 13 (46.4%) | — |
| - No. of Caucasian participants (%) | 16 (59.3%) | 13 (46.4%) | — |
| - No. of Hispanic participants (%) | 3 (11.1%) | 1 (3.6%) | — |
| - No. of other races (%) | 1 (3.7%) | 1 (3.6%) | ns |
| Age (years) | 30.2 ±9.4 | 36.6 ±6.3 | 0.005 |
| IQ (Shipley) | 110.1 ±11.8 | 107.4 ±8.2 | ns |
| Smoking status—No. regular smokers (%) | 9 (33.3%) | 25 (89.3%) | <0.0001 |
| Years of cocaine use | 0 | 9.0 ±7.2 | — |
| No. of days used in past month | 0 | 21.7 ±7.6 | — |
| No. of grams per month | 0 | 45.9 ±49.7 | — |
| Years of Alcohol use | 5.9 ±5.9 | 13.6 ±7.6 | <0.0001 |
| No. of days used in past month | 3.9 ±4.8 | 10.8 ±9.0 | 0.001 |
| No. of drinks per month | 13.1 ±13.8 | 117.1 ±127.6 | 0.001 |
| No. currently alcohol dependent (%) | 0 | 13 (46.4%) | — |
| No. lifetime alcohol dependent (%) | 0 | 12 (42.9%) | — |
| No. lifetime alcohol abusing (%) | 0 | 9 (32.1%) | — |
| No. lifetime depression (%) | 1 (3.7%) | 3 (10.7%) | ns |
| No. lifetime anxiety (incl PTSD) (%) | 2 (7.4%) | 11 (39.3%) | 0.006 |
| No. lifetime anxiety (without PTSD) (%) | 0 | 6 (21.4%) | 0.01 |
ns, not significant; PTSD, Post Traumatic Stress Disorder.
Baseline differences between cocaine dependent and socially drinking controls
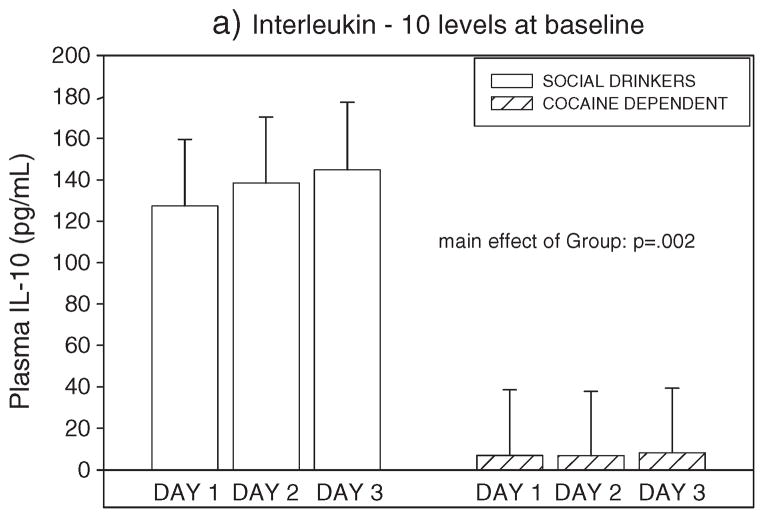
Cocaine dependent individuals demonstrated significantly lower levels of plasma IL-10 compared with social drinkers (F=11.0, p =0.002, without covariates; F = 6.1, p <0.02, with covariates) (see Figure 3).
Figure 3.
Group differences between cocaine dependent individuals and social drinkers in basal levels of Interleukin-10
Group differences in response to imagery (change from baseline)
Immune system markers
TNFα (pro-inflammatory)
The main effect of the imagery condition (F 2,495 = 6.5, p = 0.002, without covariates; F 2,495 = 6.5, p = 0.002, with covariates) indicated that the response to the neutral condition was significantly reduced compared to the response to both the stress condition (S >N, p = 0.009, without covariates; S >N, p = 0.009, with covariates) and the cue condition (C >N, p = 0.004, without covariates; C >N, p = 0.004, with covariates).
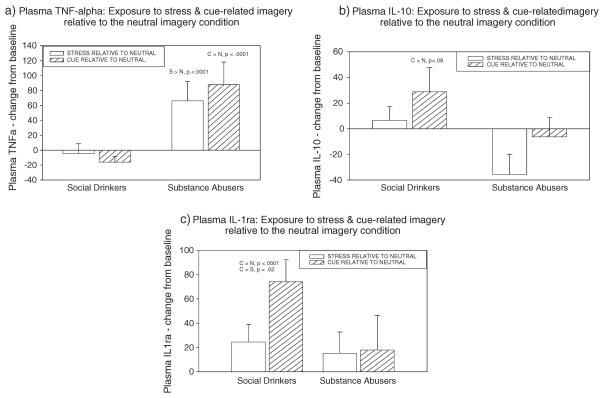
A significant group × imagery condition interaction was also observed (F 2,495 = 11.1, p <0.0001, without covariates; F 2,492 = 11.1, p <0.0001, with covariates) where the cocaine dependent individuals demonstrated higher levels of TNFα during the drug cue imagery conditions compared with the social drinkers (p <0.05, without covariates; p <0.07, with covariates). The cocaine dependent group also demonstrated significantly higher levels of TNFα when exposed to the stressful imagery and cue-related imagery compared with when they were exposed to the neutral imagery condition (p <0.0001, in all cases, with and without covariates). These stress and drug cue-induced increases in plasma TNFα were not observed in the social drinkers (see Figure 4a).
Figure 4.
Group differences between cocaine dependent individuals and social drinkers in (a) TNF alpha, (b) Interleukin-10, and (c) Interleukin-1 receptor antagonist following exposure to stress-related and cue-related imagery. Bars represent response in these conditions relative to the relaxing (control) imagery condition (means and standard errors shown). As no significant time-point interactions were observed, bars represent data collapsed across all time-points.
IL-10 (anti-inflammatory)
A group × imagery condition interaction trend was observed for IL-10 (F 2,407 = 2.7, p = 0.06 without covariates; F 2,405 = 2.7, p = 0.07, with covariates), because the social drinkers demonstrated a trend in increased IL-10 when exposed to the cue imagery condition compared with when they were exposed to the neutral imagery condition (p <0.08, with and without covariates) (see Figure 4b). This cue-induced increase in IL-10 was not observed in the cocaine dependent individuals.
IL-1ra
The main effect of imagery condition (F 2,496 = 3.6, p <0.03, without covariates; F 2,495 = 3.6, p <0.03, with covariates) indicated that the response to the cue condition was significantly higher than the response to the neutral condition (C >N, p = 0.02, without covariates; C >N, p = 0.02, with covariates). A group × imagery condition interaction was also observed (F 2,496 = 3.3, p <0.04 without covariates; F 2,495 = 3.4, p <0.04, with covariates) showing the overall cue-related effect was caused by social drinkers demonstrating an increase in IL-1ra when exposed to the cue imagery condition compared with when they were exposed to the neutral imagery condition (p <0.0001, without covariates; p <0.0001, with covariates), as well as the stress imagery condition (p <0.02, with and without covariates). This cue-induced increase in IL-1ra was not observed in the cocaine dependent individuals (see Figure 4c).
HPA system marker (salivary cortisol)
A main effect on the groups was shown in the salivary cortisol prior only to the inclusion of covariates (F 1,51 = 5.3, p <0.03 without covariate; F 2,51 = 0.9, p = ns, with covariates). The cocaine dependent individuals showed a dampened level (i.e., a greater diurnal drop) in cortisol response to all three imagery conditions compared with the social drinkers.
Extended analysis
Secondary analysis was conducted only within the cocaine dependent group to assess whether stress and cytokine variations were apparent between those meeting the criteria for lifetime anxiety and those not meeting the criteria. Mixed models were performed with the use of lifetime anxiety as a fixed factor. No significant group differences were observed, with the exception of TNFα response (F 2,212 = 18.4, p <0.0001) where cocaine dependent individuals with co-morbid lifetime anxiety showed a greater response to the cue imagery condition compared with the neutral condition (C >N, p <0.04). This was not observed in the cocaine dependent group with no lifetime history of anxiety disorders.
DISCUSSION
Current findings show that cocaine dependent individuals demonstrate increased immune system inflammation both at the baseline and in response to stress and cue imagery conditions compared with the social drinkers. Specifically, although basal inflammation in the cocaine group was characterized by significantly lower levels of the anti-inflammatory marker IL-10, phasic response to stress was marked by significantly higher levels of TNFα relative to their intra-individual relaxing baseline condition. Response to the cue imagery condition highlighted even greater the indications of inflammation, characterized by both increased intra-individual levels of TNFα not observed in the social drinkers and a dampened anti-inflammatory response highlighted by lower levels of IL-1ra. Similarly, social drinkers demonstrated a tendency for increased levels of IL-10 when exposed to the cue imagery condition compared with when they were exposed to their neutral baseline; this was not seen in the cocaine dependent group. In terms of stress system changes, compared with social drinkers, a dampened response to all three imagery conditions was observed in the cocaine dependent individuals. However, this was only observed prior to the adjustment made for the smoking status and drinking status 1 month before the treatment. Just as identical imagery paradigms are known to induce a dysregulated arousal response marked by elevated craving and negative emotion in a range of substance abusers (Fox et al., 2006, 2007, 2008, 2009; Hyman et al., 2007; Chaplin et al., 2008, 2010; Sinha et al, 2009, 2011), immune system cytokines may also represent a parallel set of biomarkers reflecting stress-related and cue-related risk factors.
Consistent with the current findings, there is some support in the literature for the existence of an inflammatory Th1 shift in cocaine dependent individuals. However, it is important to note that little research exists which focuses specifically on immune system changes during early protracted withdrawal in co-morbid cocaine dependent individuals without current pervasive health issues (Deviere et al., 1989; Masumoto et al., 1993) exists. Moreover, adaptations in cytokine cascades are highly dependent on the status of drug/ alcohol intake (Laso et al., 1996, 1997), and research has tended to focus on the acute effects of cocaine/ alcohol intake on animals or dependent humans. Despite these factors, certain preclinical studies assessing immune system changes during early protracted withdrawal show some support for current findings by documenting an up-regulation of both corticosterone and TNFa following 6 weeks (Wang et al., 1994) and 18 days (Kubera et al., 2008) of cocaine administration in mice. Intriguingly, and consistent with current basal findings, increased serum levels of anti-inflammatory IL-10 have also been shown to decline significantly in the few days after alcohol abstinence in human patients with alcohol withdrawal syndrome and who are also free of liver pathology (González-Quintela et al., 2000).
In contrast to the current findings, however, many animal studies have also documented a direct enhancement of the Th2 state—showing increases in the humoral T-dependent anti-body response (IL-10 and IL-4) following acute and prolonged cocaine exposure in mice (Stanulis et al., 1997; Gardner et al., 2004; Kubera et al., 2008). In addition, alcohol has typically been defined as an immunosuppressive agent (Gomez et al., 2010), and in vitro exposure to cocaine has shown inflammation-inhibitory and immunosuppressive responses including decreased IL-2 and IFN-y secretion from spleen cells (Falchetti et al., 1995) and reduced IL-1 and TNFα from peritoneal macrophages (Shen et al., 1994). However, again, it is important to note that discrepancy in the current findings may be related to a variation in the cytokine equilibrium associated with the specific parameters of immune system activation (Elenkov, 2008), that is, acute cocaine exposure as compared with chronic cocaine exposure. Notably, a recent study by Kubera et al. (2008) show some support for the current data by documenting increases in TNFa and a reduced production of IL-10 by splenocytes in mice following exposure to a conditioned stimuli previously paired with cocaine administration, after 10 days of withdrawal.
In the present study, cocaine dependent individuals also demonstrated a lower HPA axis drive in response to all three imagery conditions. Interestingly, however, this failure to demonstrate a typical HPA arousal in response to provocation was not apparent after controlling for effects of alcohol and nicotine consumption and is consistent with extensive prior research assessing stress system dysregulation in alcohol dependent individuals (Junghanns et al., 2003; Breese et al., 2005, 2011; Badrick et al., 2007) and alcohol dependent smokers (Fox et al., 2007; Sinha et al., 2009). A dampened HPA axis drive has also been associated with a return to early drinking in alcoholics (Breese et al., 2005; Junghanns et al., 2005), as well as being a risk marker for the development of substance use disorders in individuals with a positive family history for alcoholism (Sorocco et al., 2006). Although one of the limitations of the current study may be related to the fact that pure cocaine dependent users were not recruited, the simultaneous abuse of alcohol and nicotine is common in cocaine dependence (McCance-Katz et al., 1999; Patkar et al., 2006), and thus may reflect a more ecologically reliable participant sample. Nonetheless, future research is encouraged to clarify the relative contribution of these drug-related processes to the maintenance of cocaine use by employing additional dependent cohorts who abuse alcohol and/or other substances.
In terms of highlighting a potential inflammatory mechanism underpinning the stress system arousal in cocaine dependent individuals, it is interesting to note that the current pattern of immune system alterations are congruent with the cortisol dysregulation documented in both the current and prior research (Breese et al., 2005; Sinha et al., 2009). Just as glucocortiods up-regulate Th2 production (Ramierz et al., 1996; Blotta et al., 1997) and suppress induction of Th1 cytokines including TNFα (Elenkov and Chrousos, 1999, 2006), a dampened cortisol response to stress may account for the elevated Th1 shift observed following stress exposure in the current cocaine dependent individuals. It is important to note however that these interpretations remain tentative, as the specific interactions and parameters of such stress and immune system mechanisms have not been assessed directly in the present study, and the suppressed HPA response to provocation may be associated with concomitant substance abuse.
A clear limitation to the present research relates to the fact that it remains uncertain whether the observed adaptations relate to aspects of cocaine consumption per se or to affective changes related to withdrawal, or both. For example, activation of pro-inflammatory mediators are demonstrated in patients with mood and depressive symptomatology (Maes, 1995; Glaser et al., 2003; McNally et al., 2008; Maes et al., 2009). In addition, the treatment of both patients (Valentine et al., 1998; Gohier et al., 2003; Dunn et al., 2005) and animals (Anisman and Merali, 1999; Brebner et al., 2000; Kronfol and Remick, 2000; Bonaccorso et al., 2003; Silverman et al., 2007; Salome et al., 2008) with pro-inflammatory cytokines (TNFα; IL-1β) can produce deleterious mood-related symptoms. Moreover, pathological activation of the immune system is associated with depressive and anxiety-related symptoms in chronically ill patients (Dantzer and Kelley, 2007). In the current study, for example, high levels of cue-related TNFα were demonstrated as a function of lifetime anxiety, as well as substance abuse. Future research is encouraged to determine more thoroughly the relative contribution of these factors to the immune system changes in substance users. Despite this, the current study is one of the first to examine cytokine response to stress and cue in a relatively healthy and ecologically valid group of primary cocaine dependent individuals, who were excluded if taking medication for any current health or psychiatric problems.
The idea that maladaptive inflammatory responses may provide additional pathways contributing to stress-related risk in cocaine dependent individuals suggests that peripheral cytokines may represent efficacious new biomarkers for treatment development. Currently, there is a growing application of immunotherapy manipulations used to restore the delicate balance between anti-inflammatory and pro-inflammatory cytokines. These include administration of antibodies against specific cytokines, the administration of soluble cytokines to absorb excess cytokines, the deactivation of glial cells that produce excessive quantities of pro-inflammatory cytokines, and the use of cytokine inhibitors (Cook, 1998; Delgado, 2003; Dantzer and Kelley, 2007; Moreland, 2009). However, despite the current availability of many of these pharmacological tools, greater clarity regarding the precise cytokine mechanisms underpinning the immune and stress system interactions in substance abuse is needed before Phase 1 clinical trials can be conducted. In particular, the roles of anti-inflammatory cytokines have not been as thoroughly assessed as pro-inflammatory cytokines in terms of their effect on depression and anxiety.
In terms of assessing the applications for the current findings, the identification of an underlying inflammatory mechanism for stress-related relapse risk may hold potential for the advancement of addiction pharmacotherapies, particularly because anti-inflammatory cytokines are shown to be safe and well tolerated across a range of clinical populations. Weekly injections of recombinant IL-10 has proven to be an effective therapy for inflammatory bowel disease and psoriasis (Yamagata and Ichinose, 2006), and clinical trials are being conducted to assess its use in multiple sclerosis (phase II) and gut ischemia (phase I) (Asadullah et al., 2003). IL1ra is also being assessed for rheumatoid arthritis treatment (phase II and III) (Bresnihan, 2001; Fiocco et al., 2004). Similarly, soluble receptor medications, such as Etanercept, that bind to TNFα and decrease its role in disorders involving excess inflammation have been shown to result in only minor side effects in majority of patients (Fernandez-Botran et al., 2002). Therefore, if the stress arousal systems underpinning craving and negative reinforcing effects of drugs are shown to be characterized by chronic inflammation, as current findings would suggest, this may instigate future clinical studies addressing the applications of these targets for relapse prevention.
Acknowledgments
We wish to thank the staff at the Clinical Neuroscience Research Unit based at The Connecticut Mental Health Center, and the Hospital Research Unit of the Yale Center for Clinical Investigation at the Yale University School of Medicine for their assistance in completing this study. This study was supported in part by grants from R.S. (R0I-AA13892, PL1-SA024859, and P50-DA16556) and Yale NIH/NCRR/CTSA Program Grant (UL1 RR024139).
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no competing financial interests pertaining to the aims and results of this study.
References
- Anisman H, Merali Z. Anhedonic and anxiogenic effects of cytokine exposure. Adv Exp Med Biol. 1999;461:199–233. doi: 10.1007/978-0-585-37970-8_12. Review. [DOI] [PubMed] [Google Scholar]
- Anisman H, Poulter MO, Gandhi R, Merali Z, Hayley S. Interferon-alpha effects are exaggerated when administered on a psychosocial stressor backdrop: cytokine, corticosterone and brain monoamine variations. J Neuroimmunol. 2007;186:45–53. doi: 10.1016/j.jneuroim.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Asadullah K, Sterry W, Volk HD. Interleukin 10 therapy. Review of a new approach. Pharmacol Rev. 2003;55(2):241–269. doi: 10.1124/pr.55.2.4. Review. [DOI] [PubMed] [Google Scholar]
- Badrick E, Bobak M, Britton A, Kirschbaum C, Marmot M, Kumari M. The relationship between smoking status and cortisol secretion. J Clin Endocrinol Metab. 2007;92:819–824. doi: 10.1210/jc.2006-2155. [DOI] [PubMed] [Google Scholar]
- Bergquist KL, Fox HC, Sinha R. Self-reports of interoceptive responses during stress and drug cue related experiences in cocaine and alcohol dependent individuals. Exp Clin Psychopharmacol. 2010;18(3):229–237. doi: 10.1037/a0019451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blotta MH, DeKruyff RH, Umetsu DT. Corticosteroids inhibit IL-12 production in human monocytes and enhance their capacity to induce IL-4 synthesis in CD4+ lymphocytes. J Immunol. 1997;158:5589–5595. [PubMed] [Google Scholar]
- Bonaccorso S, Maier SF, Meltzer HY, Maes M. Behavioral changes in rats after acute, chronic and repeated administration of interleukin-1beta: relevance for affective disorders. J Affect Disord. 2003;77:143–148. doi: 10.1016/s0165-0327(02)00118-0. [DOI] [PubMed] [Google Scholar]
- Brebner K, Hayley S, Zacharko R, Merali Z, Anisman H. Synergistic effects of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha: central monoamine, corticosterone, and behavioral variations. Neuropsychopharmacology. 2000;22:566–580. doi: 10.1016/S0893-133X(99)00166-9. [DOI] [PubMed] [Google Scholar]
- Breese GR, Chu K, Dayas CV, et al. Stress enhancement of craving during sobriety: a risk for relapse. Alcohol Clin Exp Res. 2005;29(2):185–195. doi: 10.1097/01.alc.0000153544.83656.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Sinha R, Heilig M. Chronic alcohol neuroadaptation and stress contribute to susceptibility for alcohol craving and relapse. Pharmacol Ther. 2011;129(2):149–171. doi: 10.1016/j.pharmthera.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts CL, Sternberg EM. Neuroendocrine factors alter host defense by modulating immune function. Cell Immunol. 2008;252:7–15. doi: 10.1016/j.cellimm.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresnihan B. The safety and efficacy of interleukin-1 receptor antagonist in the treatment of rheumatoid arthritis. Semin Arthritis Rheum. 2001;30 (5 Suppl 2):17–20. doi: 10.1053/sarh.2001.23701. [DOI] [PubMed] [Google Scholar]
- Cahalan D, Cisin IH, Crossley HM. American drinking practices. A national study of drinking behaviors and attitudes. Monographs of the Rutgers Center of Alcohol Studies. 1969;6:260. [Google Scholar]
- Calcagni E, Elenkov I. Stress system activity, innate and T helper cytokines, and susceptibility to immune-related diseases. Ann N Y Acad Sci. 2006;1069:62–76. doi: 10.1196/annals.1351.006. [DOI] [PubMed] [Google Scholar]
- Chaplin T, Hong K-I, Bergquist K, Sinha R. Sex differences in stress and alcohol cue-induced craving and emotion in social drinkers. Alcohol Clin Exp Res. 2008;31(6):603. [Google Scholar]
- Chaplin TM, Hong K, Fox HC, Siedlarz KM, Bergquist K, Sinha R. Behavioral arousal in response to stress and drug cue in alcohol and cocaine addicted individuals versus healthy controls. Hum Psychopharmacol Clin Exp. 2010;25(5):368–376. doi: 10.1002/hup.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RT. Alcohol abuse, alcoholism, and damage to the immune system—a review. Alcohol Clin Exp Res. 1998;22:1927–1942. Review. [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Stress and immunity: an integrated view of relationships between the brain and the immune system. Life Sci. 1989;44(26):1995–2008. doi: 10.1016/0024-3205(89)90345-7. Review. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Review. de Jong IE, de Kloet ER. Glucocorticoids and vulnerability to psychostimulant drugs: toward substrate and mechanism. Ann N Y Acad Sci. 2004;1018:192–198. doi: 10.1196/annals.1296.022.
- Delgado M. Inhibition of interferon (IFN) gamma-induced Jak-STAT1 activation in microglia by vasoactive intestinal peptide: inhibitory effect on CD40, IFN-induced protein-10, and inducible nitric-oxide synthase expression. J Biol Chem. 2003;278:27620–27629. doi: 10.1074/jbc.M303199200. [DOI] [PubMed] [Google Scholar]
- Deviere J, Content J, Denys C, et al. High interleukin-6 serum levels and increased production by leucocytes in alcoholic liver cirrhosis. Correlation with IgA serum levels and lymphokines production. Clin Exp Immunol. 1989;77(2):221–225. [PMC free article] [PubMed] [Google Scholar]
- Dhillon NK, Williams R, Peng F, et al. Cocaine-mediated enhancement of virus replication in macrophages: implications for human immunodeficiency virus-associated dementia. J Neurovirol. 2007;13(6):483–495. doi: 10.1080/13550280701528684. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. The role of the interleukin-1 receptor antagonist in blocking inflammation mediated by interleukin-1. N Engl J Med. 2000;343:721–734. doi: 10.1056/NEJM200009073431011. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH, de Beaurepaire R. Cytokines as mediators of depression: what can we learn from animal studies? Neurosci Biobehav Rev. 2005;29:891–909. doi: 10.1016/j.neubiorev.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ. Neurohormonal-cytokine interactions: implications for inflammation, common human diseases and well-being. Neurochem Int. 2008;52 :40–51. doi: 10.1016/j.neuint.2007.06.037. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Chrousos GP. Stress hormones, Th1/Th2 patterns, pro/ anti-inflammatory cytokines and susceptibility to disease. Trends Endocrinol Metab. 1999;10:359–368. doi: 10.1016/s1043-2760(99)00188-5. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Chrousos GP. Stress system—organization, physiology and immunoregulation. Neuroimmunomodulation. 2006;13:257–267. doi: 10.1159/000104853. [DOI] [PubMed] [Google Scholar]
- Emanuele N, LaPaglia N, Kovacs EJ, Emanuele MA. Effects of chronic ethanol (EtOH) administration on pro-inflammatory cytokines of the hypothalamic-pituitary-gonadal (HPG) axis in female rats. Endocr Res. 2005;31:9–16. doi: 10.1080/07435800500228930. [DOI] [PubMed] [Google Scholar]
- Falchetti R, Di Francesco P, Lanzilli G, Gaziano R, Casalinuovo IA, Ravagnan G, Garaci E. In vitro effects of cocaine on cytokine secretion induced in murine splenic CD4+ T cells by antigen-specific stimulation. Cell Immunol. 1995;164(1):57–64. doi: 10.1006/cimm.1995.1142. [DOI] [PubMed] [Google Scholar]
- Falck RS, Wang J, Carlson RG, Eddy M, Siegal HA. The prevalence and correlates of depressive symptomatology among a community sample of crack-cocaine smokers. J Psychoactive Drugs. 2002;34(3):281–8. doi: 10.1080/02791072.2002.10399964. [DOI] [PubMed] [Google Scholar]
- Fernandez-Botran R, Crespo FA, Sun X. Soluble cytokine receptors in biological therapy. Expert Opin Biol Ther. 2002;2:585–605. doi: 10.1517/14712598.2.6.585. Review. [DOI] [PubMed] [Google Scholar]
- Fiocco U, Vezzù M, Cozzi L, Todesco S. [IL-1Ra (recombinant human IL-1 receptor antagonist) in the treatment of rheumatoid arthritis: the efficacy] Reumatismo. 2004;56(1 Suppl 1):62–73. doi: 10.4081/reumatismo.2004.1s.62. Review. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for the DSM-IV Axis I Disorders—Patient Edition (SCID-I/P, Version 2.0, 4/97 revision) New York State Psychiatric Institute; New York: 1997. [Google Scholar]
- Fox HC, Berquist KL, Hong KI, Sinha R. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol dependent individuals. Alcohol Clin Exp Res. 2007;31(3):395–403. doi: 10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- Fox HC, Garcia M, Kemp K, Milivojevic V, Kreek MJ, Sinha R. Gender differences in cardiovascular and corticoadrenal response to stress and drug-cue in cocaine dependent individuals. Psychopharmacology. 2006;185(3):348–357. doi: 10.1007/s00213-005-0303-1. [DOI] [PubMed] [Google Scholar]
- Fox HC, Hong KI, Siedlarz KM, Bergquist KL, Anderson GM, Kreek MJ, Sinha R. Gender dissociations in autonomic and HPA responses to stress and cues in alcohol dependent patients with cocaine abuse. Alcohol Alcohol. 2009;44(6):575–85. doi: 10.1093/alcalc/agp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Hong KI, Siedlarz KM, Sinha R. Enhanced sensitivity to stress and drug/alcohol craving in abstinent cocaine dependent individuals compared to social drinkers. Neuropsychopharmacology. 2008;33(4):796–805. doi: 10.1038/sj.npp.1301470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Sinha R. Sex differences in drug-related stress system changes: implications for treatment in substance-abusing women. Harv Rev Psychiatry. 2009;17(2):103–19. doi: 10.1080/10673220902899680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Talih M, Malison R, Anderson GM, Kreek MJ, Sinha R. Frequency of recent cocaine and alcohol use affects drug craving and associated responses to stress and to drug-related cues. Psychoneuroendocrinology. 2005;30:880–891. doi: 10.1016/j.psyneuen.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Gaab J, Rohleder N, Heitz V, et al. Stress-induced changes in LPS-induced pro-inflammatory cytokine production in chronic fatigue syndrome. Psychoneuroendocrinology. 2005;30(2):188–198. doi: 10.1016/j.psyneuen.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Gardner B, Zhu LX, Roth MD, Tashkin DP, Dubinett SM, Sharma S. Cocaine modulates cytokine and enhances tumor growth through sigma receptors. J Neuroimmunol. 2004;147(1–2):95–98. doi: 10.1016/j.jneuroim.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Gibb J, Hayley S, Gandhi R, Poulter MO, Anisman H. Synergistic and additive actions of a psychosocial stressor and endotoxin challenge: circulating and brain cytokines, plasma corticosterone and behavioral changes in mice. Brain Behav Immun. 2008;22:573–589. doi: 10.1016/j.bbi.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Glaser R, Robles TF, Sheriden J, Malarkey WB, Kiecolt-Glaser JK. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses after influenza virus vaccination in older adults. Arch Gen Psychiatry. 2003;60:1009–1014. doi: 10.1001/archpsyc.60.10.1009. [DOI] [PubMed] [Google Scholar]
- Gohier B, Goeb JL, Rannou-Dubas K, Fouchard I, Calès P, Garré JB. Hepatitis C, alpha interferon, anxiety and depression disorders: a prospective study of 71 patients. World J Biol Psychiatry. 2003;4:115–118. doi: 10.1080/15622970310029904. [DOI] [PubMed] [Google Scholar]
- Gomez M, Raju SV, Viswanathan A, et al. Ethanol upregulates glucocorticoid-induced leucine zipper expression and modulates cellular inflammatory responses in lung epithelial cells. J Immunol. 2010;184(10):5715–5722. doi: 10.4049/jimmunol.0903521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Quintela A, Dominguez-Santalla MJ, Pérez LF, Vidal C, Lojo S, Barrio E. Influence of acute alcohol intake and alcohol withdrawal on circulating levels of IL-6, IL-8, IL-10 and IL-12. Cytokine. 2000;12(9):1437–1440. doi: 10.1006/cyto.2000.0715. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Johnson AK. Stress, depression and cardiovascular dysregulation: a review of neurobiological mechanisms and the integration of research from preclinical disease models. Stress. 2009;12(1):1–21. doi: 10.1080/10253890802046281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. Review. [DOI] [PubMed] [Google Scholar]
- Hyman SM, Fox H, Hong KI, Doebrick C, Sinha R. Stress and drug cue-induced craving in opioid dependent individuals in naltrexone treatment. Exp Clin Psychopharmacol. 2007;15(2):134–143. doi: 10.1037/1064-1297.15.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghanns K, Backhaus J, Tietz U, et al. Impaired serum cortisol stress response is a predictor of early relapse. Alcohol Alcohol. 2003;38:189–193. doi: 10.1093/alcalc/agg052. [DOI] [PubMed] [Google Scholar]
- Junghanns K, Tietz U, Dibbelt L, et al. Attenuated salivary cortisol secretion under cue exposure is associated with early relapse. Alcohol Alcohol. 2005;40(1):80–85. doi: 10.1093/alcalc/agh107. [DOI] [PubMed] [Google Scholar]
- Kent S, Bluthe R-M, Kelley KW, Dantzer R. Sickness behavior as a new target for drug development. Trends Pharmacol Sci. 1992;13:24–28. doi: 10.1016/0165-6147(92)90012-u. [DOI] [PubMed] [Google Scholar]
- Koob GF. Dynamics of neuronal circuits in addiction: reward, antireward, and emotional memory. Pharmacopsychiatry. 2009;42(Suppl 1):S32–S41. doi: 10.1055/s-0029-1216356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronfol Z, Remick DG. Cytokines and the brain: implications for clinical psychiatry. Am J Psychiatry. 2000;157:683–694. doi: 10.1176/appi.ajp.157.5.683. [DOI] [PubMed] [Google Scholar]
- Kubera M, Filip M, Basta-Kaim A, et al. The effect of cocaine sensitization on mouse immunoreactivity. Eur J Pharmacol. 2004;483(2–3):309–315. doi: 10.1016/j.ejphar.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Kubera M, Filip M, Budziszewska B, et al. Immunosuppression induced by a conditioned stimulus associated with cocaine self-administration. J Pharmacol Sci. 2008;107(4):361–369. doi: 10.1254/jphs.fp0072106. [DOI] [PubMed] [Google Scholar]
- Kubera M, Kenis G, Bosmans E, et al. Plasma levels of interleukin-6, interleukin-10, and interleukin-1 receptor antagonist in depression: comparison between the acute state and after remission. Pol J Pharmacol. 2000;52 (3):237–241. [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Laso FJ, Lapeña P, Madruga JI, et al. Alterations in tumor necrosis factor-alpha, interferon-gamma, and interleukin-6 production by natural killer cell-enriched peripheral blood mononuclear cells in chronic alcoholism: relationship with liver disease and ethanol intake. Alcohol Clin Exp Res. 1997;21(7):1226–1231. [PubMed] [Google Scholar]
- Laso FJ, Madruga JI, San Miguel JF, et al. Long lasting immunological effects of ethanol after withdrawal. Cytometry. 1996;26(4):275–280. doi: 10.1002/(SICI)1097-0320(19961215)26:4<275::AID-CYTO6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Lehto SM, Niskanen L, Miettola J, Tolmunen T, Viinamäki H, Mäntyselkä P. Serum anti-inflammatory markers in general population subjects with elevated depressive symptoms. Neurosci Lett. 2010;484(3):201–205. doi: 10.1016/j.neulet.2010.08.054. [DOI] [PubMed] [Google Scholar]
- Leon LR, Kozak W, Rudolph K, Kluger MJ. An antipyretic role for interleukin-10 in LPS fever in mice. Am J Physiol. 1999;276:R81–R89. doi: 10.1152/ajpregu.1999.276.1.R81. [DOI] [PubMed] [Google Scholar]
- Liu L, Jia F, Yuan G, et al. Tyrosine hydroxylase, interleukin-1beta and tumor necrosis factor-alpha are overexpressed in peripheral blood mononuclear cells from schizophrenia patients as determined by semi-quantitative analysis. Psychiatry Res. 2010;176(1):1–7. doi: 10.1016/j.psychres.2008.10.024. [DOI] [PubMed] [Google Scholar]
- Maes M. Evidence for an immune response in major depression: a review and hypothesis. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:11–38. doi: 10.1016/0278-5846(94)00101-m. Review. [DOI] [PubMed] [Google Scholar]
- Maes M, Lin AH, Ombelet W, et al. Immune activation in the early puerperium is related to postpartum anxiety and depressive symptoms. Psychoneuroendocrinology. 2000;25(2):121–137. doi: 10.1016/s0306-4530(99)00043-8. [DOI] [PubMed] [Google Scholar]
- Maes M, Song C, Lin A, et al. The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine. 1998;10(4):313–318. doi: 10.1006/cyto.1997.0290. [DOI] [PubMed] [Google Scholar]
- Maes M, Yirmyia R, Noraberg J, et al. The inflammatory & neurode-generative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab Brain Dis. 2009;24:27–53. doi: 10.1007/s11011-008-9118-1. [DOI] [PubMed] [Google Scholar]
- Masumoto T, Onji M, Horiike N, Ohta Y. Assay of serum interleukin 8 levels in patients with alcoholic hepatitis. Alcohol Alcohol Suppl. 1993;1A:99–102. doi: 10.1093/alcalc/28.supplement_1a.99. [DOI] [PubMed] [Google Scholar]
- McAfoose J, Baune BT. Evidence for a cytokine model of cognitive function. Neurosci Biobehav Rev. 2009;33:355–366. doi: 10.1016/j.neubiorev.2008.10.005. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Carroll KM, Rounsaville BJ. Gender differences in treatment-seeking cocaine abusers—implications for treatment and prognosis. Am J Addict. 1999;8(4):300–311. doi: 10.1080/105504999305703. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Hart CL, Boyarsky B, Kosten T, Jatlow P. Gender effects following repeated administration of cocaine and alcohol in humans. Subst Use Misuse. 2005;40(4):511–528. doi: 10.1081/ja-200030693. [DOI] [PubMed] [Google Scholar]
- McNally L, Bhagwagar Z, Hannestad J. Inflammation, glutamate, and glia in depression: a literature review. CNS Spectr. 2008;13:501–510. doi: 10.1017/s1092852900016734. Review. [DOI] [PubMed] [Google Scholar]
- Mello NK, Newman JL. Discriminative and reinforcing stimulus effects of nicotine, cocaine, and cocaine + nicotine combinations in Rhesus monkeys. Exp Clin Psychopharmacol. 2011;19(3):203–14. doi: 10.1037/a0023373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer D, Woody G. Unpublished manuscript. University of Pennsylvania/ VAMC Center for Studies of Addiction; 1992. Addiction Counseling. [Google Scholar]
- Mesquita AR, Correia-Neves M, Roque S, et al. IL-10 modulates depressive-like behavior. J Psychiatr Res. 2008;43:89–97. doi: 10.1016/j.jpsychires.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Moreland LW. Cytokines as targets for anti-inflammatory agents. Ann N Y Acad Sci. 2009;1182:88–96. doi: 10.1111/j.1749-6632.2009.05072.x. [DOI] [PubMed] [Google Scholar]
- Patkar AA, Mannelli P, Peindl K, Murray HW, Meier B, Leone FT. Changes in tobacco smoking following treatment for cocaine dependence. Am J Drug Alcohol Abuse. 2006;32(2):135–148. doi: 10.1080/00952990500479209. [DOI] [PubMed] [Google Scholar]
- Preston KL, Epstein DH. Stress in the daily lives of cocaine and heroin users: relationship to mood, craving, relapse triggers, and cocaine use. Psychopharmacology (Berl) 2011;218(1):29–37. doi: 10.1007/s00213-011-2183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, He J, Hanes RN, Pluzarev O, Hong JS, Crews FT. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J Neuroinflammation. 2008;5:10. doi: 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramierz F, Fowell DJ, Puklavec M. Glucocorticoids promote a TH2 cytokine response by CD4+ T cells in vitro. J Immunol. 1996;156:2406–2412. [PubMed] [Google Scholar]
- Salome N, Tasiemski A, Dutriez I, Wigger A, Landgraf R, Viltart O. Immune challenge induces differential corticosterone and interleukin-6 responsiveness in rats bred for extremes in anxiety-related behavior. Neuroscience. 2008;151:1112–1118. doi: 10.1016/j.neuroscience.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Shen HM, Kennedy JL, Ou DW. Inhibition of cytokine release by cocaine. Int J Immunopharmacol. 1994;16(4):295–300. doi: 10.1016/0192-0561(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Silverman MN, Macdougall MG, Hu F, Pace TW, Raison CL, Miller AH. Endogenous glucocorticoids protect against TNF-alpha-induced increases in anxiety-like behavior in virally infected mice. Mol Psychiatry. 2007;12:408–417. doi: 10.1038/sj.mp.4001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist KL, Bhagwagar Z, Siedlatz K. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34(5):1198–1208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Siedlarz KA, Bergquist KT, Kreek MJ. Stress and cue-induced alcohol craving, anxiety and adrenal sensitivity are predictive of alcohol relapse outcomes. Arch Gen Psychiatry. 2011;68(9):942–52. doi: 10.1001/archgenpsychiatry.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63(3):324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Sinha R, Talih M, Malison R, Cooney N, Anderson GM, Kreek MJ. Hypothalamic-pituitary-ACDrenal axis and sympatho-ACDrenomedullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl) 2003;170:62–72. doi: 10.1007/s00213-003-1525-8. [DOI] [PubMed] [Google Scholar]
- Sorocco KH, Lovallo WR, Vincent AS, Collins FL. Blunted hypothalamic-pituitary-adrenocortical axis responsivity to stress in persons with a family history of alcoholism. Int J Psychophysiol. 2006;59:210–217. doi: 10.1016/j.ijpsycho.2005.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanulis ED, Jordan SD, Rosecrans JA, Holsapple MP. Disruption of Th1/Th2 cytokine balance by cocaine is mediated by corticosterone. Immunopharmacology. 1997;37(1):25–33. doi: 10.1016/s0162-3109(96)00167-1. [DOI] [PubMed] [Google Scholar]
- Stewart J. Stress and relapse to drug seeking: studies in laboratory animals shed light on mechanisms and sources of long-term vulnerability. Am J Addict. 2003;12(1):1–17. Review. [PubMed] [Google Scholar]
- Tilders FJ, Schmidt ED, de Goeij DC. Phenotypic plasticity of CRF neurons during stress. Ann N Y Acad Sci. 1993;697:39–52. doi: 10.1111/j.1749-6632.1993.tb49921.x. [DOI] [PubMed] [Google Scholar]
- Turnbull AV, Rivier CL. Regulation of the hypothalamic–pituitary–adrenal axis by cytokines: actions and mechanisms of action. Physiol Rev. 1999;79:1–71. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- Valentine AD, Meyers CA, Kling MA, Richelson E, Hauser P. Mood and cognitive side effects of interferon-alpha therapy. Semin Oncol. 1998;25 (1 Suppl 1):39–47. [PubMed] [Google Scholar]
- Wang Y, Dennis SH, Watson RR. In vivo and in vitro cocaine modulation of production of cytokines in C57BL/6 mice. Life Sci. 1994;54:401–411. doi: 10.1016/0024-3205(94)00698-9. [DOI] [PubMed] [Google Scholar]
- Weinstein AA, Deuster PA, Francis JL, Bonsall RW, Tracy RP, Kop WJ. Neurohormonal and inflammatory hyper-responsiveness to acute mental stress in depression. Biol Psychol. 2010;84(2):228–234. doi: 10.1016/j.biopsycho.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. Endogenous modulators for drug dependence. Biol Pharm Bull. 2008;31(9):1635–1638. doi: 10.1248/bpb.31.1635. [DOI] [PubMed] [Google Scholar]
- Yamagata T, Ichinose M. Agents against cytokine synthesis or receptors. Eur J Pharmacol. 2006;533(1–3):289–301. doi: 10.1016/j.ejphar.2005.12.046. [DOI] [PubMed] [Google Scholar]