Direct transition metal-catalyzed functionalization of C–H bonds in heterocycles serves as a powerful tool for organic synthesis. This approach offers the possibility for catalytic transformation of unreactive C–H bonds into diverse functionalities, as opposed to the traditional cross-coupling methods, employing heterocyclic halides or organometallic derivatives. In particular, direct arylation and vinylation of heterocycles has already gained widespread acceptance within the synthetic community, because of its capacity to utilize simpler and cheaper precursors for the construction of complex frameworks.1 In the last two decades, this area was rapidly growing and new types of transition metal-catalyzed direct intra- and intermolecular reactions of electron-rich1–3 and electron-defficient4 heterocycles, as well as simple arenes,4 have been developed (eq 1). These C–C bond forming reactions involve C–H arylation, heteroarylation, and vinylation. Although the majority of these methods are based on the employment of palladium catalysis in both Pd(0/II)2–4 and Pd(II/IV)5 modes, methods involving rhodium,6 platinum,7 and gold8 complexes have also been reported. Despite the vast structural complexity of the products that existing methods for direct C–H functionalization of heterocycles offer (eq 1), they are still limited to sp2–p2 carbon–carbon bond-forming reactions. Herein, we report the direct palladium-catalyzed sp2–sp carbon–carbon bond-forming reaction of electron-rich heterocycles with alkynyl halides. This conceptually new approach provides straightforward and efficient access to diverse alkynyl heterocycles (eq 2).
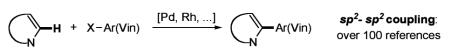 |
(1) |
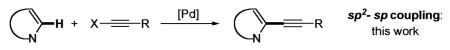 |
(2) |
It is well-established that species i serve as key electrophilic intermediates in Pd-catalyzed arylation/vinylation of electron-rich heterocycles.2 It is also known that the similar intermediate v, which forms upon oxidative addition of palladium into alkynylhalides, effectively serves as an electrophilic component in Stille9 and Suzuki10 cross-coupling reactions with the corresponding stannyl- and boryl- heterocycles. We hypothesized that, analogously to i, electrophilic species v may also undergo reaction with nonfunctionalized electron-rich heterocycles.11
 |
To test the above hypothesis, we examined a number of electron-rich N-fused heterocycles 1 in reaction with alkynyl halides 2 (eq 3). After certain optimization work, we found that 1, indeed, in the presence of 3 mol % of PdCl2(PPh3)2 and 2 equiv. of KOAc in toluene underwent smooth coupling reaction with bromoalkynes 2 (eq 3, Table 1). Remarkably, this direct alkynylation reaction appeared to be quite general with respect to the electron-rich N-fused13 heterocyclic core.14 Thus, unsubstituted- (entries 1–2) and ester-containing indolizines (entries 3–5), pyrrolo-isoquinoline (entries 6–10), densely substituted pyrroloxazole (entry 11), and pyrroloquinoline (entries 13–17) were smoothly alkynylated to give the corresponding alkynyl heterocycles 3 in good to very high yields. Notably, bis-pyrrolo-pyrimidine underwent double fold alkynylation with excess alkynyl bromide to furnish 3l in reasonable overall yield (entry 12). This alkynylation reaction also demon-strated a remarkable tolerance toward functional groups at the bromoalkyne 2.15 Indeed, bromoalkynes possessing alkyl, aryl, alkenyl, TMS, and ester groups, were nearly equally efficient in direct alkynylation (Table 1). It should be mentioned that, in contast to bromoalkynes, their chloro and iodo counterparts were much less efficient in alkynylation, providing only trace amounts of alkynylated indolizine 3c (entry 3) and quinoline 3m (entry 13).16
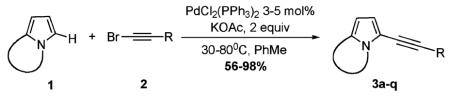 |
(3) |
Table 1.
Pd-catalyzed Alkynylation of N-Fused Heterocycles (eq 3)
| # | R | Product | Yield, %a |
|---|---|---|---|
| 1 | Ph |
|
51(59) |
| 2 | Si(CH3)3 |
|
62(71) |
| 3 | Ph |
|
76(97)b |
| 4 | nBu |
|
64(88) |
| 5 | Si(CH3)3 |
|
90(98) |
| 6 | Si(CH3)3 |
|
87(98) |
| 7 | Ph |
|
73(83) |
| 8 | nBu |
|
72(89) |
| 9 |
|
|
65(76) |
| 10 | CO2Et |
|
64(75) |
| 11 | Ph |
|
76(97) |
| 12 | Ph |
|
51(64) |
| 13 |
|
|
71(85)b |
| 14 | CO2Et |
|
63(74) |
| 15 | Si(Sh3)3 |
|
59(71) |
| 16 |
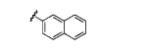
|
|
58(67) |
| 17 |
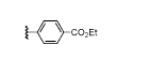
|
|
50(56)c |
Isolated yields, NMR yields are in brackets.
Trace amount of products were detected with iodo- and chloroalkynes (see Supporting Information for details).
Yield based on recovery of starting material.12
We propose that the direct Pd-catalyzed C–H alkynylation of electron-rich heterocycles operates via an electrophilic substitution pathway, analogous to that previously postulated for the palladium-(0)-catalyzed arylation of electron-rich heterocycles (Scheme 1).2 The mechanism involves a nucleophilic attack of the most electron-rich C-3 position of heterocycle 1 at alkynylpalladium intermediate v to form iminium intermediate 4. Deprotonation of the latter furnishes the PdII intermediate 5, which upon reductive elimination produces alkynyl heterocycle 3. The electrophilic nature of the process is supported by a minor kinetic isotope effect of 1.15 observed in alkynylation of the D-labeled indolizine 6 (eq 4).16 This KIE value is in the range of those reported by us2b and others2c,d in the Pd-catalyzed arylation of electron-rich heterocycles proceeding via an electrophilic pathway.17
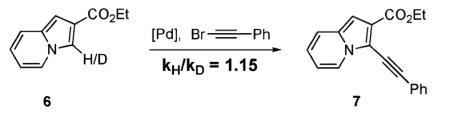 |
(4) |
Scheme 1.
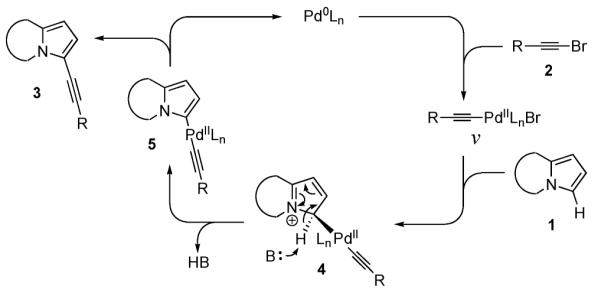
In summary, we developed a mild and effective method for the direct palladium-catalyzed C–H alkynylation of electron-rich heterocycles, including indolizine, pyrroloquinoline, pyrroloiso-quinoline, pyrrolooxazole, and bis-pyrrolo-pyrimidine. It was shown that a variety of functional groups at bromoalkyne, such as alkyl, alkenyl, aryl, silyl, and ester, were perfectly tolerated in this alkynylation reaction. This conceptually new method for sp2vsp carbonvcarbon bond-formation in heterocycles was proposed to proceed via electrophilic substitution motif.
Supplementary Material
Acknowledgment
The support of the National Institutes of Health (Grant GM-64444) is gratefully acknowledged.
Footnotes
Supporting Information Available: Preparative procedures, analytical and spectral data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).For recent reviews, see: Seregin IV, Gevorgyan V. Chem. Soc. Rev. 2007 doi: 10.1039/b606984n. published online. http://dx.doi.org/10.1039/b606984n. Alberico D, Scott ME, Lautens M. Chem. Rev. 2007;107:174. doi: 10.1021/cr0509760.
- (2).For mechanistic studies on Pd-catalyzed arylation of electron-rich heterocycles, see: Okazawa T, Satoh T, Miura M, Nomura M. J. Am. Chem. Soc. 2002;124:5286. doi: 10.1021/ja0259279. Park C-H, Ryabova V, Seregin IV, Sromek AW, Gevorgyan V. Org. Lett. 2004;6:1159. doi: 10.1021/ol049866q. Lane BS, Brown MA, Sames D. J. Am. Chem. Soc. 2005;127:8050. doi: 10.1021/ja043273t. Chiong HA, Daugulis O. Org. Lett. 2007;9:1449. doi: 10.1021/ol0702324.
- (3).For selected references on Pd-catalyzed arylation and vinylation of electron-rich heterocycles, see: Ohta A, Akita Y, Ohkuwa T, Chiba M, Fukunaga R, Miyafuji A, Nakata T, Tani N, Aoyagi Y. Heterocycles. 1990;31:1951. Desarbre E, Merour J-Y. Heterocycles. 1995;41:1987. Lavenot L, Gozzi C, Ilg K, Orlova I, Penalva V, Lemaire M. J. Organomet. Chem. 1998;567:49. Kondo Y, Komine T, Sakamoto T. Org. Lett. 2000;2:3111. doi: 10.1021/ol000183u. Glover B, Harvey KA, Liu B, Sharp MJ, Tymoschenko M. Org. Lett. 2003;5:301. doi: 10.1021/ol027266q. Mori A, Sekiguchi A, Masui K, Shimada T, Horie M, Osakada K, Kawamoto M, Ikeda T. J. Am. Chem. Soc. 2003;125:1700. doi: 10.1021/ja0289189. Li W, Nelson DP, Jensen MS, Hoerrner RS, Javadi GJ, Cai D, Larsen RD. Org. Lett. 2003;5:4835. doi: 10.1021/ol035878k. Beccalli EM, Broggini G, Martinelli M, Paladino G, Zoni C. Eur. J. Org. Chem. 2005:2091. Bressy C, Alberico D, Lautens M. J. Am. Chem. Soc. 2005;127:13148. doi: 10.1021/ja054472v.
- (4).For Pd-catalyzed arylation of electron-defficient heterocycles and simple arenes, see: Garcia-Cuadrado D, Braga AAC, Maseras F, Echavarren AM. J. Am. Chem. Soc. 2006;128:1066. doi: 10.1021/ja056165v. Lafrance M, Rowley CN, Woo TK, Fagnou K. J. Am. Chem. Soc. 2006;128:8754. doi: 10.1021/ja062509l. Campeau L-C, Rousseaux S, Fagnou K. J. Am. Chem. Soc. 2005;127:18020. doi: 10.1021/ja056800x. Leclerc J-P, Fagnou K. Angew. Chem., Int. Ed. 2006;45:7781. doi: 10.1002/anie.200602773.
- (5).For involvement of Pd(II/IV) catalytic cycle in direct C-H functionalizations, see for example: Giri R, Chen X, Yu J-Q. Angew. Chem., Int. Ed. 2005;44:2112. doi: 10.1002/anie.200462884. Zaitsev VG, Daugulis O. J. Am. Chem. Soc. 2005;127:4156. doi: 10.1021/ja050366h. Deprez NR, Kalyani D, Krause A, Sanford MS. J. Am. Chem. Soc. 2006;128:4972. doi: 10.1021/ja060809x.
- (6) (a).Lewis JC, Wu JY, Bergman RG, Ellman JA. Angew. Chem., Int. Ed. 2006;45:1589. doi: 10.1002/anie.200504289. [DOI] [PubMed] [Google Scholar]; (b) Wang X, Lane BS, Sames D. J. Am. Chem. Soc. 2005;127:4996. doi: 10.1021/ja050279p. [DOI] [PubMed] [Google Scholar]; (c) Yanagisawa S, Sudo T, Noyori R, Itami K. J. Am. Chem. Soc. 2006;128:11748. doi: 10.1021/ja064500p. [DOI] [PubMed] [Google Scholar]
- (7) (a).Liu C, Han X, Wang X, Widenhoefer RA. J. Am. Chem. Soc. 2004;126:3700. doi: 10.1021/ja031814t. [DOI] [PubMed] [Google Scholar]; (b) Furstner A, Mamane V, Seidel G, Laurich D. Org. Synth. 2006;83:103. [Google Scholar]
- (8) (a).Hashmi ASK, Schwarz L, Choi J-H, Frost TM. Angew. Chem., Int. Ed. 2000;39:2285. doi: 10.1002/1521-3773(20000703)39:13<2285::aid-anie2285>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]; (b) Li Z, Shi Z, He C. J. Organomet. Chem. 2005;690:5049. [Google Scholar]; (c) Nakamura I, Saito S, Yamamoto Y. J. Am. Chem. Soc. 2000;122:2661. [Google Scholar]
- (9) (a).Palmisano G, Santagnostino M. Synlett. 1993;10:771. [Google Scholar]; (b) Holling-worth GJ, Sweeney JB. Synlett. 1993;7:463. [Google Scholar]
- (10).Chan K-F, Wong HNC. Eur. J. Org. Chem. 2003;1:82. [Google Scholar]
- (11).For the Cu-catalyzed alkynylation of N–H bonds, see: Frederick MO, Mulder JA, Tracey MR, Hsung RP, Huang J, Kurtz KCM, Shen L, Douglas CJ. J. Am. Chem. Soc. 2003;125:2368. doi: 10.1021/ja021304j. Dunetz JR, Danheiser RL. Org. Lett. 2003;5:4011. doi: 10.1021/ol035647d.
- (12).N-fused pyrroloheterocycles are known to be among the most electron-rich heterocyclic cores. See, for example: Behnisch A, Behnisch P, Eggenweiler M, Wallenhorst T. Indolizine. Houben-Weyl. Vol. E6b/1, 2a. Thieme; Stuttgart, Germany: 1994. pp. 323–450.
- (13).Trial attempts with non-fused heterocycles, such as pyrroles, under these reaction conditions, afforded low yields of the corresponding alkynylation products. Further studies on the scope of this transformation are underway in our group.
- (14).For preparation of bromoalkynes, see Supporting Information.
- (15).See Supporting Information for details.
- (16).Carbon–carbon bond forming reactions proceeding via C-H activation motif usually exhibit much higher KIE. See, for example: Jones WD. Acc. Chem. Res. 2003;36:140. doi: 10.1021/ar020148i.
- (17).The reaction was stopped at 60% conversion to avoid thermal decomposition of the product.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


